Abstract
M. tuberculosis Antigen 85 enzymes are vital to the integrity of the highly impermeable cell-envelope and are potential therapeutic targets. Bi-substrate kinetic analysis using a label-free assay revealed both mechanistic details and a substrate profile that, in turn, allowed the design and construction of a selective in vitro mechanism-based inhibitor.
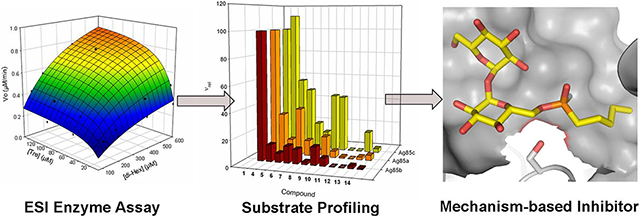
Graphical Abstract
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, is amongst the foremost causes of death and morbidity worldwide. It persists within the host with the aid of a complex and highly impermeable cell envelope containing a high content of long-chain fatty acids (mycolic acids) present as mycolate esters of arabino-galactan and of trehalose 1.1-3 Trehalose di- and mono-mycolates (TDM 2a and TMM 3a, respectively) are interconverted by the Antigen 85a,b and c (Ag85) mycolyltransferase isoforms (Figure 1).4-6 The Ag85 enzymes play a vital role in the construction and maintenance of the cell envelope.7 The Ag85 enzymes have recently been shown to display plasticity for substrates based upon the trehalose motif.8 Genetic ‘knockouts’ highlight Ag85c as the most active isoform; loss causes a 40% decrease in mycolate content of the cell wall.9 These enzymes are therefore suggested targets for novel anti-mycobacterial drugs,7 the development of which would benefit from a rapid and accurate assay of Ag85 activity and a thorough understanding of enzyme mechanism through the development of potential probes. However, despite the availability of several crystal structures and KM estimates measured under pseudo-single substrate conditions or with heterogeneous substrates, no detailed kinetic analysis or structure-activity relationship (SAR) studies have been reported. Current assays include non-specific radiometric procedures for the detection of generic mycolyltransferase activity and coupled enzymatic assays for Ag85c.4-6 Such methods preclude the possibility of readily automated screening or do not allow complete dissection of the steps of Ag85 acyltransfer, respectively. We report here the implementation of a label-free assay that allows full kinetic analysis as well as the potential for moderate-throughput screening; a panel of representative monosaccharides were used to map configurational structure-activity relationships. Together these data allowed the design of a mechanism-based inhibitor probe of Ag85c.
Figure 1.
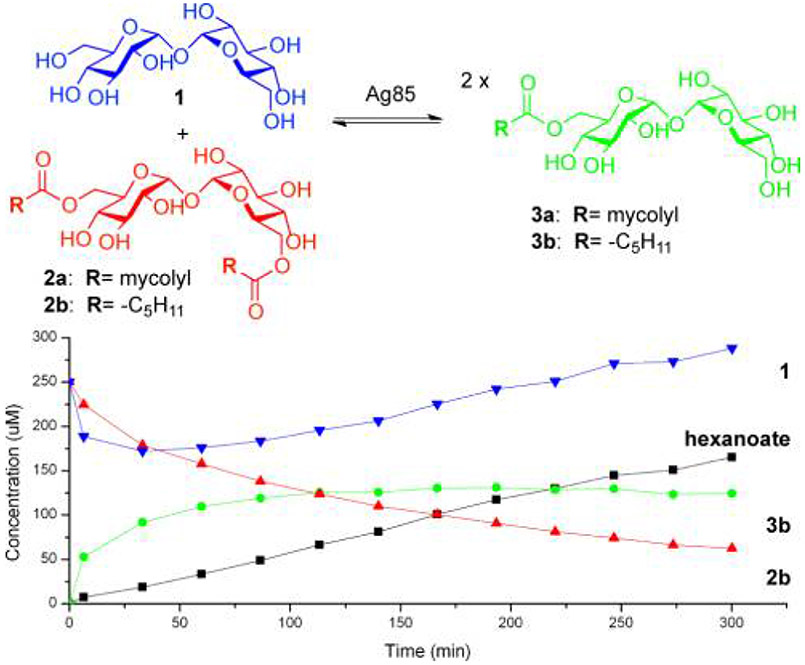
The reaction catalysed by Ag85, and a single typical time-course at high enzyme concentration. Conditions: 250 μM 1, 250 μM 2b, 2 μM Ag85c, 1 mM TEA buffer (pH 7.2), 37 °C. Full kinetics analysis utilized the full range of conditions [Tre] = 5-125 μM, [2b] = 25-600 μM, [Ag85c] = 10 nM.
Mass spectrometry (MS) is a potentially powerful enzyme assay method since unnatural substrate features/labels such as chromo/fluorophores are not necessary, it can be readily automated, and multiple natural substrates and products can be monitored simultaneously to give an unimpeded view of kinetic processes.10-13 Kinetic analysis and substrate/inhibitor profiling were enabled by the development of a precise and quantitative MS assay of Ag85 activity over time based on total ion counts (TICs) of ions corresponding to trehalose, 6,6’-di-hexanoyltrehalose 2b (TDH) and 6-hexanoyltrehalose 3b (TMH) (Figure 1). This exploited our previously described11 calibration approach with a pseudo-internal standard (here N-acetyl-D-glucosamine) injected concurrently with the sample aliquot; calibration plots for each monitored mass allowed quantitative measurements of concentration.
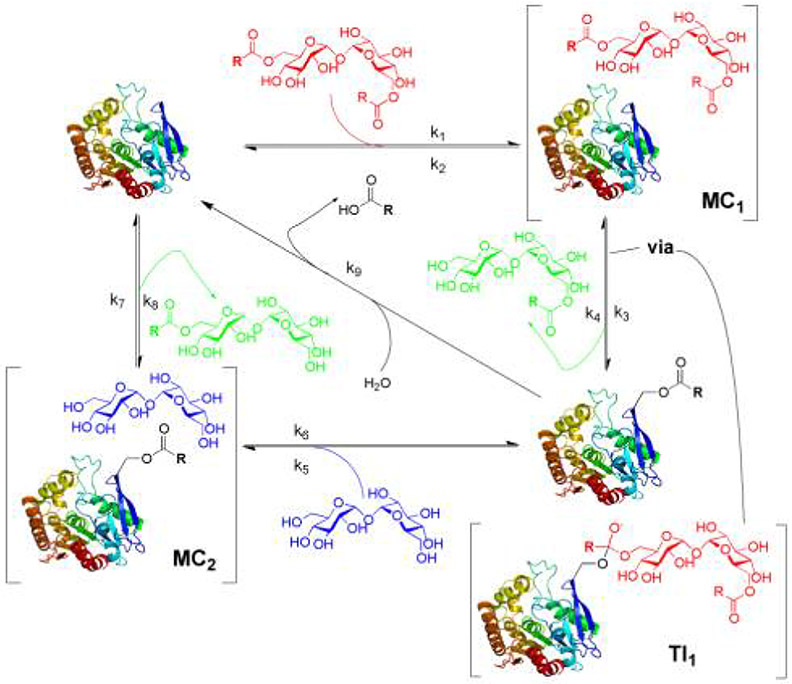
Full bisubstrate kinetic analysis was performed by determining rates of product formation varying the concentrations of both substrates (1 and 2b). Initial rates of TMH 3b formation were measured at varied TDH 2b and trehalose 1 concentrations.10-13 These data were fitted by non-linear regression methods to a range of plausible kinetic models, including modified rate equations that account for multiple acyltransfer pathways.14 Together these and double-reciprocal analysis (see SI) ruled out simple (e.g., bi-bi ping-pong) mechanisms15,16 that are typical of other acyltransferases. Nonetheless, crystal structure data show a clear Ser-His-Asp catalytic triad at the trehalose binding site of Ag85,17 the serine of which has been mutated and non-specifically modified suggesting a role as a nucleophile;18 it has also been reported that Ag85 can form a mycolyl-enzyme complex.6
Determination of the full kinetic parameters for Ag85c (Table 1) including KM values for both substrates and separate maximal reaction velocities for acyl-transfer (Vt) and hydrolysis (Vh), resolved this apparent conundrum and revealed considerable acyl-hydrolase activity (kcat 0.49s−1) in contrast with prior reports.6,19 We reasoned that this previously unreported activity of Ag85 was likely enzyme-mediated.20 Indeed, analysis using a suitably modified ping-pong cycle gave an excellent correlation with the data (R2>0.99). The implied mechanism (Figure 2) is therefore one of ping-pong formation of a hydrolytically unstable acyl-enzyme intermediate that then releases catalyst through two competitive pathways. Although rare, such mechanisms are not entirely unprecedented.21,22,23 This insight informed later inhibitor design (vide infra).
Table 1.
Bi-substrate kinetic analysis of Ag85c.
| Vt (μM/min) | 0.70 ± 0.05 | kcat (acyl-transfer) (s−1) | 1.16 |
| Vh (μM/min) | 0.29 ±0.08 | kcat (hydrolysis) (s−1) | 0.49 |
| KM(TDH) (μM) | 266.8 ±41.2 | kcat/KM(TDH) (M−1s−1) | 4.35x103 |
| K M(Tre) (μM) | 16.9 ±5.3 | kcat/KM(Tre) (M−1s−1) | 6.88x104 |
| Ki(TDH) (μM) | 355.3 ±105.6 |
Figure 2.
Mechanistic outline for Ag85.
This precise dissection of different activities highlighted a strong advantage compared with, for example single-substrate coupled assays that survey only one part of a given mechanistic cycle.19 The value for KM(Tre) correlated well with the only previous value of 8.3αM, determined under pseudo-single-substrate conditions.6 A higher KM value determined for TDH may reflect the homogenous, shorter lipid chain of 2b compared with heterogenous mycolic acid substrates. The relative acyltransferase activities of the three Ag85 isoforms were also determined under pseudo-single substrate conditions by monitoring the production of both TMH and hexanoate concurrently (Table 2), and confirmed the higher activity of Ag85c compared to Ag85a or Ag85b.24
Table 2.
Kinetic parameters for Ag85 isoforms recorded under pseudo-single substrate conditions.
| Ag85 Isoform |
Varied Substratea |
KM(app) (μM) |
kcat(app) (s−1) |
kcat(app)/KM(app) (M−1s 1) |
|---|---|---|---|---|
| a | 1 | 175 | 0.014 | 82 |
| b | 1 | 112 | 0.003 | 30 |
| c | 1 | 62 | 0.182 | 2952 |
Assay conditions: [TDH]=500 μM, [trehalose]=10-250 μM, [Ag85a/b]=500 nM, [Ag85c]=50 nM.
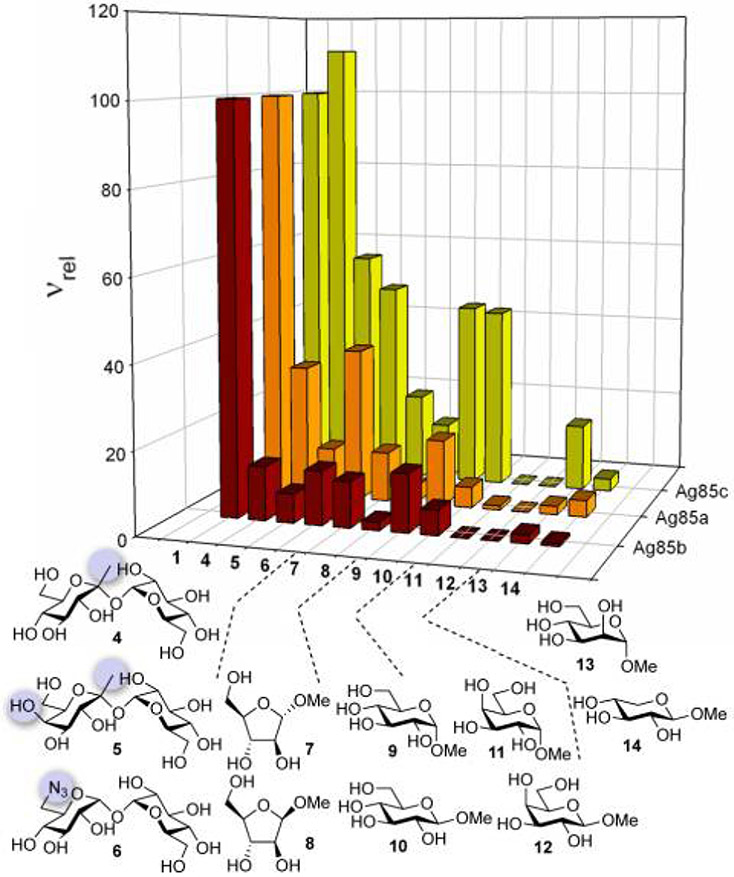
With an accurate automated assay of Ag85 activity in place we then probed substrate selectivity of the enzymes using a representative panel of monosaccharide substrates. These were chosen to evaluate the importance of the configuration of the sugar ring for substrate selectivity. We have previously shown the utility of a semi-quantititative green-amber-red (GAR) moderate-throughput screen to discover novel substrates for other bisubstrate (glycosyltransferase) enzymes.10-13 Relative reaction velocity (νrel) was determined by mass spectrometry-based GAR assay for a panel of putative substrates (Figure 3, Table S1) to evaluate their suitability as acyl acceptors. Both trehalose (1) itself and related disaccharides 4 and 5 were found to act as substrates,8 although the measured νrel for 5 was lower for Ag85a and b indicating the sensitivity of the enzymes to altering of this scaffold configuration away from that found in trehalose (here through inversion at C-4). Interestingly, 6, a known inhibitor of mycobacterial growth and previously described as an in vitro inhibitor of Ag85c activity is also a substrate for all Ag85 isoforms indicating that it acts as a competitive substrate and suggesting that some (or all) of the observed antibacterial activity may arise from downstream events after Ag85-mediated mycolyltransfer or through inhibition of other pathways.4,25
Figure 3.
a,b Substrate profiling screen and related compound structures. a: Assay conditions: 500 μM TDH, 500 μM screen compound, 2 μM Ag85 incubated at 37°C for 160 min before measurement of ν (product/substrate peak ratio). b: νrel = 100×(ν/νTre). See SI for Table
Despite this observed partial plasticity for variants of the trehalose scaffold, truncated or more dramatically altered sugar variants displayed considerably lower activity. Although trehalose is a 1,1-diglucoside, methyl D-glucosides 9 and 10 (which could be considered ‘hemi-trehaloses’) were utilised less efficiently than the disaccharides. This suggests that while some lower level turnover may occur, the enzymes all make valuable contacts with the second pyranose ring of trehalose during catalysis. Interestingly, some low level activity (up to ~20%) was also observed for the arabinofuranosides 7 and 8; this is consistent with a possible role of Ag85 in mycolate scrambling and mycolation ofMtb arabinogalactan. Notably, for these glucosides and arabinosides differential anomeric selectivity between enzyme isoforms was observed: α-anomers were preferred by Ag85a and b, with Ag85c showing little or no anomer discrimination. In keeping with this observation of tolerance at the anomeric centre, Ag85c also showed the greatest tolerance towards ketosides 4 and 5, which carry an additional methyl substituent at C-1. As for the disaccharides, configurational changes had a more dramatic effect. The very low νrel value determined for D-galactosides 11 and 12 for all three Ag85 isoforms is clear additional evidence that the epimeric configuration at C-4 is a crucial selectivity determinant. Con-versely, α-methyl mannoside 13 was slowly utilised by Ag85c indicating some degree of flexibility with respect to C-2 configuration. As expected, xyloside 14, which lacks the exocyclic hydroxymethyl of D-glucose was also poorly processed confirming that the majority of acyltransfer is OH-6. These differences in substrate recognition stand in contrast to the nearly perfectly conserved active site of these three isoforms and suggest that the enzymes may well have distinct physiological roles.18 Distal sequence variations may therefore not be simply related to immune evasion as has been previously proposed. Together these data suggested critical constitutional and configurational sugar substrate features that could be used to design a recognition motif in a probe of Ag85 or even probes that distinguished between isoforms of Ag85.
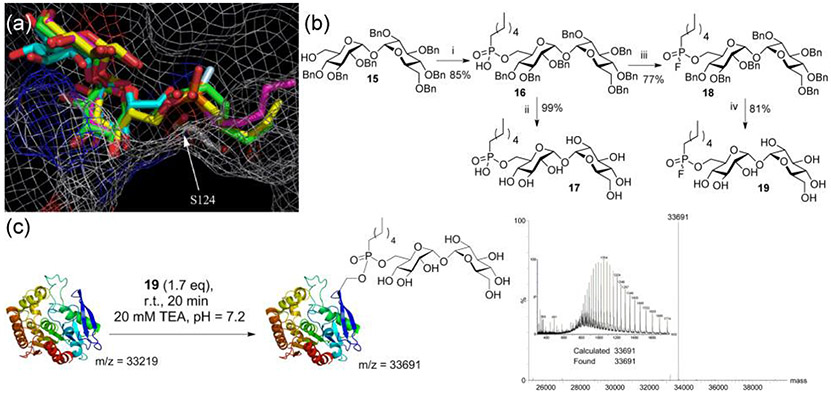
With this understanding of the substrate tolerances of the Ag85 enzymes, we sought to rationally design an effective covalent inhibitor probe. Although non-specific approaches based simply on serine modification might be considered,18 the true mechanism-based utility of this probe would be vitally dependent on high selectivity. Design was therefore essentially informed by both the unusual mechanism that we had delineated (Figure 2) as well as by the likely structures revealed by the substrate screen that would enable selectivity. These dictated an inhibitor structure that would operate by mimicking the tetrahedral intermediate (TI) formed in the first unified ‘ping-to-pong’ acylation steps rather than through intervention in the divergent pathways for catalyst regeneration by deacylation (Figure 2). Fluorophosphonate 19 (Scheme 1) was therefore based upon these key findings: a) targeting the TI of acylation (TI1) through the in situ formation of a tetrahedral mimic (Figure 2);28 b) targeting of the same TI by ensuring features that also mimic both lipid and sugar moieties (Figure 2); c) substrate preferences that highlight trehalose as a superior sugar scaffold (Figure 3). In silico evaluation supported another aspect of design with these features: molecular docking study using AutoDock Vina27 employing a rigid protein and flexible ligands, was performed to compare the binding modes that would correspond to putative Michaelis complexes (Figure 2, MC1 & Figure 4a). Lowest energy conformations of 19 (−7.3 kcal mol−1) overlaid closely with both TMH 2b (−7.0 kcal mol−1) and also with trehalose (observed in the crystal structure of Ag85b, pdb: 1F0P17), placing the acyl carbon (in 2b) and phosphoryl phosphorus (in 19) in close proximity to the targeted active site serine 124.
Figure 4.
Synthesis and evaluation of probe 19. (a) Lowest energy binding poses in the active site of Ag85c of trehalose 1 (cyan), TMH 2b (green), 17 (yellow) and 19 (magenta). The active site serine is shown with an arrow. Image produced using PyMol26 and docking poses generated with AutoDock Vina27 from pdb 1DQZ (b) Synthesis of inhibitors 17 and 19. Reagents and conditions: i) hexyldichlorophosphate, pyridine, rt, 16h; ii) 1:1 EtOH/H2O, Pd-C, H2, rt, 18h; iii) BTFFH, DIPEA, DMF, rt, 18h; iv) 1:1 EtOH/H2O, Pd-C, H2, rt, 18h. (c) Reaction of probe 19 with Ag85c indicates essentially complete conversion to covalently modified Ser124 adduct by ES-MS.
19 was readily synthesised from hepta-benzyl-trehalose 158 (Figure 4b). Direct fluorination of 16 or 17 with DAST failed, yielding a complex mixture of products. However, treatment of 16 with fluoro-N,N,N',N'-bis(tetramethylene)formamidinium hexafluorophosphate (BTFFH) afforded 18 in good yield; deprotection by catalytic hydrogenolysis gave 19 with no concomitant hydrolysis of the phosphorylfluoride.
The inhibitory potential of 19 against Ag85c was assayed using a saturating concentration of trehalose (> 25× KM) to ensure maximal turnover rate. Following incubation of Ag85c with 19 at a ratio of just 1:1.3, respectively, activity was almost entirely ablated (<7%). Phosphonate 17, an unreactive analog of 19 that cannot effectively mimic the targeted TI but has comparable sugar and lipid moieties resulted in inhibition of Ag85c only to 46% of the maximal rate.29 The kinetics of inactivation were investigated. Over the measurable concentration range, the second order rate constant kobs/Ki was determined to be 1420 (±90) min−1mM−1 (see SI); the reactivity of 19 is such that saturation could not be achieved, as has been reported for other covalent inhibitors.30 This marks 19 as a potent inhibitor based upon comparison with other fluorophosphonates.3132With inhibitory potential confirmed, critical selectivity of 19 for Ag85 over other enzymes that contain similar catalytic triads was investigated. Serine protease subtilisin from Bacillus lentus (SBL), lipase B from Candida antarctica (CalB) and Ag85c were incubated under identical conditions with 19. Detailed analysis by mass spectrometry revealed essentially complete protein modification of Ag85c at Ser124 (Figure 4c and SI), while no detectable modification or inhibition of either SBL or CalB was observed, indicating a very high selectivity.
In conclusion, mass spectrometry has provided an accurate and automated method to determine the full kinetic parameters of Ag85c, which revealed that the enzyme displays a combination of acyltransferase and acylhydrolase activities. The use of this assay method in an automated substrate profiling study has revealed that although the Ag85 enzymes show promiscuity for trehalose-based substrates,8 selectivity for differing monosaccharide and anomeric configurations is marked. Qualitative SAR investigations have revealed that glucopyranose and arabinofuranose configurations are preferred over galacto-, manno- or xylopyranose. These data provide additional evidence that Ag85 is responsible for both trehalose-mycolate scrambling and mycolylation of the Mycobacterial arabinogalactan. They also suggest that regions distal to the active sites influence activity of the three isoforms since they show differential selectivity but have near-identical active sites. The screen data indicated importantly that disaccharides are better substrates than the constituent monosaccharides, which suggests an extended active site. This in turn suggests that the optimal starting point for rational drug design may be the trehalose scaffold that was used here to design a tailored fluorophosphonate 19. This compound was a potent and highly selective covalent inhibitor probe of Ag85c that shows no reactivity towards other serine-acyltransferases using related but critically different mechanisms. The selectivity of this molecule may, for example, allow interrogation of the importance of Ag85 activity in vitro or in infected macrophages. We anticipate that this and related ‘tagged’ compounds may find use as activity-based probes33 of Ag85 function and might serve as a starting point for the design of novel anti-Mycobacterial drugs.
Supplementary Material
Acknowledgement.
This work was funded by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Disease (C.E.B.), the Rhodes Trust (K.M.B.), the Biotechnology and Biological Sciences Research Council (C.S.B.) and the Bill and Melinda Gates Foundation through the TB Drug Accelerator Program (C.E.B, B.G.D.). B.G.D. is a Royal Society Wolfson Research Merit Award recipient. We thank Colorado State "TB Vaccine Testing and Research Materials" contract for providing Antigen 85 plasmids.
Footnotes
Supporting Information. Experimental methods and supporting figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References.
- (1).Brennan PJ; Nikaido H Annu. Rev. Biochem 1995, 64, 29–63. [DOI] [PubMed] [Google Scholar]
- (2).Draper P; Daffe M In Tuberculosis and the Tubercle Bacillus; ASM Press, Washington DC: 2005. [Google Scholar]
- (3).Mills JA; Motichka K; Jucker M; Wu HP; Uhlik BC; Stern RJ; Scherman MS; Vissa VD; Pan F; Kundu M; Ma YF; McNeil MJ Biol. Chem 2004, 279, 43540–43546. [DOI] [PubMed] [Google Scholar]
- (4).Belisle JT; Vissa VD; Sievert T; Takayama K; Brennan PJ; Besra GS Science 1997, 276, 1420–1422. [DOI] [PubMed] [Google Scholar]
- (5).Kilburn JO; Takayama K; Armstrong EL Biochem. Bio-phys. Res. Commun 1982, 108, 132–139. [DOI] [PubMed] [Google Scholar]
- (6).Sathyamoorthy N; Takayama KJ Biol. Chem 1987, 262, 13417–13423. [PubMed] [Google Scholar]
- (7).Chatterjee D. Curr. Opin. Chem. Biol 1997, 1, 579–588. [DOI] [PubMed] [Google Scholar]
- (8).Backus KM; Boshoff HI; Barry CS; Boutureira O; Patel MK; D’Hooge F; Lee SS; Via LE; Tahlan K; Barry CE III; Davis BG Nature Chem. Biol 2011, 7, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Jackson M; Raynaud C; Laneelle MA; Guilhot C; Laurent-Winter C; Ensergueix D; Gicquel B; Daffe M Molec. Microbiol 1999, 31, 1573–1587. [DOI] [PubMed] [Google Scholar]
- (10).Flint J; Taylor E; Yang M; Bolam DN; Tailford LE; Martinez-Fleites C; Dodson EJ; Davis BG; Gilbert HJ; Davies GJ Nature Struct. Mol. Biol 2005, 12, 608–614. [DOI] [PubMed] [Google Scholar]
- (11).Yang M; Brazier M; Edwards R; Davis BG Chem Bio-Chem 2005, 6, 346–357. [DOI] [PubMed] [Google Scholar]
- (12).Yang M; Davies GJ; Davis BG Angew. Chem., Intl. Ed 2007, 46, 3885–3888. [DOI] [PubMed] [Google Scholar]
- (13).Yang M; Proctor MR; Bolam DN; Errey JC; Field RA; Gilbert HJ; Davis BG J. Am. Chem. Soc 2005, 127, 9336–9337. [DOI] [PubMed] [Google Scholar]
- (14).See supporting information for derivation.
- (15).See supporting information for details of double reciprocal analysis of Ag85c.
- (16).Cook PF; Cleland WW Enzyme Kinetics and Mechanism; Garland Science, London; Abingdon, 2007. [Google Scholar]
- (17).Anderson DH; Harth G; Horwitz MA; Eisenberg DJ Mol. Biol 2001, 307, 671–681. [DOI] [PubMed] [Google Scholar]
- (18).Ronning DR; Klabunde T; Besra GS; Vissa VD; Belisle JT; Sacchettini JC Nature Struct. Biol 2000, 7, 141–146. [DOI] [PubMed] [Google Scholar]
- (19).Boucau J; Sanki AK; Voss BJ; Sucheck SJ; Ronning DR Anal. Biochem 2009, 385, 120–127. [DOI] [PubMed] [Google Scholar]
- (20).Cook PF; Tai CH; Hwang CC; Woehl EU; Dunn MF; Schnackerz KD J. Biol. Chem 1996, 271, 25842–25849. [DOI] [PubMed] [Google Scholar]
- (21).Brown AJ; Snyder FJ Biol. Chem 1982, 257, 8835–8839. [PubMed] [Google Scholar]
- (22).Casals C; Acebal C; Arche R Int. J. Biochem 1984, 16, 773–778. [DOI] [PubMed] [Google Scholar]
- (23).The divergent deacylation pathways may also be related to the nature/identity of the fatty acyl chain.
- (24).νtransfer = (νTMH – νhydrolysis)/2
- (25).Kalscheuer R; Syson K; Veeraraghavan U; Weinrick B; Biermann KE; Liu Z; Sacchettini JC; Besra G; Bornemann S; Jacobs WR Nature Chem. Biol 2010, 6, 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).The PyMOL Molecular Graphics System, Version 0.99rc6, Schródinger, LLC, (http://pymol.org/pymol). [Google Scholar]
- (27).Trott O; Olson AJ J. Comput. Chem 2010, 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bachovchin DA; Ji TY; Li WW; Simon GM; Blankman JL; Adibekian A; Hoover H; Niessen S; Cravatt BF Proc. Natl Acad. Sci, U.S.A 2010, 107, 20941–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).See supporting information for details.
- (30).McCarter JD; Withers SG J. Am. Chem. Soc 1996, 118, 241–242. [Google Scholar]
- (31).Diisopropylfluorophosphonate and soman have kobs/Ki values of 140 and 9200 min−1 mM−1 respectively against human acetylcholine esterase.
- (32).Worek F; Thiermann H; Szinicz L; Eyer P Biochem. Pharamacol 2004, 68, 2237–2248. [DOI] [PubMed] [Google Scholar]
- (33).Evans MJ; Cravatt BF Chem. Rev 2006, 106, 3279–3301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.