SUMMARY
Introduction
Intranasal corticosteroids (INCs) are the first line of therapy for chronic sinonasal conditions such as rhinitis and rhinosinusitis. Among these, one of the most frequently used is beclomethasone dipropionate (BDP). Over the years many studies have evaluated the efficacy of BDP as part of therapy for chronic rhinosinusitis (CRS) and allergic rhinitis (AR) along with nasal washes, which seems to be very well tolerated.
Objective
To analyse the data in the literature regarding the various therapeutic regimens of BDP in different sinonasal disease and their efficacy and tolerability.
Materials and methods
Using different search engines, the posology, efficacy, and tolerability of BDP were reviewed and a total of 64 full-length articles were examined for eligibility. After applying inclusion and exclusion criteria, 4 articles were reviewed.
Results
BDP is among the group of INCs with significant improvement of nasal symptoms and has good efficacy and safety.
Conclusions
BDP nasal spray is one of the most frequently prescribed INC for rhinitis and rhinosinusitis. Treatment with BDP resulted in significant and clinically meaningful improvements in nasal symptoms associated with AR and CRS. BDP is well tolerated, and the safety profile is similar to that of placebo in most patients. These results, in conjunction with the significant benefit reported in subjects with CRS and AR, provide convincing evidence of the overall effectiveness of BDP for the treatment of the full spectrum of sinonasal disease.
KEY WORDS: topical nasal steroids, chronic rhinosinusitis, rhinitis, allergic rhinitis, beclomethasone dipropionate
Introduction
Allergic rhinitis (AR) is a common chronic inflammatory disease, and its prevalence is increasing worldwide in both adults and children 1. The most common symptoms are sneezing, nasal itch, rhinorrhea, nasal obstruction, and allergic conjunctivitis. There are different types of rhinitis: allergic, nonallergic, mixed, or episodic. The most appropriate treatment for rhinitis is determined by multiple factors such as the most prominent symptoms, severity, and age of the patient 2. If left untreated, AR along with rhinosinusitis may significantly affect the quality of life due to severe sleep disorders, fatigue, impaired memory and in some cases depression. There are many therapies used to treat AR and rhinosinusitis that focus on reducing airway inflammation and improving symptom control, ranging from simple nasal washes to steroids.
Under current guidelines, intranasal corticosteroids (INCs) are considered the most effective drugs, and are recommended as first-line therapy. Numerous studies have shown their effectiveness in treating allergic/non-allergic rhinitis, acute rhinosinusitis, chronic rhinosinusitis with nasal polyposis, chronic rhinosinusitis without polyposis and adenoid hypertrophy 3.
The main parameter used to assess sinonasal symptoms in AR is the Total Nasal Symptom Score (TNSS), which is derived from the sum of other marks for each of nasal congestion, sneezing, nasal itching, and rhinorrhea at each time point, using a four point scale (0-3), where a score of 0 indicates no symptoms, 1 mild symptoms that are easily tolerated, 2 awareness of symptoms which are bothersome but tolerable, and 3 is reserved for severe symptoms that are hard to tolerate and interfere with daily activity.
Another important score used in clinical practice is SNOT-22, which captures symptom severity, social and emotional impact, productivity, and sleep consequences of CRS. Items are scored from 0 (no problem) to 5 (problem as bad as it can be) and summed to form a total score of 0 to 110. The main parameter used to assess sinonasal symptoms in CRS is the ability to undergo multiple formulations, such as nasal sprays, aerosols, dry powder inhalers, and ointments that can deliver a powerful, localised anti-inflammatory effect. The intranasal administration of drugs has been increasingly widespread in recent years, both due to the availability of molecules with specific activity on the airways and the numerous technological innovations that have increased the efficiency of devices accessible in clinical practice. Among the several corticosteroid intranasal sprays, beclomethasone dipropionate (BDP) is one of the most widely prescribed and represents a possible “first line of treatment”, since this molecule has an excellent efficacy and safety profile with decades of experience. The mechanism of action is a combination of anti-inflammatory effects (by reducing pro-inflammatory gene transcription and increasing anti-inflammatory gene transcription and reducing airway inflammatory cell infiltration) and suppression of the production of pro-inflammatory mediators, cell chemotactic factors and adhesion molecules 4. Patients with nasal chronic inflammatory diseases often require long-term strategies to control symptoms: the nasal absorption from BDP improves local long-time efficacy but although the safety profiles of INCs are well established, there is some concern regarding the potential for systemic complications associated with long-term treatment. These concerns are based primarily on the systemic adverse events (AEs) that have been reported with oral and high-dose inhalation corticosteroids, including growth inhibition and hypothalamic-pituitary-adrenal (HPA) axis suppression. Nevertheless, the potential drug interaction risk of BDP is low since the drug has limited systemic bioavailability 5. The success of inflammatory disease management with intranasal medications depends on the activity of the drug and its pharmacokinetic and pharmacodynamic properties 2. For INCs, these AEs are certainly less frequent and less serious than those observed with oral steroids, but can considerably limit adherence to treatment, especially in paediatric patients, adolescents, and the elderly. The recommendation issued by the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency states that BDP has a better profile, compared with all corticosteroids, with respect to the risk of drug interactions and subsequent adrenal repercussions (Cushing’s syndrome and HPA axis suppression), which has resulted in changes to the Summary of Product Characteristics 6. With regards to the safety profile, it has been demonstrated 7 that BDP is well tolerated at the level of the nasal mucosa 8. The potential risk of drug interaction of BDP is low as the drug has limited systemic bioavailability: this has been confirmed by demonstrating lower systemic exposure with intranasal administration than with oral inhalation 2,9. Glucocorticoids remain the most effective anti-inflammatory drugs available for the treatment of allergic diseases and it has been difficult to find other therapies that are near as effective. The use of combination of INCs and topical antihistamine medications has not revealed any new safety issues. Use of INCs with topical decongestants has some limited effects of tachyphylaxis and rebound congestion 9. To date, no one has carried out a literature review regarding the duration and dosage of BDP therapy: our review aims to summarise all the publications that have dealt with this topic to understand the current state of the literature and to help determine the optimal dose of BDP and the best administration regimen for nasal disease.
Materials and methods
PubMed was searched for articles written exclusively in English, including randomised clinical trials, cohort studies, meta-analyses, case reports, and case series about efficacy and safety, duration and dosage of BDP therapy, and excluding articles about BDP hydrofluoroalkane (HFA) and articles about asthma.
Search criteria included all occurrences of the following terms in the title or abstract: BDP, intranasal corticosteroid, nasal spray, aerosol, efficacy, safety, once-daily, chronic rhinosinusitis, allergic rhinitis and randomised controlled trial. The corresponding results in the literature starting from 2010 were examined for admissibility and 64 articles were identified: 16 were assessed for eligibility. Finally, after applying the above-mentioned inclusion/exclusion criteria, 4 publications were analysed (Fig. 1) 4,10-12,13.
Figure 1.
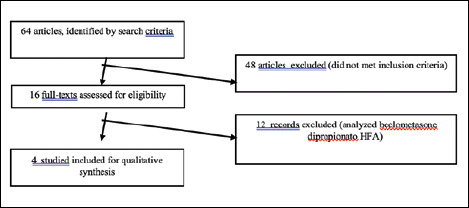
Process of selection of publications.
Results
The characteristics of the four articles included are shown in Table I. Two of the articles analysed were multicentric studies and the other two single-center studies. Three were randomised, double-blind, placebo controlled studies and one was a randomised, double-blind, clinical study. Sample sizes varied between 50 to 126. All studies included patients of both genders older than 12 years.
Table I.
Summary of the most significant studies of BPD nasal spray or aerosol in patients with sinonasal disease.
| Reference | Study design | Single centre or multicentric study | Patient’s population sample | Patient disease | Pharmaceutical form | Frequency daily | Daily dose (microgram/day) | Weeks of treatment | Parameters improved with treatment (parameters improved/total parameter of the score, p value) |
|---|---|---|---|---|---|---|---|---|---|
| Sonnemann et al., 2014 10 | Randomised, double blind, control, placebo study | Multicentre | 50 | AR | Nasal spray | 2 | 320 | 2 | TNSS (4/4, p < 0.001) |
| Weinstein et al., 2014 11 | Randomised, double blind, control, placebo study | Multicentre | 126 | AR | Aerosol | 1 | 320 | 52 | QoL (1/14, p = 0.008) |
| TNSS (4/4, p < 0.001) | |||||||||
| Chong et al., 2016 4 | Review of randomised, double blind, control, placebol trials | Single centre | 55 | CRS | Nasal spray | 2 | 400 | 26 | PNSS (4/4, p = 0.008) |
| Days on which patients required no rescue medication (p .009) | |||||||||
| Rezaeian et al., 2021 12 | Randomised, double blind, clinical trial | Single centre | 60 | CRS | Nasal spray Aerosol | 1 | 200 | 8 | Higher percentage of days with an overall nasal blockage score on waking minor of 2 (p .013) |
| Peak nasal inspiratory flow measurements (p < 0.05) | |||||||||
| - SNOT22 (p < 0.05) | |||||||||
| - Lund-Mackay score (p < 0.05) | |||||||||
| AR: allergic rhinitis; CRS: chronic rhinosinusitis; TNSS: total nasal symptoms score (nasal congestion, sneezing, nasal itching, and rhinorrhoea); PNSS: physicians nasal symptoms score. | |||||||||
Regarding treatment protocol, the total drug dose ranged between 200 μg/day to 400 μg/day administered twice daily in all studies. Two of the four studies analysed patients on BDP therapy in nasal spray formulation, one study aerosol only, and one compared aerosol and nasal spray.
In all studies, the duration of treatment ranged from 2 to 52 weeks. Two articles referred to patients with AR and two articles to patients with CRS.
The study by Sonnemann et al. found significant improvement in the TNSS of patients treated with BDP nasal spray (p < 0.001). Furthermore, QoL was evaluated with a questionnaire in which the frequency of brushing of nose parameter varied in a statistically significant way after treatment (p = 0.008), while the other 13 parameters improved but not significantly. Those parameters were: frequency of handkerchief use (p = 0.568), rubbing eyes and nose (p = 0.999), bad sleep (p = 0.878), bad work performance (p = 0.328), fatigue (p = 0.690), thirst (p = 0.178), lack of concentration (p = 0.389), general well-being (p = 0.462), headache (p = 0.081), bad temper (p = 0.549), general disconcertment (p = 0.099), frustration (p = 0.195) and reactions of others to the allergy (p = 0.377).
At the end of the study, patients assessed both efficacy and tolerability with a score of 0 (no efficacy, bad to tolerate), 1 (moderate efficacy, moderate tolerability), 2 (good efficacy, good tolerability), and 3 (very good efficacy, very good tolerability): the mean score values showed that the perception of efficacy and tolerability were good 10. The study by Weinstein et al considered TNSS as the efficacy parameter which showed improvement in nasal congestion, sneezing, nasal itching, and rhinorrhoea (p < 0.001) in patients treated with aerosol BDP compared to placebo. Furthermore, the Physician Nasal Symptom Score (PNSS), defined as the sum of the scores for four individual physician-assessed nasal symptoms obtained by questioning patients and with ear, nose, and throat examinations (using a 0-3 scale) was also improved in all 4 parameters (p = 0.008). QoL improved after 52 weeks of treatment, but was not statistically significant (p = 0.130). Regarding tolerability, the AEs related to aerosol therapy were epistaxis (10.6%), and it was concluded that the drug is safe and effective 1. The study by Chong et al. reported a significant improvement in days in which AR patients required no rescue medication (p = 0.009) and higher percentage of days with an overall “nasal blockage score on waking” of < 2 (p = 0.013). BDP nasal spray was effective based on the physician’s assessments of symptoms and polyp score at all clinic visits. In this study, safety and tolerability were measured indirectly by collecting AE data, showing that the only drug-related AE was epistaxis 4. The article by Rezaeian 12 found improvement from baseline in both groups comparing the basal BDP aerosol and spray scores of SNOT-22 and Lund-Mackay (LM) score (a radiological score assessing opacification of a paranasal sinus and the osteo-meatal complex). They were significantly decreased in the BDP-aerosol group compared with the BDP-nasal spray group (p < 0.05). It also compared the values of SNOT-22 and LM score before and after treatment with BDP aerosol and BDP nasal spray, finding a lower score in these two scores in both groups of patients, although not statistically significant (p > 0.05). However, comparing the values of LM score and SNOT-22 between the two groups of patients, it emerged that LM score was significantly lower in patients treated with BDP aerosol versus BDP nasal spray (p = 0.041) and that the changes of the LM and SNOT-22 scores in the BDP-aerosol group were higher than the BDP-nasal spray group (p < 0.05). Efficacy and tolerability were measured indirectly by collecting data relating to AEs, showing that only two patients receiving BDP aerosol complained of slight nasal obstruction and no AEs were recorded in the group of patients treated with BDP nasal spray 12.
Discussion
CRS and AR are very common pathologies which require chronic treatment and therapies that have good efficacy and safety. The first line treatment is nasal lavage combined with INCs. In the literature, studies have shown that the efficacy of different INCs is very similar 13. While this is true, there are subtle nuances that should be considered when selecting the most appropriate INC. It is therefore assumed that patients, considering their preferences, tend to change the way they use the INC spray based on sensory perceptions and efficacy. The prescription should be adapted according to bioavailability, the intranasal environment and factors influencing patient adherence. BDP has been shown to have good bioavailability and good tolerability in terms of sensory perception.
Dosage
Regarding the dosage of INC, there is no established consensus on the maximum daily dose and the number of administrations. Our review showed that any dose of BDP in the range analysed (200 μg/day to 400 μg/day administered twice daily) was well tolerated. As far as the number of daily administrations is concerned, two studies considered two administrations, and two only one administration. Since the outcomes are expressed in different ways and with different parameters, the possibilities for comparison are limited. However, comparing the two articles that used BDP at 320 microgram/day as an outcome parameter, both with one and two administrations, TNSS improved significantly.
A cycle of 20 days per month for 3 months can be repeated after 1 month interruption.
Efficacy, safety and tolerability
In the literature, the assessment of the safety profile and tolerability has usually included AEs. The efficacy is established by evaluating the improvement of the symptomatologic parameters. The studies we analysed showed that treatment with BDP is not associated with serious AEs and is characterised by good safety and tolerability.
As far as efficacy is concerned, the studies evaluated different outcomes expressed with different scores. The most widely used score was the TNSS which improved after therapy in all 4 of its subgroups: nasal congestion, sneezing, nasal itching, and rhinorrhoea. Chong et al. analysed 3 specific parameters: days on which patients required no rescue medication, higher percentage of days with an overall nasal blockage score on waking of less than 2 and peak nasal inspiratory flow measurements which improved after therapy 3.
Conclusions
All studies included in the present review showed significant improvement of nasal symptoms with both aerosol and spray BDP, which was effective and safe in all studies. This review has some limitations that did not allow us to establish what is the best dose or the ideal duration of treatment. To establish the total daily dose with the best efficacy profile and ideal duration of therapy with BDP, further studies are needed, possibly increasing the sample size, the follow-up time, and using comparable efficacy, quality of life and safety parameters (using the same symptom scores relating to different total daily doses). With a view to precision medicine, it would be useful to increase the number of pathologies analysed to allow the ideal dosage to be established for each patient, to render INC therapy usable for all while minimising side effects (although already minimal within the optimal range). In view of the fact that in AR and CRS nasal spray is the most widely used, future studies should focus on this formulation.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
The individual contributions of authors to the manuscript should be specified in this section: CZ: write the article; AG: elaborate the data; GM: reference research; AM: conclusions; PC: review the article; MB: review the article.
Ethical consideration
This study was approved by the Institutional Ethics Committee (Italian Academy of Rhinology 2132023, 2023-04-01).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Figures and tables
References
- 1.Bukstein D, Parikh R, Eid S, et al. Beclomethasone dipropionate nasal aerosol in patients with perennial allergic rhinitis (BALANCE) study: 6-month results. Allergy Asthma Proc 2016;37:121-130. https://doi.org/10.2500/aap.2016.37.3939 10.2500/aap.2016.37.3939 [DOI] [PubMed] [Google Scholar]
- 2.Ratner PH, Melchior A, Dunbar SA, et al. Pharmacokinetic profile of beclomethasone dipropionate hydrofluoroalkane after intranasal administration versus oral inhalation in healthy subjects: results of a single-dose, randomized, open-label, 3-period crossover study. Clin Ther 2012;34:1422-1431. https://doi.org/10.1016/j.clinthera.2012.04.023 10.1016/j.clinthera.2012.04.023 [DOI] [PubMed] [Google Scholar]
- 3.Fowler J, Rotenberg BW, Sowerby LJ, et al. The subtle nuances of intranasal corticosteroids. J Otolaryngol Head Neck Surg 2021;50:18. https://doi.org/10.1186/s40463-020-00480-z 10.1186/s40463-020-00480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong LY, Head K, Hopkins C, et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4:CD011996. https://doi.org/10.1002/14651858.CD011996.pub2 10.1002/14651858.CD011996.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger WE, Jacobs RL, Amar NJ, et al. Efficacy and safety of beclomethasone dipropionate nasal aerosol in children with perennial allergic rhinitis. Ann Allergy Asthma Immunol 2015;115:130-136. https://doi.org/10.1016/j.anai.2015.05.012 10.1016/j.anai.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 6.Pharmaco vigilance Risk Assessment Committee - PRAC (EMA/PRAC/700146/2016). [Google Scholar]
- 7.Klossek JM, Laliberté F, Laliberté MF, et al. Local safety of intranasal triamcinolone acetonide: clinical and histological aspects of nasal mucosa in the long-term treatment of perennial allergic rhinitis. Rhinology 2001;39:17-22. [PubMed] [Google Scholar]
- 8.Ciprandi G, Gelardi M. Trattamento delle riniti allergiche e vasomotorie: il ruolo del beclometasone dipropionato e dell’acido ialuronico (ad alto peso molecolare) [Treatment of allergic and vasomotor rhinitis: the role of beclomethasone dipropionate and hyaluronic acid (with high molecular weight)]. Recenti Prog Med 2018;109:E257-E265. https://doi.org/10.1701/2896.29199 10.1701/2896.29199 [DOI] [PubMed] [Google Scholar]
- 9.Gorica A, Zeroli C, Giorli A, et al. Aqueous nasal spray in treatment of rhinitis and rhinosinusitis: adverse event focusing on epistaxis. J Clin Res Med 2023;6:1-3. https://doi.org/10.31038/JCRM.2023612 10.31038/JCRM.2023612 [DOI] [Google Scholar]
- 10.Sonnemann U, Möller M, Bilstein A. Noninterventional open-label trial investigating the efficacy and safety of ectoine containing nasal spray in comparison with beclomethasone nasal spray in patients with allergic rhinitis. J Allergy (Cairo) 2014;2014:297203. https://doi.org/10.1155/2014/297203 10.1155/2014/297203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein SF, Andrews CP, Shah SR, et al. Long-term efficacy and safety of once-daily treatment with beclomethasone dipropionate nasal aerosol. Allergy Asthma Proc 2014;35:323-331. https://doi.org/10.2500/aap.2014.35.3767 10.2500/aap.2014.35.3767 [DOI] [PubMed] [Google Scholar]
- 12.Rezaeian A, Kargoshaei A, Rastegar Z, et al. A comparison of beclomethasone aqueous spray and aerosol delivery system in nasal polyps: a randomized control trial. Adv Biomed Res 2021;10:51. https://doi.org/10.4103/abr.abr_30_20 10.4103/abr.abr_30_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonnell J, Weller K, Pien LC. Safety of intranasal steroids: an updated perspective. Curr Allergy Asthma Rep 2020;20:69. https://doi.org/10.1007/s11882-020-00960-2 10.1007/s11882-020-00960-2 [DOI] [PubMed] [Google Scholar]


