Abstract
Intracerebral inoculation of susceptible strains of mice with Theiler’s murine encephalomyelitis virus (TMEV) results in immune-mediated demyelination. Three major T-cell epitopes have previously been identified within the VP1 (VP1233–250), VP2 (VP274–86), and VP3 (VP324–37) capsid proteins in virus-infected SJL/J mice. These epitopes appear to account for the majority (∼90%) of major histocompatibility complex class II-restricted T-cell responses to TMEV. Interestingly, the effect of immunization with synthetic peptides bearing the predominant T-cell epitopes on the course of TMEV-induced demyelination indicates that T cells reactive to the VP1 and VP2 epitopes, but not VP3, accelerate the pathogenesis of demyelination. The predominant pathogenic role of the T cells is verified by similar immunization with the fusion proteins containing the entire individual capsid proteins. The order of appearance and level of T cells specific for the individual epitopes during the course of demyelination are similar to each other. However, cytokine profiles of T cells from virus-infected mice indicate that T cells specific for the VP1 (and perhaps the VP2) epitope are Th1, whereas T cells reactive to VP3 are primarily Th2. These results suggest that Th1-type cells specific for VP1 and VP2 are involved in the pathogenesis of viral demyelination induced by TMEV. Thus, a predominance of Th1-inducing viral epitopes is likely critical for the pathogenesis of demyelination.
Although the etiology of multiple sclerosis remains unknown, epidemiological studies and investigations with experimental animal models have supported a potential role for viruses as the causative agent for demyelination (2, 10, 11). In particular, intracerebral (i.c.) injection of Theiler’s murine encephalomyelitis virus (TMEV) into susceptible strains of mice results in a chronic, progressive demyelinating disease that closely resembles human multiple sclerosis (24, 40). Demyelination induced by TMEV is associated with a persistent virus infection in the central nervous system (CNS) (4, 26, 38), and T-cell responses to viral antigens seem to play a critical role in the immunopathologic tissue damage induced after viral infection (8, 25). Demyelinating lesions are characterized by an inflammatory cell infiltrate consisting of predominantly T cells and macrophages (34), and the course of demyelinating disease correlates well with the development of a virus-specific, class II-restricted, delayed-type hypersensitivity response (8, 16). In addition, TMEV-induced demyelinating disease (TMEV- IDD) is inhibited after induction of virus-specific tolerance which down-regulates Th1-type responses (19, 32), further supporting a critical role for Th1 cells in the pathogenesis of demyelination. However, it is not clear how TMEV containing multiple T-cell epitopes can determine the overall Th1 versus Th2 responses.
The specificity of the CD4+ T-cell response induced upon TMEV infection has only recently been elucidated in the highly susceptible SJL/J mouse strain. As with most picornavirus infections, the cellular immune response is directed primarily at the structural proteins which form the icosahedral capsid structure of the virus (reviewed in reference 41). Dominant T-cell determinants have been mapped within amino acids 233 to 250 of the VP1 capsid protein (45), residues 74 to 86 of VP2 (15), and residues 24 to 37 of VP3 (44). Previous analyses of bulk T-cell populations induced by either immunization or infection with TMEV and T-cell hybridomas derived from virus-infected mice demonstrated that these three viral epitopes account for the majority of the viral epitopes recognized by T cells induced upon TMEV infection (45). Such a restriction in the CD4+ T-cell epitopes permits investigation of the role of these individual epitopes in the pathogenesis of demyelination.
Immunization with VP1233–250 or VP274–86 peptide, but not VP324–37, accelerates the onset of demyelination.
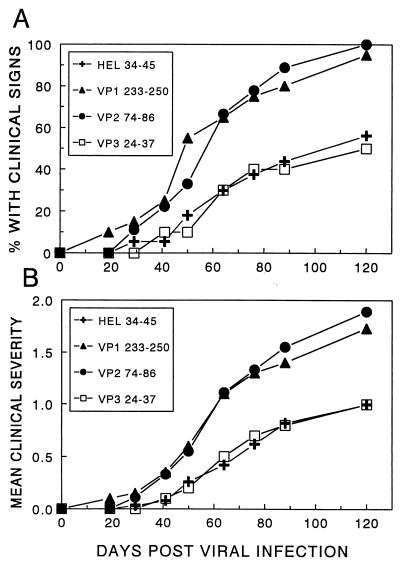
Previous studies have strongly suggested that TMEV-induced demyelination is mediated by an inflammatory CD4+ T-cell population specific for viral epitopes. To further determine the relative role of the individual epitope-specific T-cell responses in the pathogenesis of TMEV-IDD, virus-infected SJL/J mice were immunized with peptides containing the major T-cell epitopes to examine the potential acceleration of the onset and/or increase in the severity of virus-induced demyelinating disease. SJL/J mice were infected with a suboptimal i.c. dose of TMEV (BeAn 8386 strain) at 5 days prior to subcutaneous (s.c.) immunization with peptides representing VP1233–250, VP274–86, and VP324–37 or a nonspecific control peptide containing residues 34 to 45 of hen egg lysozyme (HEL). Mice were subsequently observed for the development of clinical signs of disease (Fig. 1). The data shown in Fig. 1A demonstrate that immunization with peptides containing VP1233–250 or VP274–86 can significantly accelerate the onset of TMEV-IDD. In this particular experiment, the disease incidence was 100% for the VP1233–250- and VP274–86-immunized mice at 120 days post viral infection, although the disease incidence was only 50% for the negative control, HEL34–45-immunized mice (P < 0.05 by χ2 analysis). However, immunization with a peptide containing VP324–37 did not result in a similar acceleration in the clinical symptoms of demyelination (Fig. 1). The disease incidence paralleled the clinical score, suggesting that there is no significant influence on the degree of symptoms in these mice (Fig. 1B). Additional experiments using lower peptide doses confirmed these findings (data not shown). Immunization with VP324–37 resulted in a recall T-cell proliferative response to that peptide similar to the levels in other epitope-primed mice, demonstrating that the inability to accelerate TMEV-IDD with VP324–37 was not due to the lack of priming for VP324–37-specific T cells (data not shown).
FIG. 1.
Effect of immunization with T-cell epitope-containing peptides on the development of TMEV-induced demyelination. SJL/J mice from the National Cancer Institute received an i.c. injection of a suboptimal dose (6 × 104 PFU) of BeAn 5 days prior to an s.c. injection of 25 μg of either VP1233–250 (20 mice), VP274–86 (9 mice), VP324–37 (10 mice), or nonspecific control peptide HEL34–45 (17 mice) emulsified in complete Freund’s adjuvant. Mice subsequently received booster injections with the indicated peptides (25 μg) emulsified in incomplete Freund’s adjuvant 10 days following the first s.c. injection. The amino acid sequences of the peptides are as follows: VP1233–250, SASVRIRYK K M KVFCPRP; VP274–86, QEAFSHIRIPLPH; VP324–37, PIYGKTISTPSDYM; HEL34–45, FESNFNTQATNR. (A) Mice were monitored for the development of clinical signs of demyelinating disease, which included a waddling gait, extensor spasms, paralysis, and loss of the righting reflex. Results are expressed as the percentage of mice exhibiting clinical signs of disease at the respective days post i.c. infection. The disease course (days 43 to 111) in mice immunized with VP1233–250 (P = 0.0003) and VP274–86 (P = 0.003), but not VP324–37 (P = 0.24), was significantly different from that in mice immunized with the control HEL34–45 peptide based on a two-tailed, unpaired t test. (B) The clinical severity of the above mice was assessed on a scale of 0 to 2: no signs of clinical disease, 0; mild gait abnormalities, 1; extreme gait abnormalities and loss of righting reflex, 2. To compare the severity with the above clinical incidence, the mean clinical severity of all of the mice in a group was included in the calculation.
Immunization with capsid protein VP1 or VP2 (but not VP3) accelerates the onset of demyelination.
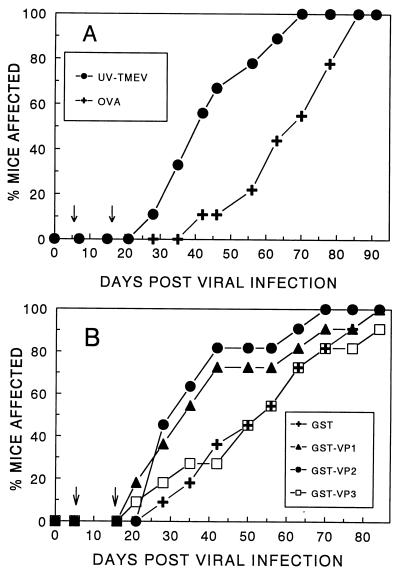
To verify that the VP1233–250 and VP274–86 epitopes are the major pathogenic epitopes of the viral capsid, susceptible SJL/J mice were immunized with UV-inactivated TMEV or glutathione S-transferase (GST) fusion proteins containing individual capsid proteins 5 and 15 days post viral infection. As previously demonstrated (9), immunization of virus-infected SJL/J mice with UV-inactivated TMEV significantly (P = 0.022) accelerated the disease onset (Fig. 2A). The day of disease onset in 50% of the mice immunized with UV-TMEV (DO50) was day 40 post i.c. inoculation, while that of control mice was day 67. Similar immunization with GST-VP1 or GST-VP2 also resulted in significant acceleration (P = 0.046 and P = 0.009, respectively) from control GST-immunized mice, altering the DO50 from day 53 to day 33 or 30 (Fig. 2B). In contrast, mice immunized with GST-VP3 showed no significant (P = 0.87) level of acceleration of disease or difference in DO50 (day 53) compared with control mice. These results clearly demonstrate that immune responses to capsid proteins VP1 and VP2, but not VP3, enhance the disease progression and are consistent with the results obtained with the individual epitopes (Fig. 1). Therefore, the immunity to the predominant Th epitopes involved in the pathogenesis of demyelination appears to represent a great majority of the Th responses for the entire viral capsid proteins.
FIG. 2.
Effect of immunization with UV-TMEV and individual capsid proteins on the onset of TMEV-induced demyelination. (A) Effect of immunization of virus-infected SJL/J mice with UV-inactivated TMEV on the progression of demyelinating disease. SJL/J mice were infected i.c. with 106 PFU of BeAn. Five and 15 days after viral infection, mice (10 per group) were immunized s.c. with either UV-inactivated, purified TMEV or control ovalbumin (OVA; 25 μg) emulsified in complete or incomplete Freund’s adjuvant, respectively. The number of clinically affected mice in each group is expressed as a percentage of the total number of mice in each group. The disease course (days 28 to 78) in mice immunized with UV-inactivated TMEV was significantly (two tailed P = 0.022) different from that of the control group based on an unpaired t test. (B) Effect of immunization of virus-infected SJL/J mice with GST-based fusion proteins containing the entire VP1, VP2, or VP3 capsid region on the progression of demyelinating disease. GST fusion proteins of three major capsid proteins of TMEV (VP1, VP2, and VP3) were generated as described previously (44, 45). SJL/J mice were similarly immunized after viral infection with 25 μg of GST-VP1 (11 mice), GST-VP2 (11 mice), GST-VP3 (10 mice), or control GST (11 mice) in complete and incomplete Freund’s adjuvant at 5 and 15 days, respectively. The disease course (days 21 to 63) in mice immunized with GST-VP1 (P = 0.046) and GST-VP2 (P = 0.009), but not GST-VP3 (P = 0.87), was significantly different from that in mice immunized with a control GST protein based on a two-tailed, unpaired t test.
Antibody responses cannot account for the differential acceleration of disease induced by immunization with capsid proteins.
To further analyze the immunity induced by GST-capsid proteins which differentially affected the course of demyelination, the levels of antibodies specific for the virus and selective major linear antibody epitopes were also assessed (data not shown). All of these immunized mice were able to produce antibodies to the linear epitopes on the respective capsid proteins equally well, including A3A on VP3 in GST-VP3-immunized mice. Therefore, the differential influence of individual capsid fusion proteins on the acceleration of demyelination is not likely due to the lack of antibody response to the respective capsid proteins.
T-cell proliferative responses to the predominant viral epitopes are similar during the course of demyelinating disease.
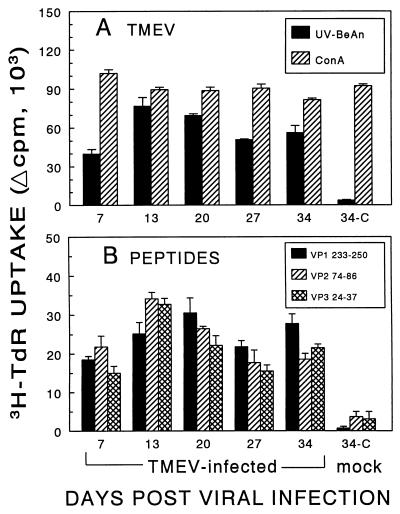
To determine whether there are differences in the T-cell responses to these major epitopes during the initial phase of TMEV-IDD, SJL/J mice were infected i.c. at different time points with the same stock of virus and then the splenic proliferative responses were determined at the same time to minimize experimental variability. As shown in Fig. 3, proliferative responses to UV-inactivated TMEV and peptides containing the predominant T-cell epitopes can be detected as early as 7 days post viral infection. The proliferative response to the whole virus, as well as to the predominant T-cell epitopes, peaked at approximately 2 to 3 weeks postinfection. However, there were no significant differences in the magnitude of the T-cell proliferative responses at any time point with 1 and 10 μM peptides (data not shown for 10 μM peptides). Thus, these results suggest that the levels of T-cell responses to these major epitopes are similar to each other during the initial phases of TMEV-IDD. However, the T-cell proliferative response levels may not directly reflect the frequencies of T-cell precursors toward the epitopes due to the differential T-cell stimulation.
FIG. 3.
Comparison of splenic proliferative responses of TMEV-infected SJL/J mice to peptides containing the predominant T-cell epitopes at various times during the early phases of TMEV-IDD. SJL/J mice were infected i.c. with 3 × 106 PFU of BeAn or Dulbecco modified Eagle medium (mock infected) at various times (x axis) prior to the assay. Spleens were removed from three mice, and pooled splenocytes (5 × 105/well) were cultured in triplicate for 4 days with 12.5-μg/ml UV-BeAn and 3-μg/ml concanavalin A (ConA) as controls (A) or a 1 μM concentration of the indicated epitope-containing peptides (B), as described previously (45). Cultures were then pulsed with [3H]thymidine (3H-TdR) 18 h before harvesting. Phosphate-buffered saline and HEL34–45 served as nonspecific negative controls. Results are expressed as the mean change in counts per minute (Δcpm) ± the standard error of the mean (mean counts per minute from UV-inactivated-BeAn-stimulated cultures minus the mean counts per minute from control cultures with phosphate-buffered saline or the mean counts per minute from peptide-stimulated cultures minus the mean counts per minute from HEL34–45-stimulated cultures). The background counts per minute were similar to each other in cultures with phosphate-buffered saline or the nonspecific peptide HEL34–45 and ranged between 3,316 and 8,642 cpm.
Frequencies of precursor T cells reactive to VP1233–250, VP274–86, and VP324–37 are similar.
To correlate the level of T-cell proliferative response with the number of T cells specific to a given epitope, the frequencies of T-cell precursors toward individual T-cell epitopes were assessed as described previously (45) at two different time points, 14 and 26 days post viral infection. The frequency of T cells reactive to VP1233–250 (1 in 41,695; 95% confidence interval, 1 in 31,505 to 1 in 61,626) appears to be somewhat lower than that of T cells reactive to VP274–86 (1 in 26,797; 1 in 19,336 to 1 in 43,633) or VP324–37 (1 in 36,559; 1 in 25,693 to 1 to 63,349) at 14 days and become slightly higher at 26 days post viral infection. Collectively, these data demonstrate that VP1233–250, VP274–86, and VP324–37 represent the predominant epitopes recognized by T cells from SJL/J mice and that there are no significant differences in the frequencies of T cells specific for these epitopes during the course of TMEV-IDD. Therefore, the level of stimulation of T cells toward the individual T-cell epitopes is not likely involved in the differential acceleration of demyelination. Taken together, neither the order nor the level of Th responses to these major epitopes appears to play a critical role in the pathogenesis of immune-mediated demyelination.
T cells reactive to VP1233–250 and VP274–86 are primarily Th1 and those to VP324–37 are Th2.
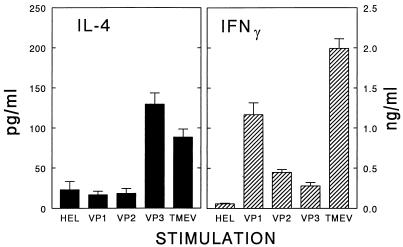
Since neither the level nor the order of T-cell responses to the major epitopes corresponded to the differential acceleration of demyelination (Fig. 3), the potential qualitative differences in T-cell responses to the individual epitopes were assessed (Fig. 4). The production of several representative cytokines by T cells from TMEV-infected mice at a preclinical stage (day 13 post viral infection) in response to peptides containing the predominant T-cell epitopes was analyzed. As shown in Fig. 4, T cells from virus-infected mice produce significant levels of a Th1 cytokine, gamma interferon (IFN-γ), in response to peptides containing the T-cell epitopes or to the whole virus. The level of IFN-γ produced in response to VP1233–250 was approximately four- to fivefold higher than the level of IFN-γ produced in response to either VP274–86 or VP324–37 at day 13. The relatively higher level (two- to threefold) of IFN-γ produced in response to the VP1233–250 peptide was maintained at 34 days post viral infection (data not shown). However, the Th1 cytokine production in response to VP274–86 was only slightly higher than that against VP324–37. The proliferative responses to these three epitopes in these experiments were also very similar (data not shown), strongly suggesting that the increased production of Th1 cytokines in response to the selective epitopes was not due to an increased expansion of the epitope-specific T cells.
FIG. 4.
Determination of IL-4 and IFN-γ levels produced by T cells from virus-infected SJL/J mice in response to UV-inactivated TMEV or synthetic peptides containing the major Th epitopes. Nylon wool-isolated splenic T cells (2 × 105 to 3 × 105/well) from three mice at 13 days after viral infection were stimulated for 72 h with peptides (1 μM) or UV-inactivated BeAn (1 μg/ml) in the presence of 5 × 104 DAS.15 cells (I-As transfectants) as antigen-presenting cells (27). Cytokine levels produced by splenic T cells in response to viral antigens were assessed by a specific cytokine enzyme-linked immunosorbent assay (Endogen, Cambridge, Mass.). Results are expressed as means ± the standard deviations.
In addition, there was no significant production of interleukin 4 (IL-4), a representative Th2 cytokine, in response to peptides containing the VP1233–250 and VP274–86 T-cell epitopes, confirming the presence of a predominant, inflammatory Th1 response against these regions in mice with viral demyelination (45). In contrast, the highest levels (greater than fivefold; P < 0.05) of IL-4 were produced in response to VP324–37, compared to the levels produced in response to VP1233–250, VP274–86, or control HEL34–45, at 13 days. The level of IL-4 was, however, significantly reduced at 28 days, while the differential IL-4 levels were maintained (data not shown). Since the IL-4 level in response to the VP3 epitope was similar to that against the whole virus, most of the IL-4 produced in response to the virus may represent the cytokine produced by VP324–37-specific T cells. These data suggest that T-cell responses against VP1233–250 and VP274–86 are predominantly Th1, whereas VP324–37-specific T cells are preferentially Th2 at the early stages of viral infection. The differential production of Th1 and Th2 cytokines in response to major T-cell epitopes was verified and expanded by using a reverse transcriptase-PCR (data not shown). The pattern of cytokine message profiles of T cells in response to the peptides and the virus was similar to the cytokine protein pattern seen with an enzyme-linked immunosorbent assay (Fig. 4). Higher levels of Th2 cytokine messages (IL-4, IL-10, and transforming growth factor β) were again observed in response to VP324–37 and the whole virus, compared to those in response to VP1233–250 and VP274–86. Taken together, the production of a high level of IFN-γ, combined with a low level of IL-4, in response to VP1233–250 and, perhaps, VP274–86 suggests a key role of these T-cell responses in the pathogenesis of this inflammatory demyelinating disease.
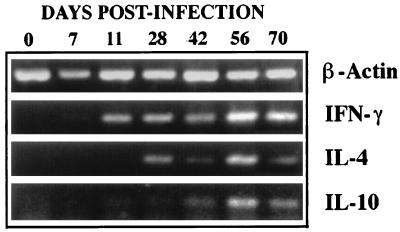
Production of Th1 cytokines precedes that of Th2 cytokines in the CNS during the development of demyelinating disease.
To further understand the T-cell types involved in the pathogenesis of demyelination, the levels of representative cytokines in the spinal cords were examined at various time intervals (0, 7, 11, 28, 42, 56, and 70 days) post viral infection (Fig. 5). The IFN-γ messages were detectable as early as 11 days and maintained until the last test time point at 70 days. On the other hand, IL-4 and IL-10 messages were detectable only after 28 days. Therefore, the production of Th2 cytokine messages in the CNS following viral infection appears to be lower than that of Th1 cytokine messages (IL-2 and IFN-γ) at the early stage. This result is consistent with our previous observation (31) confirming the initial accumulation of Th1 cytokines in the CNS of virus-infected SJL/J mice. This early accumulation of relatively higher level of Th1 cytokines in the CNS may set an environment favoring the subsequent immune-mediated inflammatory demyelination.
FIG. 5.
Comparison of Th1 and Th2 cytokines in the CNS during the course of demyelinating disease. Representative Th1 (IFN-γ) and Th2 (IL-4 and IL-10) cytokine levels in the spinal cords of SJL/J mice (three or four mice per group) were assessed by reverse transcriptase-PCR during the course of demyelinating disease. The level of Th2 cytokine messages appears to be lower than that of Th1 cytokine messages in the early stages of viral infection. The cytokine messages were assessed based on the levels of amplified PCR products (35 cycles) with appropriate primers obtained from Clontech. Total cellular RNA was isolated from the spinal cords of phosphate-buffered saline-perfused mice by using the guanidinium isothiocyanate method (7). mRNA was then reverse transcribed into cDNA by using oligo(dT)15–18 and murine leukemia virus reverse transcriptase. The relative concentrations of cDNA were equalized among the groups based on the level of β-actin amplification.
Potential mechanisms and implications of the differential Th1/Th2 responses to individual epitopes.
Our results indicate that individual major Th epitopes within a pathogen are able to induce Th1 and Th2 responses independently. However, the mechanism underlying the differential Th1 and Th2 responses to individual epitopes is not clear. Perhaps the predominant VP1 and VP2 (but not VP3) epitopes interact with high affinity to major histocompatibility complex class II molecules and/or are presented at high ligand densities that preferentially induce Th1-type responses (22, 29, 33). Alternatively, only VP324–37 among the major Th epitopes overlaps a predominant linear antibody epitope region (18, 44). Therefore, it is conceivable that T cells recognizing adjacent or overlapping determinants of antibody epitopes are more likely to interact with epitope-reactive B cells for mutual stimulation, leading to B- and T-cell activation. Such an enhanced interaction between B and T cells may preferentially promote Th2 responses, as described previously (14).
Treatment of susceptible strains of mice with antibodies to either class II or CD4 molecules can significantly suppress the demyelination induced by TMEV (13, 16, 42). The adoptive transfer of a CD4+ T-cell line specific for VP274–86 has also been shown to potentiate the demyelination induced by TMEV (16). In addition, the majority of VP1233–250- or VP274–86-specific T cells cloned from the inflammed spinal cords of TMEV-infected mice are of the Th1 type, suggesting that such T cells have infiltrated the CNS (45). It was also previously shown that downregulation of Th1 responses, but not Th2 responses, suppresses the development of TMEV-IDD (19). These data are consistent with the idea that inflammatory Th1-mediated responses are closely involved in the pathogenesis of virally induced demyelination.
T cells specific for these viral determinants may recruit and activate macrophages via their inflammatory cytokines, such as tumor necrosis factors (alpha and beta) and IFN-γ, resulting in destruction of myelin (8). The production of inflammatory cytokines by these virus-specific T cells may also mediate various other effects on the CNS cells within the microenvironment. For example, IFN-γ increases the expression of major histocompatibility complex class I and II molecules on the CNS cell types (3, 43), enabling such CNS cells to present antigenic determinants to infiltrating T cells (12). In addition, such an inflammatory Th1 cell type may also be involved in the destruction of class II-bearing CNS cells (37, 39), contributing to the pathogenesis of TMEV-IDD and subsequent autoimmunity to myelin components (28). Thus, Th1 cells specific for VP1233–250 and VP274–86, which are readily found in the CNS (34, 45), are likely to contribute to chronic inflammation, leading to virus-induced demyelination.
Certain Th2 cytokines (e.g., IL-4 and IL-10) are known to inhibit cell-mediated inflammatory Th1 responses in vivo (1, 23, 35). The reciprocal regulation of Th1 and Th2 responses is critical for the resolution or progression of certain infectious disease (17, 30). In addition, Th2 responses involving the production of IL-4 and IL-10 can suppress the development of CNS inflammation associated with autoimmune demyelination, (experimental allergic encephalomyelitis [EAE]) (6, 36), although a single cytokine effect may not be sufficient (30). Moreover, recovery from EAE is also associated with the presence of Th2-type cytokines (20, 21). Aside from the direct downregulation of pathogenic Th1 responses, Th2 cytokines can also exhibit protective effects against neuronal cell injury caused by activated microglia (5). Despite the preferential Th2 response to the VP3 epitope, the level of the Th2 response to this epitope may not be sufficient to overcome the effect of overall greater Th1 responses to other viral epitopes such as the VP1 and VP2 epitopes. Thus, it is conceivable that the ratio of Th1- to Th2-inducing epitopes may determine the initial type of Th responses to a pathogen (31) and the consequent pathogenicity of immune-mediated inflammatory disease. In addition, our preliminary studies indicating that variant viruses containing a single amino acid substitution within the VP1 epitope leading to a Th2 response are not pathogenic (data not shown) reinforce the importance of the ratio of Th1 to Th2 epitopes in the induction of immune-mediated inflammatory disease.
Acknowledgments
This work was supported by USPHS grants RO1 NS28752 and RO1 NS33008. J.P. is a postdoctoral fellow (FG1172-A-1) of the National Multiple Sclerosis Society.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Allen I, Brankin B. Pathogenesis of multiple sclerosis—the immune diathesis and the role of viruses. J Neuropathol Exp Neurol. 1993;52:95–105. doi: 10.1097/00005072-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Borrow P, Nash A A. Susceptibility to Theiler’s virus-induced demyelinating disease correlates with astrocyte class II induction and antigen presentation. Immunology. 1992;76:133–139. [PMC free article] [PubMed] [Google Scholar]
- 4.Bureau J F, Montagutelli X, Bihl F, Lefebvre S, Guenet J L, Brahic M. Mapping loci influencing the persistence of Theiler’s virus in the murine central nervous system. Nat Genet. 1993;5:87–91. doi: 10.1038/ng0993-87. [DOI] [PubMed] [Google Scholar]
- 5.Chao C C, Molitor T W, Hu S. Neuroprotective role of IL-4 against activated microglia. J Immunol. 1993;151:1473–1481. [PubMed] [Google Scholar]
- 6.Chen Y, Kuchroo V K, Inobe J, Hafler D A, Weiner H L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Clatch R J, Lipton H L, Miller S D. Characterization of Theiler’s murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986;136:920–927. [PubMed] [Google Scholar]
- 9.Crane M A, Yauch R, Dal Canto M C, Kim B S. Effect of immunization with Theiler’s virus on the course of demyelinating disease. J Neuroimmunol. 1993;45:67–73. doi: 10.1016/0165-5728(93)90165-u. [DOI] [PubMed] [Google Scholar]
- 10.Dal Canto M C, Melvold R W, Kim B S, Miller S D. Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison. Microsc Res Tech. 1995;32:215–229. doi: 10.1002/jemt.1070320305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhib-Jalbut S, McFarlin D E. Immunology of multiple sclerosis. Ann Allergy. 1990;64:433–444. [PubMed] [Google Scholar]
- 12.Fontana A, Frei K, Bodmer S, Hofer E. Immune-mediated encephalitis: on the role of antigen-presenting cells in brain tissue. Immunol Rev. 1987;100:185–201. doi: 10.1111/j.1600-065X.1987.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedmann A, Frankel G, Lorch Y, Steinman L. Monoclonal anti-I-A antibody reverses chronic paralysis and demyelination in Theiler’s virus-infected mice: critical importance of timing of treatment. J Virol. 1987;61:898–903. doi: 10.1128/jvi.61.3.898-903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajewski T F, Pinnas M, Wong T, Fitch F W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- 15.Gerety S J, Karpus W J, Cubbon A R, Goswami R G, Rundell M K, Peterson J D, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 16.Gerety S J, Rundell M K, Dal Canto M C, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 17.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue A, Choe Y K, Kim B S. Analysis of antibody responses to predominant linear epitopes of Theiler’s murine encephalomyelitis virus. J Virol. 1994;68:3324–3333. doi: 10.1128/jvi.68.5.3324-3333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpus W J, Pope J G, Peterson J D, Dal Canto M C, Miller S D. Inhibition of Theiler’s virus-mediated demyelination by peripheral immune tolerance induction. J Immunol. 1995;155:947–957. [PubMed] [Google Scholar]
- 20.Kennedy M K, Torrance D S, Picha K S, Mohler K M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 21.Khoury S J, Hancock W W, Weiner H L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon-gamma by T cells. Proc Natl Acad Sci USA. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liblau R S, Singer S M, McDevitt H O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 24.Lipton H L, Dal Canto M C. Chronic neurologic disease in Theiler’s virus infection of SJL/J mice. J Neurol Sci. 1976;30:201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- 25.Lipton H L, Dal Canto M C. Theiler’s virus-induced demyelination: prevention by immunosuppression. Science. 1976;192:62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- 26.Lipton H L, Kratochvil J, Sethi P, Dal Canto M C. Theiler’s virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology. 1984;34:1117–1119. doi: 10.1212/wnl.34.8.1117. [DOI] [PubMed] [Google Scholar]
- 27.McRae B L, Nikcevich K M, Karpus W J, Hurst S D, Miller S D. Differential recognition of peptide analogs by naive versus activated PLP 139-151-specific CD4+ T cells. J Neuroimmunol. 1995;60:17–28. doi: 10.1016/0165-5728(95)00048-7. [DOI] [PubMed] [Google Scholar]
- 28.Miller S D, Vanderlugt C L, Begolka W S, Pao W, Yauch R L, Neville K L, Katz-Levy Y, Carrizosa A, Kim B S. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 29.Murray J S, Ferrandis-Edwards D, Wolfe C J, Schountz T. Major histocompatibility complex regulation of T helper functions mapped to a peptide C terminus that controls ligand density. Eur J Immunol. 1994;24:2337–2344. doi: 10.1002/eji.1830241012. [DOI] [PubMed] [Google Scholar]
- 30.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 31.Palma J P, Park S H, Kim B S. Treatment with lipopolysaccharide enhances the pathogenicity of a low-pathogenic variant of Theiler’s murine encephalomyelitis virus. J Neurosci Res. 1996;45:776–785. doi: 10.1002/(SICI)1097-4547(19960915)45:6<776::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 32.Peterson J D, Karpus W J, Clatch R J, Miller S D. Split tolerance of Th1 and Th2 cells in tolerance to Theiler’s murine encephalomyelitis virus. Eur J Immunol. 1993;23:46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope J G, Karpus W J, VanderLugt C, Miller S D. Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease. Evidence for a CD4+ T cell-mediated pathology. J Immunol. 1996;156:4050–4058. [PubMed] [Google Scholar]
- 35.Powrie F, Menon S, Coffman R L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:2223–2229. doi: 10.1002/eji.1830230926. . (Erratum, 24:785, 1994.) [DOI] [PubMed] [Google Scholar]
- 36.Racke M K, Bonomo A, Scott D E, Cannella B, Levine A, Raine C S, Shevach E M, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reder A T, Lascola C D, Flanders S A, Maimone D, Jensen M A, Skias D D, Lancki D W. Astrocyte cytolysis by MHC class II-specific mouse T cell clones. Transplantation. 1993;56:393–399. doi: 10.1097/00007890-199308000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez M, Leibowitz J, Lampert P. Persistent infection of oligodendrocytes in Theiler’s virus induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- 39.Sun D, Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986;320:70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- 40.Theiler M. Spontaneous encephalomyelitis of mice: a new virus disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usherwood E J, Nash A A. Lymphocyte recognition of picornaviruses. J Gen Virol. 1995;76:499–508. doi: 10.1099/0022-1317-76-3-499. [DOI] [PubMed] [Google Scholar]
- 42.Welsh C J, Tonks P, Nash A A, Blakemore W F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- 43.Wong G H, Bartlett P F, Clark-Lewis I, Battye F, Schrader J W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- 44.Yauch R L, Kerekes K, Saujani K, Kim B S. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler’s virus in demyelination-susceptible SJL/J mice. J Virol. 1995;69:7315–7318. doi: 10.1128/jvi.69.11.7315-7318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yauch R L, Kim B S. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler’s virus is located within VP1(233-244) J Immunol. 1994;153:4508–4519. [PubMed] [Google Scholar]