CAPSULE SUMMARY
What is already known on this topic
The paradoxical development of psoriasis is an unintended consequence of TNFα inhibition.
What this article adds to our knowledge
Discontinuing TNFα therapy resulted in psoriasis resolution (47.7%) more often than in those who switched (36.7%) or continued (32.9%) TNFα therapy
How this information impacts clinical practice and/or changes patient care
TNFα inhibitor-induced psoriasis is often successfully managed with skin-directed therapies and in many cases does not require cessation of TNFα inhibitor treatment.
INTRODUCTION
The proinflammatory cytokine tumor necrosis factor-alpha (TNFα) has been implicated in the pathogenesis of multiple inflammatory and autoimmune conditions such as Crohn’s disease (CD), ulcerative colitis (UC), rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriasis. The development of TNFα inhibitors has dramatically improved therapeutic options for patients with these conditions. However, there are many reports in the literature of TNFα inhibitors paradoxically inducing new onset psoriasis or worsening pre-existing quiescent psoriatic disease. A study analyzing the United States Food and Drug Administration Adverse Event Reporting System from 2004–2011 found that TNFα inhibitors used in the treatment of Crohn’s Disease were associated with an increased risk of psoriasiform adverse events compared to other medications.1 There is currently no standardized approach for managing TNFα inhibitor-induced psoriasis, and there is little clarity on whether to withdraw TNFα inhibitor therapy.
The objective of this systematic review of reported cases of TNFα inhibitor-induced psoriasis was to better define the demographic, clinical, and histological features of TNFα inhibitor-induced psoriasis and determine the optimal treatment approach.
METHODS
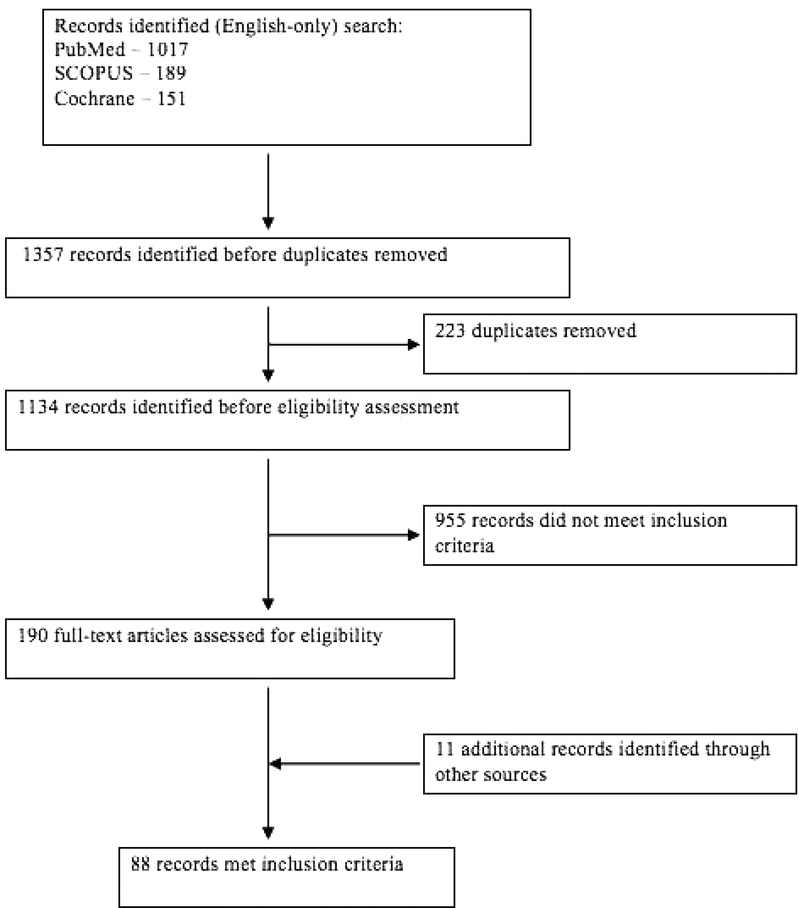
We conducted an electronic literature search to identify studies, case reports, and case series that documented new-onset psoriasiform lesions in patients being treated with a TNFα inhibitor (Fig. 1).
Figure 1:
The electronic search, selection and exclusion of studies in the systematic review.
Information Sources and Search Strategy
With the assistance of a research librarian, we identified literature on PubMed on June 18, 2013 using the terms “anti-TNF-alpha”, “TNF-alpha inhibitor”, “TNF-alpha inhibitors”, “TNF-alpha antagonist”, “TNF-alpha antagonists”, “anti-tumor necrosis factor”, “tumor necrosis factor inhibitor”, “tumor necrosis factor inhibitors”, “tumor necrosis factor antagonist”, “tumor necrosis factor antagonists”, “infliximab”, “adalimumab”, “etanercept”, “golimumab”, “certolizumab”, “psoriasis”, “psoriasiform”[MeSH], “palmoplantar”[MeSH], “pustul*”, “induce*”, “cause*”, “complicat*”, “due”, “flar*”, “exacerbat*”, “paradox*”, “induct*”, “induce*”, “advers*”, “onset”, “associat*”, “risk”, “nonpsoriatic”, “psoriasis/chemically induced”[MeSH], “Tumor Necrosis Factor-alpha/adverse effects”[MeSH]. We restricted the search results to English only records. We conducted similar computerized searches using SCOPUS and the Cochrane Database. The last search was conducted on July 1, 2014. Additionally, we reviewed citations within the identified articles and relevant reviews to locate published articles missed by database searches. We performed an EndNote function to identify and remove duplicates.
Study Selection
Two investigators (MH and MW) assessed study eligibility using title and abstract for initial screening. Two additional investigators (EW and GB) further evaluated the eligibility of the studies by reviewing the full-text publication. Any study that reported individual data of patients with new-onset psoriasis after the initiation of a TNFα inhibitor was eligible for inclusion. We excluded records that omitted specific data regarding individual patients, as well as cases in which the patient had a prior history of psoriasis.
Data Collection and Extraction
Two reviewers (EW and GB) independently extracted individual patient data from each record. We constructed a data collection spreadsheet and extracted the following data items from each record: patient demographics (including age, gender, ethnicity), age at onset of psoriasiform eruption, family history of psoriasis, prior TNFα inhibitor medication history, disease being treated, TNFα inhibitor resulting in psoriasiform eruption, time elapsed prior to onset of psoriasiform eruption, morphology of psoriasiform lesions, body areas involved, biopsy result, recurrence with TNFα inhibitor switch, concomitant immunomodulator, concomitant systemic steroids, management and response.
RESULTS
Clinical and Histopathologic Characteristics
Of the 190 full-text articles assessed for eligibility, 88 articles met inclusion criteria for the final analysis. We extracted a total of 216 cases of new-onset TNFα inhibitor-induced psoriasis from these publications. Demographic features of patients with TNFα inhibitor-induced psoriasis are summarized in Table 1.
Table 1:
Summary of demographic features of patients with TNFα inhibitor-induced psoriasis
| Demographic Characteristics | Patients (N = 216) Patients (N = 216) |
|---|---|
| Gender, N (%) | |
| Female | 156 (72.2) |
| Male | 60 (27.8) |
| Average age, years (range) | 38.5 (7–83) |
| Family history of psoriasis, N (% of 161 reported) | 19 (11.8) |
| Primary disease, N (%) | |
| Crohn’s Disease | 88 (40.7) |
| Rheumatoid arthritis | 80 (37.0) |
| Ankylosing spondylitis | 30 (13.9) |
| Ulcerative colitis | 6 (2.8) |
| Juvenile idiopathic arthritis | 5 (2.3) |
| Behcet’s disease | 3 (1.4) |
| Spondylarthropathy | 5 (2.3) |
| Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) | 2 (1.0) |
| Bilateral idiopathic panuveitis | 1 (0.5) |
| Inflammatory arthritis | 1 (0.5) |
| TNF-alpha inhibitor resulting in psoriasis eruption, N (%) | |
| Infliximab | 135 (62.5) |
| Adalimumab | 47 (21.8) |
| Etanercept | 31 (14.4) |
| Certolizumab | 2 (1.0) |
| Golimumab | 1 (0.5) |
| Concomitant immunomodulators, N (% of 95 reported) | |
| Methotrexate | 32 (33.7) |
| Azathioprine | 18 (18.9) |
| Leflunomide | 13 (13.7) |
| Sulfasalazine | 7 (7.4) |
| Hydroxychloroquine | 2 (2.1) |
| 6-mercaptopurine | 2 (2.1) |
| Mesalazine | 2 (2.1) |
| Cyclosporine | 1 (1.0) |
| Tacrolimus | 1 (1.0) |
| Concomitant systemic steroids, N | 17 |
| Clinical latency to eruption, months (range in months) | |
| Infliximab | 13.6 (1–120) |
| Adalimumab | 14.4 (1–62) |
| Etanercept | 16.2 (2–72) |
| Certolizumab | 5.0 (4–6) |
| Golimumab | 4.0 (4; N=1) |
| Morphology of skin lesions, N (%) | |
| Plaque | 90 (44.8) |
| Palmoplantar pustular psoriasis | 73 (36.3) |
| Psoriasiform | 40 (19.9) |
| Severe alopecia | 15 (7.5) |
| Generalized pustular | 22 (10.9) |
| Guttate | 16 (8.0) |
| Inverse | 7 (3.5) |
| Follicular | 2 (1.0) |
| More than one form | 54 (26.9) |
| Body areas involved, N (%) | |
| Soles | 99 (45.8) |
| Extremities | 98 (45.4) |
| Palms | 97 (44.9) |
| Scalp | 78 (36.1) |
| Trunk | 70 (32.4) |
| Face | 19 (8.8) |
| Axillae | 15 (6.9) |
| Groin | 14 (6.5) |
| Not specified | 20 (9.3) |
| Histology of lesions, N (% of 102 reported) | |
| Classic | 56 (54.9) |
| Pustular PSO | 34 (33.3) |
| Psoriasiform dermatitis | 12 (11.8) |
| Psoriatic treatment, N (% of 204 reported) | |
| Topical steroids | 156 (76.5) |
| Vitamin D Analogue | 36 (17.6) |
| Methotrexate | 35 (17.2) |
| Systemic steroids | 19 (9.3) |
| Phototherapy | 17 (8.3) |
| Cyclosporine | 11 (5.4) |
| Acitretin | 4 (2.0) |
| Coal tar | 2 (1.0) |
| Unknown | 12 |
Women comprised 72.2% of the cases, with a female predominance in RA and CD (89.9% and 63.2%, respectively). Ages of psoriasis onset ranged from 7 years to 83 years; the mean age of psoriasis onset was 38.5 years. The majority of patients received TNFα inhibitor therapy for CD (40.7%), RA (37.0%), or AS (13.9%). Patients had a positive family history of psoriasis in 11.8% (N=19) of disclosed cases (N=161), and no patients had a personal history of psoriasis or psoriatic arthritis, as those cases were excluded. There were no identified cases with known pre-existing psoriatic arthritis prior to developing psoriasis on TNFα inhibitor therapy.
The psoriasis presentations were variable, and 26.9% of patients had more than one reported morphological type. The two most commonly observed clinical presentations included plaque (44.8%) and palmoplantar pustular (36.3%) psoriasis. Other presentations included ill-defined psoriasiform dermatitis (19.9%), severe scalp involvement associated with alopecia (7.5%), and generalized pustular psoriasis (10.9%). The anatomical sites commonly involved included soles (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%).
Of the 102 cases confirmed with biopsy, 54.9% revealed plaque psoriasis and 33.3% were consistent with pustular psoriasis with the remainder interpreted as psoriasiform dermatitis. An eosinophil rich infiltrate was noted in 3 of the plaque psoriasis biopsies and 3 of the pustular psoriasis biopsies.
TNFα Inhibitor Treatment Characteristics
The majority of cases were associated with infliximab (62.5%), followed by adalimumab (21.8%) and etanercept (14.4%). Only 2 cases involved certolizumab and 1 case with golimumab. The most commonly prescribed agent was infliximab (62.5%), with the majority of patients with CD and AS receiving infliximab compared to other agents (CD 82.8%; AS 73.3%). Patients underwent TNFα inhibitor therapy for an average of 14.0 months prior to onset (infliximab 13.6; adalimumab 14.4; etanercept 16.2, certolizumab 5.0, golimumab 4.0; overall range 1 to 120 months). Although 69.9% of patients experienced the onset of psoriasis in the first year of treatment, 31.2% of cases occurred in the second year (infliximab 17.4%; adalimumab 9.8%; etanercept 13.3%). 19 patients reported prior TNFα inhibitor treatment, 3 of which had previously experienced psoriasiform eruption and resolution with withdrawal. The most common concomitant immunomodulators at the time of psoriasis onset included methotrexate (MTX; 33.7%), azathioprine (AZA; 18.9%), and leflunomide (13.7%). 17 cases reported administration of concomitant systemic steroids.
Management and Outcomes
Therapeutic management and outcomes of TNFα inhibitor-induced psoriasis are summarized in Table 2. Resolution of psoriasis (or no evidence of recurrence at time of follow-up) was reported in patients who discontinued TNF therapy (47.7%), switched to a different TNF agent (36.7%), or continued the same TNF therapy (32.9%). Improvement but incomplete resolution of psoriasis was reported in patients who continued the same TNF therapy (57.3%), discontinued TNF therapy (46.2%), and switched to a different TNF agent (18.4%). Regardless of the TNFα inhibitor treatment decision, the majority of patients received skin-directed therapy with one or more agents including topical steroids (76.5%), vitamin D analogues (17.6%), MTX (17.2%), phototherapy (8.3%), cyclosporine (5.4%), acitretin (2.0%), and coal tar (1.0%).
Table 2:
Summary of therapeutic management and outcomes of patients with TNFα inhibitor-induced psoriasis
| Total | Infliximab | Adalimumab | Etanercept | Golimumab | Certolizumab | |
|---|---|---|---|---|---|---|
| Continued on TNF therapy (N) | 82 | 55 | 14 | 12 | 1 | - |
| Resolved (%) | 27 (32.9) | 22 (40.0) | 1 (7.1) | 4 (33.3) | - | - |
| Improved (%) | 47 (57.3) | 30 (54.5) | 12 (85.7) | 4 (33.3) | 1 (100.0) | - |
| No improvement (%) | 8 (9.8) | 3 (5.5) | 1 (7.1) | 4 (33.3) | - | - |
| Discontinued off TNF therapy (N) | 65 | 32 | 19 | 12 | - | 2 |
| Resolved (%) | 31 (47.7) | 17 (53.1) | 10 (52.6) | 3 (25.0) | - | 1 (50.0) |
| Improved (%) | 30 (46.2) | 12 (37.5) | 8 (42.1) | 9 (75.0) | - | 1 (50.0) |
| No improvement(%) | 4 (6.2) | 3 (9.4) | 1 (5.3) | - | - | - |
| Switched to different TNF agent (N) | 49 | 9 | 19 | 18 | - | 3 |
| Resolved/no recurrence at follow-up | 18 (36.7) | 2 (22.2) | 8 (42.1) | 7 (38.9) | - | 1 (33.3) |
| Improved | 9 (18.4) | 2 (22.2) | 2 (10.5) | 4 (22.2) | - | 1 (33.3) |
| No improvement | 22 (44.9) | 5(55.6) | 9 (47.4) | 7 (38.9) | - | 1 (33.3) |
Management strategies and resulting outcomes were analyzed for patients with more severe presentations, including alopecia and/or generalized pustular psoriasis (Table 3). Half of the patients with alopecia had resolution of symptoms regardless of continuing or discontinuing TNF therapy (resolution in 2/4 who continued vs. 4/8 who discontinued therapy). Patients with generalized pustular psoriasis who continued therapy experienced resolution in 33.3% of cases (2/6). Of patients with generalized pustular psoriasis who discontinued therapy, 63.6% had improvement of symptoms and 27.3% had resolution of psoriasis at follow-up.
Table 3:
Summary of outcomes with severe presentations including alopecia and/or generalized pustular psoriasis
| Clinical subtype* | Clinical Outcome | Continued TNF therapy | Discontinued TNF therapy | Switched to different TNF agent |
|---|---|---|---|---|
| Alopecia | Resolved | 2/4 (50.0%) | 4/8 (50.0%) | |
| Improved | 1/4 (25.0%) | 2/8 (25.0%) | 1/1 (100.0%) | |
| No improvement | 1/4 (25.0%) | 2/8 (25.0%) | ||
| Unknown (N=2) | ||||
| Generalized pustular psoriasis | Resolved | 2/6 (33.3%) | 3/11 (27.3%) | 2/4 (50.0%) |
| Improved | 2/6 (33.3%) | 7/11 (63.6%) | 2/4 (50.0%) | |
| No improvement | 2/6 (33.3%) | 1/11 (9.1%) | ||
| Unknown (N=1) |
Note that 3 patients had both alopecia and generalized pustular psoriasis
DISCUSSION
Psoriasiform lesions are a well documented complication of TNFα inhibitor therapy which can occur at any time during the treatment course.2 However, in our analysis, we found the majority (69.9%) to occur within the first year of treatment. Furthermore, our review of published photographs suggests that many patients had generalized involvement with more than one morphological type. The typical presentations include plaque or palmoplantar pustular psoriasis, though various clinical morphologies have been reported. Severe scalp psoriasis associated with alopecia is a less common presentation observed in de novo psoriasis and the onset in a patient undergoing TNFα inhibitor therapy should prompt consideration of TNFα inhibitor-induced psoriasis.
The majority of the TNFα inhibitor-induced psoriasis biopsy samples were histologically indistinguishable from de novo psoriasis. However, in a study investigating histopathologic changes in TNFα inhibitor-induced psoriasiform clinical lesions, the authors concluded that the reactions comprise a spectrum ranging from that closely mimicking psoriasis, a sterile pustular folliculitis, or a lichenoid pattern.3 The presence of eosinophils may also be suggestive of TNFα inhibitor-induced psoriasiform dermatitis.3 A biopsy may be helpful in patients with new-onset psoriasis while on TNFα inhibitors to evaluate for these specific features. Biopsy may also be helpful in distinguishing TNFα inhibitor-induced psoriasis from lichenoid drug reaction, as both can present with clinically psoriasiform lesions. There are fewer reported TNFα inhibitor-induced lichen planus cases in the literature; however, the prognosis is comparable to TNFα inhibitor-induced psoriasis such that most patients completely resolved following agent continuation or withdrawal with concomitant skin-directed treatment.4
In this study we found that more patients had resolution of symptoms after discontinuing TNF therapy (47.7%) compared to those who continued therapy (32.9%). While the majority of patients switching to a different TNFα inhibitor had either resolution (36.7%) or improvement (18.4%) in psoriasis, 44.9% experienced no improvement or recurrence, suggesting a need for additional treatment options in these patients.
The management approach may also vary depending on the clinical presentation. In patients with more severe presentations, such as generalized pustular psoriasis, only 27.3% of patients who discontinued therapy had resolution compared to 47.7% of all patients analyzed in systematic review who discontinued therapy. However, 63.6% of patients with generalized pustular psoriasis showed improvement, but not resolution, after discontinuing therapy. Therefore, such patients may need counseling on realistic expectations on the outcome and the probability that they may experience improvement rather than complete resolution of symptoms after discontinuing therapy. In contrast, half of the patients with alopecia had resolution of symptoms despite continuing or discontinuing therapy, albeit a larger quantity of patients were managed with discontinuation of therapy. These results suggest that patients may have different outcomes based on clinical subtype of psoriasis. While the number of patients with alopecia or generalized pustular psoriasis who switched to a different TNFα agent was small, all had either resolution or improvement of symptoms, which suggests that this may be a reasonable therapeutic approach in a subset of patients.
The prevalence of new-onset psoriasis in patients treated with TNFα inhibitors is unknown. The underlying disease itself may play a predisposing role, as patients with chronic rheumatologic and gastrointestinal inflammatory diseases have a higher incidence of psoriasis.5,6 Immune dysregulation related to immunosuppression may additionally contribute; the prevalence of TNFα inhibitor-induced psoriasis in patients with RA and spondyloarthropathy (2.3–5%) 7–9 is similar to that in HIV disease (1.3–1.5%).10 However, the temporal relationship between TNFα inhibitor and psoriasis onset (most often in the first year after exposure) supports a causal association. Overall the data suggest that TNFα inhibitor-induced psoriasis is a class effect, as this adverse reaction has been observed with all TNFα inhibitors and with all diseases for which TNFα inhibitors are indicated.9,11
While the exact mechanism of TNFα inhibitor-induced psoriasis remains elusive, emerging evidence suggests that the IL-23/TH17 axis plays an important role in the pathogenesis.12 IL-23 is a proinflammatory cytokine that drives downstream TH1 and TH17 effector responses, which have been implicated in the pathogenesis of chronic inflammatory diseases.13 Genome-wide association studies have associated specific polymorphisms in the IL-23R gene with increased susceptibility to both CD and psoriasis.14,15 In a study evaluating TNFα inhibitor-induced psoriasis in pediatric patients with CD, those who developed psoriasis were more often homozygous for three specific IL-23R polymorphisms compared to disease-matched controls who did not develop psoriasis following TNFα inhibitor treatment.16 Similarly, adult patients with severe TNFα inhibitor-induced psoriasis and psoriatic alopecia were homozygous wildtype carriers for a rare IL-23R variant.17 Consistent with molecular studies, IL-12/23 antagonism with ustekinumab has been an effective treatment in several CD patients who developed TNFα inhibitor-induced psoriasis.17,18 In addition, IL-23 regulates TH17 cells, which secrete IL-17, a cytokine known to play a critical role in psoriasis. IL-17 serum and lesional levels in plaque and pustular psoriasis are higher compared to controls.19 Furthermore, IL-17-expressing T cell infiltrates have been identified in TNFα inhibitor-induced psoriatic lesions.17,20 In a study by Tillack et al, patients with CD who required transition to ustekinumab expressed high levels of infiltrating IL-17A+ cells.17 While ustekinumab is not currently FDA approved for CD, clinical trials have shown promising results.21–23 This data suggests that IL-23 and/or IL-17 antagonism may benefit a patient subset with severe TNFα inhibitor-induced psoriasis.
Disequilibrium in proinflammatory cytokine interferon alpha (IFNα) levels in the setting of TNFα suppression is also thought to contribute to psoriatic lesion development.24–26 TNFα normally attenuates IFNα levels, therefore TNFα inhibition results in elevated IFNα. IFNα stimulates TH1 lymphocytes, which play a role in the pathogenesis of psoriasis.27 Furthermore, patients with hepatitis who developed psoriasis during IFNα treatment demonstrated regression of psoriatic lesions following IFN agent withdrawal.28,29 Moreover, increased IFNα expression has been demonstrated in lesional dermal vasculature in patients with TNFα inhibitor-induced psoriasis.24 Therefore, IFNα dysregulation may contribute to the pathogenesis of TNFα inhibitor-induced psoriasis.
The primary limitation of this review was variations in data presented in the reviewed publications. Not all authors performed biopsies or disclosed treatment approach or outcome. In addition, the follow-up interval varied among papers and may not truly represent the eventual clinical outcome. Furthermore, the long latency period between TNFα inhibitor initiation and psoriasis onset does not exclude the possibility of de novo psoriasis onset occurring independently of TNFα inhibitor therapy. The data is limited by the retrospective nature and reliance on case reports and small case series. Patients with inflammatory bowel or rheumatologic disease requiring TNFα inhibitor therapy are at risk for progression of disease if effective medications are discontinued after development of psoriasis. Identifying genotypical or phenotypical features of disease or risk factors for development of TNFα inhibitor-induced psoriasis may allow us to risk stratify patients. For example, with inflammatory bowel disease, the majority of patients reported to develop psoriasis had CD rather than UC, which may be due to the stronger association of CD and psoriasis with IL23R and IL12B gene variants than is observed with UC as discussed above. In addition, the majority of affected patients are female. Beyond that, with case reports and case series, only features of affected patients are presented, and it is not clear whether there is a difference in disease location, phenotype, or activity between patients who develop psoriasis and those who do not.
CONCLUSION
The paradoxical development of psoriasis can be an unintended consequence of TNFα antagonism. TNFα inhibitor cessation may result in resolution of induced psoriasis in nearly half the cases, but there is still a substantial proportion of patients for whom lesions may persist. Decisions regarding interruption of anti TNFα therapy should be carefully considered in light of the possibility of psoriasis persistence and possible loss of efficacy for rheumatologic or gastrointestinal disease with cessation, should rechallenge become necessary. Thus, an initial approach of “treating through” with typical skin-directed therapies is reasonable except in the most severe cases. Further research is needed to clarify optimal strategies and clinical outcomes based on different clinical subtypes. While much remains to be learned about the mechanism of TNFα-induced psoriasis, emerging data on specific IL23R genetic polymorphisms have revealed potential therapeutic targets for patients with severe disease.
ACKNOWLEDGMENTS
We would like to thank Mr. Chris Stave for assisting with the literature search and Dr. Tim Berger for his critical suggestions to improve the manuscript.
Funding sources:
This work was supported in part by grants from the National Institutes of Health (R01AR065174 and K08AR057763) to W.L., National Clinical and Translational Science Institute (8KL2TR000143) to E.L., and Dermatology Foundation Career Development Awards to E.L. and A.H.
Footnotes
Conflict of Interest Disclosure:
The authors do not report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kip KE, Swoger JM, Grandinetti LM, Barrie AM 3rd, Greer JB, Regueiro MD. Tumor necrosis factor alpha antagonist-associated psoriasis in inflammatory diseases: an analysis of the FDA adverse event reporting system. Inflammatory bowel diseases. May 2013;19(6):1164–1172. [DOI] [PubMed] [Google Scholar]
- 2.Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. Jul 15 2008;59(7):996–1001. [DOI] [PubMed] [Google Scholar]
- 3.Laga AC, Vleugels RA, Qureshi AA, Velazquez EF. Histopathologic spectrum of psoriasiform skin reactions associated with tumor necrosis factor-alpha inhibitor therapy. A study of 16 biopsies. The American Journal of dermatopathology. Aug 2010;32(6):568–573. [DOI] [PubMed] [Google Scholar]
- 4.Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. Jul 2009;61(1):104–111. [DOI] [PubMed] [Google Scholar]
- 5.Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol. Jun 2003;48(6):805–821; [DOI] [PubMed] [Google Scholar]
- 6.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. Nov 2012;148(11):1244–1250. [DOI] [PubMed] [Google Scholar]
- 7.Baeten D, Kruithof E, Van den Bosch F, et al. Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis. Sep 2003;62(9):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flendrie M, Vissers WH, Creemers MC, de Jong EM, van de Kerkhof PC, van Riel PL. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis research & therapy. 2005;7(3):R666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. Aug 2005;52(8):2513–2518. [DOI] [PubMed] [Google Scholar]
- 10.Zalla MJ, Su WP, Fransway AF. Dermatologic manifestations of human immunodeficiency virus infection. Mayo Clinic proceedings. Mayo Clinic Nov 1992;67(11):1089–1108. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentino DF. [Google Scholar]
- 12.Ma HL, Napierata L, Stedman N, et al. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. Feb 2010;62(2):430–440. [DOI] [PubMed] [Google Scholar]
- 13.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annual review of pathology. Jan 24 2013;8:477–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffin KC, Krueger GG. Genetic variations in cytokines and cytokine receptors associated with psoriasis found by genome-wide association. The Journal of investigative dermatology. Apr 2009;129(4):827–833. [DOI] [PubMed] [Google Scholar]
- 15.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. Dec 1 2006;314(5804):1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. Journal of pediatric gastroenterology and nutrition. May 2013;56(5):512–518. [DOI] [PubMed] [Google Scholar]
- 17.Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-gamma-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut Apr 2014;63(4):567–577. [DOI] [PubMed] [Google Scholar]
- 18.Andrisani G, Marzo M, Celleno L, et al. Development of psoriasis scalp with alopecia during treatment of Crohn’s disease with infliximab and rapid response to both diseases to ustekinumab. European review for medical and pharmacological sciences. Oct 2013;17(20):2831–2836. [PubMed] [Google Scholar]
- 19.Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch Dermatol Res. Aug 2012;304(6):465–469. [DOI] [PubMed] [Google Scholar]
- 20.Wlodarczyk M, Sobolewska A, Wojcik B, Loga K, Fichna J, Wisniewska-Jarosinska M. Correlations between skin lesions induced by anti-tumor necrosis factor-alpha and selected cytokines in Crohn’s disease patients. World journal of gastroenterology : WJG. Jun 14 2014;20(22):7019–7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. Oct 2008;135(4):1130–1141. [DOI] [PubMed] [Google Scholar]
- 22.Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. Nov 11 2004;351(20):2069–2079. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. Oct 18 2012;367(16):1519–1528. [DOI] [PubMed] [Google Scholar]
- 24.de Gannes GC, Ghoreishi M, Pope J, et al. Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch Dermatol. Feb 2007;143(2):223–231. [DOI] [PubMed] [Google Scholar]
- 25.Grinblat B, Scheinberg M. The enigmatic development of psoriasis and psoriasiform lesions during anti-TNF therapy: a review. Seminars in arthritis and rheumatism. Feb 2008;37(4):251–255. [DOI] [PubMed] [Google Scholar]
- 26.Gilliet M, Conrad C, Geiges M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. Dec 2004;140(12):1490–1495. [DOI] [PubMed] [Google Scholar]
- 27.Funk J, Langeland T, Schrumpf E, Hanssen LE. Psoriasis induced by interferon-alpha. Br J Dermatol. Nov 1991;125(5):463–465. [DOI] [PubMed] [Google Scholar]
- 28.Ketikoglou I, Karatapanis S, Elefsiniotis I, Kafiri G, Moulakakis A. Extensive psoriasis induced by pegylated interferon alpha-2b treatment for chronic hepatitis B. Eur J Dermatol. Mar-Apr 2005;15(2):107–109. [PubMed] [Google Scholar]
- 29.Horev A, Halevy S. New-onset psoriasis following treatment with pegylated interferonalpha 2b and ribavirin for chronic hepatitis C. The Israel Medical Association journal : IMAJ. Dec 2009;11(12):760–761. [PubMed] [Google Scholar]
- 30.Adams DR, Buckel T, Sceppa JA. Infliximab associated new-onset psoriasis. J Drugs Dermatol. Feb 2006;5(2):178–179. [PubMed] [Google Scholar]
- 31.Al-Mutairi A, Elkashlan M, Al-Fayed HM, Swayed M. TNF-alpha inhibitor (adalimumab) induced psoriasis: a case report. The Australasian journal of dermatology. May 2012;53(2):157. [DOI] [PubMed] [Google Scholar]
- 32.Angelucci E, Cocco A, Viscido A, Vernia P, Caprilli R. Another paradox in Crohn’s disease: new onset of psoriasis in a patient receiving tumor necrosis factor-alpha antagonist. Inflammatory bowel diseases. Aug 2007;13(8):1059–1061. [DOI] [PubMed] [Google Scholar]
- 33.Aslanidis S, Pyrpasopoulou A, Leontsini M, Zamboulis C. Anti-TNF-alpha-induced psoriasis: case report of an unusual adverse event. International journal of dermatology. Aug 2006;45(8):982–983. [DOI] [PubMed] [Google Scholar]
- 34.Bal A, Gurcay E, Aydog E, Umay E, Tatlican S, Cakci A. Onset of psoriasis induced by infliximab. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Apr 2008;14(2):128–129; [DOI] [PubMed] [Google Scholar]
- 35.Beuthien W, Mellinghoff HU, von Kempis J. Skin reaction to adalimumab. Arthritis Rheum. May 2004;50(5):1690–1692. [DOI] [PubMed] [Google Scholar]
- 36.Bordel-Gomez MT, Sanchez-Estella J, Martinez-Gonzalez O, Cardenoso-Alvarez ME. Palmoplantar psoriasis: a paradoxical adverse reaction induced by adalimumab. J Eur Acad Dermatol Venereol. Apr 2009;23(4):444–445. [DOI] [PubMed] [Google Scholar]
- 37.Bosch RI, Amo Ndel V, Manteca CF, Cortina EL, Polo RG, Courel LG. Psoriasis induced by anti-TNF probably not so uncommon. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Apr 2008;14(2):128; [DOI] [PubMed] [Google Scholar]
- 38.Broge T, Nguyen N, Sacks A, Davis M. Infliximab-associated psoriasis in children with Crohn’s disease may require withdrawal of anti-tumor necrosis factor therapy. Inflammatory bowel diseases. Apr 2013;19(5):E75–77. [DOI] [PubMed] [Google Scholar]
- 39.Buisson A, Cuny JF, Barbaud A, et al. Methotrexate for psoriasiform lesions associated with anti-tumour necrosis factor therapy in inflammatory bowel disease. Alimentary pharmacology & therapeutics. May 2012;35(10):1175–1180. [DOI] [PubMed] [Google Scholar]
- 40.Capkin E, Karkucak M, Yayli S, Aydin Capkin A, Tosun M. Infliximab-induced palmoplantar psoriasis in a patient with ankylosing spondylitis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Aug 2011;17(5):293–294. [DOI] [PubMed] [Google Scholar]
- 41.Carter JD, Gerard HC, Hudson AP. Psoriasiform lesions induced by tumour necrosis factor antagonists: a skin-deep medical conundrum. Ann Rheum Dis. Aug 2008;67(8):1181–1183. [DOI] [PubMed] [Google Scholar]
- 42.Chen LA, Su LH, Chang YJ, Hsu YL, Tsai TH. New-onset psoriasis associated with etanercept therapy. J Dermatol. Apr 2010;37(4):378–380. [DOI] [PubMed] [Google Scholar]
- 43.Cohen JD, Bournerias I, Buffard V, et al. Psoriasis induced by tumor necrosis factor-alpha antagonist therapy: a case series. J Rheumatol. Feb 2007;34(2):380–385. [PubMed] [Google Scholar]
- 44.Conklin LS, Cohen B, Wilson L, Cuffari C, Oliva-Hemker M. Rash induced by anti-tumor necrosis factor agents in an adolescent with Crohn’s disease. Nature reviews. Gastroenterology & hepatology. Mar 2010;7(3):174–177. [DOI] [PubMed] [Google Scholar]
- 45.Costa-Romero M, Coto-Segura P, Suarez-Saavedra S, Ramos-Polo E, Santos-Juanes J. Guttate psoriasis induced by infliximab in a child with Crohn’s disease. Inflammatory bowel diseases. Oct 2008;14(10):1462–1463. [DOI] [PubMed] [Google Scholar]
- 46.Cuchacovich R, Hagan J, Khan T, Richert A, Espinoza LR. Tumor necrosis factor-alpha (TNF-alpha)-blockade-induced hepatic sarcoidosis in psoriatic arthritis (PsA): case report and review of the literature. Clin Rheumatol. Jan 2011;30(1):133–137. [DOI] [PubMed] [Google Scholar]
- 47.Dalkilic E, Bulbul Baskan E, Alkis N, et al. Tumor necrosis factor-alpha antagonist therapy-induced psoriasis in Turkey: analysis of 514 patients. Modern rheumatology / the Japan Rheumatism Association. Sep 2012;22(5):738–742. [DOI] [PubMed] [Google Scholar]
- 48.De Castro GRW, Neves FS, Pereira IA, Souza Fialho SCM, Ribeiro G, Zimmermann AF. Resolution of adalimumab-induced psoriasis after vitamin D deficiency treatment. Rheumatology international. // 2012;32(5):1313–1316. [DOI] [PubMed] [Google Scholar]
- 49.Dereure O, Guillot B, Jorgensen C, Cohen JD, Combes B, Guilhou JJ. Psoriatic lesions induced by antitumour necrosis factor-alpha treatment: two cases. Br J Dermatol. Aug 2004;151(2):506–507. [DOI] [PubMed] [Google Scholar]
- 50.Doyle LA, Sperling LC, Baksh S, et al. Psoriatic alopecia/alopecia areata-like reactions secondary to anti-tumor necrosis factor-alpha therapy: a novel cause of noncicatricial alopecia. The American Journal of dermatopathology. Apr 2011;33(2):161–166. [DOI] [PubMed] [Google Scholar]
- 51.El Shabrawi-Caelen L, La Placa M, Vincenzi C, Haidn T, Muellegger R, Tosti A. Adalimumab-induced psoriasis of the scalp with diffuse alopecia: a severe potentially irreversible cutaneous side effect of TNF-alpha blockers. Inflammatory bowel diseases. Feb 2010;16(2):182–183. [DOI] [PubMed] [Google Scholar]
- 52.English PL, Vender R. Occurrence of plantar pustular psoriasis during treatment with infliximab. Journal of cutaneous medicine and surgery. Jan-Feb 2009;13(1):40–42. [DOI] [PubMed] [Google Scholar]
- 53.Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-alpha inhibitor therapy. Clin Exp Dermatol. Aug 2012;37(6):639–641. [DOI] [PubMed] [Google Scholar]
- 54.Faillace C, Duarte GV, Cunha RS, de Carvalho JF. Severe infliximab-induced psoriasis treated with adalimumab switching. International journal of dermatology. Feb 2013;52(2):234–238. [DOI] [PubMed] [Google Scholar]
- 55.Famenini S, Wu JJ. Infliximab-induced psoriasis in treatment of Crohn’s disease-associated ankylosing spondylitis: case report and review of 142 cases. J Drugs Dermatol. Aug 2013;12(8):939–943. [PubMed] [Google Scholar]
- 56.Goldstein J, Levine J. Infliximab-induced psoriaform rash. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Oct 2010;8(10):A24. [DOI] [PubMed] [Google Scholar]
- 57.Goncalves DP, Laurindo I, Scheinberg MA. The appearance of pustular psoriasis during antitumor necrosis factor therapy. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Oct 2006;12(5):262. [DOI] [PubMed] [Google Scholar]
- 58.Grinblat B, Scheinberg M. Unexpected onset of psoriasis during infliximab treatment: comment on the article by Beuthien et al. Arthritis Rheum. Apr 2005;52(4):1333–1334; [DOI] [PubMed] [Google Scholar]
- 59.Harris MD, Richards R. First case report of adalimumab-induced psoriasis in Crohn’s disease. The American journal of gastroenterology. Mar 2009;104(3):792–793. [DOI] [PubMed] [Google Scholar]
- 60.Hawryluk EB, Linskey KR, Duncan LM, Nazarian RM. Broad range of adverse cutaneous eruptions in patients on TNF-alpha antagonists. Journal of cutaneous pathology. May 2012;39(5):481–492. [DOI] [PubMed] [Google Scholar]
- 61.Hiremath G, Duffy L, Leibowitz I. Infliximab-induced psoriasis in children with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. Feb 2011;52(2):230–232. [DOI] [PubMed] [Google Scholar]
- 62.Iborra M, Beltran B, Bastida G, Aguas M, Nos P. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a paradoxical side effect. Journal of Crohn’s & colitis. Apr 2011;5(2):157–161. [DOI] [PubMed] [Google Scholar]
- 63.Kary S, Worm M, Audring H, et al. New onset or exacerbation of psoriatic skin lesions in patients with definite rheumatoid arthritis receiving tumour necrosis factor alpha antagonists. Ann Rheum Dis. Mar 2006;65(3):405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawazoe Y, Sugita S, Yamada Y, Akino A, Miura K, Mochizuki M. Psoriasis triggered by infliximab in a patient with Behcet’s disease. Japanese journal of ophthalmology. Jan 2013;57(1):95–97. [DOI] [PubMed] [Google Scholar]
- 65.Klein RQ, Spivack J, Choate KA. Psoriatic skin lesions induced by certolizumab pegol. Arch Dermatol. Sep 2010;146(9):1055–1056. [DOI] [PubMed] [Google Scholar]
- 66.Korkmaz U, Duman AE, Dindar G, et al. Adalimumab-induced psoriasis in a patient with Crohn’s disease. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. Mar 2013;32(2):135–136. [DOI] [PubMed] [Google Scholar]
- 67.Kuhara T, Watanabe D, Iwahori Y, Tamada Y, Yamamura M, Matsumoto Y. Psoriasiform and pustular eruption induced by etanercept and infliximab. Eur J Dermatol. Jul-Aug 2009;19(4):388–389. [DOI] [PubMed] [Google Scholar]
- 68.Lee HH, Song IH, Friedrich M, et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol. Mar 2007;156(3):486–491. [DOI] [PubMed] [Google Scholar]
- 69.Lo Nigro A, Ramonda R, Alaibac M, Modesti V, Punzi L. Multiple paradoxical adverse events in a patient affected with ankylosing spondylitis treated with TNF blockers. Eur J Dermatol. Mar-Apr 2011;21(2):263–264. [DOI] [PubMed] [Google Scholar]
- 70.Lopez-Robles A, Queiro R, Alperi M, Alonso S, Riestra JL, Ballina J. Psoriasis and psoriasiform lesions induced by TNFalpha antagonists: the experience of a tertiary care hospital from northern Spain. Rheumatology international. Dec 2012;32(12):3779–3783. [DOI] [PubMed] [Google Scholar]
- 71.Manni E, Barachini P. Psoriasis induced by infliximab in a patient suffering from Crohn’s disease. Int J Immunopathol Pharmacol. Jul-Sep 2009;22(3):841–844. [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Moran C, Sanz-Munoz C, Morales-Callaghan AM, Torrero V, Miranda-Romero A. Pustular psoriasis induced by infliximab. J Eur Acad Dermatol Venereol. Nov 2007;21(10):1424–1426. [DOI] [PubMed] [Google Scholar]
- 73.Matthews C, Rogers S, FitzGerald O. Development of new-onset psoriasis while on antiTNFalpha treatment. Ann Rheum Dis. Nov 2006;65(11):1529–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medkour F, Babai S, Chanteloup E, Buffard V, Delchier JC, Le-Louet H. Development of diffuse psoriasis with alopecia during treatment of Crohn’s disease with infliximab. Gastroenterologie clinique et biologique. Feb 2010;34(2):140–141. [DOI] [PubMed] [Google Scholar]
- 75.Michaelsson G, Kajermo U, Michaelsson A, Hagforsen E. Infliximab can precipitate as well as worsen palmoplantar pustulosis: possible linkage to the expression of tumour necrosis factor-alpha in the normal palmar eccrine sweat duct? Br J Dermatol. Dec 2005;153(6):1243–1244. [DOI] [PubMed] [Google Scholar]
- 76.Mocciaro F, Renna S, Orlando A, Cottone M. Severe cutaneous psoriasis after certolizumab pegol treatment: report of a case. The American journal of gastroenterology. Nov 2009;104(11):2867–2868. [DOI] [PubMed] [Google Scholar]
- 77.Nakagomi D, Harada K, Yagasaki A, Kawamura T, Shibagaki N, Shimada S. Psoriasiform eruption associated with alopecia areata during infliximab therapy. Clin Exp Dermatol. Dec 2009;34(8):923–924. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen K, Vleugels RA, Velez NF, Merola JF, Qureshi AA. Psoriasiform reactions to anti-tumor necrosis factor alpha therapy. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Oct 2013;19(7):377–381. [DOI] [PubMed] [Google Scholar]
- 79.Osorio F, Magro F, Lisboa C, et al. Anti-TNF-alpha induced psoriasiform eruptions with severe scalp involvement and alopecia: report of five cases and review of the literature. Dermatology. 2012;225(2):163–167. [DOI] [PubMed] [Google Scholar]
- 80.Papadavid E, Gazi S, Dalamaga M, Stavrianeas N, Ntelis V. Palmoplantar and scalp psoriasis occurring during anti-tumour necrosis factor-alpha therapy: a case series of four patients and guidelines for management. J Eur Acad Dermatol Venereol. Mar 2008;22(3):380–382. [DOI] [PubMed] [Google Scholar]
- 81.Park JJ, Lee SC. A Case of Tumor Necrosis Factor-alpha Inhibitors-induced Pustular Psoriasis. Annals of dermatology. May 2010;22(2):212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peek R, Scott-Jupp R, Strike H, Clinch J, Ramanan AV. Psoriasis after treatment of juvenile idiopathic arthritis with etanercept. Ann Rheum Dis. Sep 2006;65(9):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peramiquel L, Puig L, Dalmau J, Ricart E, Roe E, Alomar A. Onset of flexural psoriasis during infliximab treatment for Crohn’s disease. Clin Exp Dermatol. Nov 2005;30(6):713–714. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Perez L, Caeiro JL, Fabeiro JM, Allegue F, Zulaica A. Induction of pustular lesions during infliximab therapy for Crohn’s disease. Acta Derm Venereol. 2008;88(3):292–293. [DOI] [PubMed] [Google Scholar]
- 85.Perman MJ, Lovell DJ, Denson LA, Farrell MK, Lucky AW. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatric dermatology. Jul-Aug 2012;29(4):454–459. [DOI] [PubMed] [Google Scholar]
- 86.Pirard D, Arco D, Debrouckere V, Heenen M. Anti-tumor necrosis factor alpha-induced psoriasiform eruptions: three further cases and current overview. Dermatology. 2006;213(3):182–186. [DOI] [PubMed] [Google Scholar]
- 87.Pontikaki I, Shahi E, Frasin LA, et al. Skin manifestations induced by TNF-alpha inhibitors in juvenile idiopathic arthritis. Clinical reviews in allergy & immunology. Apr 2012;42(2):131–134. [DOI] [PubMed] [Google Scholar]
- 88.Pourciau C, Shwayder T. Occurrence of pustular psoriasis after treatment of Crohn disease with infliximab. Pediatric dermatology. Sep-Oct 2010;27(5):539–540. [DOI] [PubMed] [Google Scholar]
- 89.Richette P, Viguier M, Bachelez H, Bardin T. Psoriasis induced by anti-tumor necrosis factor therapy: a class effect? J Rheumatol. Feb 2007;34(2):438–439. [PubMed] [Google Scholar]
- 90.Roux CH, Brocq O, Leccia N, et al. New-onset psoriatic palmoplantaris pustulosis following infliximab therapy: a class effect? J Rheumatol. Feb 2007;34(2):434–437. [PubMed] [Google Scholar]
- 91.Safa G, Martin A, Darrieux L. Exacerbation of infliximab-induced palmoplantar psoriasis under ustekinumab therapy in a patient with ankylosing spondylitis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Oct 2011;17(7):385–386. [DOI] [PubMed] [Google Scholar]
- 92.Sari I, Akar S, Birlik M, Sis B, Onen F, Akkoc N. Anti-tumor necrosis factor-α-induced psoriasis. Journal of Rheumatology. // 2006;33(7):1411–1414. [PubMed] [Google Scholar]
- 93.Sauder MB, Glassman SJ. Palmoplantar subcorneal pustular dermatosis following adalimumab therapy for rheumatoid arthritis. International journal of dermatology. May 2013;52(5):624–628. [DOI] [PubMed] [Google Scholar]
- 94.Scheinberg M, Goncalves DP, Laurindo IM. Anti-TNF agents inducing psoriasis: a recognized adverse effect. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. Apr 2008;14(2):130. [DOI] [PubMed] [Google Scholar]
- 95.Sedie AD, Bazzichi L, Bombardieri S, Riente L. Psoriasis, erythema nodosum, and nummular eczema onset in an ankylosing spondylitis patient treated with infliximab [2]. Scandinavian journal of rheumatology. // 2007;36(5):403–404. [DOI] [PubMed] [Google Scholar]
- 96.Severs GA, Lawlor TH, Purcell SM, Adler DJ, Thompson R. Cutaneous adverse reaction to infliximab: report of psoriasis developing in 3 patients. Cutis; cutaneous medicine for the practitioner. Sep 2007;80(3):231–237. [PubMed] [Google Scholar]
- 97.Sharma N, Lindsay J. Anti-TNF-Alpha-Induced Psoriasis - An Unusual Paradox. Case reports in gastroenterology. 2009;3(3):404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shelling ML, Vitiello M, Lanuti EL, Miteva M, Romanelli P, Kerdel FA. A Case of Palmoplantar Pustulosis Induced by Certolizumab Pegol: New Anti-TNF-alpha Demonstrates the Same Class Effect. The Journal of clinical and aesthetic dermatology. Aug 2012;5(8):40–41. [PMC free article] [PubMed] [Google Scholar]
- 99.Soto Lopes MS, Trope BM, Rochedo Rodriguez MP, Grynszpan RL, Cuzzi T, Ramos ESM. Paradoxical Reaction to Golimumab: Tumor Necrosis Factor alpha Inhibitor Inducing Psoriasis Pustulosa. Case reports in dermatology. 2013;5(3):326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Starmans-Kool MJ, Peeters HR, Houben HH. Pustular skin lesions in patients treated with infliximab: report of two cases. Rheumatology international. Sep 2005;25(7):550–552. [DOI] [PubMed] [Google Scholar]
- 101.Steinwurz F, Denadai R, Saad-Hossne R, Queiroz ML, Teixeira FV, Romiti R. Infliximab-induced psoriasis during therapy for Crohn’s disease. Journal of Crohn’s & colitis. Jun 2012;6(5):610–616. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi H, Hashimoto Y, Ishida-Yamamoto A, Ashida T, Kohgo Y, Iizuka H. Psoriasiform and pustular eruption induced by infliximab. J Dermatol. Jul 2007;34(7):468–472. [DOI] [PubMed] [Google Scholar]
- 103.Teraki Y, Tanaka S, Hitomi K, Izaki S. A case of generalized psoriasiform and pustular eruption induced by infliximab: evidence for skin-homing Th17 in the pathogenesis. Br J Dermatol. Dec 2010;163(6):1347–1351. [DOI] [PubMed] [Google Scholar]
- 104.Thurber M, Feasel A, Stroehlein J, Hymes SR. Pustular psoriasis induced by infliximab. J Drugs Dermatol. Jul-Aug 2004;3(4):439–440. [PubMed] [Google Scholar]
- 105.Ubriani R, Van Voorhees AS. Onset of psoriasis during treatment with TNF-{alpha} antagonists: a report of 3 cases. Arch Dermatol. Feb 2007;143(2):270–272. [DOI] [PubMed] [Google Scholar]
- 106.Umeno J, Matsumoto T, Jo Y, Ichikawa M, Urabe K, Iida M. Psoriasis during anti-tumor necrosis factor-alpha therapy for Crohn’s disease. Inflammatory bowel diseases. Sep 2007;13(9):1188–1189. [DOI] [PubMed] [Google Scholar]
- 107.Verea MM, Del Pozo J, Yebra-Pimentel MT, Porta A, Fonseca E. Psoriasiform eruption induced by infliximab. The Annals of pharmacotherapy. Jan 2004;38(1):54–57. [DOI] [PubMed] [Google Scholar]
- 108.Volpe A, Caramaschi P, Carletto A, Pieropan S, Bambara LM, Biasi D. Psoriasis onset during infliximab treatment: description of two cases. Rheumatology international. Oct 2006;26(12):1158–1160. [DOI] [PubMed] [Google Scholar]
- 109.Wegscheider BJ, El-Shabrawi L, Weger M, et al. Adverse skin reactions to infliximab in the treatment of intraocular inflammation. Eye (London, England). Apr 2007;21(4):547–549. [DOI] [PubMed] [Google Scholar]
- 110.Wollina U, Hansel G, Koch A, Schonlebe J, Kostler E, Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008;9(1):1–14. [DOI] [PubMed] [Google Scholar]
- 111.Younis S, Rimar D, Slobodin G, Rosner I. Tumor necrosis factor-associated palmoplantar pustular psoriasis treated with interleukin 6 blocker. J Rheumatol. Oct 2012;39(10):2055–2056. [DOI] [PubMed] [Google Scholar]