Abstract
Measles virus infection can result in a variety of immunologic defects. We have begun studies to determine the basis for the lack of immune responsiveness to antigen and mitogen following infection. Here we present data showing that Epstein-Barr virus-transformed B-cell lines infected with measles virus produce a soluble factor that can inhibit antigen-specific T-cell proliferation and inhibit the proliferation of uninfected B cells. The soluble factor was neither interleukin-10, transforming growth factor β, nor alpha/beta interferon. B cells infected with measles virus or treated with the soluble factor were unable to present antigen to T cells in a manner that supported antigen-specific proliferation. This could represent one mechanism of how measles virus limits T-cell expansion. However, we found that once CD4+ or CD8+ T cells were activated, their cytolytic activity was intact whether infected with measles virus or treated with soluble factor. Thus, while slow to be generated these cytoxic cells could participate in viral clearance.
Measles virus was the first virus reported to alter immune function (39). Since that time, various investigators have studied different aspects of immune dysfunction, including reduction of mitogen-induced proliferation of T cells obtained from patients following natural infection and from individuals following vaccination with measles virus (6, 13, 15, 20, 21, 23, 25, 37, 40–42) or by direct infection of lymphoid cells (36) as a means to study measles-induced immunosuppression (2, 4, 7, 16, 26–30, 43, 44). Similarly, B cells have been studied for functional alterations induced by measles virus infection. McChesney et al. found that infection of B cells led to decreased antibody production when B cells were stimulated by pokeweed mitogen (28). More recently, Ravanel et al. have shown that the nucleocapsid protein of measles virus had the ability to bind to B cells through the Fcγ receptor and inhibit immunoglobulin (Ig) synthesis (34). In contrast, measles virus-infected T cells still have the ability to produce cytokines required to help uninfected B cells differentiate into plasma cells and secrete Ig (31). Thus, measles virus infection can affect T- and B-cell function.
We have previously shown that infection of antigen-specific T-cell lines with measles virus can inhibit antigen and mitogen driven proliferation (1). We have also found that infected T cells produce a soluble factor which can also inhibit the proliferation of uninfected antigen-specific T cells in response to antigen, mitogen, and superantigen (38). By sizing experiments, the antiproliferative activity was shown to be larger than 60 kDa. Heating to 56°C for 30 min destroyed the activity. The activity was found in fractions smaller than 10 kDa after trypsin treatment (38). These data suggest that the factor may be a new cytokine with antiproliferative effects.
In this work, we examined the immunologic consequences of B-cell infection by measles virus. We demonstrate that measles virus infection of B cells at very low multiplicities of infection (MOIs) can inhibit the spontaneous proliferation of Epstein-Barr virus (EBV)-transformed B cells. This inhibitory effect was due to a soluble factor that was actively secreted by infected B cells. Further, the antiproliferative effect was not due to interleukin-10 (IL-10), transforming growth factor β (TGF-β), or alpha/beta interferon (IFN-α/β). The soluble factor was larger than 50 kDa and inactivated at temperatures above 55°C. In addition, treatment of B cells with this soluble factor inhibited their ability to act as fully competent antigen-presenting cells (APCs) for antigen-specific T-cell proliferation. The inhibition of APC function was not due to the elaboration of the antiproliferative factor. The effects of direct infection and the production of the antiproliferative factor would limit T-cell expansion. However, effector function has not been studied. To determine whether effector functions were affected, cloned cytolytic CD4+ and CD8+ T-cell lines were tested for the ability to lyse target cells. Whether infected with measles virus or treated with high concentrations of soluble factor, these clonal T-cell lines retained the ability to perform cytolytic functions. This is the first demonstration that infection of B cells by measles virus can inhibit antigen presentation and that a soluble factor can mirror the inhibitory effects of infection. However, once activated T cells are generated, they retain the ability to kill. This suggests that while slow to be generated, effector T cells, once activated and present in sufficient numbers, maintain the ability to clear virus-infected cells.
MATERIALS AND METHODS
B cells.
Peripheral blood mononuclear cells (PBMCs) were obtained from 15 ml of autologous blood. PBMCs were separated on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). Cells were collected and washed three times with RPMI 1640 (Gibco/BRL, Grand Island, N.Y.). One milliliter of a culture supernatant from IA3 cells producing EBV was added to 3 × 106 PBMCs, and cells were cultured in the presence of cyclosporin (2 μg/ml) in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 1% antibiotics in 24-well plates. Transformed B cells were observed growing out of the PBMC population within 14 days after infection. B cells were subcultured two to four times prior to being used as APCs. By flow cytometry analyses, B-cell lines were greater than 99% surface Ig positive and less than 1% CD3 positive.
Antigen-specific T-cell lines.
PBMCs from healthy volunteers were obtained. Antigen-specific T-cell lines were isolated as previously described (1, 3). Briefly, T-cell lines were generated by culturing fresh PBMCs (3 × 106 cells/well) with antigen (purified protein derivative [PPD; 5 μg/ml; Serum Institute, Copenhagen, Denmark] or myelin basic protein [MBP; 30 μg/ml]) in 24-well plates. At 1 week, these primary cultures were examined by inverted microscopy. Wells showing colonies of growing T cells were maintained with the addition of recombinant human IL-2 (20 U/ml; Chiron, Emeryville, Calif.) every 2 to 3 days and restimulated with either PPD or MBP presented by irradiated (4,000 R) autologous PBMCs as APCs at about 2-week intervals. Antigen-specific proliferation was assessed by [3H]thymidine incorporation (0.5 μCi/well) in response to stimulation with antigen. Cultured T-cell lines were CD4+ as determined by flow cytometry (Huntsman Cancer Institute Flow Cytometry Facility).
Clonal allotype-specific T-cell lines were generated as described by Gubarev et al. (14). These clonal lines were either CD4+ or CD8+ as determined by flow cytometry. T cells were either infected with measles virus at an MOI of 0.05 or treated with a 1/2 or 1/4 dilution of supernatant from uninfected or infected B cells for 18 h. Cells were washed three times with RPMI 1640 (Gibco/BRL). Cytotoxic T-lymphocyte (CTL) assays were performed as described below.
Infection, proliferation assays, and supernatant fluids.
B cells were infected with the Edmonston strain of measles virus (American Type Culture Collection, Rockville, Md.) at various MOIs for 1 h at 37°C. Control B cells were mock infected and incubated at 37°C for 1 h. Cells were then washed three times with phosphate-buffered saline, resuspended in RPMI 1640 (Gibco/BRL), supplemented with 10% FCS (Gibco/BRL) and 1% antibiotics (MediaTech, Herndon, Va.), and incubated overnight at 37°C. B cells were washed three times, and viability was determined by trypan blue exclusion. Cells were then irradiated (6,000 R) and used as APCs. For proliferation assays, and 3 × 104 to 5 × 104 cells infected as described above were added to each well in 96-well plates, pulsed with [3H]thymidine on day 2, and harvested on day 3 postinfection.
For the determination of antigen-induced proliferation, antigen-specific T-cell lines were cultured in triplicate wells, each containing 3 × 104 to 5 × 104 cells, with autologous EBV-transformed B cells (APCs) (in a 1-to-1 ratio; 3 × 104 to 5 × 104 cells/well). Antigen, either MBP (30 μg/ml) or PPD (5 μg/ml), was added to selected microwells. Cultures were assayed for proliferation by pulsing with [3H]thymidine (New England Nuclear, Wilmington, Del.) for the final 18 to 24 h of a 96-h proliferation assay.
For production of soluble factor, B cells were infected with measles virus (MOI of 0.005), washed extensively, and incubated in culture overnight. The B cells were then washed three times with Hanks balanced salt solution and counted. Cells were cultured in RPMI 1640 supplemented with 10% FCS and 1% antibiotics. HeLa and Vero cells were infected at an MOI of 0.05. Supernatants were collected on day 3 of culture. Supernatants were subjected to UV irradiation (Philips Sterileguard G36T6L at a distance of 20 cm for 1 h), filtered through a 0.2-μm-pore-size filter, and stored at −70°C until used. This treatment is able to reduce 106 PFU/ml to undetectable levels as determined by viral plaque assay. When the supernatants were plaqued on Vero cell monolayers, no infectious virus was detected. In addition, guanidinium thiocyanate extracts were prepared from supernatants from infected or mock-infected B cells, and RNA was subjected to reverse transcriptase PCR using measles virus nucleocapsid-specific primers. No band was detected with this technique, whereas RNA from gradient-purified measles virus (10) produced a unique band of appropriate size. Under the conditions used, the lower limit of detection was 250 pg of measles virus RNA. Further, supernatants from infected B cells were added to Vero cell monolayers and allowed to incubate for 1 h at 37°C. Vero cells were then cultured overnight, and RNA was extracted and subjected to reverse transcriptase PCR. Measles virus RNA was not detected.
In additional control experiments to determine whether UV-treated supernatant could induce a nonproductive or abortive measles virus infection in B cells, B cells were treated with undiluted supernatant from infected or mock-infected B cells for 24 h. At the same time, B cells were infected with measles virus (MOI of 3). B cells were then washed three times and stained for surface expression of measles virus hemagglutinin (HA), using an anti-HA monoclonal antibody (1/25 dilution) (9) or serum from a patient with subacute sclerosing panencephalitis (SSPE serum; 1/100 dilution) containing high-titered antibody to HA and fusion protein (12). The second antibodies used were a fluorescein isothiocyanate-labeled goat anti-mouse IgG (The Binding Site, Birmingham, England) add a fluorescein isothiocyanate-labeled goat anti-human IgG (Calbiochem, San Diego, Calif.). Flow cytometric analyses were performed to assess the fluorescence intensity of positive cells. No staining for HA above the control level was seen with the two measles virus antibody reagents.
For sizing experiments, supernatants were obtained from measles virus-infected (MOI of 0.05) or mock-infected B cells on day 3 postinfection. Samples were filtered through Microcon 10, 50, and 100 concentrators (molecular size cutoffs of 10, 50, and 100 kDa, respectively; Amicon Inc., Beverly, Mass.). Samples were used at a 1/2 dilution to treat B-cell cultures. After 24 h, B-cell cultures were pulsed with [3H]thymidine, and cultures were harvested the next day to measure thymidine incorporation.
To determine whether treatment of B cells permanently inhibited proliferation or whether cells could recover after treatment, B cells were cultured in the presence of supernatant from infected or uninfected B cells for 3 days. The B cells were then washed three times with Hanks balanced salt solution and cultured in RPMI 1640 supplemented with 10% FCS for 2, 6, or 8 days without any additional supernatant. At each time point, cells were counted and equivalent numbers of viable cells (5 × 104 cells) were transferred to 96 wells and pulsed with [3H]thymidine for 6 to 8 h, after which time cells were harvested and incorporation was measured.
Measles virus plaque assay and infectious center assay.
Virus was diluted in phosphate-buffered saline containing 3% FCS and 1% antibiotics (MediaTech). Tenfold serial dilutions were made, and 200 μl was added to confluent Vero cell monolayers in six-well cluster plates (Costar, Pleasanton, Calif.). Virus was allowed to adsorb for 1 h at 37°C in a humidified incubator in a 5% CO2 atmosphere. Cluster plates were rocked every 10 to 15 min, fluid was aspirated after 1 h, and 3 ml of overlay medium (medium 199 [Gibco] containing 1% antibiotics and 10% FCS) was added. Cells were incubated for 6 days at 37°C in a humidified incubator in a 5% CO2 atmosphere, at which time cell monolayers were stained with neutral red and plaques were enumerated (11).
B cells were infected at an MOI of 0.001 or 0.005 as follows. After incubation with measles virus for 1 h at 37°C, 106 B cells were washed three times with RPMI 1640, cultured overnight at 37°C in a humidified incubator containing 5% CO2, then washed three times with RPMI 1640, and counted. Infected cells were then divided into two groups. One was diluted and added to Vero cell monolayers; the second was subjected to freezing and thawing, diluted, and added to Vero cell monolayers. After 1 h of incubation at 37°C, 3 ml of overlay medium (see above) was added. Cells were incubated as described above and stained with neutral red after 6 days. The number of plaques from the freeze-thawed group was subtracted from the value for the whole-cell group, and infectious centers were enumerated.
CTL assay.
Target cell lines consisting of 2 × 103 B-lymphoblastoid cells which differed at minor HLA antigens were labeled with 51Cr (DuPont NEN, Boston, Mass.), mixed with allo-specific CD4+ or CD8+ T-cell clones at various effector/target ratios, and cultured in 96-well plates. After 4 h, plates were centrifuged, and 1/2 volume of medium was removed from each well and counted in a Packard Cobra gamma counter. Maximum release and minimum release were determined by treating labeled cells with Triton X-100 (Sigma, St. Louis, Mo.) and medium, respectively (32).
RESULTS
B cells become infected during the viremic stage of measles (8). When infected in vitro, B cells lose the ability to proliferate due to an inhibition in G1 of the cell cycle (30). The studies by McChesney et al. (30) used high MOIs where all B cells would be expected to be infected. To extend these observations and assess the effect of measles virus infection on the proliferation of EBV-transformed B-cell lines, B cells were infected at MOIs of 0.05 to 0.000005 (28). We wanted to determine how few infectious measles virions could be used to inhibit proliferation and approximate levels that would be comparable to the viremic phase of infection. It is clear from Table 1 (experiment A) that B cells infected with very few infectious virions were inhibited from proliferation. UV-inactivated measles virus had no effect on B-cell proliferation (experiment B). Thus, we have confirmed the original observation by McChesney et al. (30) and have demonstrated that only a few infectious virions were needed to inhibit proliferation.
TABLE 1.
Suppressive effect of measles virus on B-cell proliferationa
| Expt | MOI | cpm ± SEMb |
|---|---|---|
| A | 0 | 25,731 ± 1,507 |
| 0.05 | 2,369 ± 52 | |
| 0.005 | 2,096 ± 82 | |
| 0.0005 | 6,459 ± 78 | |
| 0.00005 | 13,126 ± 691 | |
| 0.000005 | 15,589 ± 49 | |
| B | 0 | 26,216 ± 543 |
| 0.05 | 28,230 ± 484 | |
| 0.005 | 25,834 ± 603 | |
| 0.0005 | 26,158 ± 747 | |
| 0.00005 | 27,836 ± 1,072 |
EBV-transformed B cells were infected at the MOIs indicated (experiment A) and washed three times. Cells were resuspended in medium, and 3 × 104 to 5 × 104 cells were added to each well. Cultures were pulsed 2 days later with [3H]thymidine, harvested, and counted the next day. The same virus preparation as used to infect B cells in experiment A was subjected to UV irradiation for 1 h, and equivalent MOIs of inactivated measles virus were used to infect B cells (experiment B). No PFU were detected after 1 h of UV irradiation. Cells were washed three times and resuspended in medium; 3 × 104 to 5 × 104 cells were added to each of 96 wells. Cultures were pulsed 2 days later with [3H]thymidine, harvested, and counted the next day.
Mean of three wells.
In these assays, it was important to determine whether MOI correlated with the actual number of infected cells. Therefore, we infected B cells at an MOI of 0.001 and performed infectious center assays. A portion of the solution used to infect the cells was also plaqued to confirm the actual number of input PFU. Three experiments were performed. The actual MOIs were 0.0014, 0.0017, and 0.0012 as determined by plaque assay. The numbers of infectious centers were 2.5/10,000, 4.3/10,000, and 4.2/10,000 cells, respectively. This corresponds to an input of 14.3 PFU/10,000 cells (average), resulting in 3 per 10,000 (average) cells actually being infected. Therefore, under the conditions used in our studies, the efficiency of infection was 21%.
We have recently demonstrated that infection of antigen-specific T cells with measles virus results in an inability to proliferate in response to antigen presented by APCs (1) and that supernatants from infected T cells contain a factor that could inhibit antigen-, mitogen- and IL-2-induced T-cell proliferation (38). To ascertain whether measles virus infection of B cells (APCs) could affect antigen-specific T-cell proliferation, B cells were infected with measles virus at various MOIs or mock infected, washed three times, and cultured for 18 h at 37°C. B cells were then collected, washed extensively, irradiated, and used as APCs. PPD-specific T cells, B cells (mock or infected), and PPD were cultured for 3 days and pulsed with [3H]thymidine. Cultures were harvested the next day (day 4, and thymidine incorporation was determined. As shown in Table 2, infection of B cells in these assays suppressed antigen-specific T-cell proliferation. Addition of as few as five infectious virions to 103 B cells resulted in a greater than 90% decrease in antigen-specific T-cell proliferation. Infectious center assays of four experiments revealed that among B cells infected at an MOI of 0.005, 3 to 7.4% of the total cells in the cultures scored positive.
TABLE 2.
Suppression of antigen-specific proliferation by infection of APCsa
| Cells | Antigen | MOI | cpm ± SEMb |
|---|---|---|---|
| T alone | None | 0 | 36 ± 10 |
| B alone | None | 0 | 3,352 ± 97 |
| T + B | None | 0 | 4,249 ± 162 |
| PPD | 0 | 42,017 ± 3,026 | |
| PPD | 0.05 | 556 ± 24 | |
| PPD | 0.005 | 1,976 ± 88 | |
| PPD | 0.0005 | 38,536 ± 1,436 | |
| PPD | 0.00005 | 32,562 ± 1,297 | |
| PPD | 0.000005 | 31,360 ± 1,934 |
B cells were infected at the indicated MOI or mock infected. Cells were washed two times and cultured overnight, washed extensively, counted, and irradiated with 6,000 R. T cells, B cells, and PPD (5 μg/ml) were all combined in 96 wells and cultured for 4 days. Cultures were pulsed with 1 μCi of [3H]thymidine for the last 18 h of culture.
Mean of three wells.
A potential explanation for the above observations is that the few infected cells secrete a cytokine or synthesize a viral product which has antiproliferative effects and/or affect the ability of B cells to present antigen. To further explore this issue, B cells were infected at an MOI of 0.005 or mock infected, cultured for 18 h, washed extensively, and further cultured for 72 h, after which supernatants were collected. The supernatants from infected and mock-infected B cells were exposed to UV irradiation and then added to uninfected cultures of antigen-specific T cells, APCs, and antigen. The data are shown in Table 3, experiment A. The supernatant from infected B cells had the ability to inhibit the proliferation of stimulated uninfected T-cell cultures. Similar inhibitory effects were observed for a different T-cell line specific for MBP generated from a different individual. In contrast, infected supernatant fluids were obtained from measles virus-infected HeLa and Vero cells. When supernatants were UV treated, filtered, and added to T-cell proliferation assays, no suppression of T-cell proliferation was observed (Table 3, experiment B).
TABLE 3.
Suppression of antigen-specific proliferation by supernatant from measles virus-infected B cells
| Expt | Cells | Antigen | Supernatant | cpm ± SEMa |
|---|---|---|---|---|
| Ab | T + PBMC | None | None | 2,765 ± 274 |
| T + PBMC | PPD | None | 29,519 ± 1,370 | |
| T + PBMC | PPD | 1/10, uninf | 35,010 ± 5,211 | |
| T + PBMC | PPD | 1/10, inf | 2,602 ± 153 | |
| T + PBMC | PPD | 1/50, inf | 8,093 ± 413 | |
| T + PBMC | PPD | 1/250, inf | 16,063 ± 363 | |
| T + PBMC | PPD | 1/1,000, inf | 21,941 ± 1,924 | |
| Bc | T alone | None | None | 86 ± 1 |
| PBMC alone | None | None | 2,792 ± 61 | |
| T + PBMC | None | None | 3,667 ± 317 | |
| T + PBMC | PPD | None | 39,564 ± 845 | |
| T + PBMC | PPD | HeLa, 1:50 | 36,009 ± 794 | |
| T + PBMC | PPD | HeLa, 1:100 | 36,597 ± 702 | |
| T + PBMC | PPD | Vero, 1:50 | 35,883 ± 627 | |
| T + PBMC | PPD | Vero, 1:100 | 37,957 ± 2,575 |
Average of three wells.
B cells were infected with measles virus at an MOI of 0.005 (inf) or mock infected (uninf), and supernatants were collected 3 days later. Supernatants were diluted and added to cultures containing uninfected T cells and autologous irradiated PBMCs and PPD (5 μg/ml). Cultures were pulsed with 1 μCi of [3H]thymidine for the last 18 h of a total culture period of 96 h. PBMCs were irradiated with 4,000 R.
HeLa and Vero cells were infected with measles virus at an MOI of 0.05, and supernatants were collected 3 days later. Supernatants were diluted and added to cultures containing uninfected T cells and autologous irradiated PBMCs and PPD (5 μg/ml). Cultures were pulsed with 1 μCi of [3H]thymidine for the last 18 h of a total culture period of 96 h. PBMCs were irradiated with 4,000 R.
To determine whether the supernatant from infected B cells could affect B-cell function, three sets of experiments were conducted. First, B cells were cultured in the presence of various dilutions of UV-treated supernatants from infected or mock-infected B-cell cultures (Table 4). Cultures were pulsed with [3H]thymidine and harvested the next day, and thymidine incorporation was measured. Proliferation was inhibited greater than 90% at a 1/25 or 1/50 dilution of supernatant from infected B cells (Table 4). Thus, a factor in the supernatant fluid inhibited proliferation of the EBV-transformed B cells.
TABLE 4.
Suppression of B-cell proliferation by supernatants from measles virus-infected B cellsa
| Supernatant | cpm ± SEMb |
|---|---|
| None | 36,858 ± 2,672 |
| 1/25, uninf | 44,887 ± 3,575 |
| 1/25, inf | 3,653 ± 393 |
| 1/50, inf | 4,322 ± 143 |
| 1/250, inf | 9,592 ± 483 |
| 1/1,000, inf | 20,124 ± 694 |
B cells were placed into culture with supernatant from mock-infected (uninf) or infected (inf; MOI of 0.005) B cells. Cultures were pulsed with 1 mCi of [3H]thymidine on day 2 and harvested on day 3.
Average of three wells.
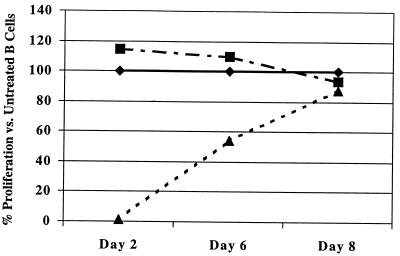
Second, since B cells were inhibited from proliferating by the supernatant factor, we wanted to determine how long the antiproliferative effect lasted. To accomplish this, B cells were treated for 3 days (equivalent to the time of our standard B-cell proliferation assay) with supernatant from infected or mock-infected B cells. After 3 days, B cells were washed and placed into culture without supernatant supplementation. On days 2, 6, and 8, equivalent numbers of B cells from each group were aliquoted into 96-well plates. Supernatant (mock infected and infected)-treated or nontreated B cells were pulsed with [3H]thymidine and harvested 6 to 8 h later, and incorporation was measured. As shown in Fig. 1, B-cell proliferation was suppressed on days 2 and 6 following treatment with supernatant from infected B cells compared to B cells not treated or treated with supernatant from uninfected B cells. By day 8, proliferation levels of the cells were comparable. These data show that even though equivalent numbers of cells were added to each well (5 × 104 cells/well), treated cells did not incorporate thymidine until day 8. Thus, B cells were able to recover and proliferate after supernatant treatment.
FIG. 1.
Proliferation of B cells after supernatant treatment. B cells were cultured with medium alone (⧫), UV supernatant from measles virus-infected B cells (▴), or supernatant from mock-infected B cells (■) for 3 days. B cells were then washed three times and cultured in medium not containing supernatant. On days 2, 6, and 8 of culture, 5 × 104 B cells were added to 96-well plates and pulsed for 6 to 18 h with [3H]thymidine.
Third, we examined whether treatment of uninfected B cells with supernatant from infected B cells could inhibit the ability of these B cells to act as APCs in antigen-specific T-cell proliferation assays. B cells were incubated with UV-treated supernatant from infected or uninfected B cells. Cells were then washed three times, counted, and irradiated with 6,000 R. These B cells were then used as APCs in antigen-specific T-cell proliferation assays. A T-cell line reactive with MBP was used. As shown in Table 5, in assays using B cells treated with supernatant from measles virus-infected B cells, no proliferation was observed. In contrast, when B cells were treated with supernatant from uninfected B cells, T-cell proliferation in response to MBP was observed.
TABLE 5.
Treatment of B Cells with soluble suppressor factor inhibits the ability of B cells to present antigena
| Cells | B-cell treatmentb | Antigen | cpm ± SEMc |
|---|---|---|---|
| T alone | None | 93 ± 3 | |
| B alone | None | None | 4,926 ± 116 |
| T + B | None | None | 5,133 ± 65 |
| None | MBP | 68,583 ± 2,903 | |
| Inf super | MBP | 6,030 ± 419 | |
| Uninf super | MBP | 62,120 ± 670 |
On day 0, B cells were cultured with a 1/8 dilution of supernatant for 3 days. On day 3, cells were washed three times and irradiated with 6,000 R. T cells and treated B cells and antigen were added to 96 wells and placed at 37°C. Cultures were pulsed on day 6 and harvested on day 7.
Inf super, supernatant collected on day 3 from measles virus-infected B cells at an MOI of 0.005; Uninf super, supernatant collected on day 3 from mock-infected B cells.
Average of three wells.
Two explanations could account for the data presented in Table 5: (i) the treated B cells elaborated additional antiproliferative factor, which could inhibit T-cell proliferation; and (ii) the treated B cells were not effective in presenting antigen to T cells. To differentiate between these two possibilities, the following experiment was performed. Uninfected B cells were treated for 24 h with supernatant from measles virus-infected or uninfected B cells. Cells were then washed three times and cultured in the absence of supernatant. The supernatants from these treated B cells were harvested after 3 days and assayed for the ability to inhibit B-cell proliferation. No inhibition of B-cell proliferation or IL-2-driven T-cell proliferation was observed in supernatants from treated B cells (data not shown). This finding indicates that treated B cells do not elaborate an antiproliferative factor, which suggests that treated B cells do not inhibit T-cell proliferation by the production of the antiproliferative factor but are unable to present antigen and do not directly inhibit T-cell proliferation.
To determine whether B cells treated with UV-irradiated supernatant from measles virus-infected B cells resulted in an abortive infection, we incubated uninfected B cells with undiluted supernatant for 24 h and then stained them for surface expression of measles virus HA. Two reagents were used to detect measles virus HA and/or fusion protein. The first was a monoclonal antibody to HA (9) and human SSPE serum containing antibody to HA and fusion protein (12). These were used at very low dilutions (1/25 and 1/100, respectively) to detect low levels of measles virus protein expression. No differences in mean fluorescence intensity were observed between B cells treated with supernatant derived from measles virus-infected B cells and B cells treated with supernatant derived from mock-infected B cells (data not shown). There was a dramatic increase in fluorescent staining in measles virus-infected B cells treated with the same reagents (positive control).
Several cytokines, including TGF-β, IL-10, IFN-α, and IFN-β (5, 22, 33, 35), have been shown to inhibit T- and B-cell proliferation. To determine whether these cytokines play a role in the inhibition of proliferation observed by supernatants from infected B cells, the supernatants were supplemented with neutralizing antibodies to the cytokines and then tested for the ability to inhibit B-cell proliferation. As shown in Table 6, anti-TGF-β, IL-10, IFN-α, and IFN-β had no effect on the supernatant’s ability to inhibit proliferation, which indicates that the antiproliferative effect mediated by the supernatant was not due to these cytokines.
TABLE 6.
Antiproliferative effects are not due to TGF-β, IL-10, or IFN-α or -βa
| Antibody | Supernatant | Mean cpm ± SEM |
|---|---|---|
| None | None | 48,708 ± 1,604 |
| Uninfected | 42,398 ± 1,501 | |
| Infected | 6,861 ± 454 | |
| Anti-TGF-β | None | 34,816 ± 2,233 |
| Infected | 8,874 ± 465 | |
| Anti-IL-10 | None | 33,595 ± 964 |
| Infected | 8,577 ± 836 | |
| Anti-IFN-α | None | 34,269 ± 2,258 |
| Infected | 9,040 ± 1,106 | |
| Anti-IFN-β | None | 32,415 ± 2,218 |
| Infected | 8,694 ± 541 |
Anti-TGF-β and anti-IL-10 were added to a concentration such that 2.5 μg of each cytokine was neutralized (according to the manufacturer’s information) per well; anti-IFN-α was at a concentration such that 5,000 U was neutralized (according to the manufacturer’s information) per well; anti-IFN-β was at a concentration such that 315 U was neutralized (according to the manufacturer’s information) per well. All supernatants were UV irradiated and filtered prior to use. The final concentration of supernatants was 1/40.
The supernatant from infected B cells was subjected to sizing membranes. B cells were then cultured with supernatant fractions larger than 100, 50 to 100, and 10 to 50 kDa. As shown in Table 7, antiproliferative activity was seen in fractions larger than 50 kDa but not in equivalent fractions from supernatant fluid from mock-infected B cells.
TABLE 7.
Antiproliferative activity is in fractions larger than 50 kDaa
| Treatment | Mean cpm ± SEM |
|---|---|
| Untreated cells | 40,301 ± 73.99 |
| Measles virus-infected B-cell supernatant | 7,251 ± 291.45 |
| Infected supernatant fraction | |
| >100 kDa | 17,719 ± 308.44 |
| 50–100 kDa | 7,791 ± 234.16 |
| 10–50 kDa | 40,174 ± 142.76 |
| Uninfected supernatant fraction | |
| >100 kDa | 40,424 ± 81.35 |
| 50–100 kDa | 40,473 ± 97.77 |
| 10–50 kDa | 40,477 ± 47.39 |
B cells were cultured in one half of each supernatant fraction in standard culture medium.
To determine whether the antiproliferative activity was stable to heating, supernatants from infected B cells were incubated at 50, 55, and 62°C. B cells were then cultured in the presence of the treated supernatant. As shown in Table 8, the antiproliferative activity was stable for 2 h at 50°C and was destroyed at 55 and 62°C.
TABLE 8.
Antiproliferative activity is labile at 55°Ca
| Treatment | Mean cpm ± SEM |
|---|---|
| None | 60,937 ± 1,411.15 |
| Infected supernatant | |
| Untreated | 4,491 ± 53.15 |
| Treated at 50°C | 5,642 ± 148.89 |
| Treated at 55°C | 44,029 ± 4,002.49 |
| Treated at 62°C | 41,205 ± 255.14 |
Supernatant from infected B cells was treated at the indicated temperatures for 2 h and then added to B-cell cultures at a final dilution of 1:2. Cultures were pulsed with [3H]thymidine 24 h after cultures were established and assessed for thymidine incorporation 24 h later.
We have shown that T cells infected with measles virus (1) or uninfected T cells treated with supernatant from infected T cells do not proliferate in response to antigen and APCs (38). In addition, we have reported that cytokine production in infected T cells is relatively intact, particularly for IL-2 production (1). Here, we provide data that B-cell APC function is inhibited. However, effector functions of T cells have not been studied. Therefore, we wanted to determine whether killing by CD4+ or CD8+ T cells was altered by measles virus infection or treatment with soluble factor from lymphoid cells. An allo-specific CD4+ CTL clone (C-7) was infected with measles virus at an MOI of 0.05 (18 h) or treated with supernatant from measles virus-infected or mock-infected T cells. Cells were cultured overnight, washed, and counted. A standard 51Cr release assay was performed on allotarget cells. Shown in Table 9 are results of two experiments examining the specific killing by the CD4+ T cells. T cells treated with 1/2 dilution of supernatant from infected T cells killed at high effector/target ratios, but killing was somewhat reduced when effector cells were in limited numbers (Table 9, experiment 1). This reduction in killing was not observed when T cells were incubated overnight with a 1/4 dilution of supernatant (Table 9, experiment 2). Allo-specific CD8+ T cells were also tested for the ability to kill allotarget cells. CD8+ T cells were infected with measles virus at an MOI of 0.05 or treated with a 1/2 or 1/4 dilution of supernatant from mock-infected B cells or measles virus-infected B cells. In experiments 3 to 5, two independent T-cell lines (F5 and F6) were tested. Whether the T cells were infected with measles virus or not, the cytotoxic activity remained intact. The first cell line (Table 9, experiment 3) was treated for 18 h with a 1/2 dilution of supernatant and then tested for cytolytic activity. At the various effector/target ratios, there was a slight decrease in the cells’ ability to lyse allotype-specific targets. However, when the same CD8+ T-cell clone was treated with a 1/4 dilution of supernatant from B cells, there was no difference between the T cells treated with infected B-cell supernatant and those treated with uninfected B-cell supernatant. Similar data were obtained with the second clone incubated at a 1/4 dilution of measles virus-infected supernatant (Table 9, experiment 5). These data corroborate the CD4+ data (experiments 1 and 2). Thus, T cells, whether infected with measles virus or supernatant from infected B cells, retain cytolytic activity.
TABLE 9.
No effect of measles virus infection or soluble factor on T-cell killinga
| Expt, clone | Treatment | % Specific lysis at effector/ target ratio of: | |||
|---|---|---|---|---|---|
| 2:1 | 1:1 | 0.5:1 | 0.25:1 | ||
| 1, C-7 | Mock infected | 111 | 93 | 75 | 70 |
| MV infected | 91 | 92 | 76 | 52 | |
| Uninfected sup | 84 | 88 | 71 | 61 | |
| Infected sup | 81 | 67 | 51 | 30 | |
| 5:1 | 2.5:1 | 1.25:1 | 0.6:1 | ||
| 2, C-7 | Mock infected | 66 | 57 | 52 | 48 |
| MV infected | 63 | 62 | 62 | 55 | |
| Uninfected sup | 52 | 53 | 50 | 43 | |
| Infected sup | 61 | 58 | 55 | 51 | |
| 10:1 | 5:1 | 2.5:1 | 1.25:1 | ||
| 3, F5 | Mock infected | 82 | 63 | 49 | 42 |
| MV infected | 79 | 72 | 67 | 49 | |
| Uninfected sup | 71 | 72 | 66 | 50 | |
| Infected sup | 58 | 47 | 39 | 29 | |
| 4, F5 | Mock infected | 90 | 75 | 57 | 50 |
| MV infected | 76 | 75 | 71 | 49 | |
| Uninfected sup | 74 | 66 | 56 | 43 | |
| Infected sup | 75 | 65 | 58 | 51 | |
| 5, F6 | Mock infected | 58 | 51 | 53 | 37 |
| MV infected | 69 | 58 | 53 | 44 | |
| Uninfected sup | 62 | 54 | 49 | 46 | |
| Infected sup | 72 | 58 | 52 | 41 | |
An allotype-specific CD4+ CTL clone (C-7) was infected with measles virus (MV) at an MOI of 0.05. Virus was allowed to absorb for 1 h at 37°C, and then cells were washed three times with RPMI 1640 and cultured for 18 h. At that time, CTL were washed three times and used as effector cells. C-7 was also treated with supernatant (Sup) from either measles virus-infected or mock-infected B cells (0.5 dilution of UV-irradiated supernatant [experiment 1]) and (0.25 dilution [experiment 2]) for 18 h. CTL were washed three times and used as effector cells in a standard CTL assay. Cloned minor HLA antigen-specific CD8+ T-cell lines (F5 and F6) were infected with measles virus at an MOI of 0.05. Virus was allowed to absorb for 1 h at 37°C, and then cells were washed three times with RPMI 1640 and cultured for 18 h. CTLs were then washed three times and used as effector cells. Similarly, CTL were treated with supernatant from either measles virus-infected or mock-infected B cells (0.5 dilution [experiment 3] and 0.25 dilution [experiments 4 and 5] of UV-irradiated supernatant) for 18 h. CTL were washed three times and used as effector cells in a standard CTL assay.
DISCUSSION
Here we demonstrate that infection of B cells can have profound effects on proliferation and ability to present antigen to T cells. We show that infection with as few as five infectious viral particles added to 10,000 EBV-transformed B cells can inhibit their proliferation. Similarly, five infectious viral particles used to infect 1,000 B cells used as APCs can reduce antigen-specific T-cell proliferation, where by infectious center assays less than 8% of total cells become infected. Therefore, only a minority of cells become infected at the time of the assays. In addition, supernatants from infected B cells (containing no detectable virus) had the ability to inhibit antigen-specific T-cell proliferation as well as the proliferation of EBV-transformed B cells. Supernatant-treated B cells did not produce additional antiproliferative factor yet were not able to act as APCs and support antigen-specific T-cell proliferation. This finding suggests that B cells that have come in contact with the factor lack the ability to present antigen to T cells.
Viruses have evolved many strategies to evade the host immune response. In the case of measles virus, direct infection of lymphoid cells leads to the inhibition of proliferation. The effect of this is to limit the number of expanding virus-specific T cells and antibody-producing B cells. This limited expansion allows the virus to replicate to sufficient numbers for spread to new hosts before the immune response can eliminate virus-infected cells and neutralize virus. We have shown that T cells infected with measles virus produce an antiproliferative cytokine that can limit clonal expansion of the antigen-specific T cells (38). Here we demonstrate that B cells can also produce an inhibitory factor, most likely the same entity since its physical properties are similar. The factor has the ability to limit the proliferation of lymphoid cells but does not affect the growth of HeLa cells (data not shown). Besides inhibiting proliferation of transformed B cells, the factor affects the ability of B cells to present antigen. This is the first example of a virus causing a cell to produce a factor that inhibits APC function.
Our data on presentation of antigen are consistent with those of Leopardi et al. (24), who studied the ability of measles virus-infected monocytes to present antigen. In experiments using an MOI of 5, they found that major histocompatibility complex class II was up-regulated by infection and that monocytes retained the ability to present measles virus antigens but were unable to present other exogenous antigens to T lymphocytes. A difference between our study and theirs is that we used 104-fold less virus. In addition, we studied the effects on proliferation and presentation of determined antigens by B cells and treatment with soluble factor.
Unlike human immunodeficiency virus, measles virus is ultimately cleared by the host’s immune response. Jacobson et al. found that much of the CTL response in humans to measles virus was major histocompatibility complex class II restricted (18), and they identified the restricting element as HLA-DR2 (17). They suggested that the CTL response was skewed to the internal viral proteins such as the nucleocapsid protein (19). Our data indicate that while proliferative responses are reduced and APC function is hindered, the ability to kill is not abrogated by infection or treatment with soluble factor. We find that CD4+ and CD8+ T-cell killing is intact (Table 9) and have data indicating that measles virus-infected MBP-specific T cells can still kill MBP-pulsed B cells (data not shown). We speculate that while slow to be generated, virus-specific CTLs once activated following infection, can still kill virus-infected cells. This ability would eventually contribute to viral clearance, but only after the virus had spread to other susceptible individuals.
ACKNOWLEDGMENTS
We thank Kristie M. Parker for excellent technical help. Kathleen Borick was instrumental in manuscript preparation. We thank Michel Doyle (Chiron, Emeryville, Calif.) for the recombinant human IL-2 and Deming Sun for advice and supplying some of the PPD used in this study.
This work was supported by grant AI 35198 (R.S.F.) and by a merit review grant from the Veterans Affairs Research Service (J.B.B.).
REFERENCES
- 1.Bell A F, Burns J B, Fujinami R S. Measles virus infection of human T cells modulates cytokine generation and IL-2 receptor alpha chain expression. Virology. 1997;232:241–247. doi: 10.1006/viro.1997.8577. [DOI] [PubMed] [Google Scholar]
- 2.Borysiewicz L K, Casali P, Rogers B, Morris S, Sissons J G P. The immunosuppressive effects of measles virus on T cell function—failure to affect IL-2 release or cytotoxic T cell activity in vitro. Clin Exp Immunol. 1985;59:29–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Burns J, Rosenzweig A, Zweiman B, Lisak R P. Isolation of myelin basic protein-reactive T-cell lines from normal human blood. Cell Immunol. 1983;81:435–440. doi: 10.1016/0008-8749(83)90250-2. [DOI] [PubMed] [Google Scholar]
- 4.Casali P, Rice G P, Oldstone M B A. Immune balance in the cytomegalovirus-infected host: in vitro studies of virus-lymphocyte interactions and the effects on specific lymphocyte function. Birth Defects. 1984;20:149–159. [PubMed] [Google Scholar]
- 5.Finbloom D S, Larner A C. Induction of early response genes by interferons, interleukins, and growth factors by the tyrosine phosphorylation of latent transcription factors. Implications for chronic inflammatory diseases. Arthritis Rheum. 1995;38:877–889. doi: 10.1002/art.1780380702. [DOI] [PubMed] [Google Scholar]
- 6.Finkel A, Dent P B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973;6:41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- 7.Fireman P, Friday G, Kumate J. Effect of measles vaccine on immunologic responsiveness. Pediatrics. 1969;43:264–272. [PubMed] [Google Scholar]
- 8.Forthal D N, Aarnaes S, Blanding J, de la Maza L, Tilles J G. Degree and length of viremia in adults with measles. J Infect Dis. 1992;166:421–424. doi: 10.1093/infdis/166.2.421. [DOI] [PubMed] [Google Scholar]
- 9.Fujinami R S, Norrby E, Oldstone M B A. Antigenic modulation induced by monoclonal antibodies: antibodies to measles virus hemagglutinin alters expression of other viral polypeptides in infected cells. J Immunol. 1984;132:2618–2621. [PubMed] [Google Scholar]
- 10.Fujinami R S, Oldstone M B A. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980;125:78–85. [PubMed] [Google Scholar]
- 11.Fujinami R S, Oldstone M B A. Failure to cleave measles virus fusion protein in lymphoid cells: a possible mechanism for viral persistence in lymphocytes. J Exp Med. 1981;154:1489–1499. doi: 10.1084/jem.154.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujinami R S, Sissons J G P, Oldstone M B A. Immune reactive measles virus polypeptides on the cell’s surface: turnover and relationship of the glycoproteins to each other and to HLA determinants. J Immunol. 1981;127:935–940. [PubMed] [Google Scholar]
- 13.Griffin D E, Johnson R T, Tamashiro V G, Moench T R, Jauregui E, Lindo de Soriano I, Vaisberg A. In vitro studies of the role of monocytes in the immunosuppression associated with natural measles virus infections. Clin Immunol Immunopathol. 1987;45:375–383. doi: 10.1016/0090-1229(87)90090-0. [DOI] [PubMed] [Google Scholar]
- 14.Gubarev M I, Jenkin J C, Leppert M F, Buchanan G S, Otterud B E, Guilbert D A, Beatty P G. Localization to chromosome 22 of a gene encoding a human minor histocompatibility antigen. J Immunol. 1996;157:5448–5454. [PubMed] [Google Scholar]
- 15.Hussey G D, Goddard E A, Hughes J, Ryon J J, Kerran M, Carelse E, Strebel P M, Markowitz L E, Moodie J, Barron P, Latief Z, Sayed R, Beatty D, Griffin D E. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J Infect Dis. 1996;173:1320–1326. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 16.Ilonen J, Salonen R, Marusyk R, Salmi A. Measles virus strain-dependent variation in outcome of infection of human blood mononuclear cells. J Gen Virol. 1988;69:247–252. doi: 10.1099/0022-1317-69-1-247. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson S, Nepom G T, Richert J R, Biddison W E, McFarland H F. Identification of a specific HLA DR2 Ia molecule as a restriction element for measles virus-specific HLA class II-restricted cytotoxic T cell clones. J Exp Med. 1985;161:263–268. doi: 10.1084/jem.161.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson S, Richert J R, Biddison W E, Satinsky A, Hartzman R J, McFarland H F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984;133:754–757. [PubMed] [Google Scholar]
- 19.Jacobson S, Rose J W, Flerlage M L, McFarlin D E, McFarland H F. Induction of measles virus-specific human cytotoxic T cells by purified measles virus nucleocapsid and hemagglutinin polypeptides. Viral Immunol. 1987;1:153–162. doi: 10.1089/vim.1987.1.153. [DOI] [PubMed] [Google Scholar]
- 20.Kadowaki J I, Nihira M, Nakao T. Reduction of phytohemagglutinin-induced lymphocyte transformation in patients with measles. Pediatrics. 1970;45:508–509. [PubMed] [Google Scholar]
- 21.Kiepiela P, Coovadia H M, Coward P. T helper cell defect related to severity in measles. Scand J Infect Dis. 1987;19:185–192. doi: 10.3109/00365548709032397. [DOI] [PubMed] [Google Scholar]
- 22.Larner A C, Finbloom D S. Protein tyrosine phosphorylation as a mechanism which regulates cytokine activation of early response genes. Biochim Biophys Acta. 1995;1266:278–287. doi: 10.1016/0167-4889(95)00015-k. [DOI] [PubMed] [Google Scholar]
- 23.Leon M E, Ward B, Kanashiro R, Hernandez H, Berry S, Vaisberg A, Escamilla J, Campos M, Bellomo S, Azabache V, et al. Immunologic parameters 2 years after high-titer measles immunization in Peruvian children. J Infect Dis. 1993;168:1097–1104. doi: 10.1093/infdis/168.5.1097. [DOI] [PubMed] [Google Scholar]
- 24.Leopardi R, Ilonen J, Mattila L, Salmi A A. Effect of measles virus infection on MHC class II expression and antigen presentation in human monocytes. Cell Immunol. 1993;147:388–396. doi: 10.1006/cimm.1993.1078. [DOI] [PubMed] [Google Scholar]
- 25.Lin C Y, Hsu H C. Histopathological and immunological studies in spontaneous remission of nephrotic syndrome after intercurrent measles infection. Nephron. 1986;42:110–115. doi: 10.1159/000183647. [DOI] [PubMed] [Google Scholar]
- 26.Lucas C J, Ubels-Postma J, Galama J M D, Rezee A. Studies on the mechanism of measles virus-induced suppression of lymphocyte functions in vitro: lack of a role for interferon and monocytes. Cell Immunol. 1978;37:448–458. doi: 10.1016/0008-8749(78)90212-5. [DOI] [PubMed] [Google Scholar]
- 27.McChesney M B, Altman A, Oldstone M B A. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J Immunol. 1988;140:1269–1273. [PubMed] [Google Scholar]
- 28.McChesney M B, Fujinami R S, Lampert P W, Oldstone M B A. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986;163:1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 29.McChesney M B, Fujinami R S, Lerche N W, Marx P A, Oldstone M B A. Virus-induced immunosuppression: infection of peripheral blood mononuclear cells and suppression of immunoglobulin synthesis during natural measles virus infection of rhesus monkeys. J Infect Dis. 1989;159:757–760. doi: 10.1093/infdis/159.4.757. [DOI] [PubMed] [Google Scholar]
- 30.McChesney M B, Kehrl J H, Valsamakis A, Fauci A S, Oldstone M B A. Measles virus infection of B lymphocytes permits cellular activation but blocks progression through the cell cycle. J Virol. 1987;61:3441–3447. doi: 10.1128/jvi.61.11.3441-3447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McChesney M B, Oldstone M B A. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279–304. doi: 10.1146/annurev.iy.05.040187.001431. [DOI] [PubMed] [Google Scholar]
- 32.Mickelson E M, Beatty P G, Storb R, Hansen J A. Immune responses in an untransfused patient with aplastic anemia: analysis of cytolytic and proliferative T cell clones. Hum Immunol. 1984;10:189–201. doi: 10.1016/0198-8859(84)90039-9. [DOI] [PubMed] [Google Scholar]
- 33.Moore K W, O’Garra A, deWaal-Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 34.Ravanel K, Castelle C, Defrance T, Wild T F, Charron D, Lotteau V, Rabourdin-Combe C. Measles virus nucleocapsid protein binds to FcγRII and inhibits human B cell antibody production. J Exp Med. 1997;186:269–278. doi: 10.1084/jem.186.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravitz M J, Wenner C E. Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-beta. Adv Cancer Res. 1997;71:165–207. doi: 10.1016/s0065-230x(08)60099-8. [DOI] [PubMed] [Google Scholar]
- 36.Schlender J, Schnorr J J, Spielhoffer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaheen S O, Aaby P, Hall A J, Barker D J, Heyes C B, Shiell A W, Goudiaby A. Cell mediated immunity after measles in Guinea-Bissau: historical cohort study. Br Med J. 1996;313:969–974. doi: 10.1136/bmj.313.7063.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Burns J B, Howell J M, Fujinami R S. Suppression of antigen-specific T cell proliferation by measles virus infection: role of a soluble factor in suppression. Virology. 1998;246:24–33. doi: 10.1006/viro.1998.9186. [DOI] [PubMed] [Google Scholar]
- 39.von Pirquet C. Das Verhalten der kautanen Tuberculin-reaktion wahrend der Masern. Deutsche Med Wochenschr. 1908;34:1297–1298. [Google Scholar]
- 40.Ward B J, Griffin D E. Changes in cytokine production after measles virus vaccination: predominant production of IL-4 suggests induction of a Th2 response. Clin Immunol Immunopathol. 1993;67:171–177. doi: 10.1006/clin.1993.1061. [DOI] [PubMed] [Google Scholar]
- 41.Ward B J, Johnson R T, Vaisberg A, Jauregui E, Griffin D E. Cytokine production in vitro and the lymphoproliferative defect of natural measles virus infection. Clin Immunol Immunopathol. 1991;61:236–248. doi: 10.1016/s0090-1229(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 42.Whittle H C, Bosseter J, Oduloju A, Bryesson D M, Greenwood B M. Cell mediated immunity during natural measles infection. J Clin Invest. 1978;62:678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanagi Y, Cubitt B A, Oldstone M B A. Measles virus inhibits mitogen-induced T cell proliferation but does not directly perturb the T cell activation process inside the cell. Virology. 1992;187:280–289. doi: 10.1016/0042-6822(92)90316-h. [DOI] [PubMed] [Google Scholar]
- 44.Zweiman B. In vitro effects of measles virus on proliferating human lymphocytes. J Immunol. 1971;106:1154–1158. [PubMed] [Google Scholar]