Abstract
INTRODUCTION:
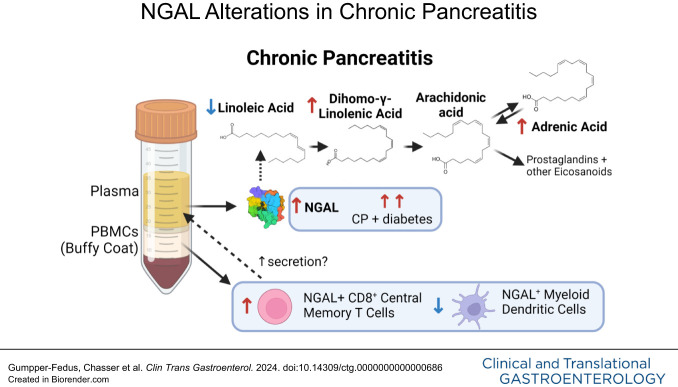
Chronic pancreatitis (CP) is a progressive fibroinflammatory disorder lacking therapies and biomarkers. Neutrophil gelatinase-associated lipocalin (NGAL) is a proinflammatory cytokine elevated during inflammation that binds fatty acids (FAs) such as linoleic acid. We hypothesized that systemic NGAL could serve as a biomarker for CP and, with FAs, provide insights into inflammatory and metabolic alterations.
METHODS:
NGAL was measured by immunoassay, and FA composition was measured by gas chromatography in plasma (n = 171) from a multicenter study, including controls (n = 50), acute and recurrent acute pancreatitis (AP/RAP) (n = 71), and CP (n = 50). Peripheral blood mononuclear cells (PBMCs) from controls (n = 16), AP/RAP (n = 17), and CP (n = 15) were measured by cytometry by time-of-flight.
RESULTS:
Plasma NGAL was elevated in subjects with CP compared with controls (area under the curve [AUC] = 0.777) or AP/RAP (AUC = 0.754) in univariate and multivariate analyses with sex, age, body mass index, and smoking (control AUC = 0.874; AP/RAP AUC = 0.819). NGAL was elevated in CP and diabetes compared with CP without diabetes (P < 0.001). NGAL+ PBMC populations distinguished CP from controls (AUC = 0.950) or AP/RAP (AUC = 0.941). Linoleic acid was lower, whereas dihomo-γ-linolenic and adrenic acids were elevated in CP (P < 0.05). Linoleic acid was elevated in CP with diabetes compared with CP subjects without diabetes (P = 0.0471).
DISCUSSION:
Elevated plasma NGAL and differences in NGAL+ PBMCs indicate an immune response shift that may serve as biomarkers of CP. The potential interaction of FAs and NGAL levels provide insights into the metabolic pathophysiology and improve diagnostic classification of CP.
KEYWORDS: lipocalin 2, body mass index, smoking, mass CyTOF, peripheral blood mononuclear cells, linoleic acid
INTRODUCTION
Pancreatitis is an inflammatory disease that ranges from acute (AP) to recurrent acute (RAP) to chronic pancreatitis (CP), and cases have increased by 13.3% in the past 2 decades (1). Patients with CP are at risk of long-term complications, including chronic abdominal pain, diabetes, exocrine pancreatic dysfunction (EPD), osteopathy, and pancreatic cancer (2). Our understanding of CP pathophysiology remains limited, leading to extensive testing, delayed diagnosis, and a lack of biomarkers and effective therapies (3–5).
Our systematic review investigating potential CP biomarkers indicated that neutrophil gelatinase-associated lipocalin (NGAL/lipocalin 2) was elevated in pancreatic juice of CP patients, warranting further evaluation as a potential biomarker of CP (5). NGAL is a secreted protein in the lipocalin family that binds hydrophobic ligands, including fatty acids (FAs) such as linoleic acid, and siderophores, as part of an acute-phase inflammatory response (6–11). Increased NGAL expression promotes lipolysis and FA oxidation, releasing FAs from intracellular storage during inflammation (12). NGAL contributes to pancreatic cancer pathogenesis by regulating inflammation in pancreatic tumors and is increased in the blood of subjects with pancreatic cancer compared with controls (13). Increased NGAL expression in immune cell subtypes within the peripheral blood mononuclear cells (PBMCs), such as B cells in chronic lymphocytic leukemia (14) and CD4+ T cells in acute kidney injury (15), is associated with apoptosis inhibition and enhanced proinflammatory or profibrotic effects (14,16). Although studies have assessed NGAL as a potential biomaker for CP in various biofluids, the studies were small, lacked well-controlled sample acquisition and clinical criteria, and focused on a comparison between CP and pancreatic cancer instead of a control group (13,17–19).
We evaluated whether NGAL levels or NGAL+ PBMCs could serve as diagnostic biomarkers of CP and gain insights into inflammatory alterations associated with CP. Furthermore, we investigated whether FAs known to interact with NGAL were altered in CP to better understand the metabolic alterations in this disease.
METHODS
Study design
This study used well-defined human samples from the prospective multicenter study Prospective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED) (NCT03099850), a study of the Chronic Pancreatitis, Diabetes, and Pancreatic Cancer consortium (20,21). Subjects selected for the current analysis were enrolled into PROCEED, from June 2017 to September 2020 and included a random sampling of healthy controls (n = 50), AP/RAP (n = 71), and CP (n = 50). Sample size was determined by a small pilot study using a separate set of samples from the Ohio State University. PBMCs from controls (n = 16), AP/RAP (n = 17; 1 subject with AP), and CP (n = 15) subjects were selected from the PROCEED biorepository based on sample availability. AP and RAP were diagnosed using the revised Atlanta criteria (22), and CP was diagnosed as previously described (20) based on pancreatic calcifications and/or advanced Cambridge stage. Inclusion and exclusion criteria for the PROCEED cohort included in this study are previously published and summarized in Supplementary Table 1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/B85) (20,21). Samples (plasma, urine, and PBMCs) were collected 1 month after an acute attack to prevent bias from acute inflammation. Data for sex, age, body mass index (BMI), etiology, presence of EPD, diabetes status, and smoking history were retrieved from PROCEED (20).
Enzyme-linked immunosorbent assay
Plasma and urine NGAL concentrations were measured using an enzyme-linked immunosorbent assay (DLCN20; R&D Systems, Minneapolis, MN) following the manufacturer's recommended protocol. All samples were measured in duplicate and reported in nanograms per milliliter determined from a standard curve. The personnel and study team were blinded to group assignment.
Mass cytometry by time-of-flight
PBMCs were thawed and processed as previously described (21). PBMCs were stained using the Maxpar Direct Immune Profiling Assay kit (PN 400286 B1; Standard BioTools, San Francisco, CA) with the addition of CD11b (Standard BioTools) and CD33 (BioLegend, San Diego, CA) antibodies (see Supplementary Table 2, Supplementary Digital Content 1, http://links.lww.com/CTG/B85) (23). After surface staining, the cells were fixed, permeabilized, and intracellularly stained for NGAL (ab224264; Abcam, Cambridge, UK), which was conjugated to metal isotope 159Tb, using the Maxpar X8 Antibody Labeling Kit, 159Tb-4 RXN (Standard BioTools, see Supplementary Table 2, Supplementary Digital Content 1, http://links.lww.com/CTG/B85). The stained PBMCs were analyzed on a Fluidigm Helios Mass Cytometer. Data concatenation and normalization were performed using the Cytobank software v7.0 (Beckman Coulter). Samples with more than 100,000 events were used for analysis. PBMC populations and subsets were gated based on phenotypic markers of each population (see Supplementary Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/B85) (23). Differences in NGAL+ of PBMC populations were calculated as the number of cells positive for NGAL within an immune population divided by the number of live PBMCs positive for NGAL. Differences in NGAL+ PBMC subsets were calculated as the number of cells positive for NGAL within an immune subpopulation divided by the number of cells positive for NGAL within the respective parent population (see Supplementary Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/B85).
FA composition analysis
Total lipids were extracted from plasma samples using the 2:1 chloroform:methanol method and methylated (24,25) as we previously described (26–29), blinded to disease group. The methyl ester form of the FAs was analyzed using gas chromatography run on a 30-m Omegawax TM 320 fused silica capillary column (Supelco, Bellefonte, PA), with the oven temperature and carrier gas flow rate settings as previously described (29,30). Results were compared with purchased standards for each FA methyl ester (Matreya, LLC, Pleasant Gap, PA; Nu-Check Prep, Elysian, MN). The data are presented as the relative composition of all identified FAs (% area), as recommended by experts in the field for clinical studies (30,31).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA), JMP 16 (SAS Institute, Cary, NC), and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Relative frequencies were reported and compared for categorical variables using Fisher exact test or χ2 tests of independence, whereas mean values and SDs were reported and compared by study group for the continuous age variable using 1-way ANOVA. Because EPD was an exclusion criterion for the control group in the PROCEED study and none of our control subjects had diabetes, we did not include controls when comparing the difference in these clinical factors using χ2 tests between AP/RAP and CP. Propensity score matching on the covariates of sex, age, BMI, and smoking was used to calculate the average treatment effect for NGAL when comparing control with CP, control with AP/RAP, and AP/RAP with CP. Receiver operator characteristic curves were generated, the area under the curve (AUC) was calculated to assess the ability of NGAL concentration to distinguish between study groups, and a multiple logistic regression was used to control for clinical characteristics. A 2-way ANOVA with Tukey's testing for pairwise comparisons on log-transformed NGAL concentrations compared NGAL across groups by categorical subject characteristics. Because age is a continuous variable, we performed a correlation analysis between age and NGAL and assessed differences in the correlation slopes and intercepts. Nonparametric Kruskal-Wallis tests were performed on the percentages of NGAL+ PBMC populations and subsets and on overall PBMC populations and subsets to compare the differences between controls, AP/RAP, and CP. FA composition was compared among the disease groups using 1-way ANOVA parametric test, or Kruskal-Wallis nonparametric test, with Holm-Šidák correction for multiple testing. T-test or Mann-Whitney nonparametric test was used to assess differences in FA composition in CP based on diabetes.
RESULTS
Study group characteristics
All groups had similar sex distributions. On average, the CP group was older, leaner, more likely to smoke, and more likely to have EPD compared with the AP/RAP group and, where applicable, the control group. Idiopathic and hypertriglyceridemia etiologies were more common in the AP/RAP group, whereas alcoholic and genetic etiologies were more common in the CP group (Table 1, top). In the subset of subjects with available PBMCs, age, etiology, EPD, and smoking were all different between the controls and disease groups (Table 1, bottom).
Table 1.
Comparison of patient characteristics by PROCEED study group
| Plasma/urine | Control n = 50 |
AP/RAP n = 71 |
CP n = 50 |
P value |
| Age, yr, mean (SD) | 51.4 (14.1) | 48.7 (13.8)a | 55.5 (11.9)a | 0.028 |
| Sex, n (%) | 0.915 | |||
| Male | 29 (58.0) | 42 (59.2) | 31 (62.0) | |
| Female | 21 (42.0) | 29 (40.8) | 19 (38.0) | |
| BMI, kg/m2, n (%) | <0.001 | |||
| <24.9 | 14 (28.0) | 27 (38.0) | 34 (68.0) | |
| 25–29.9 | 26 (52.0) | 26 (36.6) | 9 (18.0) | |
| >30 | 10 (20.0) | 18 (25.4) | 7 (14.0) | |
| Etiology, n (%) | 0.006 | |||
| Idiopathic | N/A | 41 (57.7) | 17 (34.0) | |
| Alcoholic | 18 (25.4) | 26 (52.0) | ||
| Genetic | 1 (1.4) | 3 (6.0) | ||
| Obstructive | 2 (2.8) | 1 (2.0) | ||
| Hypertriglyceridemia | 9 (12.7) | 1 (2.0) | ||
| Postnecrotic | 0 (0.0) | 1 (2.0) | ||
| Other | 0 (0.0) | 1 (2.0) | ||
| EPD present, n/total n (%) | N/A | 10/49 (20.4)b | 24/40 (60.0)b | <0.001 |
| Diabetes present, n/total n (%) | N/A | 18/71 (25.4) | 19/49 (38.8)b | 0.118 |
| Smoking, n (%) | <0.001 | |||
| Current | 2 (4.0) | 13 (18.3) | 25 (50.0) | |
| Past | 10 (20.0) | 23 (32.4) | 13 (26.0) | |
| Never | 38 (76.0) | 35 (49.3) | 12 (24.0) |
| PBMCs | Control n = 16 |
AP/RAP n = 17 |
CP n = 15 |
P value |
| Age, yr, mean (SD) | 45.7 (11.9) | 40.2 (11.2) | 54.5 (10.4) | 0.006 |
| Sex, n (%) | 0.608 | |||
| Male | 9 (56.3) | 11 (64.7) | 8 (53.3) | |
| Female | 7 (53.7) | 8 (35.3) | 7 (46.7) | |
| BMI, kg/m2, n (%) | 0.069 | |||
| <24.9 | 6 (37.5) | 4 (23.5) | 8 (53.3) | |
| 25–29.9 | 7 (43.8) | 3 (17.7) | 4 (26.7) | |
| >30 | 3 (18.8) | 10 (58.8) | 3 (20.0) | |
| Etiology, n (%) | 0.030 | |||
| Idiopathic | N/A | 9 (52.9) | 7 (46.7) | |
| Alcoholic | 4 (23.5) | 8 (53.3) | ||
| Genetic | 0 (0.0) | 0 (0.0) | ||
| Obstructive | 0 (0.0) | 0 (0.0) | ||
| Hypertriglyceridemia | 4 (23.5) | 0 (0.0) | ||
| Postnecrotic | 0 (0.0) | 0 (0.0) | ||
| Other | 0 (0.0) | 0 (0.0) | ||
| EPD present, n (%) | N/A | 3/11 (27.3)b | 10/13 (76.9)b | 0.038 |
| Diabetes present, n (%) | N/A | 5 (29.4) | 9 (60.0) | 0.080 |
| Smoking, n (%) | 0.001 | |||
| Current | 0 (0.0) | 5 (29.4) | 9 (60.0) | |
| Past | 3 (18.8) | 5 (29.4) | 4 (26.7) | |
| Never | 13 (81.3) | 7 (41.2) | 2 (13.3) |
Results for subjects with available plasma and urine are shown at the top, whereas results for subjects with available PBMCs are shown at the bottom. Subject age was analyzed using 1-way ANOVA with Tukey's HSD for multiple comparisons. Subject sex, BMI, pancreatitis etiology, EPD, diabetes status, and cigarette use were analyzed using χ2 analysis.
AP, acute pancreatitis; BMI, body mass index; CP, chronic pancreatitis; EPD, exocrine pancreatic dysfunction; HSD, honestly significant difference; PBMC, peripheral blood mononuclear cell; RAP, recurrent acute pancreatitis.
P < 0.05 between AP/RAP and CP.
Missing data from some of the cases.
Plasma NGAL is elevated in CP
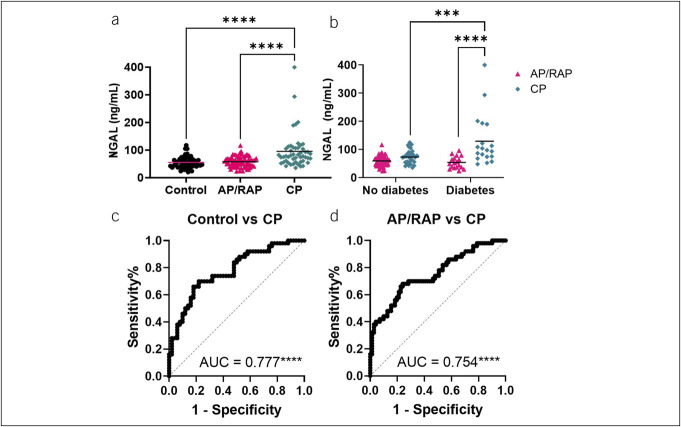
Plasma NGAL levels were higher, on average, in CP compared with control and AP/RAP (Figure 1a). Two-way ANOVAs assessing the interaction between study group and the clinical characteristics of sex, BMI, pancreatitis etiology, EPD, and smoking history and a correlation analysis between NGAL and age demonstrated that NGAL remained higher, on average, in CP compared with control and AP/RAP within the subgroups of each of the clinical features, indicating the differences in NGAL are attributable to disease group rather than these clinical features (see Supplementary Figure 1A–F, Supplementary Digital Content 1, http://links.lww.com/CTG/B85). NGAL was significantly elevated in subjects with both CP and diabetes, compared with CP subjects without diabetes (P = 0.0009) (Figure 1b). We did not observe any significant difference in the urine NGAL levels between any of the groups (see Supplementary Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/B85). We generated receiver operator characteristic curves to assess NGAL's potential as a biomarker and found that NGAL distinguished CP from control (AUC = 0.777; P < 0.0001) (Figure 1c) and CP from AP/RAP (AUC = 0.754; P < 0.0001) (Figure 1d). Propensity score matching to mitigate the possible effect of clinical features (age, sex, BMI, and smoking) on the difference in NGAL levels between groups continued to provide evidence of significantly higher NGAL levels in subjects with CP compared with controls (P = 0.011) and AP/RAP (P = 0.007) (Table 2). The matching resulted in similar AUCs for NGAL differentiating CP vs control or AP/RAP (Table 2).
Figure 1.
NGAL expression increases in the plasma of subjects with CP. (a) NGAL measured by ELISA from the multicenter (PROCEED) study discovery set. Statistical significance was determined by a non-parametric 1-way ANOVA with Tukey's multiple comparisons correction. (b) AP/RAP and CP samples were subdivided by diabetes status and compared for plasma NGAL concentration. Statistical significance was determined by 2-way ANOVA with Tukey's multiple testing correction. (c) ROCs with AUCs of plasma NGAL comparing control vs CP and (d) AP/RAP vs CP. ***P < 0.001, ****P < 0.0001. AP, acute pancreatitis; CP, chronic pancreatitis; ELISA, enzyme-linked immunosorbent assay; NGAL, neutrophil gelatinase-associated lipocalin; RAP, recurrent acute pancreatitis.
Table 2.
Propensity score matching for plasma NGAL in CP
| Disease | ATT (SE) | t statistic | ROC AUC |
| CP (n = 50) vs control (n = 21) | 42.06 (16.56) | 2.54* | 0.785*** |
| CP (n = 50) vs AP/RAP (n = 27) | 35.83 (13.33) | 2.69** | 0.739*** |
| AP/RAP (n = 71) vs control (n = 34) | 6.27 (4.83) | 1.30 |
ATT, average treatment effect on the disease group; AP, acute pancreatitis; AUC, area under the curve; CP, chronic pancreatitis; RAP, recurrent acute pancreatitis; ROC, receiver operator characteristic.
*P < 0.05, **P < 0.01, ***P < 0.001.
To evaluate the predictive performance of NGAL in combination with clinical features, multivariate models to predict CP (vs control and vs AP/RAP) were fit using NGAL, age, sex, BMI, and smoking as predictors. Diabetes and EPD were excluded because these are complications of CP that may cause an overestimation of NGAL's discriminative ability. The regression results (Table 3) showed that a model that includes clinical features in addition to NGAL improved AUC over NGAL alone for CP compared with control (AUC = 0.874; P < 0.0001) and for CP compared with AP/RAP (AUC = 0.819; P < 0.0001). In the multivariable models, NGAL significantly contributed to the differentiation of CP and controls (P < 0.001) and CP and AP/RAP (P < 0.001) after controlling for these clinical features. History of smoking and low BMI also made a significant contribution to the differentiation of CP vs controls or AP/RAP.
Table 3.
Multiple logistic regressions using plasma NGAL (top) or NGAL+ PBMC subpopulations (bottom) to predict CP
| Odds ratio | 95% CI | P value | AUC | |
| Plasma NGAL | ||||
| Control vs CP | 0.874**** | |||
| Intercept | 5.52 | 0.20–170.4 | 0.316 | |
| NGAL (1 ng/mL) | 1.05 | 1.02–1.08 | <0.001 | |
| Sex (male) | 0.63 | 0.20–1.85 | 0.405 | |
| Age (yr) | 1.01 | 0.97–1.05 | 0.809 | |
| BMI (kg/m2) | 0.87 | 0.77–0.96 | 0.012 | |
| Smoking (never) | 0.14 | 0.04–0.38 | <0.001 | |
| AP/RAP vs CP | 0.819**** | |||
| Intercept | 0.56 | 0.03–10.5 | 0.703 | |
| NGAL (1 ng/mL) | 1.04 | 1.02–1.07 | <0.001 | |
| Sex (male) | 0.89 | 0.35–2.27 | 0.809 | |
| Age (yr) | 1.03 | 1.0–1.07 | 0.096 | |
| BMI (kg/m2) | 0.87 | 0.78–0.95 | 0.003 | |
| Smoking (never) | 0.42 | 0.16–1.05 | 0.069 | |
| NGAL+ PBMCs | ||||
| Control vs CP | 0.950**** | |||
| Intercept | 10.39 | 0.002–93833 | 0.585 | |
| CD8+ central memory T cells | 1.59 | 1.13–2.82 | 0.033 | |
| CD8+ effector T cells | 0.98 | 0.89–1.08 | 0.750 | |
| Naive CD4+ T cells | 0.95 | 0.85–1.04 | 0.273 | |
| Memory resting B cells | 0.83 | 0.60–0.96 | 0.137 | |
| Myeloid dendritic cells | 0.94 | 0.82–1.05 | 0.298 | |
| AP/RAP vs CP | 0.941**** | |||
| Intercept | 10.64 | 0.001–645744 | 0.631 | |
| CD8+ central memory T cells | 1.63 | 1.14–3.15 | 0.051 | |
| CD8+ effector T cells | 1.06 | 0.94–1.22 | 0.392 | |
| Naive CD4+ T cells | 1.04 | 0.92–1.19 | 0.565 | |
| Memory resting B cells | 0.90 | 0.65–1.19 | 0.447 | |
| Myeloid dendritic cells | 0.88 | 0.75–0.98 | 0.044 |
PROCEED study NGAL+ PBMC subpopulations that were significantly different between CP and either control or AP/RAP were considered.
AP, acute pancreatitis; BMI, body mass index; CP, chronic pancreatitis; NGAL, neutrophil gelatinase-associated lipocalin; PBMC, peripheral blood mononuclear cell; RAP, recurrent acute pancreatitis.
****ROC AUC P < 0.0001.
Profiling PBMCs identified altered NGAL+ immune cell populations in CP
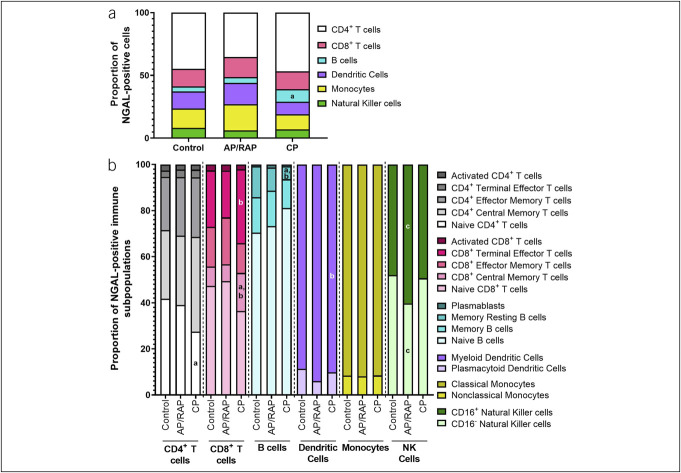
Immune cells are one of several sources of systemic NGAL, particularly in inflammatory conditions (15). Therefore, we performed mass cytometry by time-of-flight (CyTOF) to determine whether there were differences in NGAL+ PBMCs for patients with CP. When assessing NGAL+ cells as a percentage of live PBMCs, there were more NGAL+ B cells in CP compared with controls (P = 0.021) (Figure 2a), with similar results in the overall PBMC populations (see Supplementary Figure 3A, Supplementary Digital Content 1, http://links.lww.com/CTG/B85). We observed differences in NGAL+ PBMC subpopulations as a percentage of the NGAL+ parent population between controls, AP/RAP, and CP (Figure 2b). Compared with controls, CP subjects had less NGAL+ naive CD4+ T cells (P = 0.037) and memory B cells (P = 0.019) and more NGAL+ CD8+ central memory T cells (P = 0.009). Compared with AP/RAP, the CP group had fewer NGAL+ memory resting B cells (P = 0.027) and myeloid dendritic cells (P = 0.042) and more NGAL+ CD8+ central memory T cells (P = 0.001) and CD8+ effector T cells (P = 0.041). There were fewer NGAL+ CD16− and more NGAL+ CD16+ natural killer cells (P = 0.034) in subjects with AP/RAP compared with control subjects (Figure 2b). Some of these shifts occurred in the overall PBMCs immune cell subpopulations (see Supplementary Figure 3B, Supplementary Digital Content 1, http://links.lww.com/CTG/B85). These differences in immune cells suggest a shift away from an innate immune response and toward an adaptive immune response during CP that is enhanced in NGAL+ cells.
Figure 2.
NGAL+ immune cells within PBMC populations and subsets tends to increase in the adaptive immune cells of subjects with CP. PBMCs from multicenter (PROCEED) discovery set (healthy controls, AP/RAP, and subjects with CP) collected during enrollment visit were analyzed by CyTOF. (a) Proportion of NGAL+ immune populations (b) and subpopulations. a P < 0.05 between control and CP; b P < 0.05 between AP/RAP and CP; c P < 0.05 between control and AP/RAP; ****P < 0.001. AP, acute pancreatitis; CP, chronic pancreatitis; CyTOF, mass cytometry by time-of-flight; NGAL, neutrophil gelatinase-associated lipocalin; PBMC, peripheral blood mononuclear cell; RAP, recurrent acute pancreatitis.
Multiple logistic regressions of the immune cell populations that were significantly different between CP and either the control or AP/RAP groups were fit to determine the utility of this combination of NGAL+ PBMC subpopulations for distinguishing CP from either controls or AP/RAP. A panel of NGAL+ CD8+ central memory T cells, CD8+ effector T cells, naive CD4+ T cells, memory resting B cells, and myeloid dendritic cells differentiated control from CP (AUC = 0.950; P < 0.0001) and AP/RAP from CP (AUC = 0.941; P < 0.0001) (Table 3). By contrast, the same overall PBMC subpopulations resulted in lower AUCs comparing control with CP (AUC = 0.883; P < 0.0001) and AP/RAP with CP (AUC = 0.890; P < 0.0001) (see Supplementary Table 6, Supplementary Digital Content 1, http://links.lww.com/CTG/B85). This suggests that a subset of circulating NGAL+ immune cells could be used as a potential biomarker panel for CP.
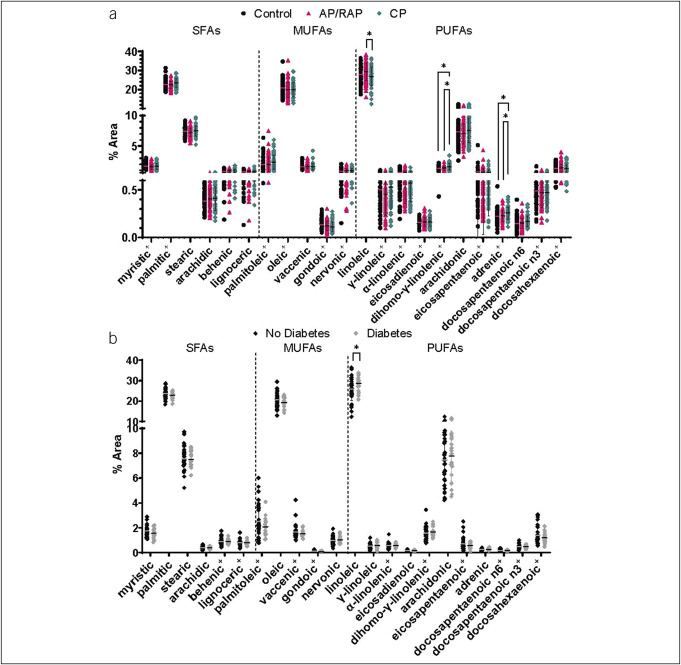
FA composition is altered in CP
Because NGAL binds FAs such as linoleic acid, we measured FA composition in the plasma of the same subjects for whom we measured NGAL levels. Three polyunsaturated FAs (PUFAs) were differentially affected in CP compared with controls or AP/RAP. Linoleic acid was lower, whereas dihomo-γ-linolenic and adrenic acids were higher in CP compared with AP/RAP (Figure 3a). Because several FAs interact with NGAL and NGAL is modulated by both CP and diabetes, we assessed whether FA composition was also different in subjects with CP and diabetes compared with those without diabetes. Interestingly, like NGAL, plasma linoleic acid was also significantly higher in subjects with diabetes and CP compared with those with CP without diabetes (P = 0.0471) (Figure 3b). Overall, the differences observed suggest a dysregulation of PUFAs in CP.
Figure 3.
PUFA composition is altered in the plasma of subjects with CP. (a) Plasma FAs ordered by saturation and carbon chain number compared by 1-way ANOVA with multiple testing for significance. (b) Plasma FAs from subjects with CP subdivided by diabetes compared by t tests. +Nonparametric Kruskal-Wallis or Mann-Whitney *P < 0.05. AP, acute pancreatitis; CP, chronic pancreatitis; FA, fatty acid; MUFA, monounsaturated FA; PUFA, polyunsaturated FA; RAP, recurrent acute pancreatitis; SFA, saturated FA.
DISCUSSION
One of the key distinctions between AP/RAP and CP is the constant proinflammatory state in CP, leading to complications such as fibrosis and calcification. However, these complications are typically observable by imaging once CP is severe as the spectrum of pancreatitis lacks biomarkers to discern between disease stages. In this study, we demonstrated elevated plasma NGAL levels in patients with CP, especially those with diabetes. NGAL emerged as a promising biomarker for CP, particularly when coupled with easily accessible clinical characteristics. Furthermore, our investigation revealed alterations in NGAL+ PBMCs, suggesting a transition from a naive state to memory and effector cell types in CP. These differences could potentially form a panel for distinguishing CP from controls or AP/RAP. Furthermore, we identified differences in plasma linoleic acid composition in CP and CP combined with diabetes The changes observed in NGAL and PUFAs known to interact with NGAL in CP reflect metabolic changes that may explain some of the pathophysiologic features of CP.
NGAL regulates inflammation, particularly in cancers such as pancreatic cancer, and has been suggested to have clinical value in diagnosing the spectrum of pancreatic diseases, including AP, CP, and pancreatic cancer (7). However, previous studies assessing NGAL as a biomarker of pancreatic diseases mainly compared CP with pancreatic cancer or AP to controls (5,7). These studies had low sample sizes, different criteria to define CP, and did not account for the impact the timing of sample collection, and clinical characteristics could have had on plasma NGAL concentrations. The multicenter PROCEED discovery sample set used in this study enabled us to compare larger numbers of well-defined pancreatitis subjects to healthy controls, allowing us to better adhere to the phase 1 of the prospective-specimen-collection, retrospective-blinded-evaluation study design for biomarker discovery (20,32,33). A recent study using the Olink technology in a similar sample set from the PROCEED study identified IL-17 as another potential biomarker of CP (NGAL was not a part of that cytokine panel) (34). Interestingly, IL-17 can promote expression of NGAL (35). Combining NGAL with other potential mechanistic markers such as IL-17 may improve accuracy for CP compared with controls or AP/RAP (36,37). Urine NGAL is currently being investigated as a biomarker for acute kidney injury (38,39), diabetic nephropathy, or diabetic kidney disease (40,41), and others are working on ways to test plasma NGAL using automated assays (42). Because we did not see differences in NGAL levels in the urine, urine NGAL may be specific to kidney injury and not CP. It is important to note that we do not expect plasma NGAL to be a differential diagnostic marker between pancreatic and kidney disease or even other inflammatory disorders. Rather, persistent elevated plasma NGAL with other indicators of pancreatic disease may be markers of CP inflammation in patients suspected of having a pancreatic disease, but for whom the diagnosis is unclear.
Diabetes is a common complication of CP and often represents a progression in severity (2). Although many patients presenting with CP already have diabetes, those who do not are likely to develop type 3c diabetes as pancreatitis progresses (43). There is conflicting evidence on whether NGAL promotes insulin resistance or counteracts diabetes (44,45). However, several studies suggest NGAL is elevated in diabetes associated with microvascular complications (neuropathy and nephropathy) (40,41,46). Here, NGAL levels were higher in subjects with both CP and diabetes compared with CP alone, although we were unable to compare with a control group with diabetes because of a limitation in the PROCEED study design. Our analysis does not provide definitive certainty regarding whether NGAL concentrations in the CP and diabetes participants reflect the influence of the diabetic state or if it represents a more advanced clinical phenotype of disease (i.e., CP with endocrine insufficiency). Based on these data, future studies would include additional controls with diabetes to better understand how exocrine and endocrine diseases influence plasma NGAL levels.
NGAL is essential for innate and adaptive immune functions, with roles in iron homeostasis (10,47), T-cell and B-cell development and proliferation (48), antibody production (49), and dendritic cell antigen presentation (50). Although the processing and storage methods used in PROCEED (21) caused neutrophil degradation, an established source of NGAL (9,51,52), we detected a shift in NGAL+ PBMCs of subjects with CP away from the naive state and toward memory and effector cell types. Unlike other studies, we did not observe a difference in circulating monocytes (53) or lymphocytes (54), and we saw an increase in CD8+ central memory T cells that conflicts with earlier studies (55). The differences are likely due to variations in detection method (e.g., flow cytometry (54,56,57), barcoding cells for CyTOF (53), and metal-labeled antibodies for CyTOF like the one used in this study), immune cell source (tissue (53,57) vs circulating (54,58)), and time after an acute attack (53,54,56,58). The increase observed in NGAL+ B cells and NGAL+ B-cell subpopulations detected in subjects with CP in our study results contrasts with a study that found B cells to only be increased in AP (and further increased in severe AP), compared with healthy subjects (54). However, since the samples in the aforementioned study were collected on admission rather than 1 month after an AP attack (as is the case for PROCEED), differences in these findings may be due to acute rather than chronic disease response. Overall, the differences in NGAL+ PBMCs point toward a rise in a cell-mediated immune response through cytotoxic mechanisms (14,59) that could lead to increased tissue immune infiltration and inflammation of subjects with CP. Furthermore, our data suggest that a small panel of NGAL+ immune cell populations may be developed as another effective diagnostic biomarker tool for CP compared with the expensive and less widely available CyTOF technology; however, this will need to be validated.
NGAL interacts with several hydrophobic ligands such as linoleic, palmitic, and oleic acids, and these interactions have been associated with endothelial dysfunction and promoting inflammation (11). Arachidonic acid, a downstream metabolite of linoleic and dihomo-γ-linolenic acids, is further metabolized into eicosanoids, which are elevated in the pancreatic juice of subjects with CP (60) and can mediate pain sensation, inflammation, and promote fibrosis, all of which are present in CP (61,62). It is possible that excessive NGAL in CP may be binding and trafficking linoleic acid into cells for use and metabolism; however, because we do not have dietary information, we cannot rule out the impact of an altered diet in subjects with CP. In subjects with both CP and diabetes, we observed an increase in linoleic acid, which was surprising because higher levels of linoleic acid have been correlated with a decreased incidence of type 2 diabetes mellitus (63). Further mechanistic studies will be required to tease out this complex interaction between CP and diabetes in modulating NGAL and linoleic acid to determine whether this interaction is targetable for future therapeutic development for pancreatitis.
This study on NGAL is strengthened over previous studies (5,7) from the use of an extensive set of blood and urine samples with associated clinical data gathered from multiple clinical centers using standardized procedures (21). We also conducted a comprehensive assessment of NGAL+ immune populations and subpopulations from PBMCs. Our study enabled a direct comparison of circulating NGAL in subjects with CP to subjects with AP/RAP and control subjects. Finally, we identified a potential association between alterations in NGAL levels and its hydrophobic ligands, particularly in CP and diabetes. In subsequent studies, we will explore whether binding and trafficking of linoleic acid by NGAL in CP contributes to pathophysiologic processes such as inflammation, fibrosis, and pain in the progression of pancreatic disease from acute pancreatitis to pancreatic cancer and validate NGAL as a biomarker of CP. Overall, this study presents a meticulous analysis of the diagnostic utility of NGAL in CP and identifies a potential biological relevance of elevated NGAL and its associated FAs in pancreatitis.
CONFLICTS OF INTEREST
Guarantor of the article: Zobeida Cruz-Monserrate, PhD.
Specific author contributions: K.G.-F. and K.C.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. V.P.-G.: acquisition of data; analysis and interpretation of data; administrative, technical, or material support; and critical revision of the manuscript for important intellectual content. M.T. and T.P.: acquisition of data; administrative, technical, or material support. T.A.M.: acquisition of data; analysis and interpretation of data; and critical revision of the manuscript for important intellectual content. R.M.C. and M.A.B.: acquisition of data; analysis and interpretation of data; administrative, technical, or material support; and critical revision of the manuscript for important intellectual content. S.C.: analysis and interpretation of data; administrative, technical, or material support; critical revision of the manuscript for important intellectual content; and statistical analysis. P.A.H.: analysis and interpretation of data; administrative, technical, or material support; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. S.G.K., L.F.L., M.L.R., W.F., E.L.F., C.E.F., L.L., S.P., W.G.P., J.S., S.K.V.D.E., S.S.V., and D.Y.: administrative, technical, or material support; critical revision of the manuscript for important intellectual content. D.L.C.: analysis and interpretation of data; administrative, technical, or material support; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. Z.C.-M.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; and study supervision.
Financial support: Research in this publication was supported by The National Cancer Institute (NCI) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) under award numbers: U01DK108328 (CDMC), U01DK108323 (IU), U01DK108306 (UPMC), U01DK108327 (OSU), U01DK108314 (CSMC), U01DK108300 (Stanford), U01DK108332 (KPNC), U01DK108320 (UF), U01DK108288 (Mayo), U01DK108326 (Baylor), the National Center for Advancing Translational Sciences Award TL1TR002735 (KG-F), National Cancer Institute T32 Tumor Immunology Fellowship 5T32CA09223 (KC), the Pelotonia Scholarship Program (VP-G), start-up funds from the Comprehensive Cancer Center at The Ohio State University (OSUCCC) (ZC-M), the National Institutes of Health (NIH) P30CA016058, an IDEA Award Diversity Supplement from the intramural research program at OSUCCC (KC), and the Pelotonia Institute of Immuno-Oncology (PIIO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the PIIO. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the Pelotonia Fellowship Program or The Ohio State University.
Potential competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest unless otherwise stated below. M.A.B.: research funding from Soy Nutrition Institute, not related to the study. S.G.K.: grant (investigator initiated) support from Maun Kea Technologies. Not related to the study presented. C.E.F.: research funding from AbbVie. Consultant for Nestle. Not related to the study presented. W.G.P.: research funding from AbbVie. Consultant for Nestle, Arctx Medical, Ariel Medicine, CapsoVision, Olympus, and Pfizer. Not related to the study presented. D.Y.: consultant for Pfizer. Not related to the study presented.
Ethical approval: The use of human samples was approved by the Ohio State University Institutional Review Board.
Study Highlights.
WHAT IS KNOWN
✓ Chronic pancreatitis (CP) is an inflammatory disease of the pancreas with significant morbidity and mortality.
✓ There are no diagnostic biomarkers for CP.
✓ The pathophysiology of CP remains poorly understood.
WHAT IS NEW HERE
✓ Plasma neutrophil gelatinase-associated lipocalin (NGAL) in combination with clinical characteristics is a potential biomarker for CP.
✓ NGAL+ immune cells associated with CP that suggest a shift in the adaptive immune response in CP.
✓ NGAL and its binding partner linoleic acid are altered in subjects with CP and diabetes.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the participants in the PROCEED study and the clinical coordinators at all sites for enrolling and collecting patient samples. This research was possible through resources, expertise, and support provided by the Pelotonia Institute for Immuno-Oncology (PIIO), which is funded by the Pelotonia community and the OSUCCC. We thank the PIIO and the Immune Monitoring and Discovery Platform for their help with the CyTOF instrumentation.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/B85.
Kristyn Gumpper-Fedus and Kaylin Chasser contributed equally to this work.
Contributor Information
Kristyn Gumpper-Fedus, Email: kristyn.gumpper-fedus@osumc.edu.
Kaylin Chasser, Email: kaylin.chasser@osumc.edu.
Valentina Pita-Grisanti, Email: valentina.pita-grisanti@osumc.edu.
Molly Torok, Email: mollytorok17@gmail.com.
Timothy Pfau, Email: timpfau@mail.mvnu.edu.
Thomas A. Mace, Email: thomas.mace@osumc.edu.
Rachel M. Cole, Email: Cole.311@osu.edu.
Martha A. Belury, Email: Belury.1@osu.edu.
Stacey Culp, Email: Stacey.Culp@osumc.edu.
Phil A. Hart, Email: philip.hart@osumc.edu.
Somashekar G. Krishna, Email: somashekar.krishna@osumc.edu.
Luis F. Lara, Email: luis.lara@osumc.edu.
Mitchell L. Ramsey, Email: mitchell.ramsey@osumc.edu.
William Fisher, Email: wfisher@bcm.edu.
Evan L. Fogel, Email: efogel@iu.edu.
Chris E. Forsmark, Email: chris.forsmark@medicine.ufl.edu.
Liang Li, Email: lli15@mdanderson.org.
Stephen Pandol, Email: stephen.pandol@cshs.org.
Walter G. Park, Email: wgpark@stanford.edu.
Jose Serrano, Email: serranoj@extra.niddk.nih.gov.
Stephen K. Van Den Eeden, Email: Stephen.vandeneeden@kp.org.
Santhi Swaroop Vege, Email: Vege.Santhi@mayo.edu.
Dhiraj Yadav, Email: yadavd@upmc.edu.
Darwin L. Conwell, Email: darwin.conwell@uky.edu.
REFERENCES
- 1.Ouyang G, Pan G, Liu Q, et al. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. BMC Med 2020;18(1):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey ML, Conwell DL, Hart PA. Complications of chronic pancreatitis. Dig Dis Sci 2017;62(7):1745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez-Munoz JE, Drewes AM, Lindkvist B, et al. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology 2018;18(8):847–54. [DOI] [PubMed] [Google Scholar]
- 4.Hart PA, Conwell DL. Chronic pancreatitis: Managing a difficult disease. Am J Gastroenterol 2020;115(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Monserrate Z, Gumpper K, Pita V, et al. Biomarkers of chronic pancreatitis: A systematic literature review. Pancreatology 2021;21(2):323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 2012;1826(1):129–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumpper K, Dangel AW, Pita-Grisanti V, et al. Lipocalin-2 expression and function in pancreatic diseases. Pancreatology 2020;20(3):419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santiago-Sanchez GS, Pita-Grisanti V, Quinones-Diaz B, et al. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int J Mol Sci 2020;21(12):4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjeldsen L, Johnsen AH, Sengelov H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268(14):10425–32. [PubMed] [Google Scholar]
- 10.Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: An emerging player in iron homeostasis and inflammation. Annu Rev Nutr 2017;37:103–30. [DOI] [PubMed] [Google Scholar]
- 11.Song E, Fan P, Huang B, et al. Deamidated lipocalin-2 induces endothelial dysfunction and hypertension in dietary obese mice. J Am Heart Assoc 2014;3(2):e000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Zhu Y, Jadhav K, et al. Lipocalin-2 protects against diet-induced nonalcoholic fatty liver disease by targeting hepatocytes. Hepatol Commun 2019;3(6):763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Chou SB, Swidnicka-Siergiejko AK, Badi N, et al. Lipocalin-2 promotes pancreatic ductal adenocarcinoma by regulating inflammation in the tumor microenvironment. Cancer Res 2017;77(10):2647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauvois B, Pramil E, Jondreville L, et al. Relation of neutrophil gelatinase-associated lipocalin overexpression to the resistance to apoptosis of tumor B cells in chronic lymphocytic leukemia. Cancers (Basel) 2020;12(8):2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SA, Noel S, Kurzhagen JT, et al. CD4(+) T cell-derived NGAL modifies the outcome of ischemic acute kidney injury. J Immunol 2020;204(3):586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonafine M, Martinez-Martinez E, Jaisser F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin Sci (Lond) 2018;132(9):909–23. [DOI] [PubMed] [Google Scholar]
- 17.Kaur S, Baine MJ, Guha S, et al. Neutrophil gelatinase-associated lipocalin, macrophage inhibitory cytokine 1, and carbohydrate antigen 19-9 in pancreatic juice: Pathobiologic implications in diagnosing benign and malignant disease of the pancreas. Pancreas 2013;42(3):494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogendorf P, Durczynski A, Skulimowski A, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) concentration in urine is superior to CA19-9 and Ca 125 in differentiation of pancreatic mass: Preliminary report. Cancer Biomark 2016;16(4):537–43. [DOI] [PubMed] [Google Scholar]
- 19.Moniaux N, Chakraborty S, Yalniz M, et al. Early diagnosis of pancreatic cancer: Neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer 2008;98(9):1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav D, Park WG, Fogel EL, et al. PROspective Evaluation of Chronic Pancreatitis for EpidEmiologic and Translational StuDies: Rationale and study design for PROCEED from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47(10):1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher WE, Cruz-Monserrate Z, McElhany AL, et al. Standard operating procedures for biospecimen collection, processing, and storage: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47(10):1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 23.Thomas J, Torok MA, Agrawal K, et al. The neonatal Fc receptor is elevated in monocyte-derived immune cells in pancreatic cancer. Int J Mol Sci 2022;23(13):7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226(1):497–509. [PubMed] [Google Scholar]
- 25.Stoffel W, Chu F, Ahrens EH. Analysis of long-chain fatty acids by gas-liquid chromatography. Anal Chem 1959;31(2):307–8. [Google Scholar]
- 26.Gumpper-Fedus K, Hart PA, Belury MA, et al. Altered plasma fatty acid abundance is associated with cachexia in treatment-naive pancreatic cancer. Cells 2022;11(5):910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumpper-Fedus K, Crowe O, Hart PA, et al. Changes in plasma fatty acid abundance related to chronic pancreatitis: A pilot study. bioRxiv 2023. doi: 10.1101/2023.01.05.522899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole RM, Angelotti A, Sparagna GC, et al. Linoleic acid-rich oil alters circulating cardiolipin species and fatty acid composition in adults: A randomized controlled trial. Mol Nutr Food Res 2022;66(15):e2101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belury MA, Cole RM, Bailey BE, et al. Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol Nutr Food Res 2016;60(5):1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold LE, Young AS, Belury MA, et al. Omega-3 fatty acid plasma levels before and after supplementation: Correlations with mood and clinical outcomes in the omega-3 and therapy studies. J Child Adol Psychop 2017;27(3):223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenna JT, Plourde M, Stark KD, et al. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr 2018;108(2):211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93(14):1054–61. [DOI] [PubMed] [Google Scholar]
- 33.Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J Natl Cancer Inst 2008;100(20):1432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee B, Jones EK, Manohar M, et al. Distinct serum immune profiles define the spectrum of acute and chronic pancreatitis from the multi-center PROCEED study. Gastroenterology 2023;165(1):173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J Biol Chem 2010;285(19):14088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Huang Y, Patil D, et al. Covariate adjustment in continuous biomarker assessment. Biometrics 2023;79(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Considine EC. The search for clinically useful biomarkers of complex disease: A data analysis perspective. Metabolites 2019;9(7):126.doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soni SS, Cruz D, Bobek I, et al. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol 2010;42(1):141–50. [DOI] [PubMed] [Google Scholar]
- 39.Bolgeri M, Whiting D, Reche A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker of renal injury in patients with ureteric stones: A pilot study. J Clin Urol 2021;14(1):21–8. [Google Scholar]
- 40.Ali H, Abu-Farha M, Alshawaf E, et al. Association of significantly elevated plasma levels of NGAL and IGFBP4 in patients with diabetic nephropathy. BMC Nephrol 2022;23(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He P, Bai M, Hu JP, et al. Significance of neutrophil gelatinase-associated lipocalin as a biomarker for the diagnosis of diabetic kidney disease: A systematic review and meta-analysis. Kidney Blood Press Res 2020;45(4):497–509. [DOI] [PubMed] [Google Scholar]
- 42.Hansen YB, Damgaard A, Poulsen JH. Evaluation of NGAL TestTM on Cobas 6000. Scand J Clin Lab Invest 2014;74(1):20–6. [DOI] [PubMed] [Google Scholar]
- 43.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016;1(3):226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007;56(10):2533–40. [DOI] [PubMed] [Google Scholar]
- 45.Mosialou I, Shikhel S, Luo N, et al. Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes. J Exp Med 2020;217(10):e20191261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Shen X, Zhou W, et al. The association of elevated serum lipocalin 2 levels with diabetic peripheral neuropathy in type 2 diabetes. Endocr Connect 2021;10(11):1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432(7019):917–21. [DOI] [PubMed] [Google Scholar]
- 48.Cherayil BJ. Iron and immunity: Immunological consequences of iron deficiency and overload. Arch Immunol Ther Exp (Warsz) 2010;58(6):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Konishi A, Fujita Y, et al. Lipocalin 2 bolsters innate and adaptive immune responses to blood-stage malaria infection by reinforcing host iron metabolism. Cell Host Microbe 2012;12(5):705–16. [DOI] [PubMed] [Google Scholar]
- 50.Watzenboeck ML, Drobits B, Zahalka S, et al. Lipocalin 2 modulates dendritic cell activity and shapes immunity to influenza in a microbiome dependent manner. PLoS Pathog 2021;17(4):e1009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Summers C, Rankin SM, Condliffe AM, et al. Neutrophil kinetics in health and disease. Trends Immunol 2010;31(8):318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diks AM, Bonroy C, Teodosio C, et al. Impact of blood storage and sample handling on quality of high dimensional flow cytometric data in multicenter clinical research. J Immunol Methods 2019;475:112616. [DOI] [PubMed] [Google Scholar]
- 53.Manohar M, Jones EK, Rubin SJS, et al. Novel circulating and tissue monocytes as well as macrophages in pancreatitis and recovery. Gastroenterology 2021;161(6):2014–29.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei X, Yao W, Li H, et al. B and NK cells closely correlate with the condition of patients with acute pancreatitis. Gastroenterol Res Pract 2019;2019:7568410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee B, Adamska JZ, Namkoong H, et al. Distinct immune characteristics distinguish hereditary and idiopathic chronic pancreatitis. J Clin Invest 2020;130(5):2705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grundsten M, Liu GZ, Permert J, et al. Increased central memory T cells in patients with chronic pancreatitis. Pancreatology 2005;5(2–3):177–82. [DOI] [PubMed] [Google Scholar]
- 57.Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 2015;6:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, et al. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol 2006;12(33):5344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shashidharamurthy R, Machiah D, Aitken JD, et al. Differential role of lipocalin 2 during immune complex-mediated acute and chronic inflammation in mice. Arthritis Rheum 2013;65(4):1064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu Dayyeh BK, Conwell D, Buttar NS, et al. Pancreatic juice prostaglandin e2 concentrations are elevated in chronic pancreatitis and improve detection of early disease. Clin Transl Gastroenterol 2015;6(1):e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang Y, Kim M, Hwang SW. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J Neuroinflammation 2020;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li K, Zhao J, Wang M, et al. The roles of various prostaglandins in fibrosis: A review. Biomolecules 2021;11(6):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belury MA, Cole RM, Snoke DB, et al. Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins Leukot Essent Fatty Acids 2018;132:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]