Abstract
Purpose:
While most small renal masses (SRM) < 4 cm have an excellent prognosis following resection, the impact of adverse T3a pathologic features on oncologic outcomes of SRMs remains unclear. We sought to compare clinical outcomes for surgically resected pT3a versus pT1a SRMs at our institution.
Materials and Methods:
We retrospectively reviewed records of patients who underwent radical or partial nephrectomy (RN, PN) for renal tumors < 4 cm at our institution between 2010–2020. We compared features and outcomes of pT3a vs pT1a SRMs. Continuous and categorical variables were compared using Student’s T and Pearson’s Chi-squared tests, respectively. Post-operative outcomes of interest including overall, cancer-specific, and recurrence-free survival (OS, CSS, RFS) were analyzed using Kaplan-Meier method, Cox proportional hazard regression, and competing risk analysis. Analyses were performed using R statistical package (R Foundation, v4.0).
Results:
We identified 1,837 patients with malignant SRMs. Predictors of post-operative pT3a upstaging included higher renal score, larger tumor size, and presence of radiologic features concerning for T3a disease (OR = 5.45, CI 3.92 – 7.59, p < 0.001). On univariable modeling, pT3a SRMs had higher positive margin rates (9.6% vs 4.1%, p < 0.001), worse OS (HR = 2.9, 95% CI 1.6–5.3, p = 0.002), RFS (HR 9.32, 95% CI 2–40.1, p = 0.003), and CSS (HR = 3.6, 95% CI 1.5 – 8.2, p = 0.003). On multivariable modeling, pT3a status remained associated with worse RFS (HR = 2.7, 95% CI 1.04 – 7, p = 0.04), but not OS (HR 1.6, 95% CI = 0.83 – 3.1, p = 0.2); multivariable modeling was deferred for CSS due to low event rates.
Conclusions:
Adverse T3a pathologic features portend worse outcomes for SRMs, highlighting the crucial role of preoperative planning and case selection. These patients have relatively poor prognosis, and should be monitored more closely and counseled for consideration of adjuvant therapy or clinical trials.
Funding:
National Institutes of Health Cancer Center Support Grant (NIH, NCI; 2P30CA008748–48)
Keywords: Small renal mass, adverse pathology, T3 stage, T3a stage
SUMMARY
Objectives:
We sought to compare clinical outcomes for patients with pT3a versus pT1a small renal masses (SRMs) < 4 cm that underwent resection at our institution, to determine the impact of adverse T3a pathologic features on oncologic outcomes in SRMs.
Methods and Materials:
We retrospectively reviewed records of patients who underwent radical or partial nephrectomy (RN, PN) for renal tumors < 4 cm at our institution between 2010–2020. We compared features and outcomes of pT3a vs pT1a SRMs. Continuous and categorical variables were compared using Student’s T and Pearson’s Chi-squared tests, respectively. Post-operative outcomes of interest including overall, cancer-specific, and recurrence-free survival (OS, CSS, RFS) were analyzed using Kaplan-Meier method, Cox proportional hazard regression, and competing risk analysis. Analyses were performed using R statistical package (R Foundation, v4.0).
Results:
We identified 1,837 patients with malignant SRMs. Predictors of post-operative pT3a upstaging included higher renal score, larger tumor size, and presence of radiologic features concerning for T3a disease (OR = 5.45, CI 3.92 – 7.59, p < 0.001). On univariable modeling, pT3a SRMs had higher positive margin rates (9.6% vs 4.1%, p < 0.001), worse OS (HR = 2.9, 95% CI 1.6–5.3, p = 0.002), RFS (HR 9.32, 95% CI 2–40.1, p = 0.003), and CSS (HR = 3.6, 95% CI 1.5 – 8.2, p = 0.003). On multivariable modeling, pT3a status remained associated with worse RFS, but worse RFS (HR = 2.7, 95% CI 1.04 – 7, p = 0.04), but not OS (HR 1.6, 95% CI = 0.83 – 3.1, p = 0.2); multivariable modeling was deferred for CSS due to low event rates.
Conclusions:
Adverse T3a pathologic features portend worse outcomes for SRMs, highlighting the crucial role of pre-operative planning and case selection. These patients have relatively poor prognosis, and should be monitored more closely and counseled for consideration of adjuvant therapy or clinical trials.
INTRODUCTION
Most renal masses, particularly small renal masses (SRMs), are now incidentally detected on imaging studies usually performed for nonspecific abdominal or musculoskeletal complaints1. Following surgical resection, the management and follow-up of malignant SRMs are largely dependent on pathological staging2,3. However, while pathologic staging for pT1-pT2 RCC is driven by tumor size (≤ 7 cm for pT1, > 7 cm for pT2), renal tumors of any size may be upstaged to pT3a if they exhibit specific adverse pathologic features, including tumor extension into the renal vein or its segmental branches, or peri-renal or renal sinus fat4.
Several retrospective studies have evaluated the influence of upstaging to pT3a disease on oncologic outcomes in cT1 renal masses (≤ 7 cm)5–12, with a recent meta-analysis of these studies concluding that upstaging from cT1 to pT3a portends worse overall, cancer-specific, and recurrence-free survival (OS, CSS, RFS) for patients13. However, the incidence and influence of pathologic upstaging to T3a disease on oncologic outcomes in SRMs (< 4cm) remain unclear.
In this study, we retrospectively reviewed records of patients with SRMs who underwent surgery at our institution over a 10-year period. Our primary objectives were to compare the peri-operative features as well as oncologic outcomes of pT3a and pT1a SRMs, and identify the predictors of post-operative pathologic upstaging to pT3a disease in SRMs.
METHODS
Study Population and Data Collection
Following institutional review board approval, we retrospectively reviewed our institutional kidney cancer surgical database for patients who underwent RN or PN using open or minimally invasive surgical (MIS) techniques for suspected renal cortical tumors between 1/1/2010 and 05/10/2021. Exclusion criteria included masses > 4 cm, benign masses, known or suspected advanced (≥ pT3b and/or N1/M1) disease preoperatively and incomplete pathology or radiology reports. Following surgery, oncological follow-up schedule was in alignment with the AUA guideline on management of renal masses2,14.
Pre-operative variables of interest included age, gender, BMI, Charlson comorbidity index (CCI), preoperative tumor size (maximum tumor diameter on imaging), enrollment in active surveillance, and adverse radiologic features (ARFs), defined as RFs concerning for cT3 disease noted on pre-operative cross-sectional imaging, such as tumor abutment or extension into the renal sinus, hilar vessels, or perirenal fat. RENAL nephrometry scores15 were also retrospectively calculated by the authors (SK, BD, PKL) for all pT3a masses and using a representative subset of pT1a masses (280, 16.8%) that were previously assessed using our institutional Arterial Based Complexity (ABC) nephrometry score16, a CT-based score developed and applied for all renal masses seen at our institution since August 2015 to estimate the morbidity of PN. The authors were not blinded to pT stage or the ABC score prior to RENAL nephrometric scoring. Our primary outcomes of interest included OS, CSS, and RFS, the latter including both local recurrence and distant metastases.
Statistical Analysis
Continuous and categorical variables were compared among SRM groups using Student’s T and Pearson’s Chi-squared tests, respectively. Potential predictors of post-operative upstaging to pT3a were examined using univariable and multivariable logistic regression modeling, with multivariable LRM utilizing predictors with p ≤ 0.1 on univariable LRM. OS was analyzed using Kaplan-Meier (K-M) and Cox-Proportional Hazard Regression (Cox-PHR) modeling, while CSS and RFS were assessed in terms of cumulative incidence of kidney cancer (KC)-related death and recurrence, respectively, using competing risk analysis (CR) models17–19 to account for censoring by competing events that may have precluded detecting the event of interest, namely death unrelated to KC for CSS, and death due to any cause for RFS; CR subdistribution hazard ratios were used to assess potential predictors for CSS and RFS. Multivariable analyses were performed for OS, and RFS using clinically relevant variables, but deferred for CSS due to the low event rate. All analyses were performed using R statistical package (R Foundation, v4.0).
RESULTS
Overview of Cohort
We identified 2,130 patients with SRMs that underwent PN or RN between 1/1/2010 and 05/10/2021, of which 293 (13.76%) were benign and excluded from further analyses. The characteristics of the remaining 1,837 patients with malignant SRMs are summarized in Table 1. The median age at nephrectomy was 60 years (IQR 52–68), with most patients undergoing PN (94.7%). Median tumor size was 2.5 cm (IQR 1.9 – 2.6 cm), with a 4.6% positive surgical margin (PSM) rate. Clear-cell renal cell carcinoma (cc-RCC) was the most common histology (62.6%), followed by papillary RCC (15.8%), and chromophobe RCC (9.7%). The proportion of pT3a SRMs was 9.04% (166).
Table 1.
Peri-operative features of cohort.
| Characteristic | Value* |
|---|---|
|
| |
| Mean age at nephrectomy | 59 ± 11 |
|
| |
| Male gender | 1205 (65.6%) |
|
| |
| BMI | 30 ± 6.1 |
|
| |
| Charlson comorbidity Index | 0.83 ± 1.3 |
|
| |
| Previously on active surveillance | 119 (6.5%) |
|
| |
| R.E.N.A.L nephrometry score | 7.19 ± 1.8 |
|
| |
| Adverse radiologic features | 341 (18.6%) |
|
| |
| Underwent radical nephrectomy | 97 (5.3%) |
|
| |
| Surgery done via minimally invasive approach | 771 (42%) |
|
| |
| Tumor size (max diameter) on pathology | 2.6 ± 0.9 |
|
| |
| Positive margin | 85 (4.6%) |
|
| |
| Tumor histology predominantly cc-RCC | 1,149 (62.6%) |
|
| |
| Nuclear grade | |
| G1/2 | 827 (45%) |
| G3/4 | 672 (36.6%) |
| NA | 338 (18.4%) |
Mean ± SD; n (%)
Peri-operative Features of pT3a and pT1a SRMs
Compared to patients with pT1a SRMs, patients with pT3a SRMs were older and more likely to be male (median age at nephrectomy 62 vs 59, p < 0.001; 76% vs 58.7%, p = 0.003), but did not differ significantly in their BMI or Charlson Comorbidity Index (Table 2). Compared to pT1a SRMs, pT3a SRMs had higher mean RENAL nephrometry score15, mean tumor size (7.82 vs 6.83, p < 0.001; 3.0 vs 2.5 cm, p < 0.001, respectively), ISUP grade (G3/4) tumors (63 vs 34%, p < 0.001), and PSM rates (9.6% vs 4.1%, p < 0.001) (Table 2). pT3a SRMs had a higher incidence of ARFs on pre-operative imaging (49% vs 15.5%; OR 6.1, 95% CI 4.3 – 8.7, p < 0.001). Of these, the most commonly noted ARF was contact with the renal sinus (47.6% in pT3a vs 15.1% in pT1a, OR 5.1, p < 0.001; Supp Table 1). pT3a masses were more likely to undergo RN (20% vs 3.8%, p < 0.001), but with no significant difference in the odds of intra-op conversion from PN to RN relative to pT1a SRMs (1.8% vs 0.9%, respectively, p = 0.2).
Table 2.
Comparison of peri-operative features between pT1a and pT3a SRMs
| Characteristic | pT1a* N = 1,671 |
pT3a* N = 166 |
p-value** |
|---|---|---|---|
|
| |||
| Mean age at nephrectomy | 59 ± 11 | 62 ± 12 | < 0.001 |
|
| |||
| Male gender | 1,079 (58.7%) | 126 (76%) | 0.003 |
|
| |||
| BMI | 30 ± 6.1 | 30.7 ± 6.2 | 0.2 |
|
| |||
| Charlson comorbidity Index | 0.82 ± 1.29 | 0.98 ± 1.4 | 0.1 |
|
| |||
| Previously on active surveillance | 106 (6.3%) | 14 (8.4%) | 0.3 |
|
| |||
| R.E.N.A.L nephrometry score | 6.83 ± 1.7 | 7.82 ± 1.9 | < 0.001 |
|
| |||
| Adverse radiologic features | 259 (15.5%) | 82 (49%) | < 0.001 |
|
| |||
| Underwent radical nephrectomy | 64 (3.8%) | 35 (20%) | < 0.001 |
|
| |||
| Surgery done via minimally invasive approach | 703 (42.1%) | 68 (41%) | 0.8 |
|
| |||
| Tumor size (max diameter) on pathology | 2.5 ± 0.9 | 3.0 ± 0.9 | < 0.001 |
|
| |||
| Positive surgical margin | 69 (4.1%) | 16 (9.6%) | < 0.001 |
|
| |||
| Tumor histology predominantly ccRCC | 1,039 (62.2%) | 110 (66%) | 0.3 |
|
| |||
| Nuclear grade | < 0.001 | ||
| G1/2 | 786 (47%) | 41 (25%) | |
| G3/4 | 568 (34%) | 104 (63%) | |
| NA | 317 (19%) | 21 (13%) | |
Mean ± SD; n (%)
Student’s T-test; Pearson’s Chi-squared test
Predictors of Postoperative pT3 Upstaging
On univariable LRM (u-LRM), statistically significant predictors of post-operative pathologic upstaging of SRMs to pT3a included older age (OR = 1.03, CI = 1.02 – 1.05, p < 0.001), male gender (OR = 1.76, 1.23 – 2.57, p = 0.003), presence of ARFs (OR = 5.45, CI 3.92 – 7.59, p < 0.001), higher RENAL score (OR = 1.37, CI = 1.22 – 1.54, p < 0.001), and larger tumor size (OR = 1.92, CI 1.55 – 2.41, p < 0.001); these variables remained statistically significant on multivariable LRM (m-LRM). BMI and CCI were not significantly associated with upstaging to pT3a disease on u-LRM (p = 0.2 and 0.13, respectively) and were thus excluded from m-LRM (Table 3).
Table 3. Predictors of post-operative upstaging to pT3 on univariable and multivariable logistic regression modeling (LRM).
Variables with p ≤ 0.1 on univariable LRM were applied to multivariable LRM. OR = odds radio; CI = confidence interval
| Univariable LRM | Multivariable LRM | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI* | p-value |
| Mean age at nephrectomy | 1.03 | 1.02 - 1.05 | < 0.001 | 1.02 | 1.00 – 1.04 | 0.016 |
| Male gender | 1.73 | 1.21 - 2.53 | 0.004 | 2.31 | 1.44 – 3.78 | < 0.001 |
| R.E.N.A.L nephrometry score | 1.38 | 1.22 - 1.55 | < 0.001 | 1.17 | 1.02 – 1.35 | 0.022 |
| Adverse radiologic features | 5.32 | 3.82 - 7.42 | < 0.001 | 1.58 | 1.58 – 4.3 | < 0.001 |
| Tumor size (max diameter) on pre-op imaging | 1.95 | 1.56 – 2.44 | < 0.001 | 2.6 | 1.17 – 2.16 | 0.003 |
| BMI | 1.02 | 0.99 – 1.05 | 0.2 | Excluded from multivariable model | ||
| Charlson comorbidity Index | 1.09 | 0.97 – 1.21 | 0.13 | Excluded from multivariable model | ||
Post-operative outcomes of pT1a vs pT3a masses
Median follow-up was 3.5 years. The overall incidence of all and kidney cancer-related deaths were 72 patients (3.9%) and 7 patients (0.4%), respectively (Supp Table 2), with higher incidence in pT3a vs pT1a SRMs (overall mortality 7.2% vs 3.6%, p=0.02; cancer-specific mortality 1.8% vs 0.2%, p = 0.02). Similarly, the rate of disease recurrence was higher in pT3a compared to pT1a SRMs (4.2% vs 1.5%, p = 0.02).
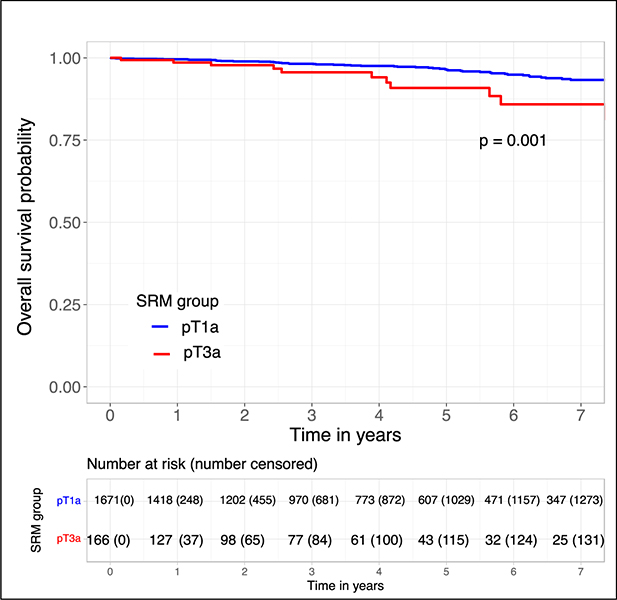
Overall survival was worse in pT3a compared to pT1a SRMs (log-rank p = 0.001; Figure 1). On univariable Cox-PHR, pT3a features were associated with worse OS (HR = 2.9, 95% CI 1.6–5.3, p = 0.002), along with CCI, age at nephrectomy, and tumor size (Table 4). On multivariable Cox-PHR, pT3a features were not significantly associated with OS (HR 1.6, 95% CI = 0.83 – 3.1, p = 0.2), adjusting for ARFs, Charlson CI, tumor size, and age at nephrectomy (Supp Table 3).
Figure 1.
Kaplan-Meier curve analysis for overall survival comparing pT1a to pT3a SRMs
Table 4. Univariable survival analysis for predictors of OS, CSS, and RFS.
Cox proportional hazard modeling was used for OS, while competing risk subdistribution hazard modeling was used for CSS and RFS.
| OS | CSS | RFS | ||||
|---|---|---|---|---|---|---|
| Predictor | HR & 95% CI | p-value | HR & 95% CI | p-value | HR & 95% CI | p-value |
| pT3a features | 2.7 (1.5 - 5.1) | 0.005 | 9.4 (2.14, 41.27) | 0.003 | 3.58 (1.55, 8.26) | 0.003 |
| Adverse RFs | 1.8 (1.04 – 3.02) | 0.046 | 2.08 (0.4, 10.8) | 0.38 | 1.43 (0.62, 3.3) | 0.4 |
| Radical nephrectomy | 4.7 (2.4 – 9.22) | < 0.001 | 4.43 (0.55, 35.88) | 0.17 | 2.59 (0.79, 8.51) | 0.12 |
| Age at nephrectomy | 1.1 (1.06 - 1.11) | < 0.001 | 1.2 (1.05, 1.38) | 0.01 | 1.05 (1.02, 1.09) | 0.005 |
| Tumor size on pathology (max diameter in cm) | 1.45 (1.14 - 1.84) | 0.003 | 1.95 (1.18, 3.22) | 0.01 | 1.64 (1.13, 2.39) | 0.009 |
| ccRCC histology | 1.32 (0.81 - 2.17) | 0.26 | 0.81 (0.18, 3.64) | 0.79 | 0.9 (0.45, 1.83) | 0.78 |
| Positive surgical margins | 1.01 (0.37 - 2.78) | 0.98 | 2.74 (0.31, 24.18) | 0.36 | 1.15 (0.27, 4.82) | 0.85 |
| Charlson Comorbidity Index | 1.39 (1.24 - 1.56) | < 0.001 | 1.69 (1.28, 2.23) | <0.001 | 1.53 (1.32, 1.78) | <0.001 |
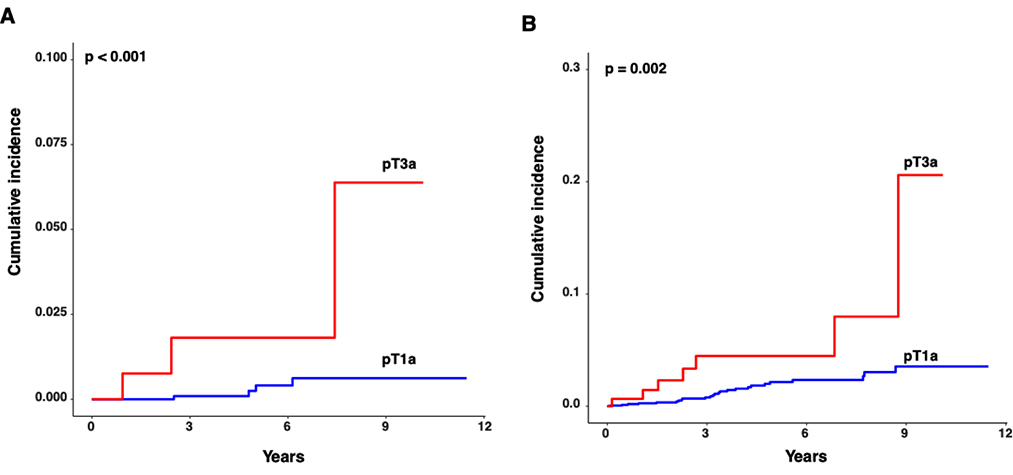
Similarly, pT3a stage was associated with worse CSS and RFS compared to pT1a on univariable CR analysis (HR 9.4, 95% CI 2.1 – 41.3, p = 0.003: HR = 3.6, 95% CI 1.6 – 8.3, p = 0.003, respectively; Table 4 and Figure 2). On multivariable modeling for predictors of RFS, pT3a status was significantly associated with worse RFS (HR 2.7, 95% CI 1.04 – 7, p = 0.041), adjusting for histology (ccRCC or non-ccRCC), tumor size, PSM, and nephrectomy type (RN or PN) (Supp Table 4). Multivariable modeling was deferred for CSS due to the low event rate.
Figure 2.
Comparison of (a) cancer specific and (b) recurrence-free survival between pT3a and pT1a SRMs, assessed in terms of cumulative incidence of kidney cancer deaths and disease recurrences, respectively, using competing risk model analyses.
DISCUSSION
The management of SRMs has evolved over the past two decades due to several factors, including the increasing incidental detection of SRMs on radiologic workup for unrelated complaints rather than the historic delayed diagnosis of kidney tumors when patients presented with classic symptoms of hematuria, flank pain, and flank mass 1. The resultant size and stage migration lead to the expansion of kidney sparing operations and evidence for equivalent oncological outcomes between PN and RN in the management of SRMs20–23. The important added advantage of PN was renal functional preservation and avoidance or delay in the onset of chronic kidney disease and associated cardiovascular morbidity and mortality24,25.
Furthermore, as advances in histopathologic diagnosis revealed large variation in the oncologic implications of different tumor histologies, they also noted that certain adverse pathologic features predicted worse survival, even in masses that would have been staged pT1/pT2 based on size alone. Bonsib et al26 noted tumor invasion into the renal sinus, branched muscular renal veins and perinephric fat portended a worse prognosis regardless of tumor size27,28. These findings questioned the AJCC size-based staging system for pT1/pT2 masses, prompting the 6th and following editions of the AJCC staging system to redefine pT3a stage based on the presence of these features, independent of tumor size4,29.
In this study, we demonstrate that pT3a status remains an independent poor prognostic factor even in SRMs. pT3a SRMs were twice as likely to have positive margins (9.6 vs 4.1%, p < 0.001), and pT3a status was significantly associated with worse OS, RFS, and CSS on univariable models. On multivariable modeling, we found pT3a status to remain associated with worse RFS (HR = 2.7, 95% CI 1.04 – 7, p = 0.041, respectively) but not OS (HR 1.6, 95% CI = 0.83 – 3.1, p = 0.2). Comparing preoperative features of pT1a vs pT3a SRMs, pT1a were smaller (mean size 2.5 ± 0.9 cm vs 3.1 ± 0.9 cm; p < 0.001) and had lower RENAL nephrometry scores (mean score 6.83 ± 1.7 vs 7.82 ± 1.9), but did not differ from their pT3a counterparts in proportion of ccRCC (66% vs 62.2%, p = 0.3), although pT3a tumors had a higher ratio of ISUP grade G3/4 tumors (63% vs 34%, p < 0.001).
Limitations of our study include its retrospective nature and the relatively short median follow-up time (3.5 years), and the relatively low incidence of our outcomes of interest, which may be related to our short follow-up, thereby limiting our statistical assessment of oncologic outcomes. RENAL scores were also calculated retrospectively for all pT3a masses and only a representative subset of pT1a masses that were previously assessed using our institutional ABC nephrometry score16, and the lack of blinding of authors to final pathology and ABC score may have biased their RENAL score assessments. However, our findings are in agreement with prior findings by Chevinsky et al30, which found pT3 stage to predict fourfold risk of disease recurrence compared to pT1/pT2 masses, even after adjusting for tumor size, with similar association noted when examining the SRM subsets of pT3 vs pT1/T2 masses in the cohort (HR 4.4, 95% CI 1.4 – 13.6, p = 0.01)30.
Our findings emphasize careful pre-operative planning and vigilance in the management of SRMs, through careful examination of pre-operative imaging for adverse RFs associated with pT3a features, such as tumor abutment of the renal sinus and perinephric fat31, as well as muscular branch vein invasion32,33. Despite marked improvements in imaging technology, the sensitivity of CT and MRI modalities for pT3a features remains somewhat limited with high levels of inter-reader variability and discrepancy34–36, with one series noting radiologic staging based on axial imaging (CT or MRI) to have the lowest sensitivity for stage III RCC - 27.7%, versus 90.7%, 67.3%, and 64.2% for stages I, II, and IV, respectively34. Similarly, while pT3a SRMs had a significantly higher incidence of adverse RFs compared to pT1a masses in our series (49% vs 15.5%; OR 6.1, 95% CI 4.3 – 8.7, p < 0.001), 51% of pT3a masses did not exhibit any concerning radiologic features preoperatively. This issue of “unexpected pathologic upstaging” of seemingly cT1 masses to pT3a post-operatively has been shown to portend worse OS, CSS, and RFS compared to cT1/pT1a masses5–13. Such issues may again be mitigated by the use of additional imaging modalities such as pre- and intra-op renal ultrasound (RUS), which are routinely performed at our institution in addition to pre-operative cross-sectional imaging with CT or MRI, to detect otherwise unrecognized branched vein or renal sinus fat invasion37. In our series, we found the presence of adverse RFs concerning for T3a disease on pre-operative imaging to be a significant predictor of upstaging to pT3a disease on both univariable and multivariable LRM, along with higher RENAL scores (univariable LRM OR = 5.32 and 1.38, respectively; multivariable LRM OR = 1.58 and 1.17; all with p < 0.001). pT3a tumors were more likely to undergo RN than their pT1a counterparts (21% vs 3.8% rates of RN) with no significant difference in the odds of intra-op conversion from PN to RN relative to pT1a SRMs (p = 0.2), suggesting that the operating surgeons were aware of these adverse features as they designed their operations. Interestingly, male gender was also a predictor of upstaging to pT3a disease (univariable LRM OR 1.73, p = 0.004; multivariable LRM OR 2.3, p < 0.001), a finding noted on previous analyses of surgically resected tumors in SEER38, NCDBI39, and the Dutch PALGA40 registries, which found female gender to be associated with smaller size and lower stage and grade on presentation38–40, although the etiology underlying this difference remains unclear.
In summary, our findings emphasize that pT3a stage portends worse survival outcomes in SRMs, a subgroup of renal masses where current guidelines on renal cell carcinoma such as the EAU41, NCCN3, and AUA42 guidelines list active surveillance as a management option. It is critical to note that this recommendation of active surveillance as a management option is restricted to cT1a SRMs in the latter two guidelines (NCCN3 and AUA)42, i.e., SRMs with no adverse cT3 RFs. Therefore, as with surgical management of SRMs, the decision to proceed with active surveillance cannot be solely made based on tumor size alone, and requires a similar level of vigilance with regards to thorough examination of imaging for features suggestive of T3a stage, which would warrant an interventional rather than surveillance approach. However, given the variable and limited sensitivity of imaging modalities for pT3a features, which allow for discordance between radiologic and pathologic staging 34, and the equivalence in oncologic outcomes for PN and RN in appropriate patients43,44, the presence of adverse RFs may not necessitate a more aggressive surgical approach upfront. Post-operatively, our findings are in alignment with current guideline recommendations for closer surveillance, and potentially referral to medical oncology to discuss adjuvant therapy or clinical trials in patients with pT3 SRMs given their worse outcomes compared to pT1a45–48.
CONCLUSIONS
Adverse T3a pathologic features portend worse cancer-specific and recurrence-free survival outcomes for patients with SRMs. Our findings emphasize the crucial role of pre-operative planning, case selection, and a multidisciplinary approach to post-surgical management for T3a masses, regardless of their size, given their poorer prognosis compared to lower stage masses.
Supplementary Material
Acknowledgments
Our study was funded in part by the National Institutes of Health Cancer Center Support Grant (NIH, NCI; 2P30CA008748-48).
Footnotes
Conflict of Interest
The authors do not have any direct conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nguyen MM, Gill IS and Ellison LM: The Evolving Presentation of Renal Carcinoma in the United States: Trends From the Surveillance, Epidemiology, and End Results Program. J. Urol. 2006. Available at: https://www.auajournals.org/doi/abs/10.1016/j.juro.2006.07.144, accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 2.S C, RG U, ME A, et al. : Renal Mass and Localized Renal Cancer: AUA Guideline. J. Urol. 2017; 198. Available at: https://pubmed.ncbi.nlm.nih.gov/28479239/, accessed January 16, 2022. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Memorial Sloan Kettering Cancer Center Þ, Jonasch E, et al. : NCCN Guidelines Version 1.2021 Kidney Cancer.; 2020. Available at: https://www2.tri-kobe.org/nccn/guideline/urological/english/kidney.pdf, accessed September 8, 2021.
- 4.Amin MB, Greene FL, Edge SB, et al. : The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA. Cancer J. Clin. 2017; 67: 93–99. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28094848, accessed February 9, 2022. [DOI] [PubMed] [Google Scholar]
- 5.Nayak JG, Patel P, Saarela O, et al. : Pathological Upstaging of Clinical T1 to Pathological T3a Renal Cell Carcinoma: A Multi-institutional Analysis of Short-term Outcomes. Urology 2016; 94: 154–160. Available at: https://www.sciencedirect.com/science/article/pii/S0090429516300103?casa_token=BS1tlhyvIygAAAAA:A-SH2pIdOVMd0iHDlMj5RLy2KYF70JovRh_nqvS0K6QPrJ5eadotj1ghV9NscnUODGLxvlp1pA, accessed August 1, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Nayak JG, Patel P, Bjazevic J, et al. : Clinical outcomes following laparoscopic management of pT3 renal masses: A large, multi-institutional cohort. Can. Urol. Assoc. J. 2015; 9: 397–402. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26788228, accessed February 22, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veccia A, Antonelli A, Minervini A, et al. : Upstaging to pT3a disease in patients undergoing robotic partial nephrectomy for cT1 kidney cancer: Outcomes and predictors from a multi-institutional dataset. Urol. Oncol. Semin. Orig. Investig. 2020; 38: 286–292. Available at: https://www.sciencedirect.com/science/article/pii/S1078143919305253, accessed June 19, 2022. [DOI] [PubMed] [Google Scholar]
- 8.Gorin MA, Ball MW, Pierorazio PM, et al. : Outcomes and Predictors of Clinical T1 to Pathological T3a Tumor Up-Staging after Robotic Partial Nephrectomy: A Multi-Institutional Analysis. J. Urol. 2013; 190: 1907–1911. Available at: http://www.jurology.com/doi/10.1016/j.juro.2013.06.014, accessed August 1, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Mouracade P, Kara O, Dagenais J, et al. : Perioperative morbidity, oncological outcomes and predictors of pT3a upstaging for patients undergoing partial nephrectomy for cT1 tumors. World J. Urol. 2017; 35: 1425–1433. Available at: http://link.springer.com/10.1007/s00345-017-2004-x, accessed June 19, 2022. [DOI] [PubMed] [Google Scholar]
- 10.Ramaswamy K, Kheterpal E, Pham H, et al. : Significance of Pathologic T3a Upstaging in Clinical T1 Renal Masses Undergoing Nephrectomy. Clin. Genitourin. Cancer 2015; 13: 344–349. Available at: https://www.sciencedirect.com/science/article/pii/S1558767315000038?casa_token=3WpwiopWIvEAAAAA:zENSvQU5j2PY-GmtLFuwD4pQQWuNiZCqlF3g0kPLReb66NK1DIWIuhRufuRuZxvx_SZTGmA5GA, accessed August 1, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Russell CM, Lebastchi AH, Chipollini J, et al. : Multi-institutional Survival Analysis of Incidental Pathologic T3a Upstaging in Clinical T1 Renal Cell Carcinoma Following Partial Nephrectomy. Urology 2018; 117: 95–100. Available at: https://www.sciencedirect.com/science/article/pii/S0090429518303200, accessed June 19, 2022. [DOI] [PubMed] [Google Scholar]
- 12.Jeong S, Kim JK, Park J, et al. : Pathological T3a Upstaging of Clinical T1 Renal Cell Carcinoma: Outcomes According to Surgical Technique and Predictors of Upstaging. Edited by Chuu C-P. PLoS One 2016; 11: e0166183. Available at: https://dx.plos.org/10.1371/journal.pone.0166183, accessed June 19, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veccia A, Falagario U, Martini A, et al. : Upstaging to pT3a in Patients Undergoing Partial or Radical Nephrectomy for cT1 Renal Tumors: A Systematic Review and Meta-analysis of Outcomes and Predictive Factors. Eur. Urol. Focus 2021; 7: 574–581. Available at: https://www.sciencedirect.com/science/article/abs/pii/S2405456920301486, accessed August 1, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Campbell SC, Novick AC, Belldegrun A, et al. : Guideline for Management of the Clinical T1 Renal Mass. J. Urol. 2009. Available at: https://www.auajournals.org/doi/10.1016/j.juro.2009.07.004, accessed June 19, 2022. [DOI] [PubMed] [Google Scholar]
- 15.Kutikov A and Uzzo RG: The R.E.N.A.L. Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J. Urol. 2009; 182: 844–853. Available at: http://www.jurology.com/doi/10.1016/j.juro.2009.05.035, accessed August 1, 2021. [DOI] [PubMed] [Google Scholar]
- 16.Spaliviero M, Poon BY, Karlo CA, et al. : An Arterial Based Complexity (ABC) Scoring System to Assess the Morbidity Profile of Partial Nephrectomy. Eur. Urol. 2016; 69: 72–79. Available at: https://www.sciencedirect.com/science/article/pii/S0302283815007514, accessed November 1, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray RJ: A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988; 16: 1141–1154. Available at: https://projecteuclid.org/journals/annals-of-statistics/volume-16/issue-3/A-Class-of-K-Sample-Tests-for-Comparing-the-Cumulative/10.1214/aos/1176350951.full, accessed March 22, 2022. [Google Scholar]
- 18.Fine JP and Gray RJ: A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999; 94: 496–509. Available at: http://www.tandfonline.com/doi/abs/10.1080/01621459.1999.10474144, accessed March 22, 2022. [Google Scholar]
- 19.Scrucca L, Santucci A and Aversa F: Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010; 45: 1388–1395. Available at: https://www.nature.com/articles/bmt2009359, accessed March 26, 2022. [DOI] [PubMed] [Google Scholar]
- 20.LEE CT, KATZ J, SHI W, et al. : Surgical management of renal tumors 4 cm or less in a contemporary cohort. J. Urol. 2000; 163: 730–736. Available at: http://www.jurology.com/doi/10.1016/S0022-5347%2805%2967793-2, accessed April 11, 2022. [PubMed] [Google Scholar]
- 21.Fergany AF, Hafez KS and Novick AC: Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year follow up. J. Urol. 2000; 163: 442–445. Available at: https://www.sciencedirect.com/science/article/pii/S0022534705678962, accessed October 10, 2022. [PubMed] [Google Scholar]
- 22.Gregg JR, Cookson MS, Phillips S, et al. : Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J. Urol. 2011; 185: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LEIBOVICH BC, BLUTE ML, CHEVILLE JC, et al. : Nephron Sparing Surgery for Appropriately Selected Renal Cell Carcinoma Between 4 and 7 Cm Results in Outcome Similar to Radical Nephrectomy. J. Urol. 2004. Available at: https://www.auajournals.org/doi/10.1097/01.ju.0000113274.40885.db, accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 24.Weight CJ, Larson BT, Fergany AF, et al. : Nephrectomy Induced Chronic Renal Insufficiency is Associated With Increased Risk of Cardiovascular Death and Death From Any Cause in Patients With Localized cT1b Renal Masses. J. Urol. 2010. Available at: https://www.auajournals.org/doi/10.1016/j.juro.2009.12.030, accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 25.Kim SP, Thompson RH, Boorjian SA, et al. : Comparative Effectiveness for Survival and Renal Function of Partial and Radical Nephrectomy for Localized Renal Tumors: A Systematic Review and Meta-Analysis. J. Urol. 2012; 188: 51–57. Available at: https://www.sciencedirect.com/science/article/pii/S0022534712030091, accessed October 11, 2022. [DOI] [PubMed] [Google Scholar]
- 26.Bonsib SM, Gibson D, Mhoon M, et al. : Renal sinus involvement in renal cell carcinomas. Am. J. Surg. Pathol. 2000; 24: 451–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10716160, accessed April 11, 2022. [DOI] [PubMed] [Google Scholar]
- 27.Feifer A, Savage C, Rayala H, et al. : Prognostic Impact of Muscular Venous Branch Invasion in Localized Renal Cell Carcinoma Cases. J. Urol. 2011. Available at: https://www.auajournals.org/doi/full/10.1016/j.juro.2010.08.084, accessed April 11, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson RH, Leibovich BC, Cheville JC, et al. : Is renal sinus fat invasion the same as perinephric fat invasion for pT3a renal cell carcinoma. J. Urol. 2005. Available at: https://www.auajournals.org/doi/full/10.1097/01.ju.0000173942.19990.40, accessed October 11, 2022. [DOI] [PubMed] [Google Scholar]
- 29.Swami U, Nussenzveig RH, Haaland B, et al. : Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann. Transl. Med. 2019; 7: S18. Available at: http://www.ncbi.nlm.nih.gov/pubmed/31032299, accessed February 21, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevinsky M, Imnadze M, Sankin A, et al. : Pathological Stage T3a Significantly Increases Disease Recurrence across All Tumor Sizes in Renal Cell Carcinoma. J. Urol. 2015; 194: 310–315. Available at: http://www.jurology.com/doi/10.1016/j.juro.2015.02.013, accessed September 8, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkassem AA, Allen BC, Sharbidre KG, et al. : Update on the Role of Imaging in Clinical Staging and Restaging of Renal Cell Carcinoma Based on the AJCC 8th Edition, From the AJR Special Series on Cancer Staging. Am. J. Roentgenol. 2021: 1–15. Available at: https://www.ajronline.org/doi/10.2214/AJR.21.25493, accessed July 28, 2021. [DOI] [PubMed] [Google Scholar]
- 32.Karlo CA, Donati OF, Marigliano C, et al. : Role of CT in the Assessment of Muscular Venous Branch Invasion in Patients With Renal Cell Carcinoma. Am. J. Roentgenol. 2013; 201: 847–852. Available at: http://www.ajronline.org/doi/10.2214/AJR.12.10496, accessed April 11, 2022. [DOI] [PubMed] [Google Scholar]
- 33.Karlo CA, Di Paolo PL, Donati OF, et al. : Renal Cell Carcinoma: Role of MR Imaging in the Assessment of Muscular Venous Branch Invasion. Radiology 2013; 267: 454–459. Available at: http://pubs.rsna.org/doi/10.1148/radiol.13121555, accessed October 11, 2022. [DOI] [PubMed] [Google Scholar]
- 34.Ucer O, Muezzinoglu T, Ozden E, et al. : How accurate is radiological imaging for perirenal fat and renal vein invasion in renal cell carcinoma? Int. J. Clin. Pract. 2021; 75: e14359. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ijcp.14359, accessed March 25, 2022. [DOI] [PubMed] [Google Scholar]
- 35.Kathrins M, Caesar S, Mucksavage P, et al. : Renal mass size - concordance between pathology and radiology. Curr. Opin. Urol. 2013; 23: 389–393. Available at: https://journals.lww.com/00042307-201309000-00002, accessed July 28, 2021. [DOI] [PubMed] [Google Scholar]
- 36.Sokhi HK, Mok WY and Patel U: Stage T3a renal cell carcinoma: staging accuracy of CT for sinus fat, perinephric fat or renal vein invasion. Br. J. Radiol. 2015; 88: 20140504. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25410425, accessed August 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhosale PR, Wei W, Ernst RD, et al. : Intraoperative Sonography During Open Partial Nephrectomy for Renal Cell Cancer: Does It Alter Surgical Management? Am. J. Roentgenol. 2014; 203: 822–827. Available at: https://www.ajronline.org/doi/10.2214/AJR.13.12254, accessed November 12, 2022. [DOI] [PubMed] [Google Scholar]
- 38.Aron M, Nguyen MM, Stein RJ, et al. : Impact of Gender in Renal Cell Carcinoma: An Analysis of the SEER Database. Eur. Urol. 2008; 54: 133–142. Available at: https://www.sciencedirect.com/science/article/pii/S0302283807015400?casa_token=cXxWL8B7HjEAAAAA:z81w3UF6UAk3G_a2u9cbeNf74GH3TTTXdR9vXvdpIb1e7lOYBfttcJPU3xVbByjHx00JgS70iLY, accessed November 12, 2022. [DOI] [PubMed] [Google Scholar]
- 39.Woldrich JM, Mallin K, Ritchey J, et al. : Sex Differences in Renal Cell Cancer Presentation and Survival: An Analysis of the National Cancer Database, 1993–2004. J. Urol. 2008; 179: 1709–1713. Available at: https://www.sciencedirect.com/science/article/pii/S002253470800027X, accessed November 12, 2022. [DOI] [PubMed] [Google Scholar]
- 40.Hew MN, Zonneveld R, Kümmerlin intan PED, et al. : Age and Gender Related Differences in Renal Cell Carcinoma in a European Cohort. J. Urol. 2012; 188: 33–38. Available at: https://www.sciencedirect.com/science/article/pii/S0022534712029722?casa_token=WQg8eCeENQIAAAAA:-TbZnUfmQetuHJCmICq6×4IiE8te-BrfVJxdGn1t8nGLQWTBaPEQOHzoHAxzWu3Kh8t-DWCsM6Y, accessed November 12, 2022. [DOI] [PubMed] [Google Scholar]
- 41.Ljungberg B, Albiges L, Abu-Ghanem Y, et al. : European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022; 82: 399–410. Available at: http://www.ncbi.nlm.nih.gov/pubmed/35346519, accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 42.Campbell SC, Clark PE, Chang SS, et al. : Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021; 206: 199–208. Available at: http://www.jurology.com/doi/10.1097/JU.0000000000001911, accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 43.Deng H, Fan Y, Yuan F, et al. : Partial nephrectomy provides equivalent oncologic outcomes and better renal function preservation than radical nephrectomy for pathological T3a renal cell carcinoma: A meta-analysis. Int. braz j urol 2021; 47: 46–60. Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1677-55382021000100046&tlng=en, accessed April 24, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerrato C, Patel D, Autorino R, et al. : Partial or radical nephrectomy for complex renal mass: a comparative analysis of oncological outcomes and complications from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group. World J. Urol. 2023; 41: 747–755. Available at: https://link.springer.com/10.1007/s00345-023-04279-1, accessed April 24, 2023. [DOI] [PubMed] [Google Scholar]
- 45.Oza B, Frangou E, Smith B, et al. : RAMPART: A phase III multi-arm multi-stage trial of adjuvant checkpoint inhibitors in patients with resected primary renal cell carcinoma (RCC) at high or intermediate risk of relapse. Contemp. Clin. Trials 2021; 108: 106482. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1551714421002184, accessed January 28, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powles T, Tomczak P, Park SH, et al. : Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022; 23: 1133–1144. Available at: https://www.sciencedirect.com/science/article/pii/S1470204522004879, accessed October 23, 2022. [DOI] [PubMed] [Google Scholar]
- 47.Motzer RJ, Russo P, Grünwald V, et al. : Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet (London, England) 2023; 401: 821–832. Available at: http://www.ncbi.nlm.nih.gov/pubmed/36774933, accessed April 24, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas NB, Manola J, Uzzo RG, et al. : Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016; 387: 2008–2016. Available at: https://www.sciencedirect.com/science/article/pii/S0140673616005596?via%3Dihub, accessed January 6, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.