Abstract
Objectives
CDKL5 developmental and epileptic encephalopathy (CDKL5-DEE) is a rare X-linked dominant genetic disorder. Family-centered Early Intervention (EI) programs, which promote axonal plasticity and synaptic reorganization through exposure to an enriched environment, should be integrated into clinical practice. However, there is presently a dearth of dedicated EI protocols for patients with CDKL5-DEE and cerebral visual impairment (CVI).
Methods
We present a girl with a deletion of the CDKL5 gene (MIM*300203). At the age of 2 months, the child presented with severe epilepsy. The neurologic examination was abnormal, and she had severe CVI. At the first assessment, at 5 months old, her Developmental Quotient (DQ) on the Griffiths Mental Developmental Scales III (GMDS-III) was equivalent to 3-month-old skills (95% CI). The child was enrolled in an EI program for 6 months.
Results
At 12 months of age, the DQ score was 91. There has been improvement in the neurovisual functions. The findings from the scales show a gradual improvement in neuromotor and psychomotor development, which is in contrast to the expected outcome of the disease.
Discussion
The case study shows that a family-centered EI and prompt assessment of CVI can promote and enhance neurodevelopment.
PRACTICAL IMPLICATIONS
Assessing visual function early on and introducing patients to family-centered intervention programs with specialized experts in early rehabilitation can aid in the recovery and support of patients with DEEs and CVI in achieving neurodevelopmental milestones.
Introduction
CDKL5 developmental and epileptic encephalopathy (CDKL5-DEE) is an X-linked genetic disorder, caused by loss of function variants in CDKL5 gene. CDKL5-DEE is characterized by severe epileptic seizures with onset in the first 6 months of life, hypotonia, poor eye contact, and delayed neurocognitive development.1 Cerebral visual impairment (CVI) has been shown to be associated with delayed achievement of developmental milestones and may be considered an early marker of a neurodevelopmental disorder.2 According to Wojcik and collaborators, infants and children with genetic diagnoses, such as those with DEEs, are less likely to be referred to EI programs.3 EI aims to “promote child health and well-being, enhance emerging competencies, minimize developmental delays, remediate existing or emerging disabilities, prevent functional deterioration, and promote adaptive parenting and overall family function.”4 EI can be considered a “neuroprotective” strategy that stimulates brain development5 and neuroplasticity. Exposure to an “Enriched Environment” has been shown to accelerate the development of the visual system6 and to support both sensitive parenting and the child's development in the presence of CVI.7 To date, few studies consider EI programs for patients with CDKL5-DEE and CVI.8 The study highlights the effectiveness of multidisciplinary EI and early assessment of CVI.
Methods
History of Present Illness
A 5-month-old infant with a history of maternal HIV infection and prophylactic zidovudine during delivery was referred for evaluation due to focal motor and/or dyscognitive seizures with an autonomic component. Born at 39-week gestation with a 9–10 APGAR score, the infant experienced seizures at 2 months characterized by rhythmic limb movements and loss of consciousness. A genetic panel for DEEs using next-generation sequencing (NGS) was performed and yielded negative results. The parents also reported an ongoing issue with constipation.
Neurologic Examination
At 6 months of age, the child (height of 61 cm, weight in the 75th–90th percentile, and head circumference in the 88th percentile) showed abnormal eye movements and inconsistent eye contact. Spontaneous movements appeared jerky and lacked fluidity, and axial hypotonia was observed. Reflexes were normal, but difficulties were noted in lifting the head and supporting weight while prone. Bilateral rolling to the side was not observed, and parachute reactions were absent.
Laboratory Tests, Imaging, and Genetic Testing
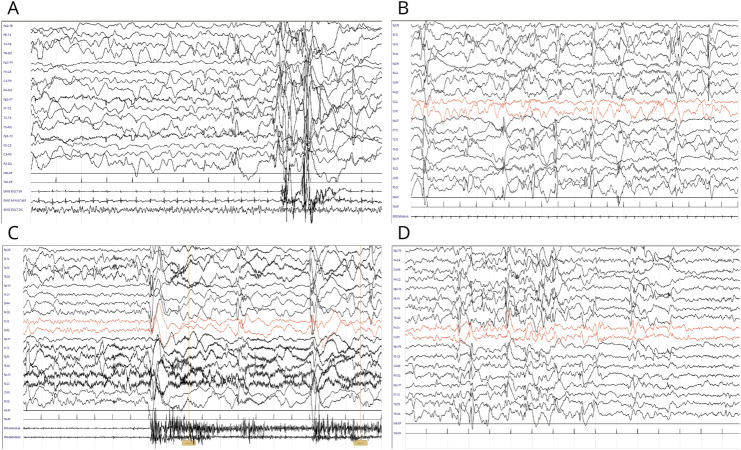
A MRI scan showed small hemosiderin deposits in some regions of the right hemisphere without indications of CSF obstruction. Serial EEG investigations revealed diffuse polyspikes, wave discharges, and epileptiform abnormalities during sleep. At 10 and 15 months, EEG displayed clusters of epileptic spasms with characteristic polyspike waves and subsequent slow wave activity as illustrated in Figure 1. The patient did not experience a seizure-free “honeymoon” phase.
Figure 1. EEGs at 5 (A), 7 (B), 10 (C), and 12 (D) Months.
Multiple antiepileptic drugs were administered, with limited efficacy, including levetiracetam 200 mg/d, valproic acid (VPA) 250 mg/d, and vigabatrin (VGB) 750 mg/d. At 10 months old, dosages of VPA and VGB were reduced to 100 mg/d and 500 mg/d, respectively. At 12 months of age, the VGB dose was further decreased to 250 mg/d. Array-comparative genomic hybridization (array-CGH) revealed a de novo 2.2 Mb deletion on the short arm of the X chromosome at Xp22.2p22.13, inclusive of the NHS gene completely (MIM*300457) and partially (MIM*300203) the CDKL5 gene. Segregation analysis confirmed the de novo origin, and X-chromosome inactivation analysis showed no skewed inactivation.
Neurodevelopmental Evaluations
The child underwent evaluations at 6, 9, and 12 months using the Griffiths Scales for Child Development 3rd Edition (GMDS-III).9 Visual functions were assessed using the Ricci et al.10 protocol. Additional assessments included the Teller Acuity Cards11 and Hiding-Heidi Low Contrast Face Cards.12
Family-Centered “Early Intervention” Program
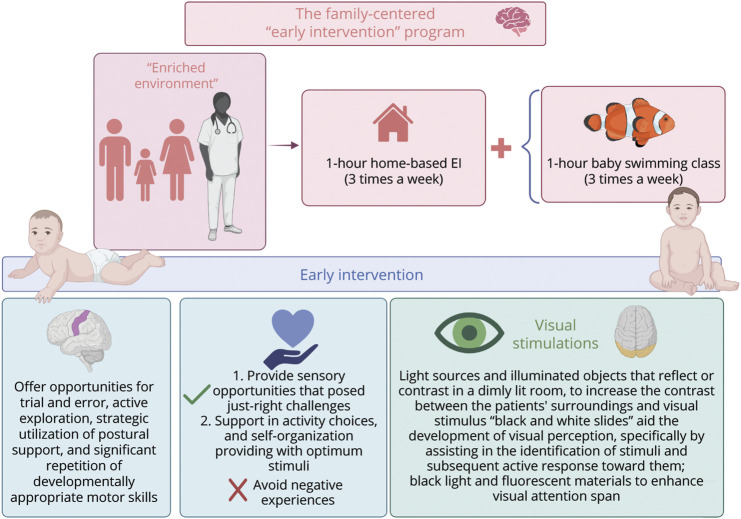
The patient participated in a 6-month intensive multisensory rehabilitation program (Figure 2) from October 2022 to April 2023. The EI sessions were led by a specialized neurodevelopmental therapist and involved both parents. The program used strategies aimed at trial and error, active exploration, postural support, and repetition of motor skills and involves the use of light sources, illuminated objects, and black and white slides to enhance visual perception.13
Figure 2. Summary Diagram of a Multisensory Early Intervention Program.
Created with BioRender.com.
Results
Follow-Up and Outcomes
The GMDS-III
Table 1 presents the GMDS-III composite score changes at 9 and 12 months. Before treatment, the child's developmental level was comparable with that of a 3-month-old; the child's development reached the expected range at 9 months (3 months after treatment) with a developmental quotient (DQ) of 92. At 12 months, the DQ was 91. See GMDS-III manual for normative data. Videos 1, 2, and 3 exhibit gross and fine motor skill achievements. Videos 4 and 5 show her neurodevelopmental skills at 11 and 12 months of age.
Table.
Griffiths-III Change in Composite Scores of the Patient at 9 and 12 Months
| Age (mo) | Foundations of learning | Language | Eye-hand coordination | Personal-social-emotional | Gross-motor functions | ||||||||||
| CS | P | DQ | CS | P | DQ | CS | P | DQ | CS | P | DQ | CS | P | DQ | |
| 9 | 10 | 4 | 75 | 11 | 23 | 90 | 16 | 70 | 109 | 14 | 19 | 87 | 13 | 32 | 94 |
| 12 | 14 | 9 | 81 | 12 | 13 | 87 | 17 | 27 | 92 | 16 | 5 | 76 | 14 | 12 | 83 |
Abbreviations: CS = composite score; DQ = developmental quotient; P = percentile.
Patient at 5 months (before treatment). Download Supplementary Video 1 (8.5MB, mp4) via http://dx.doi.org/10.1212/200287_Video_1
Patient at 7 months during treatment, prone position, and crawling. Download Supplementary Video 2 (19.2MB, mp4) via http://dx.doi.org/10.1212/200287_Video_2
Patient at 9 months. Stabilization of crawling. Download Supplementary Video 3 (11.9MB, mp4) via http://dx.doi.org/10.1212/200287_Video_3
Patient at 11 months. Finger grasp acquisition. Download Supplementary Video 4 (35.5MB, mp4) via http://dx.doi.org/10.1212/200287_Video_4
Patient at 12 months. Standing position with support. Download Supplementary Video 5 (7MB, mp4) via http://dx.doi.org/10.1212/200287_Video_5
Visual Functions
At 6 months, the patient's visual assessment revealed severe CVI evidenced by incomplete horizontal and vertical tracking, no arc tracking, altered discrimination of striped targets (spatial frequency of 0.86 cycles/degree at 38 cm), and limited attention at distance (refer to Ricci et al. for normative data). At 12 months, CVI persisted, but improvements in visual acuity and tracking, particularly with high-contrast objects, were observed. Visual acuity at 55 cm was 2.4–3.1, with slightly decreasing values over time. Contrast sensitivity was altered.
Discussion
The importance of neurodevelopmental assessment and neurorehabilitation in CDKL5-DEE is emphasized.14 This case report exemplifies the utility of family-centered EI in a 5-month-old infant with CDKL5-DEE and CVI over 6 months. Although developmental milestones were achieved, they were not reflected in GMDS-III scores, indicating potential limitations in assessment tools. The severity of symptoms in the patients may be influenced by X-chromosome inactivation.15 Our patient did not exhibit X-skewed inactivation. This suggests that despite the developmental delay, the child acquired abilities that were not typically associated with the described pathology in the literature. Fehr et al.16 conducted a study on 127 individuals diagnosed with CDKL5-DEE. The study showed that 10% of patients achieved independent sitting by the age of 10 months, while three-quarters were able to sit independently by the age of 5 years. In our case, the infant reached the sitting position independently at 9 months. Severe CVI is linked to neurodevelopmental delay.2 However, the presented clinical case indicates that EI for CVI may improve developmental milestones.
Assisting families in responding to their child's needs has significant implications for the nervous system development of patients with CDKL5-DEE.
The study conducted by Demarest et al.17 on the “lived experience” of 37 families revealed that parents and caregivers often experience negative emotions that can have a detrimental effect on the quality of the parent/caregiver-child relationship. Therefore, we recognized the importance of considering the roles of parents and caregivers in all sessions of our home-based EI protocol. However, the study did not gather information on the parents/caregiver's perceived levels of anxiety, nor did it collect data on their quality of life. A potential area for future research would be to investigate the effect of EI on the anxiety and stress levels of parents and caregivers both before and after the intervention.
This study has limitations because a single case report does not provide sufficient evidence. Furthermore, establishing a genotype-phenotype relationship poses considerable challenges because multiple factors may have contributed to the patient's manifestation of a mild phenotype. The assessments were conducted within a restricted timeframe; it would have been beneficial to evaluate the patient's condition before and after treatment using the Severity Assessment Scale,18 specifically designed for patients with CDKL5-DEE. The positive aspects discovered in the study are based on clinical observations made during multiple sessions. Since this study only involves 1 mother-child dyad, it is difficult to predict how other children with CDKL5 would respond to print referencing interventions. Nevertheless, this study outlines the characteristics of a neuroprotective strategy that promotes gross and fine motor skills, CVI, and communication. In addition, it offers valuable insights for designing future studies for all patients with DEEs.
This case study highlights the significance of timely interventions during early neurodevelopment stages in patients with CDKL5-DEE. It demonstrates the positive effect of EI on dyadic regulation in daily caregiving settings. Although future controlled trials may provide quantitative insights into intervention efficacy, this study highlights the importance of family-centered strategies in rehabilitating children with DEEs and CVI.
Patient Perspective
The “Early Intervention” practiced by the neurodevelopmental disorder therapist has facilitated the establishment of a close relationship with our child. It is of paramount importance for children with neurologic disorders, particularly those with epilepsy. Although parents should not substitute therapists, thanks to this intervention, we possess resources that aid in our child's development during times when seizures prevent therapy. This has instilled a sense of security and reduced confusion.
Consent for Publication
Written informed consent was obtained from the patient's parent for publication of this case report and any accompanying images and videos. A copy of written consent is available for review by the Editor of this Journal. Anonymized data not published within this article will be made available by request from any qualified investigator.
Ethical Compliance
Procedures followed were following the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgment
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018–2022 (legge 232 del 2016). Research supported by PNRR-MUR-M4C2 PE0000006 Research Program “MNESYS”—A multiscale integrated approach to the study of the nervous system in health and disease. IRCCS 'G. Gaslini' is a member of ERN-Epicare. We are also thankful to the patient's parents for their kind cooperation in sharing their experiences.
Appendix. Authors
| Name | Location | Contribution |
| Martina Giorgia Perinelli, MSc | Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa, Italy | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Cecilia Naboni, MSc | IRCCS Fondazione Mondino, Pavia, Italy | Major role in the acquisition of data |
| Ganna Balagura, MD, PhD | Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa, Italy | Drafting/revision of the manuscript for content, including medical writing for content |
| Elisabetta Amadori, MD | IRCCS Istituto Giannina Gaslini, Genoa, Italy | Major role in the acquisition of data |
| Maria Stella Vari, MD | IRCCS Istituto Giannina Gaslini, Genoa, Italy | Major role in the acquisition of data |
| Valeria Capra, MD | IRCCS Istituto Giannina Gaslini, Genoa, Italy | Major role in the acquisition of data |
| Camelia Lentoiou, MD | Epilepsy Monitoring Unit, Emergency University Hospital Bucharest, Bucharest, Romania | Analysis or interpretation of data |
| Thomas Foiadelli, MD | IRCCS Policlinico San Matteo, Pavia, Italy | Major role in the acquisition of data |
| Fabio Sirchia, MD | IRCCS Policlinico San Matteo; Department of Molecular Medicine, University of Pavia, Italy | Major role in the acquisition of data |
| Antonella Luparia, MSc | IRCCS Fondazione Mondino, Pavia, Italy | Major role in the acquisition of data |
| Gianluigi Merseglia, MD | IRCCS Policlinico San Matteo, Pavia, Italy | Major role in the acquisition of data |
| Luca A. Ramenghi, MD, PhD | Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa; IRCCS Istituto Giannina Gaslini, Genoa, Italy | Analysis or interpretation of data |
| Pasquale Striano, MD, PhD | Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa; IRCCS Istituto Giannina Gaslini, Genoa, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Leonard H, Downs J, Benke TA, Swanson L, Olson H, Demarest S. CDKL5 deficiency disorder: clinical features, diagnosis, and management. Lancet Neurol. 2022;21(6):563-576. doi: 10.1016/S1474-4422(22)00035-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock D, Fidell A, Thomas J, Juarez-Colunga E, Benke TA, Demarest S. Cerebral visual impairment in CDKL5 deficiency disorder correlates with developmental achievement. J Child Neurol. 2021;36(11):974-980. doi: 10.1177/08830738211019284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wojcik MH, Stewart JE, Waisbren SE, Litt JS. Developmental support for infants with genetic disorders. Pediatrics. 2020;145(5):e20190629. doi: 10.1542/peds.2019-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chorna O, Cioni G, Guzzetta A. Principles of early intervention. Handb Clin Neurol. 2020;174:333-341. doi: 10.1016/B978-0-444-64148-9.00024-7 [DOI] [PubMed] [Google Scholar]
- 5.Cioni G, Inguaggiato E, Sgandurra G. Early intervention in neurodevelopmental disorders: underlying neural mechanisms. Dev Med Child Neurol. 2016;58(suppl 4):61-66. doi: 10.1111/dmcn.13050 [DOI] [PubMed] [Google Scholar]
- 6.Nithianantharajah J, Hannan A. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697-709. doi: 10.1038/nrn1970 [DOI] [PubMed] [Google Scholar]
- 7.Fazzi E, Micheletti S, Calza S, Merabet L, Rossi A, Galli J, Early Visual Intervention Study Group. Early visual training and environmental adaptation for infants with visual impairment. Dev Med Child Neurol. 2021;63(10):1180-1193. doi: 10.1111/dmcn.14865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demarest S, Olson HE, Moss A, et al. CDKL5 deficiency disorder: relationship between genotype, epilepsy, cortical visual impairment and development. Epilepsia 2019;60(8):1733-1742. doi: 10.1111/epi.16285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green E, Stroud L, O'Connell R, et al. Griffiths Scales of Child Development 3rd Ed. Part II: Administration and Scoring. Hogrefe; 2016. [Google Scholar]
- 10.Ricci D, Cesarini L, Groppo M, et al. Early assessment of visual function in full term newborns. Early Hum Dev. 2008;84(2):107-113. doi: 10.1016/j.earlhumdev.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 11.Teller DY, McDonald MA, Preston K, Sebris SL, Dobson V. Assessment of visual acuity in infants and children: the acuity card procedure. Dev Med Child Neurol. 1986;28(6):779-789. doi: 10.1111/j.1469-8749.1986.tb03932.x [DOI] [PubMed] [Google Scholar]
- 12.Chen AH, Mohamed D. New paediatric contrast test: Hiding Heidi low-contrast 'face' test. Clin Exp Ophthalmol. 2003;31(5):430-434. doi: 10.1046/j.1442-9071.2003.00691.x [DOI] [PubMed] [Google Scholar]
- 13.Hutchon B, Gibbs D, Harniess P, et al. Early intervention programmes for infants at high risk of atypical neurodevelopmental outcome. Dev Med Child Neurol. 2019;61(12):1362-1367. doi: 10.1111/dmcn.14187 [DOI] [PubMed] [Google Scholar]
- 14.Amin S, Monaghan M, Aledo-Serrano A, et al. International consensus recommendations for the assessment and management of individuals with CDKL5 deficiency disorder. Front Neurol. 2022;13:874695. doi: 10.3389/fneur.2022.874695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakimiec M, Paprocka J, Śmigiel R. CDKL5 deficiency disorder-a complex epileptic encephalopathy. Brain Sci. 2020;10(2):107. doi: 10.3390/brainsci10020107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr S, Leonard H, Ho G, et al. There is variability in the attainment of developmental milestones in the CDKL5 disorder. J Neurodev Disord. 2015;7(1):2. doi: 10.1186/1866-1955-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarest S, Marsh R, Treat L, et al. The lived experience of parents' receiving the diagnosis of CDKL5 deficiency disorder for their child. J Child Neurol. 2022;37(6):451-460. doi: 10.1177/08830738221076285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demarest S, Pestana-Knight EM, Olson HE, et al. Severity assessment in CDKL5 deficiency disorder. Pediatr Neurol. 2019;97:38-42. doi: 10.1016/j.pediatrneurol.2019.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient at 5 months (before treatment). Download Supplementary Video 1 (8.5MB, mp4) via http://dx.doi.org/10.1212/200287_Video_1
Patient at 7 months during treatment, prone position, and crawling. Download Supplementary Video 2 (19.2MB, mp4) via http://dx.doi.org/10.1212/200287_Video_2
Patient at 9 months. Stabilization of crawling. Download Supplementary Video 3 (11.9MB, mp4) via http://dx.doi.org/10.1212/200287_Video_3
Patient at 11 months. Finger grasp acquisition. Download Supplementary Video 4 (35.5MB, mp4) via http://dx.doi.org/10.1212/200287_Video_4
Patient at 12 months. Standing position with support. Download Supplementary Video 5 (7MB, mp4) via http://dx.doi.org/10.1212/200287_Video_5