Abstract
Blood flow restriction exercise (BFRE) appears to provide a unique opportunity to preserve lower limb muscle and function in patients with an Achilles tendon rupture. The purpose of this study was to investigate the feasibility of BFRE in patients with an Achilles tendon rupture. Additionally, to evaluate muscle volume and patient-reported ankle function, symptoms, complications, and physical activity following 12 weeks of BFRE. Feasibility was measured by adherence to training sessions, drop-out rate, intervention acceptability, ankle pain exacerbation (NRS), and adverse events. At baseline and 12-weeks follow-up, patients completed the Achilles Tendon Total Rupture Score questionnaire and had their thigh and calf circumference measured. At follow-up, patients’ ability to perform a single-leg heel rise was tested. Sixteen of 18 patients completed the intervention and for those, adherence to training sessions was 88% ±16%. The mean NRS following BFRE sessions was 1.1 (95%CI: 1; 1.2). Three adverse events occurred during the 12 weeks. Two re-ruptures after completion of the BFRE program and one deep venous thrombosis following cast removal. BFRE was found to be feasible in a subset of patients with an Achilles tendon rupture. However, with three adverse events in a population of 18 patients, the effectiveness and safety of BFRE warrants further investigation.
Keywords: Achilles tendon rupture, blood flow restriction exercise, case series, non-surgical treatment, rehabilitation
INTRODUCTION
Achilles tendon rupture is a common injury associated with prolonged sick leave, impaired physical function, and reduced physical activity (13, 26, 28). In recent years early ankle joint loading using weight-bearing and mobilization has been introduced to patients treated for Achilles tendon rupture (23). Despite allowing early weight-bearing post injury, the ankle joint is usually cast-immobilized, and load-restricted during the first two to eight weeks, ultimately rendering the lower limb muscles in the affected limb extremely vulnerable to muscle disuse atrophy (30). The deficits in muscle function persist even at and beyond 12 months post injury, with side-to-side differences in triceps surae muscle cross-sectional area of 9–25%, and plantar flexion of 10–35% (16). Thus, introducing an exercise method to prevent these consequences, and avoid re-rupture without violating load restrictions is essential.
Exercise using blood flow restriction by pneumatic cuff compression has been demonstrated to mitigate muscle disuse atrophy in musculoskeletal patients, with beneficial effects on strength and muscle mass (17, 35). Studies have shown that blood flow restriction combined with low-intensity aerobic exercises such as walking, can improve physical function and lower limb muscle mass (2, 8, 29, 33). Furthermore, when blood flow restriction is combined with resistance training at low external load intensities, in the range of 10–30% of 1 repetition maximum, it results in improvements in muscle mass, muscle strength, and lower limb function that are comparable to traditional resistance training (5, 11, 18).
Research on Blood Flow Restriction Exercise (BFRE) in tendon pathologies is scarce, but preliminary evidence is promising, showing positive effects on tendon cross-sectional area and tendon stiffness, in both patients with patellar tendinopathy and individuals with healthy Achilles tendons (6). BFRE provides unique opportunities to prevent muscle degeneration during early Achilles tendon rupture rehabilitation since it can be introduced at low load intensities when patients are weight-bearing (9).
This study aims to investigate the feasibility and safety of applying BFRE in the early rehabilitation phase for patients treated non-surgical for an Achilles tendon rupture. Furthermore, we intended to evaluate thigh and calf circumference, patient-reported ankle function, symptoms, physical activity, and complications following 12 weeks of BFRE.
METHODS
Participants
Patient enrolment is shown in Figure 1. Patients were identified at the emergency departments at Aarhus University Hospital and Gødstrup Regional Hospital. Patients presenting with an Achilles tendon rupture were screened for eligibility and invited to participate if they met the following criteria:
Figure 1.
Flowchart of patient enrollment. DVT: Deep venous thrombosis * Two patients identified at their initial visit in the emergency department were later excluded because they did not have an Achilles tendon rupture.
>18 years of age and diagnosed with a total Achilles tendon rupture.
Able to read and understand Danish.
Initially treated with a cast within 24 hours of injury.
Exclusion criteria were:
Previous Achilles tendon rupture in either leg.
Previous thrombosis.
Reduced leg function caused by other conditions than total Achilles tendon rupture.
Treated with quinolones or corticosteroids in the previous six months.
Medically treated for diabetes, and/or severe atherosclerosis in leg or foot.
Patients were recruited from October 2022 through January 2023. Patients were provided with oral and written study information and gave written informed consent prior to inclusion. The study protocol was approved by the Central Denmark Region Committees on Health Research Ethics (1-10-72-139-22) and registered at the Central Denmark Region’s internal list of scientific projects (1-16-02-380-22). Data was managed according to the General Data Protection Regulation (GDPR). The study was conducted in accordance with the declaration of Helsinki and carried out fully in accordance with the ethical standards of the International Journal of Exercise Science (25).
Protocol
Design
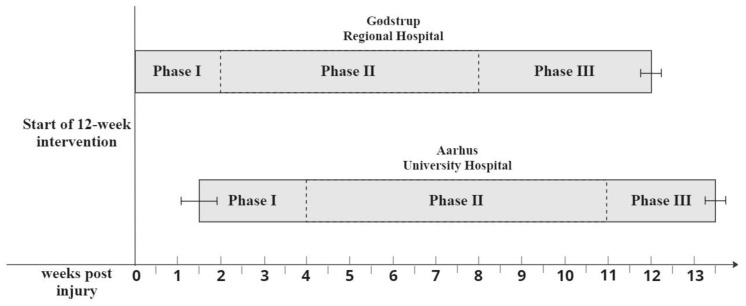
This study is a two-center prospective case series feasibility study with a 12-week intervention period conducted at Aarhus University Hospital and Gødstrup Regional Hospital in Denmark. Patients were assessed at baseline mean (SD) seven (SD 7) days post injury and assessed at follow-up mean 12.4 (SD 0.6) weeks post baseline assessment. During the 12-week intervention period, patients followed local hospital rehabilitation guidelines. At Aarhus University Hospital, guidelines consisted of four weeks in an equinus cast, followed by seven weeks in an orthopedic boot. During the initial ten weeks post injury, weight-bearing was prohibited. At Gødstrup Regional Hospital, initial treatment consisted of two weeks in an equinus cast, followed by six weeks in an orthopedic boot, and partial weight-bearing was allowed in weeks three to five transitioning to full weight-bearing six weeks post injury.
Intervention
The intervention consisted of 12 weeks of BFRE with three weekly sessions. The intervention period was divided into three phases. The duration of each phase depended on local hospital guidelines (Figure 2). Phase I consisted of seated knee extensions with concurrent blood flow restriction applied to the proximal portion of the affected leg at 40% of limb occlusion pressure (LOP) and deflated between each set. Seated knee extensions were performed in four sets of 30, 15, 15, and >15 repetitions. The last set consisted of as-many-repetitions-as-possible. When more than 15 repetitions were completed in the last set, the load was increased by adding weight to a bucket hanging from the ankle of the affected leg (Figure 3). After the final set, patients performed a venous drainage exercise consisting of two minutes of air cycling while lying supine without blood flow restriction to promote venous outflow of the lower limb. In Phase II, BFRE was performed with occlusion corresponding to 80% LOP and in four sets of 30, 15, 15, >15. The load was increased using the same scheme as in Phase I. Cuff pressure was maintained during rest periods. In Phase III, when patients were allowed full weight-bearing on the affected limb, blood flow restricted walking was added and performed after the seated knee extension exercise. Blood flow restricted walking consisted of five two-minute intervals of walking with concurrent blood flow restriction applied to the affected leg corresponding to 80% LOP. Patients rested for one minute without occlusion between each interval. All exercise variables are presented in Table 1.
Figure 2.
The three intervention phases in relation to time post injury for patients at the two hospitals. Brackets indicate the standard deviation of the time point for intervention initiation and follow-up testing.
Figure 3.
Demonstration of Seated knee extension in a patient post immobilization restrictions. 3A shows the starting position, with the bucket hanging from the foot wrist and the cuff properly placed on the thigh of the affected leg. 3B shows end-range position with the knee of the affected leg extended.
Table 1.
Intervention variables
| Cuff width (cm) | 11.7 |
|
| |
| Limb occlusion pressure (%) | |
| Phase I | 40 |
| Phase II & III | 80 |
|
| |
| Intervention period (weeks) | 12 |
|
| |
| Exercises | |
| Phase I & II | BFR seated leg extension |
| Phase III | BFR seated leg extension & BFR walking |
|
| |
| BFR Seated leg extension | |
| Sets (n) | 4 |
| Repetitions (n) | 30, 15, 15, >15 |
| Pause (s) | 30 |
|
| |
| BFR walking | |
| Intervals (n) | 5 |
| Duration of intervals (s) | 120 |
| Pause (s) | 60 |
BFR: Blood flow restriction.
Prior to each exercise, the patient placed the occlusion cuff around the most proximal part of the thigh of the injured leg. The cuff was inflated to the target pressure in a seated position prior to the knee extension exercise and while standing in a neutral position prior to the walking exercise. Pressure was released immediately after completion of each set of knee extensions in Phase I, after the final repetition in the fourth set of knee extensions in Phases II and III and after each individual interval during blood flow restricted walking. In Phases I and II the exercises were performed in the orthopedic boot at all times to prevent excessive dorsal flexion. Furthermore, in Phases I and II, an elastic band attached to the bucket and secured around the heel cap was occasionally used to prevent bucket movement.
Determination of occlusion pressure
LOP was determined at baseline by seating the patient on an examination table and placing an 11.7 cm pneumatic cuff (Occlude Aps, Aarhus Denmark) on the proximal part of the affected leg (20). An ultrasound Doppler (Edan SD3 Vascular Ultrasonic Pocket Doppler, EdanUSA, 2018) was used to identify the arterial pulse in the tibial posterior artery, and the cuff was incrementally inflated (starting at 100-mmHg with gradual step-increases of 20-mmHg) until the peripheral arterial pulse was eliminated, defining the pulse elimination pressure as LOP (31). If patients had a cast on the injured leg, LOP was determined on the opposite leg. If patients experienced pain and discomfort or were unable to perform the predetermined number of sets and repetitions at 80% LOP, the pressure was decreased by 20 mmHg (n=2) (7).
Outcome measures
Characteristics including gender, age, height, weight, BMI, and LOP were obtained at baseline. Primary outcome measures were continuously collected through patients’ exercise diaries and patient-reported feedback. Secondary outcome measures were obtained at baseline and at 12-week follow-up. The primary outcome measure of this study was the feasibility of the BFRE intervention, including adherence to training sessions, drop-out rate, patients’ acceptability of BFRE, adverse events, and ankle pain exacerbation.
Primary outcome measure: Adherence to training sessions was measured for each patient. High adherence was a priori defined as more than 75% of the total number of training sessions completed. The drop-out rate was defined as the percentage of patients not completing the 12-week training protocol. Failure to complete the training protocol for any reason was counted as a drop-out. Patients’ acceptability of BFRE was evaluated by two feasibility questions: Q1) “Based on your current knowledge and experience, how likely is it that you will choose BFRE if you experience an Achilles tendon rupture tomorrow?”; Q2) “How likely are you, based on your current knowledge and experience, to recommend BFRE to friends and family?” (21). The feasibility questions made it possible for patients to evaluate their experience with BFRE and perception of the extent to which BFRE should be introduced in the general population of patients experiencing Achilles tendon ruptures. High acceptability was a priori defined as 75% of patients reporting a 4 or 5 on a 5-point Likert scale for both questions. The Likert scale was categorized as follows: 1= not at all likely, 2= not likely, 3= neither/nor, 4= likely and 5= much likely.
Adverse events were defined as unintended and unexpected injuries, symptoms, or other events from inclusion until follow-up. Pain exacerbation was reported by patients at every training session before and after exercise and recorded in training diaries. Patients rated ankle pain and general muscle soreness separately on a 10-point numerical rating scale (NRS), where 0 = no pain and 10 = worst imaginable pain, categorized into three groups: ≤5: mild to acceptable pain, 6–7: moderate pain, and ≥8: severe pain (3).
Secondary outcome measures: The secondary outcome measures included the circumference of the thigh and calf, the Achilles tendon Total Rupture Score (ATRS), and the ability to perform a Single-Leg Heel-Rise (SLHR). Thigh and calf circumference for both legs were measured with the patient seated in a 90-degree position. Thigh circumference was measured 10 cm proximal from the base of the patella (34). Calf circumference was measured at the thickest part of the muscle belly (34). At baseline, calf circumference was omitted for the injured leg if the patient presented with a cast. The ATRS is a validated patient-reported, injury-specific questionnaire regarding physical activity and symptoms (10). The ATRS consists of 10 items scored from 0 (major limitations) to 10 (no limitations). At baseline, patients were asked to complete the ATRS based on their habitual state prior to injury. At follow-up, patients completed the ATRS based on their current state. At the 12-week follow-up, patients’ short-term recovery of function was evaluated by testing their ability to perform a SLHR (27). The patients were standing on a flat surface with the ankle in a neutral position and were asked to perform one repetition of a SLHR on their affected leg. Patients were allowed to lightly touch the wall with a finger on each hand for balance. Patients failed the test if they for any reason were unable to perform one SLHR repetition.
Statistical Analysis
Descriptive statistics are presented as means with standard deviation (SD) or using numbers (n) when appropriate. Patients’ adherence to training sessions, drop-out rate, and acceptability are presented as n (%). Changes from baseline to follow-up were analyzed with a paired t-test analysis and presented as means with 95% confidence intervals [95%CI]. Normal distribution of outcome measures was assessed by Q-Q plots. Adherence to the total number of training sessions was calculated as “the number of sessions completed” divided by “the total number of sessions planned (n=36)”. Drop-out rate was calculated by the number of patients dropping out prior to completion of the intervention divided by the number of patients included in the study (n=18). No power calculations were performed as the primary outcome of this study was the feasibility of the BFRE intervention. The statistical analysis software STATA 17.0 (StataCorp LLC, TX, USA) was used for all statistical analyses.
RESULTS
Eighteen of 23 eligible patients were enrolled in the study (Figure 1), resulting in an inclusion rate of 78%. Sixteen out of 18 patients completed the intervention. Patient characteristics are presented in Table 2. Fourteen were males, mean age was 51.8 (SD 15.5) years, mean BMI was 26.4 (SD 2.6), and 12 had injured the right leg. Hospital guidelines required 11 weeks of ankle immobilization for 11 of the patients (Aarhus University Hospital) while the remaining seven patients were immobilized for eight weeks (Gødstrup Regional Hospital).
Table 2.
Baseline characteristics of patients prior to intervention (n=18)
| Sex, male/female, n | 14/4 |
| Age in years, mean (SD) | 51.8 (15.5) |
| Height in cm, mean (SD) | 177.7 (6.8) |
| Weight in kg, mean (SD) | 83.3 (8.9) |
| BMI (kg/m2), mean (SD) | 26.4 (2.6) |
| Index leg: | |
| Right/left, n | 12/6 |
| Weeks in cast/walker: | |
| 8/11 weeks | 7/11 |
| Limb occlusion pressure (LOP) in mmHg, mean (SD) | 226.1 (20.6) |
| 40% LOP in mmHg, mean (SD) | 90.4 (8.2) |
| 80% LOP in mmHg, mean (SD)* | 180.9 (16.5) |
SD: Standard deviation. BMI: Body Mass Index. LOP: Limb Occlusion Pressure.
Primary outcome measures
For the 16 patients who completed the intervention, adherence to training sessions was 88% [79; 96%]. Intervention acceptability was excellent with 92% responding they were likely (4/5) or much likely (5/5) to recommend BFRE to others and to use BFRE if they experienced a new Achilles tendon rupture tomorrow with only one patient reporting <4 to both questions.
Three adverse events were reported during the intervention period. Two patients, from separate hospitals, sustained a re-rupture of the Achilles tendon following the 12th week of the intervention period prior to a scheduled follow-up at the hospital. One re-rupture occurred during usual care rehabilitation unrelated to the study intervention, while the other re-rupture occurred during a work event. Both patients were able to complete the follow-up test and are therefore not presented as drop-outs. A third patient developed a Deep Venous Thrombosis (DVT) in the period following cast removal with symptoms starting prior to introducing BFRE at 80% LOP, resulting in the patient dropping out. In total, two patients dropped out; one had a DVT three weeks post injury and the other initiated a quinolone antibiotic treatment in week five.
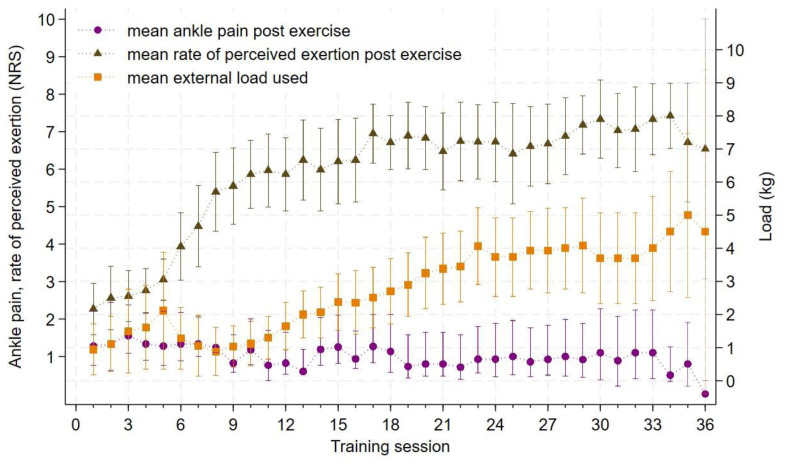
Data on mean NRS ankle pain post exercise, mean rate of perceived exertion and mean external load are illustrated in Figure 4. Mean NRS ankle pain following exercise was 1 [0.9; 1.2]. NRS ankle pain was ≤5 in 98% of the training sessions with four patients reporting NRS pain >5 in a total of eight sessions. Mean rate of perceived exertion during sessions 12–36 was 7.2 [6.9; 7.4].
Figure 4.
Mean NRS ankle pain, rate of perceived exhaustion and mean external load used for each training session (n=16). NRS: Numerical rating scale, kg: Kilogram(s), Mean ankle pain post exercise and mean rate of perceived exertion post exercise is read on the y-axis on the left, Mean external load used is read on the y-axis on the right, Brackets represents 95% confidence intervals, Generally, occlusion pressure was increased from 40% LOP to 80% LOP at ‘Training session 6’ (Gødstrup Regional Hospital) or ‘Training session 12’ (Aarhus University Hospital).
Secondary outcome measures
Secondary outcomes are presented in Table 3 and Table 4. Thigh and calf circumferences did not differ between non-injured and injured leg at baseline or follow-up. Thigh circumference on injured leg was not reduced at follow-up compared to baseline. Change in calf circumference on injured leg was measured in six patients with a mean change of −0.7 cm [−1.3; −0.03]. Mean change in ATRS was −42.8 [−60.8; −24.7]. Two of 16 patients were able to perform one repetition of the SLHR; three patients did not wish to complete the SLHR.
Table 3.
Secondary outcome measures at baseline and 12-week follow-up
| Outcome | Baseline Mean (SD) | Follow-up Mean (SD) | Difference Mean [95% CI] |
|---|---|---|---|
| Thigh circumference (cm) – Injured leg (n=16) | 45.7 (4) | 46.2 (4.2) | 0.5 [−0.4; 1.5] |
| Calf circumference (cm) – Injured leg (n=6)a | 39.6 (1.6) | 38.4 (1.3) | −1.2 [−2.3; −0.1] |
| ATRS (n=16) | 85.3 (19.2) | 38.1 (18.2) | −47.2 [−62.4; −32] |
|
| |||
| n (%) | |||
| Number of patients able to perform one repetition of SLHR (n=16) | 2 (13.3%) | ||
SD: Standard deviation. ATRS: Achilles total Tendon Rupture Score. SLHR: Single-Leg Heel-Rise.
Missing data on patients from Aarhus University Hospital using a cast at baseline.
Table 4.
Thigh and calf circumferences between non-injured and injured legs
| Outcome | Non-injured leg Mean (SD) | Injured leg Mean (SD) | Difference Mean [95% CI] |
|---|---|---|---|
| Thigh circumference (cm) – Baseline (n=16) | 45.4 (4.3) | 45.7 (4) | 0.3 [−0.4; 1] |
| Thigh circumference (cm) – Follow-up (n=16) | 46.4 (4.7) | 46.2 (4.2) | −0.2 [−0.6; 0.9] |
| Calf circumference (cm) – Baseline (n=6)a | 38.3 (2.1) | 39.6 (1.6) | 1.3 [−0.1; 2.6] |
| Calf circumference (cm) – Follow-up (n=15)b | 39.7 (2.9) | 39.2 (2.8) | −0.4 [−0.4; 1.2] |
SD: Standard deviation.
Missing data on patients from Aarhus University Hospital using a cast at baseline.
Missing data on one patient.
DISCUSSION
The main finding of this present study is that BFRE is feasible in patients treated non-surgically for an Achilles tendon rupture. The drop-out rate was low (11%), a moderate number of adverse events was observed, though similar to previous findings (36). In those who completed the intervention, adherence to training sessions was high (88%) and acceptability of the intervention was 92%. Notably, no decrease in thigh circumference was observed following the intervention, in contrast to the expected decrease usually observed after lower limb immobilization (14).
Adherence to training sessions
For patients who completed the 12-week intervention period adherence was 88%. The findings are consistent with results from previous feasibility studies by Høgsholt et al. (19) and Bentzen et al. (2) reporting training adherence of 96% and 93% when investigating the feasibility of BFRE in patients with gluteal tendinopathy and intermittent claudication. Moreover, the drop-out rates of 13% and 6% observed in these studies were in line with the drop-out rate of 11% in the present study.
Patient acceptability
Patient acceptability was measured using two feasibility questions previously described in a study by Larsen et al. (21), studying the feasibility of three weeks of BFR-E in patients with ankle fractures. They observed that all the patients included reported high acceptability to the intervention protocol, comparable to the 92% acceptability in the present study. Combined with the high adherence to training observed in our study, findings collectively suggest that the patients in the present study were highly motivated to exercise and improve their condition.
Adverse events
Three adverse events occurred during the intervention period: Two re-ruptures of the Achilles tendon and one DVT. This re-rupture rate is within the range of 3.9–13% reported for patients conservatively treated in a recent systematic review by Seow et al. (36). The two reruptures occurred separately from the study intervention in the final week just prior to follow-up. One patient developed a DVT, aligning with rates of 1–6% reported in previous Achilles tendon rupture studies (4, 15, 32). For the patient in this study, symptoms began after cast removal in week 2, while occlusion pressure was low at 40% LOP, and intermittent blood flow restriction was applied. This suggests that the DVT developed prior to increasing occlusion pressure to 80% LOP. BFRE has not been associated with an increased risk of DVT (1), and the occlusion was applied intermittently with a relatively low pressure in this case, so the event seems unlikely to be attributed to BFRE. It does, however, warrant further investigation into the effect of blood flow restriction on the risk of DVT.
Pain exacerbation
Post-exercise ankle pain was ≤5 in 98% of the training sessions, indicating that the pain associated with BFRE is acceptable for patients with an Achilles tendon rupture. The findings are in line with an ankle fracture case study by Mortensen et al. (24), reporting that NRS pain during and following BFRE was ≤5 in all sessions. Additionally, mean rate of perceived exertion following week 12 when occlusion pressure was at 80% LOP was at 7.2, suggesting that BFRE is feasible in terms of pain exacerbation even at high-intensity exercise.
Achilles Total tendon Rupture Score
The mean ATRS at 12-week follow-up was 36 (SD 20). This is similar to the score of 39 (SD 17) observed in a study by Olsson et al. (27), where ATRS was evaluated at 12 weeks following early active rehabilitation of patients with an Achilles tendon rupture. Short-term (<4 months post injury) measurement of ATRS can, however, be inconsistent when compared to one-year results (12). Therefore, further investigation on BFRE in patients with an Achilles tendon rupture should include additional follow-up times to properly evaluate the patient-reported outcome measure following BFRE.
Circumference
Mean thigh circumference was similar at follow-up compared to baseline. Thus, if any atrophy was to happen following Achilles tendon rupture, the BFRE intervention may have prevented this. Calf circumference decreased by −1.2 [−2.3; −0.1] cm, a 3% reduction, which may be attributed to prolonged ankle immobilization. This decrease aligns with findings by Maffulli et al. (22), who reported a 3.5 % decrease in circumference two years post injury in both surgical and non-surgical cases. Caution is needed when comparing our results, as they are based on a subset of six patients. Tendon Elongation was not evaluated in this present study but is associated with calf muscle mass and strength decline, warranting further investigation in future BFRE trials for patients with an Achilles tendon rupture.
Single leg heel rise
Olsson et al. suggested the ability to perform one SLHR as an early recovery milestone following an Achilles tendon rupture, with 40 out of 81 patients achieving this at 12 weeks post injury (27). In contrast, our study saw only two of 16 patients accomplishing a SLHR. However, it is important to note that Olsson et al.’s patients were allowed full weightbearing from the start contrary to load restrictions of 11 and eight weeks of ankle immobilization for patients in our study. Therefore, it may be hypothesized that full weight bearing early after injury may be an important determinant for the ability to perform a SLHR.
There are some limitations to be considered when interpreting the results of this study: Firstly, the case series design without a control group prevents causal inference between the BFRE intervention and the outcomes measured. Secondly, the small sample size limits the statistical power of tests. Thirdly, the study was conducted on two separate sites with different guidelines on Achilles tendon rehabilitation. Finally, the training protocol was adjusted to adhere to local hospital guidelines, resulting in slightly different programs at each site. Future studies on BFRE in Achilles tendon ruptures should explore the effects of a standardized exercise protocol.
BFRE is feasible in terms of adherence to training sessions, drop-out rate, intervention acceptability, and ankle pain exacerbation. Despite three adverse events, BFRE appears to be as safe as usual care for this patient group. However, the effectiveness and safety of BFRE compared to usual care, need to be further investigated to determine whether BFRE is a viable method for preventing loss of function and muscle disuse atrophy, while maintaining weight-bearing restrictions.
REFERENCES
- 1.Angelopoulos P, Mylonas K, Tsigkas G, Tsepis E, Billis E, Fousekis K. Blood Flow Restriction Training in Cardiovascular Disease Patients. Contemp Adv Sports Sci. 2021:21–34. doi: 10.22540/JFSF-08-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentzen A, Nisgaard LB, Mikkelsen RBL, Høgh A, Mechlenburg I, Jørgensen SL. Blood flow restricted walking in patients suffering from intermittent claudication: a case series feasibility and safety study. Ann Med Surg (Lond) 2023;85(5):1430–1435. doi: 10.1097/MS9.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boonstra AM, Stewart RE, Köke AJA, Oosterwijk RFA, Swaan JL, Schreurs KMG, et al. Cut-Off Points for Mild, Moderate, and Severe Pain on the Numeric Rating Scale for Pain in Patients with Chronic Musculoskeletal Pain: Variability and Influence of Sex and Catastrophizing. Front Psychol. 2016;7:1466. doi: 10.3389/fpsyg.2016.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braithwaite I, Dunbar L, Eathorne A, Weatherall M, Beasley R. Venous thromboembolism rates in patients with lower limb immobilization after Achilles tendon injury are unchanged after the introduction of prophylactic aspirin: audit. J Thromb Haemost. 2016;14(2):331–335. doi: 10.1111/jth.13224. [DOI] [PubMed] [Google Scholar]
- 5.Bryk FF, Dos Reis AC, Fingerhut D, Araujo T, Schutzer M, de Cury RP, et al. Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1580–1586. doi: 10.1007/s00167-016-4064-7. [DOI] [PubMed] [Google Scholar]
- 6.Burton I, McCormack A. Blood Flow Restriction Resistance Training in Tendon Rehabilitation: A Scoping Review on Intervention Parameters, Physiological Effects, and Outcomes. Front Sports Act Living. 2022;4:879860. doi: 10.3389/fspor.2022.879860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerqueira MS, Costa EC, Santos Oliveira R, Pereira R, Brito Vieira WH. Blood Flow Restriction Training: To Adjust or Not Adjust the Cuff Pressure Over an Intervention Period? Front Physiol. 2021;12:678407. doi: 10.3389/fphys.2021.678407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarkson MJ, Conway L, Warmington SA. Blood flow restriction walking and physical function in older adults: A randomized control trial. J Sci Med Sport. 2017;20(12):1041–1046. doi: 10.1016/j.jsams.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Conceição MS, Ugrinowitsch C. Exercise with blood flow restriction: an effective alternative for the non-pharmaceutical treatment for muscle wasting. J Cachexia Sarcopenia Muscle. 2019;10(2):257–262. doi: 10.1002/jcsm.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganestam A, Barfod K, Klit J, Troelsen A. Validity and Reliability of the Achilles Tendon Total Rupture Score. J Foot Ankle Surg. 2013;52(6):736–739. doi: 10.1053/j.jfas.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Giles L, Webster KE, McClelland J, Cook JL. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double-blind randomised trial. Br J Sports Med. 2017;51(23):1688–1694. doi: 10.1136/bjsports-2016-096329. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MS, Kristensen MT, Budolfsen T, Ellegaard K, Hölmich P, Barfod KW. Reliability of the Copenhagen Achilles length measure (CALM) on patients with an Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2020;28(1):281–290. doi: 10.1007/s00167-019-05672-3. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MS, Christensen M, Budolfsen T, Østergaard TF, Kallemose T, Troelsen A, et al. Achilles tendon Total Rupture Score at 3 months can predict patients’ ability to return to sport 1 year after injury. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1365–1371. doi: 10.1007/s00167-015-3974-0. [DOI] [PubMed] [Google Scholar]
- 14.Hardy EJO, Inns TB, Hatt J, Doleman B, Bass JJ, Atherton PJ, et al. The time course of disuse muscle atrophy of the lower limb in health and disease. J Cachexia Sarcopenia Muscle. 2022;13(6):2616–2629. doi: 10.1002/jcsm.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyes GJ, Tucker A, Michael AL, Wallace RG. The incidence of deep vein thrombosis and pulmonary embolism following cast immobilisation and early functional bracing of Tendo Achilles rupture without thromboprophylaxis. Eur J Trauma Emerg Surg. 2015;41(3):273–276. doi: 10.1007/s00068-014-0408-5. [DOI] [PubMed] [Google Scholar]
- 16.Hoeffner R, Svensson RB, Bjerregaard N, Kjær M, Magnusson SP. Persistent Deficits after an Achilles Tendon Rupture: A Narrative Review. Transl Sports Med. 2022;2022:1–7. doi: 10.1155/2022/7445398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 18.Hughes L, Rosenblatt B, Haddad F, Gissane C, McCarthy D, Clarke T, et al. Comparing the Effectiveness of Blood Flow Restriction and Traditional Heavy Load Resistance Training in the Post-Surgery Rehabilitation of Anterior Cruciate Ligament Reconstruction Patients: A UK National Health Service Randomised Controlled Trial. Sports Med (Auckland, NZ) 2019;49(11):1787–1805. doi: 10.1007/s40279-019-01137-2. [DOI] [PubMed] [Google Scholar]
- 19.Høgsholt M, Jørgensen SL, Rolving N, Mechlenburg I, Tønning LU, Bohn MB. Exercise With Low-Loads and Concurrent Partial Blood Flow Restriction Combined With Patient Education in Females Suffering From Gluteal Tendinopathy: A Feasibility Study Front Sports Act Living. 2022;4 doi: 10.3389/fspor.2022.881054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jørgensen SL, Bohn MB, Aagaard P, Mechlenburg I. Efficacy of low-load blood flow restricted resistance EXercise in patients with Knee osteoarthritis scheduled for total knee replacement (EXKnee): protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10(10):e034376. doi: 10.1136/bmjopen-2019-034376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen P, Platzer OJ, Lollesgaard L, Pedersen SK, Nielsen PK, Rathleff MS, et al. Blood-flow restricted exercise following ankle fractures - A feasibility study. Foot Ankle Surg. 2022;28(6):726–731. doi: 10.1016/j.fas.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Maffulli N, Oliva F, Migliorini F. Check-rein technique for Achilles tendon elongation following conservative management for acute Achilles tendon ruptures: a two-year prospective clinical study. J Orthop Surg Res. 2021;16(1):690. doi: 10.1186/s13018-021-02830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormack R, Bovard J. Early functional rehabilitation or cast immobilisation for the postoperative management of acute Achilles tendon rupture? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2015;49(20):1329–1335. doi: 10.1136/bjsports-2015-094935. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen L, Mechlenburg I, Langgård Jørgensen S. Low-Load Blood-Flow-Restricted Exercise to Prevent Muscle Atrophy and Decline in Functional Performance in a Patient Recovering From a Malleolus Fracture. A Case Report Clin J Sport Med. 2023;33(1):97–100. doi: 10.1097/JSM.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 25.Navalta JW, Stone WJ, Lyons TS. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson L, Thorlund JB, Kjær IL, Kazlauskas A, Christensen M. Long-term follow-up after acute achilles tendon rupture - Does treatment strategy influence functional outcomes? Foot (Edinb) 2021;47:101769. doi: 10.1016/j.foot.2020.101769. [DOI] [PubMed] [Google Scholar]
- 27.Olsson N, Karlsson J, Eriksson BI, Brorsson A, Lundberg M, Silbernagel KG. Ability to perform a single heel-rise is significantly related to patient-reported outcome after Achilles tendon rupture. Scand J Med Sci Sports. 2014;24(1):152–158. doi: 10.1111/j.1600-0838.2012.01497.x. [DOI] [PubMed] [Google Scholar]
- 28.Olsson N, Nilsson-Helander K, Karlsson J, Eriksson BI, Thomée R, Faxén E, et al. Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1385–1393. doi: 10.1007/s00167-011-1511-3. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki H, Sakamaki M, Yasuda T, Fujita S, Ogasawara R, Sugaya M, et al. Increases in thigh muscle volume and strength by walk training with leg blood flow reduction in older participants. J Gerontol A Biol Sci Med Sci. 2011;66(3):257–263. doi: 10.1093/gerona/glq182. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Lee HS, Young KW, Seo SG. Treatment of Acute Achilles Tendon Rupture. Clin Orthop Surg. 2020;12(1):1–8. doi: 10.4055/cios.2020.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front Physiol. 2019;10:533. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen MH, Wahlsten LR, Grønborg H, Gislason GH, Petersen MM, Bonde AN. Symptomatic Venous Thromboembolism After Achilles Tendon Rupture: A Nationwide Danish Cohort Study of 28,546 Patients With Achilles Tendon Rupture. Am J Sports Med. 2019;47(13):3229–3237. doi: 10.1177/0363546519876054. [DOI] [PubMed] [Google Scholar]
- 33.Petersson N, Langgård Jørgensen S, Kjeldsen T, Mechlenburg I, Aagaard P. Blood flow restricted walking in elderly individuals with knee osteoarthritis: a feasibility study. J Rehabil Med. 2022;54 doi: 10.2340/jrm.v54.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross M, Worrell TW. Thigh and calf girth following knee injury and surgery. J Orthop Sports Phys Ther. 1998;27(1):9–15. doi: 10.2519/jospt.1998.27.1.9. [DOI] [PubMed] [Google Scholar]
- 35.Saraf A, Goyal M, Goyal K. Blood Flow Restriction Training-An Overview and Implication in New Generation Physical Therapy: A Narrative Review. J Lifestyle Med. 2022;12(2):63–68. doi: 10.15280/jlm.2022.12.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seow D, Islam W, Randall GW, Azam MT, Duenes ML, Hui J, et al. Lower rerupture rates but higher complication rates following surgical versus conservative treatment of acute achilles tendon ruptures: A systematic review of overlapping meta-analyses. Knee Surg Sports Traumatol Arthrosc. 2023:1–13. doi: 10.1007/s00167-023-07411-1. [DOI] [PubMed] [Google Scholar]