Abstract
Type 1 and 2 cytokine mRNA responses were measured at various time periods and in various lymphoid compartments during the acute stage (first 4 months) of feline immunodeficiency virus (FIV) infection in laboratory cats. Cytokine responses were correlated with virus replication. Virus was detected in plasma and tissue from day 14 postinfection (p.i.) onward, peaked at 56 to 70 days, and declined greatly by 70 days. Virus replication was highest in the thymus, followed by spleen, mesenteric lymph nodes, and cervical lymph nodes. Baseline cytokine levels were highest in the mesenteric lymph nodes and lowest in the cervical lymph nodes. Cytokine upregulation after FIV infection was most dramatic in the cervical lymph nodes, with the greatest increase in interleukin-10 (IL-10) and gamma interferon (IFN-γ). Cytokine transcription in the mesenteric lymph node increased above baseline by day 14 p.i. for IFN-γ, IL-12p40, IL-4, and IL-10, while elevations in the spleen were mainly for IFN-γ, IL-12p40 and IL-10. An increase in IFN-γ, IL-10, and IL-12p40 occurred in the thymus at day 56 p.i., concomitant with the onset of thymitis. In general, type 2 cytokines (IL-4 and IL-10) were increased greater than 1 log over baseline, while the elevations in type 1 cytokines were less than 1 log. In the tissues tested, CD4+ cells were the primary source of IL-2, IL-4, and IL-10. Both CD4+ and CD8+ cells produced IFN-γ, while no cytokine mRNA was detected in B cells. These results demonstrate the presence of a heterogeneous cytokine response in lymphoid tissues during the primary stage of FIV infection. The nature and intensity of the response differed from one compartment to the other and, in the case of the thymus, also with inflammatory changes. Although limited in scope, the present study confirms the usefulness of the FIV infection model in studying early cytokine events that lead to the secondary subclinical carrier state typical of most lentivirus infections.
The immunodeficiency-inducing lentiviruses, including human, feline, and simian immunodeficiency viruses (HIV, FIV, and SIV), all share a disease course, starting with an acute primary phase, progressing to a prolonged subclinical stage, and terminating in AIDS. It is generally assumed that the primary phase of immunodeficiency virus infections is critical in determining the overall disease course, i.e., whether it proceeds to AIDS over months, years, or never (1, 4, 9, 29, 34, 35). A strong cellular immune response seems to be important in maintaining the asymptomatic phase observed in long-term nonprogressors in both HIV and FIV infections (8, 18, 20, 21, 27, 28, 30). If immune reactions occurring during primary illness are essential in determining the ultimate disease outcome, then studies of type 1 and type 2 cytokine responses during this period may help elucidate favorable and unfavorable patterns of developing immunity. Indeed, numerous studies have been performed to determine the role of cytokines on the immune response to a lentivirus and the effect of cytokines on the life cycle of the virus. Results have often been contradictory and confusing but have revealed the complexity of the cytokine network. The confusion may be explained by the variety of experimental approaches, the clinical stage of disease, and methodology used to measure cytokine levels. The phenotype and anatomic location of cells used to measure cytokine responses may also have a major impact. The anatomic compartmentalization of the immune system into tonsils, superficial and deep lymph nodes, thymus, spleen, bone marrow, mucosa-associated lymphoid tissues, etc., may also be associated with a compartmentalization of immune responses and cytokine profiles.
In the study reported herein, we used the FIV infection model in domestic cats to test the degree of heterogeneity in cytokine responses in various compartments of the immune system during primary FIV infection. We have quantified gamma interferon (IFN-γ), interleukin-4 (IL-4), IL-10, and IL-12 transcriptional levels in four lymphoid compartments (spleen, mesenteric lymph nodes [MLN], superficial cervical lymph nodes [CLN], and thymus) at a number of time points during the primary stage of FIV infection. The results demonstrate distinct cytokine profiles that differ qualitatively and quantitatively but are apparently ineffective in controlling viral replication. These results suggest that the choice and evaluation of therapeutic and vaccine strategies targeting cytokines should take into consideration the unique response of all relevant lymphoid tissues.
MATERIALS AND METHODS
Experimental design, animals, and virus inoculum.
The experimental design and animals used have previously been described in detail (34). Specific-pathogen-free cats, age 3 to 4 months, were obtained from the breeding colony of the Feline Nutrition Laboratory, University of California, Davis. Whole blood (1 ml) from a cat chronically infected with the biologic isolate FIV-Petaluma was injected intraperitoneally into cats as described elsewhere (34). Two FIV-infected cats each were sacrificed at 14, 28, 42, 56, 70, 83, 98, and 140 days postinoculation (p.i.). Two age-matched control cats were sacrificed at 0, 56, 83, and 140 days after sham inoculation. Peripheral blood was collected by venipuncture from all surviving animals every 2 weeks and prior to euthanasia.
RNA extraction.
Fresh tissues collected into cold tissue culture medium were disassociated into a single-cell suspension in phosphate-buffered saline using a cell dissociator sieve (Sigma Chemical Company, St. Louis, Mo.). The cells were pelleted (2,000 × g) for 10 min and then resuspended in 1 ml of Trisolv (Biotecx Laboratories, Inc., Houston, Tex.) for total RNA extraction by the procedure recommended by the manufacturer. RNA was resuspended in 40 to 60 μl of diethylpyrocarbonate-treated water with 20 U of RNase inhibitor and 1 U of DNase. The extracted RNA was incubated for 1 h at 37°C to remove DNA and then heated for 5 min at 99°C to inactivate DNase. RNase inhibitor was added to samples before storage at −70°C. RNA concentration was determined spectrophotometrically by measuring the A260 in a microcuvette (GeneQuant II; Pharmacia, Piscataway, N.J.). The samples were prepared for quantification by diluting RNA to 1 μg/5 μl or 0.5 μg/5 μl.
QC-RT-PCR of cytokine mRNA and FIV RNA.
Cytokine mRNA and FIV RNA were quantified as previously described (10, 34). Briefly, quantitative competitive reverse transcription-PCR (QC-RT-PCR) was performed with a nonhomologous competitor RNA. A known quantity of competitive RNA template was added to 1 μg of RNA from unknown samples, reverse transcribed, amplified by PCR, and then analyzed by densitometry after electrophoretic separation on a 3% MetaPhore agarose gel (FMC BioProducts, Rockland, Maine). The number of cytokine transcripts was interpolated from a standard curve generated by performing RT-PCR on a series of samples containing 10-fold dilutions of native RNA template with a constant amount of competitor RNA. Data presented represent average cytokine mRNA molecule numbers from the tissues of two different animals except where indicated.
Quantitation of provirus-containing cells.
Mononuclear cells from peripheral blood were harvested by density gradient centrifugation through Ficoll-Hypaque (density, 1.077; Sigma) or from lymph nodes as described above. The number of provirus-containing cells was quantified by endpoint dilution of cells followed by nested PCR as previously described (11). Data presented represent average numbers of provirus-containing cells from two different animals except at 42 days p.i., when only one animal was evaluated.
Immunophenotypic analysis of peripheral blood lymphocytes.
Peripheral blood in EDTA was used to determine CD4+ and CD8+ T-lymphocyte percentages as previously described (12). Briefly, 50 μl of blood was combined with 25 μl each of mouse monoclonal antibodies Fel7 (phycoerythrin conjugated) and fCD8 (fluorescein isothiocyanate conjugated). Samples were processed on a Q-Prep whole-blood lysis workstation (Coulter Corp., Hialeah, Fla.) and analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, Calif.) with standard two-color analysis configuration. Complete blood counts and differentials were determined by standard methods (1).
Cell sorting.
CD4+, CD8+, and CD21+ cells were purified by immunomagnetic cell sorting as previously described (11). Purity of sorted populations was verified by flow cytometry and was >97% for all samples.
RESULTS
Hematological responses.
The hematological responses of the cats used in this study have previously been reported and were similar to responses reported in previous studies using the biologic isolate FIV-Petaluma (1, 11, 34). The peripheral CD4/CD8 T-cell ratio declined in infected cats beginning at day 28 p.i., significantly decreased (P < 0.05) from 42 days p.i. on, and continued to decline throughout the study period. This was due to a decline in CD4+ lymphocytes, as CD8+ cells remained constant throughout the study (data not shown).
Virologic evaluation.
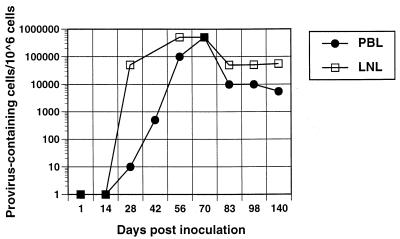
Plasma virus and serum antibodies against FIV were detected in 15 of 16 animals from day 14 p.i. and at each subsequent time point. The animal that did not have detectable plasma viremia or seroconversion was excluded from further analysis. Proviral DNA was found in peripheral blood mononuclear cells (PBMC) and MLN lymphocytes at 28 days p.i. Provirus levels in these two lymphoid compartments, as determined by PCR, peaked at 56 to 70 days p.i., and provirus was found in more than 10% of cells in PBMC and MLN populations. The proviral burden declined approximately 10 fold after 70 days p.i. (Fig. 1).
FIG. 1.
Number of peripheral blood lymphocytes (PBL) or mesenteric lymph node lymphocytes (LNL) containing provirus, quantified by endpoint dilution and seminested PCR. Each data point represents the mean of the two cats sacrificed at that time point except at 42 days p.i., which represents a single cat.
FIV replication.
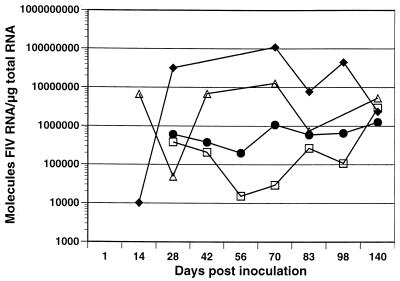
Viral replication was detected by nested RT-PCR in thymus, spleen, MLN, and superficial CLN by 14 days p.i. However, levels were below the QC-RT-PCR quantitative threshold (1,000 copies/μg of RNA) in MLN and CLN (Fig. 2). At day 14 p.i., viral RNA levels were higher in spleen than in the other three tissues. During the clinically symptomatic time period of 42 to 84 days p.i., viral RNA was highest in thymus, followed by spleen, MLN, and CLN. While FIV RNA levels among the four tissues spanned 4 logs during this clinical stage, by the onset of the asymptomatic phase at 140 days p.i., FIV RNA levels were similar in all tissues.
FIG. 2.
Viral replication in spleen (▵), MLN (•), thymus (⧫), and CLN (□), determined using by QC-RT-PCR. Each data point represents the mean of the two cats sacrificed at that time point except at 42 days p.i., which represents a single cat.
Cytokine response in lymphoid tissues.
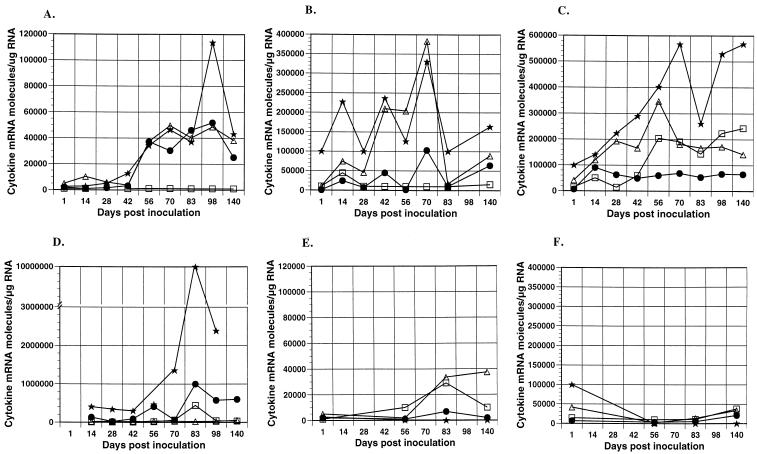
The thymus is a primary lymphoid organ and is responsible for production of mature T lymphocytes. The thymus had the lowest level of cytokine mRNA for all cytokines tested at time zero among the various tissues (Fig. 3A). Prior to 56 days p.i., we observed small increases in IFN-γ but no trends for the other three cytokines. At 56 days p.i., a dramatic upregulation occurred in IFN-γ, IL-12p40, and IL-10 mRNA and was maintained throughout the experimental period. The unique kinetics observed in the thymus can be explained in light of previously reported results which demonstrated a marked influx of inflammatory cells (including B lymphocytes and mature CD4+ and CD8+ T cells) that occurred at 56 days p.i. (34). Apparently the high levels of viral replication did not induce cytokine upregulation before 56 days p.i., and the inflammation and increased cytokine production had no effect on viral replication thereafter. Control animals showed an increase in IL-4 and IL-12p40 at 83 and 140 days p.i., suggesting these cytokines may be involved in thymus maturation and possibly involution (Fig. 3E).
FIG. 3.
Cytokine expression in lymphoid tissues during primary FIV infection. Cytokine (□, IL-4; •, IL-10; ▵, IL-12; ★, IFN-γ) mRNA expression was determined by QC-RT-PCR for FIV-infected cats in thymus (A), spleen (B), MLN (C), and CLN (D). Cytokine mRNA expression in thymus (E) and MLN (F) is also shown for uninfected, age-matched control cats. Each data point represents the mean of the two cats sacrificed at that time point except at 42 days p.i., which represents a single cat.
The spleen is a secondary lymphoid organ with an incompletely understood role in the immune response. However, it is clear that the immunologic function of the spleen is distinct from that of lymph nodes due in part to a difference in lymphocyte and antigen-presenting cell trafficking (5, 33). Viral replication in the spleen was less than the thymus but greater than the lymph nodes evaluated. Initial levels of IL-4, IL-12p40, and IFN-γ were higher than in the other tissues (Fig. 3B). Of the four tissues, upregulation of IL-12p40 was greatest in the spleen. Additionally, IFN-γ and IL-10 were markedly increased. Despite the predominance of IL-12p40, viral replication was unaffected. Cytokine levels in control animals remained near levels observed at 1 day p.i. (data not shown).
Two lymph nodes, the superficial CLN and MLN, were evaluated because of the different tissue types they drain. The afferent lymphatics to the superficial CLN and MLN are primarily from skin and small intestine, respectively. Cytologically, the MLN node is always mildly to moderately reactive due to the constant influx of foreign antigens from the gut, while the CLN is quiescent in a clinically healthy animal. This cytologic difference was reflected in the time zero levels of cytokines, which were at least 1 log lower in the superficial CLN (Fig. 3C and D). Cytokine transcription in the MLN was upregulated for all cytokines evaluated, and the increases were apparent by 14 days p.i. However, while type 2 cytokines were increased greater than a log, the elevations in the type 1 cytokines were less than a log. Cytokine upregulation after FIV infection was most dramatic in the superficial CLN, with the greatest increase observed in IL-10 and IFN-γ mRNA levels. This response has previously been described for peripheral lymph nodes of HIV-infected people and SIV-infected macaques (31, 36). Cytokine levels in control animals remained stable throughout the study period for both MLN and CLN (Fig. 3F and data not shown).
Phenotype of cytokine-producing cells.
To determine the phenotype of lymphocytes producing cytokine mRNA, MLN lymphocytes were sorted by using immunomagnetic beads and monoclonal antibodies against feline CD4, CD8, or CD21 (B cells). Cells were purified to greater than 97%, and each population was analyzed by nonquantitative RT-PCR. The results were nearly identical for four FIV-infected cats evaluated and showed that CD4+ cells are the predominant source of IL-2, IL-4, and IL-10 mRNAs. Both CD4+ and CD8+ cells produced IFN-γ mRNA, while B cells did not express mRNA for any of the cytokines measured (Table 1). No IL-12p40 was found in the purified lymphocyte populations, but IL-12p40 was present in the unfractionated mononuclear cells population. It is likely that mononuclear phagocytes were the source for the IL-12p40 in the unfractionated population. Because cytokine mRNA was not quantitated, no inference can be made regarding the relative amounts of IFN-γ produced by CD4+ and CD8+ cells.
TABLE 1.
Cytokine expression by various cell types
| Cell type | No. positive/4 tested
|
||||
|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-10 | IL-12 | |
| CD4 | 4 | 4 | 4 | 4 | 0 |
| CD8 | 4 | 0 | 0 | 0 | 0 |
| B cell | 0 | 0 | 0 | 0 | 0 |
| Totala | 4 | 4 | 4 | 4 | 4 |
Unfractionated lymph node mononuclear cell population.
DISCUSSION
In the study presented here, we sought to determine the kinetics of transcriptional changes in selected type 1 and type 2 cytokines that occur during the primary phase of FIV infection within different lymphoid compartments. We also investigated how viral replication correlated to tissue type and cytokine response. Finally, we collected preliminary data on the cellular source of cytokine mRNA. The results demonstrate that there is both a qualitative and a quantitative difference in transcriptional regulation of IFN-γ, IL-4, IL-10, and IL-12p40 among the different lymphoid compartments evaluated during primary FIV infection.
The cytokine response in FIV-infected lymph nodes was similar to those found in SIV and HIV studies of PBMC or peripheral lymph node. In SIV- or HIV-infected PBMC and peripheral lymph nodes, it was shown that levels of constitutive expression of tumor necrosis factor alpha, IFN-γ, and IL-10 were increased, IL-12 mRNA levels were decreased, and IL-2 and IL-4 levels were decreased or undetectable (16, 31, 36). During the primary phase of FIV infection, elevations in IFN-γ and IL-10 mRNA levels were predominant. A transient peak of IL-4 upregulation was observed at the point of most severe clinical symptoms, and minimal changes in IL-12p40 were noted. The kinetics of the response correlated well with the clinical symptoms, increasing gradually and then subsiding with the onset of the clinically asymptomatic phase. The spike of IL-4 transcripts may have been unique to the two animals evaluated at 83 days p.i. or may be related to the regulation of IL-4 production. Results from peripheral lymph node evaluation have been assumed to be representative of the immune system as a whole, and general conclusions have been drawn regarding the cytokine and immune response to immunodeficiency-inducing lentiviruses. However, in FIV-infected cats, the cytokine response in other tissues was quite different, suggesting the lymph node response is not representative of the entire immune system.
There was no strict type 1 versus type 2 cytokine dichotomy observed in FIV-infected cats, although there were higher relative elevations in type 2 cytokines. It has previously been shown that HIV-infected individuals produce less IL-12 and supplementation, in vitro, improves immune function (16, 14, 25). However despite early increases in IL-12p40 mRNA in the MLN and spleens of FIV-infected cats, viral replication rapidly increased. It is possible elevations in the type 2 cytokines counteracted the effects of IL-12 in these tissues, or perhaps upregulation in IL-12p40 did not result in increased bioactive IL-12p70. Both spleen and MLN also had increases in IFN-γ mRNA that may have been upregulated by IL-12. Conversely, IFN-γ elevations in superficial CLN exceeded elevations in spleen and MLN despite an absence of IL-12p40 upregulation in this tissue. With respect to type 2 cytokines in the spleen, IL-10 but not IL-4 increased, while in the MLN both type 2 cytokines increased dramatically. Thus, each tissue evaluated demonstrated a unique cytokine profile.
In this study, we demonstrated that FIV replication was highest in the thymus. The cytokine kinetics in the thymus suggest that upregulation of cytokine mRNA was secondary to inflammation and not a response to viral replication per se. Overall, the thymus produced much lower levels of cytokines than the other lymphoid organs evaluated. It is clear that cytokine expression is important for the normal maturation of thymic T cells, but the regulation of cytokines within the thymus is poorly understood (15, 23). We have previously demonstrated severe shifts in thymocyte subpopulations with a depletion of least mature cells and a marked decline in CD4+ cells (34). Whether depletion of CD4+ cells is mediated directly by the virus or indirectly through inflammation and cytokine dysregulation is not clear.
The four organs evaluated in this study each demonstrated a distinct cytokine profile with variable concentrations of cytokine mRNA produced, yet none of these responses were successful at controlling viral replication. In fact, by the endpoint of the study, all organs had similar levels of viral replication. The only commonality suggested by this study and many other reports to have a positive impact on viral replication, would be the upregulation of IL-10 (2, 3, 7, 24, 31, 36).
In this study, CD4+ cells were the predominant source of most cytokines evaluated, including IL-2, IL-4, IL-10, and IFN-γ. Several studies have shown CD8+ cells to be a source of IFN-γ (17, 22, 25), and that finding was confirmed here. No cytokines were detected in the B-cell population, although B cells are reported to be a potential source of IL-10. IL-12p40 was not detected in any lymphoid population, suggesting that this cytokine is produced by nonlymphoid cells. Additional studies are required for a complete determination of the cellular source of each cytokine.
What is the significance of the tissue-specific cytokine profiles reported here? It is unlikely that the cytokine profile seen in these FIV-infected cats is specific for FIV infection alone. Other studies that we have performed on cats infected with Serratia marcescens, Listeria monocytogenes, feline coronavirus, or attenuated strains of FIV have not duplicated the observations herein (references 13 and 26 and unpublished observations). Ultimately, appreciation of the significance of cytokine changes will require a better understanding of the complex interaction between virus, cells, and cytokines. Future experiments using gene knockout animals or anticytokine antibodies in vivo may be useful in this endeavor.
In summary, we have used FIV infection of domestic cats as a model for HIV infection of people to determine the cytokine response in several lymphoid tissues during the primary phase of infection. The FIV model is useful in investigating the role of cytokines during lentivirus infection, particularly the primary phase, and can be exploited to eliminate many of the confounding factors in human studies. The advantages of the FIV model include availability of specific-pathogen-free animals, ability to infect animals simultaneously with a known virus strain and titer, and opportunity to evaluate many tissues from the same individual. The results of this study demonstrate both qualitative and quantitative differences in transcriptional regulation of cytokines among different lymphoid compartments during primary FIV infection. Such a heterogeneous response may be important in evaluations of therapeutic and vaccine strategies targeting cytokines.
ACKNOWLEDGMENTS
We acknowledge Jennifer Woo, Alora LaVoy, Jeff Carlson, Tobie Faith, Joanne Higgins, and Carol Oxford for expert assistance.
This study was supported by NIAID grants AI01262 (G.A.D.) and AI36189 (N.C.P.).
REFERENCES
- 1.Barlough J E, Ackley C D, George J W, Levy N, Acevedo R, Moore P F, Rideout B A, Cooper M D, Pedersen N C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquired Immune Defic Syndr. 1991;4:219–227. [PubMed] [Google Scholar]
- 2.Benveniste O, Vaslin B, Le G R, Cheret A, Matheux F, Theodoro F, Cranage M P, Dormont D. Comparative interleukin (IL-2)/interferon IFN-gamma and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SICmac251 virus. Proc Natl Acad Sci USA. 1996;93:3658–3663. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blauvelt A, Chougnet C, Shearer G M, Katz S I. Modulation of T cell responses to recall antigens presented by Langerhans cells in HIV-discordant identical twins by anti-interleukin (IL)-10 antibodies and IL-12. J Clin Investig. 1996;97:1550–1555. doi: 10.1172/JCI118578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callanan J J, Thompson H, Toth S R, O’Neil B, Lawrence C E, Willett B, Jarrett O. Clinical and pathological findings in feline immunodeficiency virus experimental infection. Vet Immunol Immunopathol. 1992;35:3–13. doi: 10.1016/0165-2427(92)90116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalina M D, Carroll M C, Arizpe H, Takashima A, Estess P, Siegelman M H. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerici M, Lucey D R, Berzofsky J A, Pinto L A, Wynn T A, Blatt S P, Dolan M J, Hendrix C W, Wolf S F, Shearer G M. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 7.Clerici M, Wynn T A, Berzofsky J A, Blatt S P, Hendrix C W, Sher A, Coffman R L, Shearer G M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Investig. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connick E, Marr D G, Zhang X Q, Clark S J, Saag M S, Schooley R T, Curiel T J. HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res Hum Retroviruses. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 9.Cooper D A, Gold J, Maclean P, Donovan B, Finlayson R, Barnes T G, Michelmore H M, Brooke P, Penny R. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985;i:537–40. doi: 10.1016/s0140-6736(85)91205-x. [DOI] [PubMed] [Google Scholar]
- 10.Dean G A, Higgins J, LaVoy A, Fan Z, Pedersen N C. Measurement of feline cytokine gene expression by quantitative-competitive RT-PCR. Vet Immunol Immunopathol. 1998;63:73–82. doi: 10.1016/s0165-2427(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 11.Dean G A, Reubel G H, Moore P F, Pedersen N C. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J Virol. 1996;70:5165–5169. doi: 10.1128/jvi.70.8.5165-5169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean G A, Quackenbush S L, Ackley C D, Cooper M D, Hoover E A. Flow cytometric analysis of T-lymphocyte subsets in cats. Vet Immunol Immunopathol. 1991;28:327–335. doi: 10.1016/0165-2427(91)90124-u. [DOI] [PubMed] [Google Scholar]
- 13.Dean, G. A., J. Bernales, and N. C. Pedersen. Effect of feline immunodeficiency virus on cytokine response to Listeria monocytogenes in vivo. Vet. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 14.Gazzinelli R T, Bala S, Stevens R, Baseler M, Wahl L, Kovacs J, Sher A. HIV infection suppresses type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. J Immunol. 1995;155:1565–1574. [PubMed] [Google Scholar]
- 15.Godfrey D I, Kennedy J, Gately M K, Hakimi J, Hubbard B R, Zlotnik A. IL-12 influences intrathymic T cell development. J Immunol. 1994;152:2729–2735. [PubMed] [Google Scholar]
- 16.Graziosi C, Pantaleo G, Gantt K R, Fortin J P, Demarest J F, Cohen O J, Sekaly R P, Fauci A S. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 17.Graziosi C, Gantt K R, Vaccarezza M, Demarest J F, Daucher M, Saag M S, Shaw G M, Quinn T C, Cohen O J, Welbon C C, Pantaleo G, Fauci A S. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 19.Israel Z R, Dean G A, Maul D H, O’Neil S P, Dreitz M J, Mullins J I, Fultz P N, Hoover E A. Early pathogenesis of disease caused by SIVsmmPBj14 molecular clone 1.9 in macaques. AIDS Res Hum Retroviruses. 1993;9:277–286. doi: 10.1089/aid.1993.9.277. [DOI] [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legrand E, Pellegrin I, Neau D, Pellegrin J L, Ragnaud J M, Dupon M, Guillemain B, Fleury H J. Course of specific T lymphocyte cytotoxicity, plasma and cellular viral loads, and neutralizing antibody titers in 17 recently seroconverted HIV type 1-infected patients. AIDS Res Hum Retroviruses. 1997;13:1383–1394. doi: 10.1089/aid.1997.13.1383. [DOI] [PubMed] [Google Scholar]
- 22.Maggi E, Manetti R, Annunziato F, Cosmi L, Giudizi M G, Biagiotti R, Galli G, Zuccati G, Romagnani S. Functional characterization and modulation of cytokine production by CD8+ T cells from human immunodeficiency virus-infected individuals. Blood. 1997;89:3672–3681. [PubMed] [Google Scholar]
- 23.Moore N C, Anderson G, Smith C A, Owen J J, Jenkinson E J. Analysis of cytokine gene expression in subpopulations of freshly isolated thymocytes and thymic stromal cells using semiquantitative polymerase chain reaction. Eur J Immunol. 1993;23:922–927. doi: 10.1002/eji.1830230424. [DOI] [PubMed] [Google Scholar]
- 24.Navikas V, Link J, Persson C, Olsson T, Hojeberg B, Ljungdahl A, Link H, Wahren B. Increased mRNA expression of IL-6, IL-10, TNF-alpha, and perforin in blood mononuclear cells in human HIV infection. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:484–489. [PubMed] [Google Scholar]
- 25.Paganin C, Frank I, Trinchieri G. Priming for high interferon-gamma production induced by interleukin-12 in both CD4+ and CD8+ T cell clones from HIV-infected patients. J Clin Investig. 1995;96:1677–1682. doi: 10.1172/JCI118209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen N C, Dean G A, Bernales J, Sukura A, Higgins J. Listeria monocytogenes and Serratia marcescens infections as model for Th1/Th2 immunity in laboratory cats. Vet Immunol Immunopathol. 1998;63:83–103. doi: 10.1016/s0165-2427(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin J L, Ragnaud J M, Bernard N, Fleury H J. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Riviere Y, McChesney M B, Porrot F, Tanneau S F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, et al. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retroviruses. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 29.Sharma D P, Anderson M, Zink M C, Adams R J, Donnenberg A D, Clements J E, Narayan O. Pathogenesis of acute infection in rhesus macaques with a lymphocyte-tropic strain of simian immunodeficiency virus. J Infect Dis. 1992;166:738–746. doi: 10.1093/infdis/166.4.738. [DOI] [PubMed] [Google Scholar]
- 30.Song W, Collisson E W, Billingsley P M, Brown W C. Induction of feline immunodeficiency virus-specific cytolytic T-cell responses from experimentally infected cats. J Virol. 1992;66:5409–5417. doi: 10.1128/jvi.66.9.5409-5417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Than S, Hu R, Oyaizu N, Romano J, Wang X, Sheikh S, Pahwa S. Cytokine pattern in relation to disease progression in human immunodeficiency virus-infected children. J Infect Dis. 1997;175:47–56. doi: 10.1093/infdis/175.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Tsai C C, Follis K E, Grant R F, Nolte R E, Wu H, Benveniste R E. Infectivity and pathogenesis of titered dosages of simian immunodeficiency virus experimentally inoculated into longtailed macaques (Macaca fascicularis) Lab Anim Sci. 1993;43:411–416. [PubMed] [Google Scholar]
- 33.Williams M B, Butcher E C. Homing of naive and memory T lymphocyte subsets to Peyer’s patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 34.Woo J C, Dean G A, Pedersen N C, Moore P F. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J Virol. 1997;71:8632–8641. doi: 10.1128/jvi.71.11.8632-8641.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto J K, Sparger E, Ho E W, Andersen P R, O’Connor T P, Mandell C P, Lowenstine L, Munn R, Pedersen N C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1258. [PubMed] [Google Scholar]
- 36.Zou W, Lackner A A, Simon M, Durand G I, Galanaud P, Desrosiers R C, Emilie D. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]