Abstract
Background
Arteriovenous fistula represents the preferred vascular access for patients with kidney failure requiring hemodialysis. Surgeons have traditionally used physical examination to identify the most suitable vessels. This meta-analysis aims to evaluate whether ultrasound mapping should be routinely performed before arteriovenous fistula creation.
Methods
Medline, Scopus, Web of Science and CENTRAL were systematically searched from inception to November 1, 2022. Randomized controlled trials and cohort studies comparing routine ultrasound mapping to physical examination in terms of arteriovenous fistula patency were included. Meta-analysis was performed by fitting random-effects models. The study protocol has been prospectively registered in PROSPERO (CRD42023402390).
Results
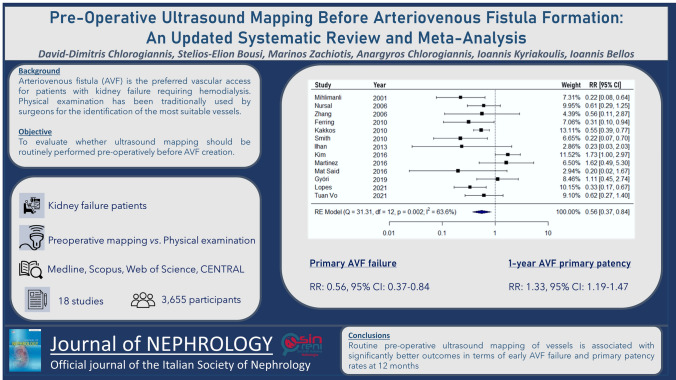
Overall, 18 studies were included, comprising 3655 participants. Routine pre-operative ultrasound mapping was associated with significantly lower rates of primary arteriovenous fistula failure (Risk Ratio-RR: 0.56, 95% confidence intervals-CI: 0.37–0.84, low certainty). A significant outcome was observed by separately pooling randomized controlled trials (RR: 0.37, 95% CI: 0.25–0.54). Routine ultrasound mapping was also associated with significantly higher rates of 1-year primary arteriovenous fistula patency (RR: 1.33, 95% CI: 1.19–1.47, moderate certainty). This effect remained significant in the analysis of randomized controlled trials (RR: 1.26, 95% CI: 1.02–1.56).
Conclusions
Implementing routine pre-operative ultrasound mapping of vessels is associated with significantly better outcomes in terms of early arteriovenous fistula failure and primary patency rates at 12 months. Further research should confirm the long-term benefits of routine ultrasound examination and evaluate its cost-effectiveness in different populations.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40620-023-01814-6.
Keywords: Ultrasound, Doppler, Mapping, Arteriovenous fistula, Dialysis
Introduction
Kidney failure on renal replacement therapy affects more than 4 million people worldwide and hemodialysis represents the main form of kidney replacement therapy [1]. The creation of an arteriovenous fistula is recommended as the primary vascular access option for the initiation of hemodialysis [2]. The successful creation of an arteriovenous fistula depends on several factors, with the incidence of 1-year primary failure rate being reported as high as 34% [3]. In addition, patients may require multiple salvage percutaneous or open interventions or even repeat vascular access procedures, which exacerbates cost-effectiveness and reduces the quality of life.
To identify a suitable candidate vessel and increase patency rates, preoperative ultrasound mapping of the vessels of the upper limbs versus conventional physical examination alone is recommended by the Spanish Clinical Guidelines for Vascular Access [2]. Rather than routine vascular mapping, the National Kidney Foundation (The Kidney Disease Outcomes Quality Initiative (KDOQI)) suggests selective preoperative ultrasound evaluation in patients at high risk of AV access failure [4]. Sonography allows for more precise measurement of internal vessel diameters, with a minimum of 2 mm of arteries and veins typically recommended. At the same time, it also provides valuable information concerning internal vascular lesions, which cannot be detected by physical examination alone [5, 6]. However, the level of evidence of such guidelines relies on randomized controlled studies which include small sample size, while there is a paucity of data concerning the value of preoperative ultrasound in arteriovenous fistula long-term patency, since their quantitative synthesis did not include one-year patency.
This systematic review and meta-analysis aims to analyze the updated clinical evidence concerning the value of routine preoperative sonographic mapping for arteriovenous fistula formation and its long-term primary failure rates in comparison to physical examination or selective ultrasound alone.
Materials and methods
Study design
The meta-analysis was designed following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [7]. The study protocol has been prospectively registered in PROSPERO (CRD42023402390). Ethical approval was omitted since no new patients were involved in this study.
Eligibility criteria
The population of the meta-analysis consisted of adult patients with kidney failure undergoing first-time arteriovenous fistula creation for dialysis initiation. Routine pre-operative Doppler ultrasound evaluation was compared to physical examination with or without selective ultrasound. The outcomes of interest were primary arteriovenous fistula failure (immediate arteriovenous fistula failure, early thrombosis or maturation failure) and primary patency at 12 months after surgery (functional arteriovenous fistula for hemodialysis without the need for intervention). Cohort studies (both prospective and retrospective) and randomized controlled trials (RCTs) were deemed eligible. Case–control, cross-sectional, descriptive, animal and in vitro studies were excluded.
Literature search
The systematic literature search was conducted on Medline (via PubMed), Scopus, Web of Science, CENTRAL (Cochrane Central Register of Controlled Trials); the full reference lists of the retrieved studies were also searched to identify additional articles (“snowball” method [8]). Databases were searched from inception until November 1, 2022. The search was based on a combination of keywords and MeSH (Medical Subject Headings) terms. The main applied algorithm was the following: ((venous mapping) OR (vessel mapping) OR (ultrasound) OR (pocus) OR (sonography) OR (point-of-care-ultrasound) OR (Doppler) OR (“Ultrasonography”[Mesh])) AND ((dialysis) OR (hemodialysis) OR (“Renal Dialysis”[Mesh])) AND ((fistula) OR (arteriovenous fistula) OR (AVF vascular access) OR (“Fistula”[Mesh])). No language restrictions were applied. The search was performed independently by two researchers and any discrepancies were resolved by consensus.
Study selection
The study selection followed three consecutive steps. Firstly, the titles and abstracts of all electronic records were screened for potential eligibility. Subsequently, the articles considered as possibly eligible were retrieved as full-texts. Then, any studies that did not report the outcomes of interest or fulfilled any of the exclusion criteria were excluded. The selection of studies was independently performed by two researchers, resolving any potential disagreement with the consensus of all authors.
Data extraction
Dedicated forms were used for extraction of the following data from the included studies: year of publication, country, study period, design, sample size, eligibility criteria, participants’ median age and sex, as well as the percentage of patients with hypertension and diabetes mellitus. Information regarding the outcomes of interest (early arteriovenous fistula failure and 12-month primary patency) was also collected. Two authors collected data, resolving any potential discrepancies after a discussion with a third author.
Quality assessment
The risk of bias of the included RCTs was evaluated using the RoB-2 tool [9], assessing the following domains: randomization, deviations from intended interventions, missing outcome data, measurement of outcomes and selection of the reported results. Risk of bias evaluation of cohort studies was performed using the ROBINS-I tool [10], which takes into account the following domains: confounding, selection of participants classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of the reported results. The risk of bias was judged as low, moderate, serious or critical. The process of quality assessment was conducted independently by two researchers, blinded to each other, and any discrepancy was resolved through the consensus of all authors.
Statistical analysis
Data analysis was performed in R-4.0.5 (package “metafor” [11]). Statistical significance was defined by a two-sided p-value threshold of 0.05. Random-effects models using the maximum likelihood method were fitted to provide estimates of risk ratio (RR) along with their 95% confidence intervals (CI). The degree of statistical heterogeneity was quantified by the inconsistency index (I2), with values > 50% denoting remarkable heterogeneity [12]. Additionally, the 95% predictive intervals were calculated to provide estimates of the effects to be expected by future studies [13]. Subgroup analysis was performed by categorizing studies based on study location, year of publication (≤ 2010 vs. > 2010), sample size (≤ 150 vs. > 150 patients), study design (cohort vs. RCT) and risk of bias. Publication bias was assessed by the visual inspection of funnel plots, while the trim-fill method was applied to account for potentially missing studies after statistical imputation [14]. The Egger’s regression test was used to evaluate funnel plot asymmetry in the case of at least 10 included studies [15].
Certainty of evidence
The GRADE approach was used to evaluate the certainty of evidence per outcome. Specifically, the certainty of evidence was judged as very low, low, moderate or high by taking into account the following domains: study limitations, inconsistency, indirectness, imprecision and publication bias [16].
Results
Study selection
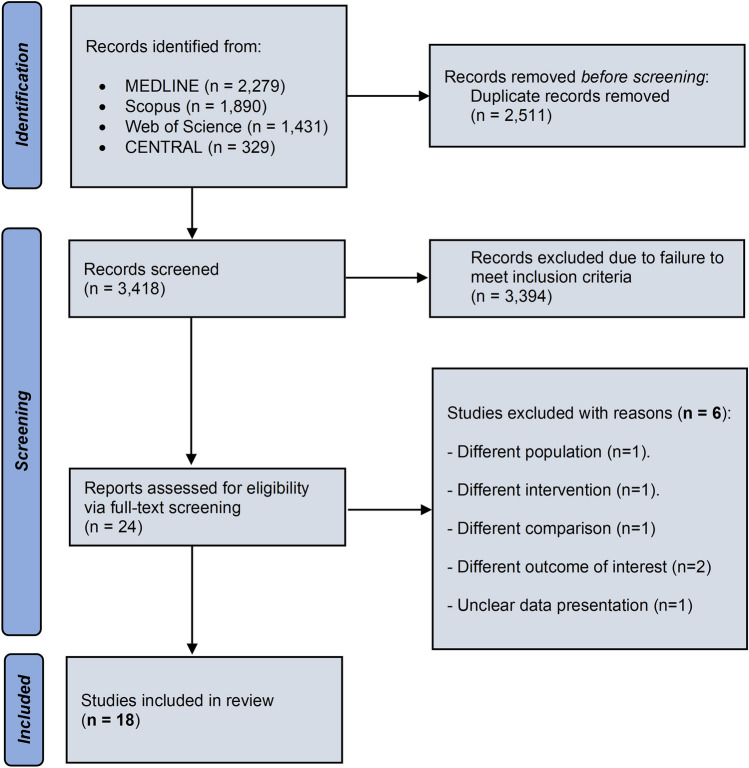
The study selection process is schematically depicted in Fig. 1. The literature search resulted in a total of 5929 records. After the removal of duplicates, 3418 articles were screened for eligibility and 24 of them were retrieved in full-text form. Subsequently, 6 studies were excluded due to the following reasons: different population (n = 1) [17], different intervention (n = 1) [18], different comparison (n = 1) [19], different outcome of interest (n = 2) [20, 21] and unclear data presentation (n = 1)[22]. As a result, a total of 18 studies were included in the meta-analysis, comprising 3,655 participants [23–40]. In 1693 of them, routine pre-operative ultrasound examination was performed, while in 1962 individuals arteriovenous fistula formation was based on physical examination.
Fig. 1.
Search plot diagram
Included studies
The methodological characteristics of the included studies are presented in Table 1. Six studies were RCTs [23, 36–40], 3 were prospective cohorts [25, 29, 34] and 9 studies followed a retrospective cohort design. [24, 26–28, 30–33, 35] Nine studies were conducted in Asia, 6 in Europe, 2 in North America and 1 in South America (Table 1). In the vast majority of studies, both arterial and venous ultrasound mapping were performed. The risk of bias in RCTs was judged to be moderate due to uncertainty and heterogeneity in the domain of outcomes measurement (Suppl. Figure 1). Regarding observational studies, the overall risk of bias was evaluated to be low in 1 study, moderate in 8 studies and serious in 3 studies. No study was judged to be at critical risk of bias (Suppl. Table 1). The main source of bias concerns was the domain of confounding reflecting the differences in the baseline characteristics of participants. In addition, bias in the domain of participant selection was recognized in studies where the non-randomized design was assessed to significantly affect the allocation of patients to routine or selective ultrasound mapping.
Table 1.
Methodological characteristics of the included studies
| Study | Country | Sample size | Study design | Exclusion criteria | Arterial/venous mapping | Median age (years) † | Male sex (%) † | Hypertension (%) † | Diabetes mellitus (%) † | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| 2001; Mihmanli | Turkey | 124 | RCT | Age > 74 years, previous AVF | Both | NR | 58.3 vs. 51.9 | NR | NR | Moderate |
| 2006; Nursal | Turkey | 66 | RCT | External venous diameter < 1 mm, visible vein length < 5 cm, inadequate pulsatile force, arm edema | Both | 56.5 vs. 56.5 | 51.4 vs. 54.3 | 94.1 vs. 82.9 | 44.1 vs. 37.1 | Moderate |
| 2006; Zhang | China | 66 | RCT | NR | Both | NR | NR | NR | NR | Moderate |
| 2007; Karakayali | Turkey | 280 | Retrospective cohort | NR | Both | 52.8 vs. 46.9* | 54.6 vs. 56.3 | NR | 35 vs. 35.1 | Moderate |
| 2010; Ferring | UK | 208 | RCT | > 1 previous AVF, previous AVG | Both | 69 vs. 67 | 61.6 vs. 66.0 | 78.4 vs. 75.2 | 43.2 vs. 34.3 | Moderate |
| 2010; Kakkos | USA | 467 | Prospective cohort | NR | Both | 62 vs. 64 | 52.7 vs. 59.3 | NR | 50 vs. 45 | Serious |
| 2010; Smith | UK | 77 | RCT | Previous vascular intervention on target limb, thrombophilia | Both | 64.3 vs. 65.3 | NR | 63.8 vs. 57.4 | 31.9 vs. 31.9 | Moderate |
| 2013; Ilhan | Turkey | 118 | Retrospective cohort | Central vein stenosis, outflow vein occlusion, arm edema | Both | 56.4 vs. 54.5 | 57.1 vs. 57.9 | 50.7 vs. 44.7 | 38 vs. 34.2 | Moderate |
| 2016; Kim | South Korea | 469 | Retrospective cohort | Central vein stenosis, outflow vein occlusion | Both | 55.6 vs. 55.8 | 52.5 vs. 65.2* | 85.6 vs. 90.1 | 60.2 vs. 43.9* | Moderate |
| 2016; Martinez | Spain | 81 | Retrospective cohort | Previous AVF in same extremity | Both | 68.3 vs. 64.8 | 67 vs. 60 | 90 vs. 86 | 64 vs. 47 | Moderate |
| 2016; Mat Said | Malaysia | 158 | Prospective cohort | Previous AVG, AVF re-intervention | Both | 52.9 vs. 52.5 | 45.5 vs. 57 | 91.1 vs. 87.3 | 59.5 vs. 55.7 | Moderate |
| 2016; Giannikouris | Italy | 102 | Retrospective cohort | Non-diabetic patients, previous AVF/AVG | Both | 65 vs. 69 | 64.7 vs. 70.6 | NR | NR | Moderate |
| 2018; Hossain | Canada | 316 | Retrospective cohort | Loop AVG, AVF for plasmapheresis | Venous | 59 vs. 60 | 73 vs. 71 | 88 vs. 90 | 48 vs. 57 | Moderate |
| 2018; Kim | South Korea | 250 | Retrospective cohort | Arterial stenosis/occlusion | Both | 56.3 vs. 56.9 | 62.7 vs. 66.1 | 81 vs. 69.4* | 62.7 vs. 54.8 | Moderate |
| 2019; Györi | Austria | 331 | Retrospective cohort | NR | Both | 60.7 vs. 58.7 | 70.2 vs. 58.1* | 65.8 vs. 73.7 | 22.8 vs. 31.3 | Serious |
| 2019; Torres | Spain | 178 | Prospective cohort | Unavailable ultrasound, AVF site indicated by clinical events | Both | 64 vs. 68.4* | 63 vs. 61 | 92 vs. 92 | 55 vs. 63 | Serious |
| 2021; Lopes | Brazil | 206 | RCT | Central vein stenosis, arterial stenosis/occlusion | Both | 56 vs. 57.5 | 65 vs. 71 | 46.5 vs. 53.5 | 40.4 vs. 39.5 | Moderate |
| 2022; Tuan Vo | Vietnam | 158 | Retrospective cohort | No start of hemodialysis | Both | 54.1 vs. 55.4 | 43 vs. 32.9 | 88.6 vs. 88.6 | 36.7 vs. 29.1 | Low |
NR not reported, RCT randomized controlled trial, AVF arteriovenous fistula, AVG arteriovenous graft
†Comparisons of routine ultrasound group vs. physical examination group
*p value < 0.05
Primary arteriovenous fistula failure
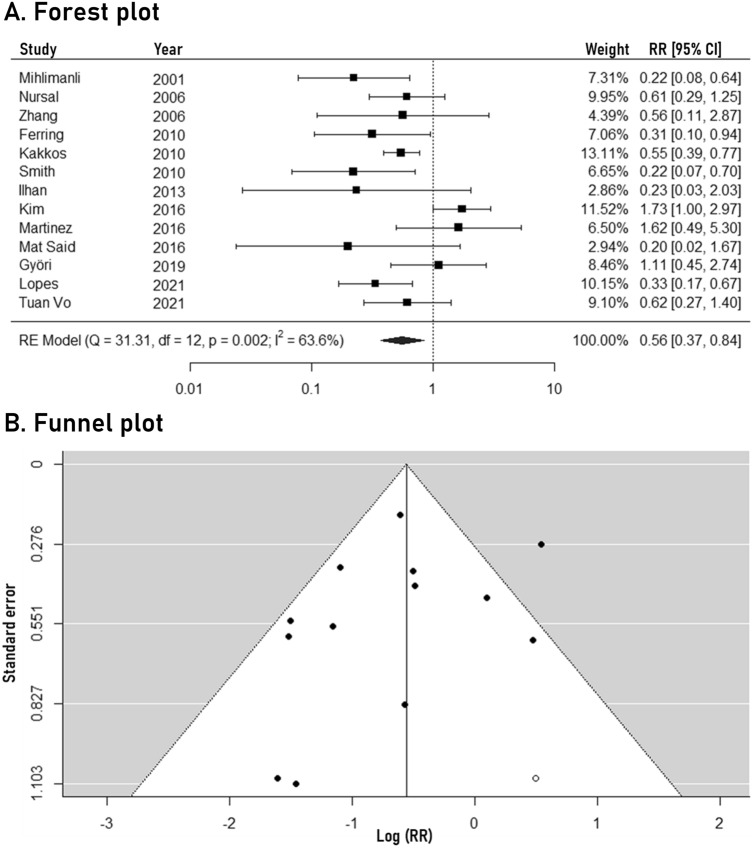
The meta-analysis outcomes regarding primary arteriovenous fistula failure are depicted in Fig. 2. Routine pre-operative ultrasound mapping was associated with significantly lower rates of primary arteriovenous fistula failure (RR: 0.56, 95% CI: 0.37 to 0.84, 13 studies). Moderate statistical heterogeneity was noted (I2: 63.6%), while the 95% predictive intervals ranged from 0.18 to 1.75. No significant funnel plot asymmetry was observed (Egger’s p-value: 0.181). The trim-fill method identified one potentially missing study, not affecting the significance of the pooled outcome (new RR: 0.57, 95% CI: 0.39 to 0.86). The results of the subgroup analysis are presented in Table 2. Importantly, the separate analysis of RCTs indicated a significant association between routine ultrasound mapping and lower primary arteriovenous fistula failure rates (RR: 0.37, 95% CI: 0.25 to 0.54). Study location, sample size, year of publication and risk of bias did not significantly influence the overall outcome. The certainty of evidence was evaluated as low due to downgrading in the domains of study limitations and inconsistency.
Fig. 2.
Forest plot (A) and funnel plot (B) of the pre-operative ultrasound mapping effects on primary arteriovenous fistula failure
Table 2.
Outcomes of the subgroup analysis
| Subgroup | Early AVF failure | 12-months primary patency |
|---|---|---|
| Overall | 0.56 (0.37–0.84) | 1.33 (1.19–1.47) |
| Study location | ||
| Europe | 0.59 (0.32–1.08) | 1.35 (1.12–1.63) |
| Asia | 0.57 (0.30–1.09) | 1.22 (1.12–1.31) |
| North America | – | 1.55 (1.32–1.82) |
| South America | 0.33 (0.17–0.67) | – |
| P value for subgroup effect | 0.757 | 0.351 |
| Sample size | ||
| ≤ 150 patients | 0.46 (0.24–0.88) | 1.22 (0.99–1.50) |
| > 150 patients | 0.62 (0.37–1.06) | 1.34 (1.19–1.51) |
| P value for subgroup effect | 0.468 | 0.590 |
| Year of publication | ||
| ≤ 2010 | 0.45 (0.32–0.64) | 1.33 (1.14–1.55) |
| > 2010 | 0.75 (0.40–1.39) | 1.33 (1.16–1.52) |
| P value for subgroup effect | 0.091 | 0.964 |
| Study design | ||
| Cohort study | 0.83 (0.50–1.38) | 1.34 (1.19–1.51) |
| Randomized controlled trial | 0.37 (0.25–0.54) | 1.26 (1.02–1.56) |
| P value for subgroup effect | 0.013 | 0.744 |
| Risk of bias | ||
| Low | 0.62 (0.27–1.40) | 1.15 (0.99–1.35) |
| Moderate | 0.49 (0.28–0.86) | 1.31 (1.18–1.46) |
| Serious | 0.69 (0.36–1.31) | 1.48 (1.00–2.19) |
| P value for subgroup effect | 0.783 | 0.412 |
Routine ultrasound examination is compared to physical examination (reference group). Data presented as risk ratio (95% confidence intervals). Bold text indicates statistical significance
AVF arteriovenous fistula
One-year primary arteriovenous fistula patency
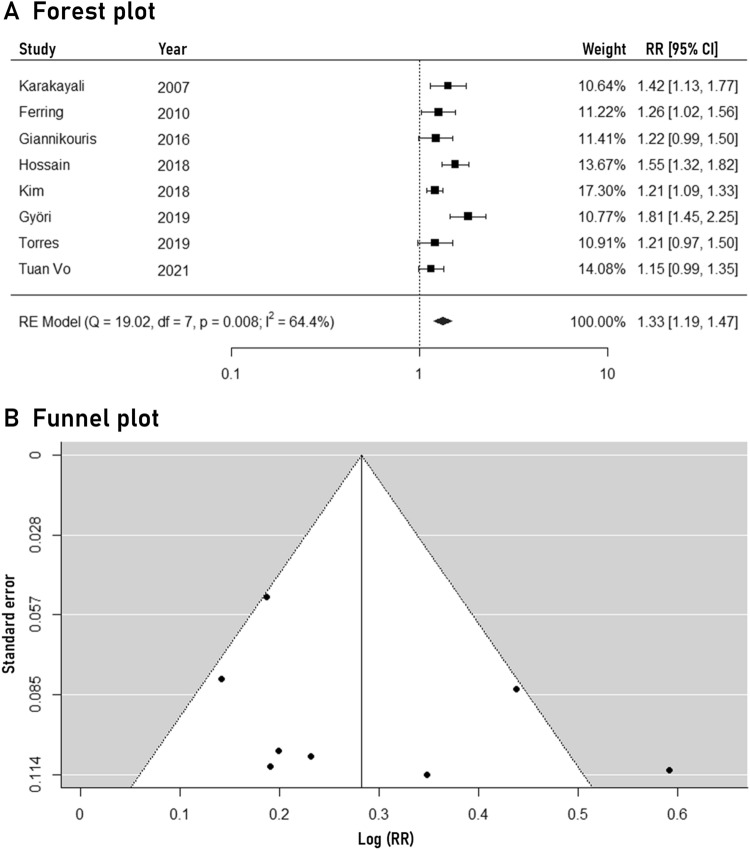
The outcomes of the meta-analysis regarding the primary arteriovenous fistula patency at 12 months are illustrated in Fig. 3. Routine ultrasound mapping was associated with significantly higher rates of 1-year primary arteriovenous fistula patency (RR: 1.33, 95% CI: 1.19–1.47, 8 studies). Statistical heterogeneity was moderate (I2: 64.4%). The 95% predictive intervals remained significant (1.03–1.71). No apparent funnel plot asymmetry was recognized and the trim-fill method identified no missing studies. The subgroup analysis revealed no significant heterogeneity based on study location, sample size, year of publication, design and risk of bias (Table 2). The certainty of evidence was evaluated to be moderate, due to downgrading only in the domain of study limitations.
Fig. 3.
Forest plot (A) and funnel plot (B) of the pre-operative ultrasound mapping effects on 1-year primary arteriovenous fistula patency
Discussion
By including eighteen studies with a total of 3655 patients, the present meta-analysis provides an updated evaluation of both early and one-year patency rates of arteriovenous fistulas created following routine pre-operative ultrasound mapping versus selective ultrasound or physical examination alone. Routine ultrasound examination was associated with significantly better outcomes in terms of early arteriovenous fistula failure, which was even more pronounced when only RCTs were included in the analysis. In addition, the implementation of routine pre-operative mapping was linked to better long-term outcomes, as reflected by the significantly higher rates of 1-year primary patency.
The findings of the present study are in contrast with the results of a previous meta-analysis which concluded that there was no statistically significant difference in favor of pre-operative ultrasound examination. However, the meta-analysis by Wong et al. included only three RCTs reporting only the early maturation rates, and information on the long-term events was lacking, highlighting the need for larger, long-term trials in order to provide more robust results [41]. This consideration was shared by a subsequent meta-analysis by Kosa et al. that concluded that there was no benefit of pre-operative mapping on fistula outcomes compared to physical examination alone [42]. The meta-analysis, however, was based on studies with a high risk of bias which, in combination with the reported low pooled sample size, make these results inconclusive.
Conversely, a meta-analysis by Georgiades et al. [43] highlighted a reduced immediate failure rate in the routine ultrasound group, with the odds of immediate failure being almost three times less. Still, the study did not report on the long-term outcomes and also did not include the results of the RCT by Lopez et al. which enrolled 228 patients and reported a significantly higher primary failure rate in the group which underwent clinical examination only (13.6%) compared to the group assessed by ultrasound (4.4%) [23]. Overall, these results indicate the need for routine sonographic evaluation of the veins in both arms to select the best candidate vessels. These benefits have been further confirmed in populations in which physical examination usually fails to identify the best candidate vessels, such as obese patients [6].
The present study has several strengths. A comprehensive literature search was conducted by screening 4 databases without date restrictions. Both RCTs and cohort studies were evaluated separately and together. Extensive subgroup analysis was performed, aiming to explore the potential sources of inter-study heterogeneity, while the calculation of the 95% predictive intervals provided an assessment of the effects to be expected by future studies. Furthermore, the certainty of outcomes was critically appraised following the standardized GRADE approach.
On the other hand, this systematic review and meta-analysis has limitations. Firstly, the analysis was conducted with study-level data and not patient-level data in order to adjust for baseline demographic characteristics that could contribute to confounding bias. Also, the observational studies that were included were rated as harboring a high risk for bias and introduced significant heterogeneity when pooled together with the randomized controlled trials, which had different inclusion criteria and assessed different primary outcomes. It is likewise important to consider that the reporting of access outcomes in hemodialysis trials is very heterogeneous, with limited patient-reported outcomes and great need for standardized outcome measures. In this context, a systematic review by Viecelli et al. identified 168 relevant trials with a total of 1426 access-related outcome measures [44]. Access function was assessed in 489 different ways including “mean access blood flow (mL/min)” and “number of thromboses” being used in 27% and 22% of the trials, respectively. Of note, the authors reported that a very limited number of trials included patient-related outcomes with pain and quality-of-life being reported in only 11% and 3% of the studies, respectively. Lastly, the inclusion of patients from different countries and socioeconomic backgrounds may contribute to the high heterogeneity of the pooled estimates and uncertainty for the generalization of the meta-analysis results.
Overall, the findings of this meta-analysis point toward better arteriovenous fistula patency rates with the use of routine pre-operative ultrasound vessel mapping. However, clinicians should be aware of the low to moderate certainty of the existing evidence, mainly due to inter-study heterogeneity resulting from different definitions of endpoints. To this end, future research should focus on the implementation of standardized outcome measures in order to promote consistency and improve the relevance of trial evidence. Specifically, several outcome measures have been suggested, such as uninterrupted arteriovenous fistula use or the ability to receive 2-needle canulation without the need for intervention. In addition, it is important for future studies to include patient-reported outcomes, aiming to take into account quality of life, as well as patients’ preferences and values [45]. Various dialysis-specific questionnaires have been developed to establish patient-reported outcome measures, such as the Kidney Disease Questionnaire (KDQ) [46] and the Hemodialysis Access-Related Quality-of-life (HARQ) tools [47]. It is also important to conduct cost-effectiveness analyses aiming for a reliable economic evaluation of access-related costs, including the need for hospitalizations and surgical procedures.
Conclusions
The present meta-analysis indicates that the implementation of routine pre-operative ultrasound mapping in addition to physical examination may be associated with beneficial outcomes in terms of both primary failure and 1-year primary patency of arteriovenous fistulas. This effect was apparent even when randomized controlled trials were separately pooled, while the overall credibility of evidence was evaluated to be low to moderate. Further research is needed to confirm these outcomes, as well as to elucidate whether a multidisciplinary approach to vascular access creation involving the routine use of ultrasound is able to affect long-term patency rates, reduce health costs related to access failure complications and improve patients’ quality of life.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by HEAL-Link Greece.
Declarations
Conflict of interest
None declared.
Ethical approval
Ethical approval was not required since no new patients were recruited. The study was a meta-analysis based exclusively on already published data.
Research involving human participants and/or animals
Not applicable. The present study is a literature-based meta-analysis, involving no new participants.
Informed consent
Not applicable. No new participants were recruited.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bello AK, Okpechi IG, Osman MA, Cho Y, Htay H, Jha V, Wainstein M, Johnson DW. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18:378–395. doi: 10.1038/s41581-022-00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidli J, Widmer MK, Basile C, de Donato G, Gallieni M, Gibbons CP, Haage P, Hamilton G, Hedin U, Kamper L, Lazarides MK, Lindsey B, Mestres G, Pegoraro M, Roy J, Setacci C, Shemesh D, Tordoir JHM, van Loon M, Guidelines Committee ESVS, Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Lindholt J, Naylor R, Vega de Ceniga M, Vermassen F, Verzini F, Guidelines Reviewers ESVS, Mohaupt M, Ricco J-B, Roca-Tey R. Editor’s choice—vascular access: 2018 clinical practice guidelines of the European society for vascular surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:757–818. doi: 10.1016/j.ejvs.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Voorzaat BM, Janmaat CJ, van der Bogt KEA, Dekker FW, Rotmans JI. Patency outcomes of arteriovenous fistulas and grafts for hemodialysis access: a trade-off between nonmaturation and long-term complications. Kidney. 2020;1:916–924. doi: 10.34067/KID.0000462020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RP, National Kidney Foundation KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4 Suppl 2):1–164. doi: 10.1053/j.ajkd.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Brooke BS, Griffin CL, Kraiss LW, Kim J, Nelson R. Cost-effectiveness of repeated interventions on failing arteriovenous fistulas. J Vasc Surg. 2019;70:1620–1628. doi: 10.1016/j.jvs.2019.01.085. [DOI] [PubMed] [Google Scholar]
- 6.Vassalotti JA, Falk A, Cohl ED, Uribarri J, Teodorescu V. Obese and non-obese hemodialysis patients have a similar prevalence of functioning arteriovenous fistula using pre-operative vein mapping. Clin Nephrol. 2002;58:211–214. doi: 10.5414/CNP58211. [DOI] [PubMed] [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/BMJ.N71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331:1064–1065. doi: 10.1136/bmj.38636.593461.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: A revised tool for assessing risk of bias in randomised trials. The BMJ. 2019 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 10.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 12.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi L, Lin L. The trim-and-fill method for publication bias. Medicine. 2019;98:e15987. doi: 10.1097/MD.0000000000015987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, deBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/J.JCLINEPI.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Siribumrungwong B, Tomtitchong P, Kanpirom K. Role of preoperative vascular ultrasonography in hemodialysis vascular access operation. J Med Assoc Thai. 2010;93(Suppl 7):S177–S182. [PubMed] [Google Scholar]
- 18.Aragoncillo Sauco I, Ligero Ramos JM, Vega Martínez A, Morales Muñoz ÁL, Abad Estébanez S, Macías Carmona N, Ruiz Chiriboga D, García Pajares R, Cervera Bravo T, López-Gómez JM, Manzano Grossi S, Menéndez Sánchez E, Río Gomez J, García Prieto AM, Linares Grávalos T, Garcia Boyano F, Reparaz Asensio LM, Albalate Ramón M, de Sequera OP, Gil Casares B, Ampuero Mencía J, Castellano S, Martín Pérez B, Conty JLM, Santos Garcia A, Luño Fernandez J. Consulta de acceso vascular: resultados antes y después de la instauración de un programa multidisciplinar con realización de ecografía doppler de rutina. Nefrologia. 2018;38:616–621. doi: 10.1016/j.nefro.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Rozenberg I, Benchetrit S, Raigorodetsky M, Fajer S, Shnaker A, Nacasch N, Einbinder Y, Zitman-Gal T, Cohen-Hagai K. Clinical outcomes of vascular accesses in hemodialysis patients. Isr Med Assoc J. 2022;24:514–519. [PubMed] [Google Scholar]
- 20.Lee K-G, Chong T-T, Goh N, Achudan S, Tan Y-L, Tan R-Y, Choong H-L, Tan C-S. Outcomes of arteriovenous fistula creation, effect of preoperative vein mapping and predictors of fistula success in incident haemodialysis patients: a single-centre experience. Nephrology. 2017;22:382–387. doi: 10.1111/nep.12788. [DOI] [PubMed] [Google Scholar]
- 21.Silva-González M, Mijangos WF, Carbajal-Robles V, Santillán-Aguayo E, Olivares-Cruz S, Sierra-Juárez MÁ. Mapeo ultrasonográfico preoperatorio para optimizar tiempo de maduración de fístulas arteriovenosas. Revista Mexicana de Angiologia. 2021 doi: 10.24875/RMA.21000008. [DOI] [Google Scholar]
- 22.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013–2020. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopes JRA, de Marques ALB, Correa JA. Randomised clinical study of the impact of routine preoperative Doppler ultrasound for the outcome of autologous arteriovenous fistulas for haemodialysis. J Vasc Access. 2021;22:107–114. doi: 10.1177/1129729820927273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karakayali F, Ekici Y, Görür SK, Arat Z, Boyvat F, Karakayali H, Haberal M. The Value of preoperative vascular imaging in the selection and success of hemodialysis access. Ann Vasc Surg. 2007;21:481–489. doi: 10.1016/j.avsg.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Kakkos SK, Haddad GK, Stephanou A, Haddad JA, Shepard AS. Routine preoperative venous and arterial mapping increases both, construction and maturation rate of upper arm autogenous arteriovenous fistulae. Vasc Endovascular Surg. 2011;45:135–141. doi: 10.1177/1538574410391819. [DOI] [PubMed] [Google Scholar]
- 26.Ílhan G, Esi E, Bozok Ş, Yürekli Í, Özpak B, Özelçi A, Destan B, Gürbüz A. The clinical utility of vascular mapping with doppler ultrasound prior to arteriovenous fistula construction for hemodialysis access. J Vasc Access. 2013;14:83–88. doi: 10.5301/jva.5000097. [DOI] [PubMed] [Google Scholar]
- 27.Kim SM, Han Y, Kwon H, Hong HS, Choi JY, Park H, Kwon T-W, Cho Y-P. Impact of a preoperative evaluation on the outcomes of an arteriovenous fistula. Ann Surg Treat Res. 2016;90:224. doi: 10.4174/astr.2016.90.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannikouris IE, Bacchini GM. Maximizing native arteriovenous fistulae rates in patients with diabetes mellitus. Is routine color Doppler vascular mapping in preoperative planning of value? Hippokratia. 2016;20:252. [PMC free article] [PubMed] [Google Scholar]
- 29.Mat Said N, Musa KI, Mohamed Daud MA, Haron J. The combination of sonography and physical examination improves the patency and suitability of hemodialysis arteriovenous fistula in vascular access. Malays J Med Sci. 2016;23:26–32. doi: 10.21315/mjms2016.23.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez Carnovale L, Esteve Simó V, Yeste Campos M, Artigas Raventós V, Llagostera Pujol S. Utilidad del mapeo ecográfico preoperatorio para los accesos vasculares de hemodiálisis. Angiologia. 2016;68:478–483. doi: 10.1016/j.angio.2016.05.001. [DOI] [Google Scholar]
- 31.Hossain S, Sharma A, Dubois L, DeRose G, Duncan A, Power AH. Preoperative point-of-care ultrasound and its impact on arteriovenous fistula maturation outcomes. J Vasc Surg. 2018;68:1157–1165. doi: 10.1016/j.jvs.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Kim KT, Ryu JW, Seo PW, Ryu KM. Clinical results of arteriovenous fistulas constructed using autologous vessels in end-stage renal disease patients on hemodialysis. Korean J Thorac Cardiovasc Surg. 2018;51:122–129. doi: 10.5090/kjtcs.2018.51.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Györi GP, Eilenberg W, Dittrich L, Neumayer C, Roka S, Berlakovich GA. Preoperative ultrasound improves patency and cost effectiveness in arteriovenous fistula surgery. J Vasc Surg. 2019;69:526–531. doi: 10.1016/j.jvs.2018.05.217. [DOI] [PubMed] [Google Scholar]
- 34.Mateos Torres E, Collado Nieto S, Cao Baduell H, Lacambra Peñart M, Velescu A, Clará Velasco A. Utilidad de la valoración ecográfica previa a la realización del primer acceso vascular para hemodiálisis. Nefrologia. 2019;39:539–544. doi: 10.1016/j.nefro.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Vo AT, Van NT, Kieu SM, Kieu AQ, Huynh KT, Nguyen QNH, Nguyen TTT, Nguyen TC. Revisiting the role of ultrasound mapping in arteriovenous fistula formation: a single-center experience. J Vasc Ultrasound. 2022;46:160–164. doi: 10.1177/15443167221113867. [DOI] [Google Scholar]
- 36.Mihmanli I, Besirli K, Kurugoglu S, Atakir K, Haider S, Ogut G, Numan F, Canturk E, Sayin AG. Cephalic vein and hemodialysis fistula: surgeon’s observation versus color Doppler ultrasonographic findings. J Ultrasound Med. 2001;20:217–222. doi: 10.7863/jum.2001.20.3.217. [DOI] [PubMed] [Google Scholar]
- 37.Nursal TZ, Oguzkurt L, Tercan F, Torer N, Noyan T, Karakayali H, Haberal M. Is routine preoperative ultrasonographic mapping for arteriovenous fistula creation necessary in patients with favorable physical examination findings? results of a randomized controlled trial. World J Surg. 2006;30:1100–1107. doi: 10.1007/s00268-005-0586-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Wang X, Zhang Z, Du G, Wang L, Yang J, et al. Hemodynamic evaluation of native arteriovenous fistulas for chronic hemodialysis with color Doppler ultrasound. Chin J Med Imaging Technol. 2006;22:718–721. [Google Scholar]
- 39.Ferring M, Claridge M, Smith SA, Wilmink T. Routine preoperative vascular ultrasound improves patency and use of arteriovenous fistulas for hemodialysis. Clin J Am Soc Nephrol. 2010;5:2236–2244. doi: 10.2215/CJN.02820310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith GE, Barnes R, Chetter IC. Randomized clinical trial of selective versus routine preoperative duplex ultrasound imaging before arteriovenous fistula surgery. Br J Surg. 2014;101:469–474. doi: 10.1002/bjs.9435. [DOI] [PubMed] [Google Scholar]
- 41.Wong CS, McNicholas N, Healy D, Clarke-Moloney M, Coffey JC, Grace PA, Walsh SR. A systematic review of preoperative duplex ultrasonography and arteriovenous fistula formation. J Vasc Surg. 2013;57:1129–1133. doi: 10.1016/j.jvs.2012.11.094. [DOI] [PubMed] [Google Scholar]
- 42.Kosa SD, Al-Jaishi AA, Moist L, Lok CE. Preoperative vascular access evaluation for haemodialysis patients. Cochrane Database of Sys Rev. 2015 doi: 10.1002/14651858.CD007013.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgiadis GS, Charalampidis DG, Argyriou C, Georgakarakos EI, Lazarides MK. The necessity for routine pre-operative ultrasound mapping before arteriovenous fistula creation: a meta-analysis. Eur J Vasc Endovasc Surg. 2015;49:600–605. doi: 10.1016/j.ejvs.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Viecelli AK, O'Lone E, Sautenet B, Craig JC, Tong A, Chemla E, Hooi LS, Lee T, Lok C, Polkinghorne KR, Quinn RR, Vachharajani T, Vanholder R, Zuo L, Irish AB, Mori TA, Pascoe EM, Johnson DW, Hawley CM. Vascular access outcomes reported in maintenance hemodialysis trials: a systematic review. Am J Kidney Dis. 2018;71(3):382–391. doi: 10.1053/j.ajkd.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Viecelli AK, Tong A, O'Lone E, Ju A, Hanson CS, Sautenet B, Craig JC, Manns B, Howell M, Chemla E, Hooi LS, Johnson DW, Lee T, Lok CE, Polkinghorne KR, Quinn RR, Vachharajani T, Vanholder R, Zuo L, Hawley CM. Report of the standardized outcomes in nephrology-hemodialysis (SONG-HD) consensus workshop on establishing a core outcome measure for hemodialysis vascular access. Am J Kidney Dis. 2018;71(5):690–700. doi: 10.1053/j.ajkd.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Rothrock NE, Kaiser KA, Cella D. Developing a valid patient-reported outcome measure. Clin Pharmacol Ther. 2011;90(5):737–742. doi: 10.1038/clpt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordyke RJ, Nicholson G, Gage SM, Lithgow T, Himmelfarb J, Rivara MB, Hays RD, Woo K, Peipert JD. Vascular access-specific health-related quality of life impacts among hemodialysis patients: qualitative development of the hemodialysis access-related quality of life (HARQ) instrument. BMC Nephrol. 2020;21(1):16. doi: 10.1186/s12882-020-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.