Hypoglycemia is a common and life-threatening complication of type 1 diabetes (T1D), with 1 in 20 patients hospitalized annually for a severe event (1). Low blood glucose promotes impaired awareness of hypoglycemia and loss of counterregulatory hormone responses in T1D. Identifying patients with impaired counterregulation is essential, as they are at ∼25-fold risk of severe hypoglycemia (2). Insulin clamp studies are not clinically scalable, and additional tools are needed to stratify hypoglycemia risk. Here, we show time below range (TBR) (glucose <70 mg/dL) on continuous glucose monitoring (CGM) predicts impaired epinephrine response during hypoglycemic clamp. CGM TBR may thus enable rapid identification of patients at highest risk for severe hypoglycemia in the clinical setting.
Methods for this study were previously described by our group (3). Twenty-two participants representing a general population with T1D wore a blinded professional CGM (FreeStyle Libre Pro; Abbott Diabetes Care, Alameda, CA) for 14 days to assess baseline glycemia. Participant ages ranged from 28–51 years, with median diabetes duration of 19 years, median A1C 6.7% (50 mmol/mol), median Clarke score of 3, and median TBR of 14% (interquartile range 8–24%). Prior to any treatment (3), participants completed a hypoglycemic clamp using infusion with 30 mU/m2/min regular insulin and variable-rate 20% dextrose to reduce serum glucose to 50 mg/dL over 20 min. Serum glucose was clamped at 50 mg/dL for 40 min, after which insulin was discontinued and dextrose infusion maintained until reaching euglycemia.
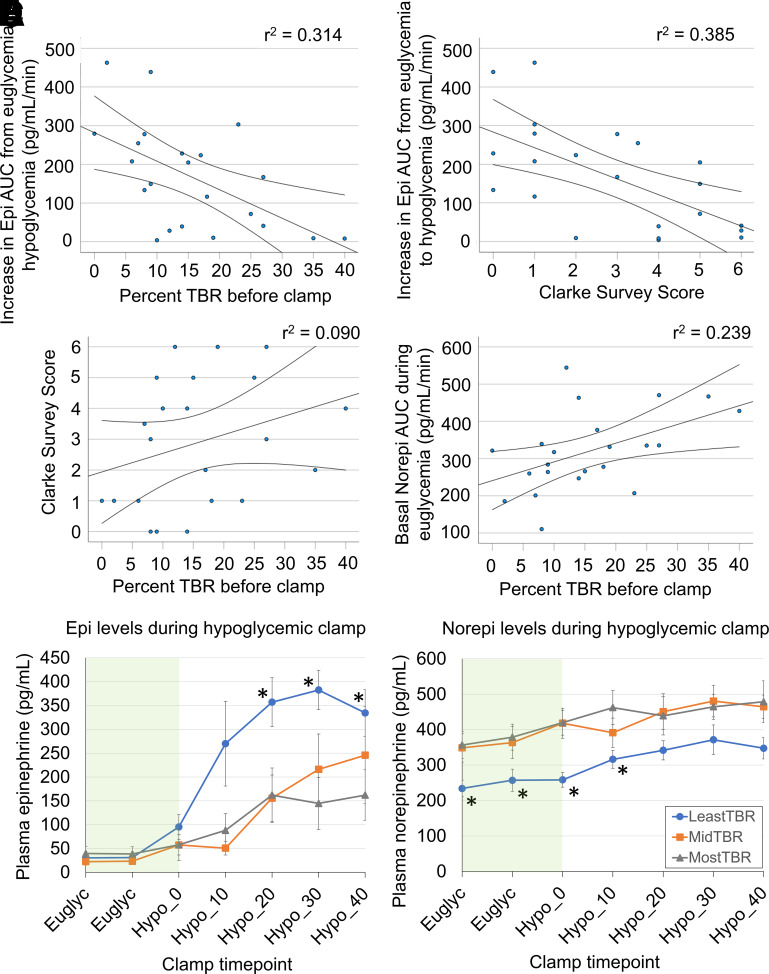
This study provided a unique opportunity to determine if CGM metrics can predict physiologic response to hypoglycemia. Indeed, Spearman analysis showed that greater CGM TBR was associated with reduced epinephrine response (−0.555, ρ = 0.007). Decreased epinephrine response was also associated with greater Clarke score (−0.602, ρ = 0.003), age (−0.542, ρ = 0.009), and duration of T1D (−0.470, ρ = 0.027). Epinephrine response did not correlate with A1C, CGM average glucose, BMI, or CGM time in range. Interestingly, greater TBR was associated with increased basal norepinephrine levels (0.538, ρ = 0.010) during euglycemia.
Linear regression (Fig. 1A and B) demonstrated that greater TBR and increasing Clark score both correlated strongly with decreased epinephrine response to hypoglycemia (r2 = 0.314 and r2 = 0.385, respectively). This was expected, as the Clarke score is a validated measure of hypoglycemia awareness. However, TBR and Clarke measures (Fig. 1C) showed poor linear correlation (r2 = 0.090) when compared with one another. In addition, greater TBR correlated with higher norepinephrine levels (Fig. 1D) during euglycemia (r2 = 0.239). To compare catecholamine levels, participants were divided into tertiles using TBR (least, mid-level, and most TBR). Mean catecholamine concentrations were plotted for each group during hypoglycemic clamps (Fig. 1E and F). At the start of hypoglycemia, patients in the lowest tertile for TBR (0–9%) showed a robust increase in epinephrine, but epinephrine response was significantly blunted in the tertile with greatest TBR (>19%). Again, patients with the greatest TBR exhibited significantly higher basal norepinephrine levels.
Figure 1.
TBR on CGM predicts impaired epinephrine response during hypoglycemic clamp. A and B: Epinephrine response is reduced with increased TBR (<70 mg/dL) or increased Clarke survey score. AUC, area under the curve; Epi, epinephrine; Norepi, norepinephrine. C: TBR and Clarke scores are not strongly correlated. D: Baseline plasma norepinephrine level during euglycemia increases with greater TBR (95% CI are shown). E: Greater TBR (grouped by TBR tertile) blunts epinephrine response to hypoglycemia on hyperinsulinemic-hypoglycemic clamp. F: Greater TBR is also associated with higher baseline norepinephrine levels. Data are shown as mean ± SEM. Green bands mark the euglycemic clamp period, while hypoglycemia time, in minutes, is marked on the x-axis. *P < 0.05.
Severe hypoglycemia remains common and life-threatening among patients living with T1D. We show here that CGM TBR can identify patients with impaired counterregulatory response to hypoglycemia. Preventing severe hypoglycemia in T1D may thus benefit from greater focus on minimizing TBR. Additionally, TBR and Clarke score do not closely correlate, and TBR thus appears to provide complementary information about hypoglycemia risk. Regarding limitations of our study, the Freestyle Libre Pro has been shown to report higher rates of hypoglycemia than capillary glucose testing. This limitation was discussed at length in our previous article (3), and we expect that modern CGM will corroborate the relationships demonstrated here. Widespread availability, ease of assessment, focus on recent trends, and power to predict impaired counterregulation make CGM TBR a strong addition for clinical hypoglycemia risk stratification. If a patient presents with high TBR, the clinician should prioritize precautionary interventions, including access to glucagon, implementing hybrid closed-loop technology, insulin dose adjustment, and lifestyle interventions (e.g., exercise education and limiting alcohol).
While increased exposure to hypoglycemia was associated with reduced epinephrine response, we found that basal norepinephrine levels paradoxically increased with greater TBR. Discordance between norepinephrine and epinephrine responses to hypoglycemia has been previously reported but without CGM data (4). More recent studies have implicated iatrogenic hypoglycemia as a trigger for sympathetic hyperactivation, norepinephrine release, and lethal cardiac arrhythmias in T1D (5). Our findings point to the concerning possibility that recurrent hypoglycemia may blunt a protective epinephrine response while increasing norepinephrine activity and cardiovascular complications.
A key question that our study raises is what degree of TBR is acceptable. Current guidelines recommend <4% based on the 2019 Advanced Technologies and Treatments for Diabetes (ATTD) consensus to keep TBR <1 h daily. Although there is no universally agreed-upon normal epinephrine cutoff, our data suggest that for every 3% reduction in TBR there would be a ∼6% relative increase in the epinephrine area under the curve response in similar patients. However, the question of whether strict avoidance of hypoglycemia can improve autonomic response and hypoglycemia awareness remains unanswered. Thankfully, a multinational National Institutes of Health consortium is currently developing a large, prospective study (https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-21-020.html) to answer this critical question.
Article Information
Acknowledgments. J.H.P. is an editor of Diabetes Care but was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Funding. The project described was partially supported by the National Institutes of Health grant UL1TR001442, National Institutes of Health T32 Training Grant in Endocrinology and Metabolism grant 2T32DK007044-41, and JDRF grant 2-SRA-2018-606-M-B. J.M.G. acknowledges additional support from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K23DK123392), Vanderbilt Diabetes Research and Training Center (DK020593), and a JDRF Career Development Award (5-ECR-2020-950-A-N).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or JDRF.
Duality of Interest. J.H.P. has served as an advisory board member or consultant for Sanofi, Novo Nordisk, Lilly, and MannKind Corporation and consultant for Carmot Therapeutics, Inc., and Diasome Pharmaceuticals. S.C.B has served as a consultant for Cecelia Health and provided research support to Dexcom, Inc., REMD Biotherapeutics, and Eli Lilly and Company. J.M.G. has served as an advisory board member for Eli Lilly, Medtronic, Dompe, vTv Therapeutics, Sanofi, and MannKind Corporation and in data and safety monitoring roles for vTv Therapeutics and Medtronic. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.L.T., J.M.G., and J.H.P. researched data, wrote the first draft of the manuscript, and contributed to the discussion. S.C.B., V.H., and E.R.G. researched data and reviewed and edited the manuscript. All authors approved the final version of the manuscript. R.L.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A preliminary abstract of this data was presented at the 83rd American Diabetes Society Scientific Sessions, San Diego, CA, 23–26 June 2023 (6).
Handling Editors. The journal editor responsible for overseeing the review of the manuscript was Mark A. Atkinson.
Funding Statement
The project described was partially supported by the National Institutes of Health grant UL1TR001442, National Institutes of Health T32 Training Grant in Endocrinology and Metabolism grant 2T32DK007044-41, and JDRF grant 2-SRA-2018-606-M-B. J.M.G. acknowledges additional support from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K23DK123392), Vanderbilt Diabetes Research and Training Center (DK020593), and a JDRF Career Development Award (5-ECR-2020-950-A-N).
Footnotes
J.M.G. and J.H.P. served as co-senior authors on this work.
References
- 1. Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–2227 [DOI] [PubMed] [Google Scholar]
- 2. International Hypoglycaemia Study Group . Minimizing hypoglycemia in diabetes. Diabetes Care 2015;38:1583–1591 [DOI] [PubMed] [Google Scholar]
- 3. Boeder SC, Gregory JM, Giovannetti ER, Pettus JH. SGLT2 inhibition increases fasting glucagon but does not restore the counterregulatory hormone response to hypoglycemia in participants with type 1 diabetes. Diabetes 2022;71:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolli GB, Tsalikian E, Haymond MW, Cryer PE, Gerich JE. Defective glucose counterregulation after subcutaneous insulin in noninsulin-dependent diabetes mellitus. Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions. J Clin Invest 1984;73:1532–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia–induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013;62:3570–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas RL, Gregory JM, Giovannetti ER, Boeder SC, Pettus J. 148-OR: CGM time below range predicts epinephrine response to hypoglycemia in patients with type 1 diabetes. Diabetes 2023;72(Supplement_1):148-OR [DOI] [PubMed] [Google Scholar]