Abstract
Human thymocytes are readily infected with human immunodeficiency virus type 1 (HIV-1) in vivo and in vitro. In this study, we found that the kinetics of replication and cytopathic effects of two molecular isolates, NL4-3 and JR-CSF, in postnatal thymocytes are best explained by the distribution of chemokine receptors used for viral entry. CXCR4 was expressed at high levels on most thymocytes, whereas CCR5 expression was restricted to only 0.1 to 2% of thymocytes. The difference in the amount of proviral DNA detected after infection of fresh thymocytes with NL4-3 or JR-CSF correlated with the levels of CXCR4 and CCR5 surface expression. Anti-CCR5 blocking studies showed that low levels of CCR5 were necessary and sufficient for JR-CSF entry in thymocytes. Interleukin-2 (IL-2), IL-4, and IL-7, cytokines normally present in the thymus, influenced the expression of CXCR4 and CCR5 on thymocytes and thus increased the infectivity and spread of both NL4-3 and JR-CSF in culture. NL4-3 was produced by both immature and mature thymocytes, whereas JR-CSF production was restricted to the mature CD1−/CD69+ population. Although CXCR4 and CCR5 distribution readily explained viral entry in mature CD69+ and immature CD69− cells, and correlated with proviral DNA distribution, we found that viral production was favored in CD69+ cells. Therefore, while expression of CD4 and appropriate coreceptors are essential determinants of viral entry, factors related to activation and stage-specific maturation contribute to HIV-1 replication in thymocyte subsets. These results have direct implications for HIV-1 pathogenesis in pediatric patients.
Human immunodeficiency virus (HIV) infection of the thymus leads to loss of thymocytes and eventual thymic atrophy (8, 29, 50, 53). While the role of the thymus in regeneration of the immune system of HIV-infected adults has not been established, the thymus is required for T-cell generation in children (18, 39). Therefore, HIV infection of thymocytes and thymic emigrants may have an impact on disease progression in children. We and others have previously shown that NL4-3, a molecularly cloned highly cytopathic CXCR4-tropic virus, as well as certain pediatric HIV type 1 (HIV-1) isolates, are able to replicate in immature and mature thymocyte subsets, while JR-CSF, a relatively noncytopathic CCR5-tropic isolate, and selected pediatric isolates have a more restricted tropism for mature thymocyte subsets (27, 33, 64, 71, 73a). In addition, interleukin-2 (IL-2), IL-4, and IL-7, cytokines implicated in thymic subset expansion and maturation, have distinct effects on HIV-1 replication (69, 70, 72, 73, 78). NL4-3 and some pediatric isolates from rapid disease progressors replicated faster in the presence of IL-4 plus IL-7 than in the presence of IL-2 plus IL-4. In contrast, JR-CSF and isolates obtained from pediatric patients with a slow disease progression replicated faster in the presence of IL-2 plus IL-4 (20, 71, 72, 73a).
Surface expression of CD4 and of specific chemokine coreceptors allows HIV-1 entry into cells (13, 15, 17, 21). HIV-1 primary isolates can use CXCR4, CCR5, both receptors (dualtropic isolates), or a number of other reported seven-transmembrane, G-protein-coupled chemokine receptors (3–5, 12, 14, 21, 34, 36, 60, 80). In adults, the critical role of CCR5 in transmission and disease progression has been suggested by genetic studies correlating resistance or delay of HIV-1 infection with the presence of CCR5 mutations that result in no or low expression of CCR5 (25, 37, 55, 61). In children, the role of CCR5 in transmission and disease progression has been assessed in a cross-sectional study of children born to mothers seropositive for HIV-1. Heterozygosity for CCR5Δ32 was not associated with transmission but was associated with a slower development of HIV-related disease in children (42). Consistent with reports of studies of HIV-1-infected adults (12), viral isolates obtained from children at early disease stages were CCR5 tropic, while those from later stages of disease used CXCR4 as a coreceptor (56). Early acquisition of CXCR4 usage by these viral isolates was associated with rapid disease progression (12, 56).
In the thymus, where CD4 is expressed on more than 95% of the cells, the distribution of HIV coreceptors would be expected to be an important determinant of tropism. Wide distribution of CXCR4 surface expression on fetal thymocytes has been recently reported (31), while expression of the coreceptors CCR5, CCR8, and STLR-33/GPR15 on total thymocytes has been reported at the mRNA level (36, 46, 51, 54, 68). Other chemokine receptors, such as CCR4 (49), not yet identified as HIV coreceptors, are also present in the thymus. Finally, three unique thymic orphan chemokines, macrophage-derived cytokine (MDC), thymus- and activation-regulated cytokine (TARC), and thymus-expressed cytokine (TECK), whose as yet unidentified receptors could potentially support HIV-1 entry have been detected (19, 24, 77).
After viral entry, the activation state of the target cell determine its ability to reverse transcribe, integrate, and support HIV replication (63, 65, 82). In peripheral blood mononuclear cells (PBMC), for example, full reverse transcription requires at least progression to the G1b phase of the cell cycle and therefore is dependent on the activation state of the cell (32, 82). Thymocytes are a heterogeneous population of cells in terms of differentiation and activation. In this study, we examined HIV replication in thymocyte subsets defined by the expression of surface molecules that are commonly used as markers of T-cell development: CD1, CD69, and CD45RA. The CD1 molecule is expressed at high levels in CD3−/low thymocytes and therefore identifies immature thymocytes (7). Downregulation of CD1 correlates with acquisition of functional maturation of thymocytes (52). During the process of positive selection, the activation marker CD69 is expressed on 10% of CD4+/CD8+ double-positive thymocytes and at high levels on mature single-positive CD4+ and CD8+ cells (67). However, CD69 expression is absent on thymocytes that emigrate from the thymus to the periphery (52, 76). By contrast, the CD45RA antigen, a marker of naive cells in the periphery, is expressed only on mature CD3+/high/CD1− thymocytes that are ready to leave the thymus (66).
We took advantage of the differential tropism of JR-CSF and NL4-3 for thymocyte subsets to study the distribution and the usage of CCR5 and CXCR4 as HIV coreceptors on freshly isolated postnatal thymocytes. The chemokine receptors CCR5 and CXCR4 have been reported as coreceptors for JR-CSF and NL4-3, respectively, in PBMC and transfected cell lines (17, 60, 80). We found that postnatal thymocytes expressed high levels of CXCR4 and low levels of CCR5. In postnatal thymocytes, CXCR4 was broadly distributed on immature and mature subsets, as previously reported for fetal thymocytes (31). Nevertheless, CCR5 expression on a low percentage of thymocytes is necessary and sufficient to support replication of a CCR5-tropic isolate. We also demonstrate that both CXCR4 and CCR5 support viral entry into CD69+ and CD69− cells, whereas only the CD69+ thymocyte subset sustained a highly productive infection. These results help explain the reported HIV-1-induced pathogenesis of the thymus by distinct HIV-1 tropic isolates.
MATERIALS AND METHODS
Reagents and monoclonal antibodies.
Recombinant human IL-2 (1.5 × 106 U/ml) and IL-4 (0.7 mg/ml) were provided by Amgen, Inc. (Thousand Oaks, Calif.). Recombinant human IL-7 (100 μg/ml) was a gift from Immunex Corp. (Seattle, Wash.). 7-Amino-actinomycin D (7-AAD) was obtained from Sigma (St. Louis, Mo.). Actinomycin D (AD) was obtained from Boehringer Mannheim (Indianapolis, Ind.). Normal mouse immunoglobulin G (IgG; 3 mg/ml) was obtained from Caltag (Burlingame, Calif.). Monoclonal antibodies to CD8, CD4, CD3, CD45RA, and CD69 conjugated with fluorescein (FITC), phycoerythrin (PE), or peridinin chlorophyll protein (PerCP) and goat anti-mouse IgG-FITC were obtained from Becton Dickinson Immunocytometry Systems (BDIS; San Jose, Calif.). The antibodies KC57-FITC and KC57-PE, which identify intracellular HIV p24gag antigen expression (10, 40), CD1-PE, CD45RA-PE, and the unconjugated antibodies CD45RA and CD69, used for thymocyte subset separations, were obtained from Coulter/Immunotech (Hialeah, Fla.). Unconjugated CXCR4 and CXCR4-PE (12G5) were obtained from Pharmingen (San Diego, Calif.). Unconjugated monoclonal antibodies to the chemokine receptors CCR-3 (7B11) (21, 23) and CCR-5 (2D7) (79) were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. CXCR-4 (12G5) was a gift from James Hoxie (16). The monoclonal antibody to CCR-5 (3A9) was a gift from LeukoSite, Inc. (80). CD4-IgG was a gift from Genentech (San Francisco, Calif.).
Freshly isolated, nonstimulated PBMC were used as the positive control for CCR5 detection. In the same PBMC adult donors, CCR5 was present in 14 to 20% of the lymphocytes stained with 2D7 but only 2 to 3% of cells stained with 3A9. However, staining with antibodies 3A9 and 2D7 gave similar results on monocytes from these donors and in freshly isolated postnatal thymocytes. To rule out the possibility that the epitopes recognized by these antibodies were not exposed on the surface of thymocytes, cells were permeabilized and stained intracellularly with antibody 2D7-PE (58). The intracellular level of CCR5 was below the detection level on thymocytes but was detectable in PBMC, although at lower levels than on the cell surface.
HIV infection and thymocyte cultures.
Normal pediatric thymuses were obtained in the course of corrective cardiac surgery. Single-cell suspensions and nylon wool purification were done as previously described, and thymocytes were cultured at 1 × 107 to 2 × 107 cells/ml in serum-free medium (albumin-transferrin-IMDM [Iscove’s modified Dulbecco’s medium]; Irvine Scientific, Santa Ana, Calif.) supplemented with delipidated bovine serum albumin (BSA; Sigma) at 1,100 μg/ml, transferrin (Sigma) at 85 μg/ml, glutamine at 2 mM (0.3 mg/ml), and penicillin-streptomycin at 25 U/ml–25 μg/ml (73, 78). Thymocytes were cultured in the presence or absence of the cytokines IL-2 (20 U/ml), IL-4 (20 ng/ml), and IL-7 (200 U/ml).
Two molecular clones of HIV-1, the non-syncytium-inducing, CCR5-tropic clone JR-CSF (33) and the syncytium-inducing, CXCR-4-tropic hybrid clone NL-4-3, were used for these studies (1). Virus stocks of JR-CSF were prepared from 24-h harvests of supernatants from PBMC infected with the supernatant of COS cells electroporated with plasmid pYKJR-CSF. Virus stocks of NL4-3 were prepared from 24-h harvests of supernatants from CEM cells (CCRF-CEM) infected with the supernatant of COS cells electroporated with plasmid pNL4-3. Virus stocks were stored at −70°C and treated with DNase (2 μg/ml; Worthington, Lakewood, N.J.) for 30 min at room temperature in the presence of 0.01 M MgCl2 before infections. Heat-inactivated controls were obtained by incubating DNase-treated viruses at 65°C for 45 min. All infections were standardized by determining infectious units (IU) in limiting dilution studies using phytohemagglutinin (PHA)-stimulated PBMC (81, 82). For thymocyte infections, JR-CSF was used at 10- to 20-fold higher multiplicity of infection (MOI) than NL4-3 unless otherwise indicated.
Thymocytes were infected and cultured as previously described (72). Briefly, virus infection was accomplished by incubating thymocytes with 30 to 200 ng of viral p24/107 cells in the presence of Polybrene (10 μg/ml; Sigma) for 1 to 2 h at 37°C. Control thymocytes were sham infected in the presence of Polybrene with supernatant from uninfected cells that were used for preparing the virus stocks. After infection, the cells were washed extensively in A-IMDM and resuspended in serum-free medium in the presence of cytokines. On day 1 postinfection and weekly thereafter, the supernatant was removed and the cells were fed with fresh medium and cytokines. Virus expression was assessed by measuring p24 antigen in the supernatant by enzyme-linked immunosorbent assay (Coulter, Hialeah, Fla.).
Blocking studies using antibodies to chemokine receptors.
Thymocytes were preincubated with antibodies to CCR5 (2D7; 1 to 5 μg/107 cells) and/or CXCR4 (12G5; 5 to 10 μg/107 cells) or CD4-IgG (100 μg/107 cells) at 4°C for 1 to 2 h before infection. The antibodies and CD4-IgG were present during infection and throughout the duration of the experiment. On day 1 and weekly thereafter, the medium was removed and fresh medium containing the antibody was added, while CD4-IgG was added on days 1 and 7 only.
Isolation of thymocyte subsets.
Magnetic beads were used to isolate thymocyte subsets. In initial experiments, magnetic beads coated with goat anti-mouse IgG-plus-IgM antibody (Kirkegaard & Perry, Gaithersburg, Md.) were used (73, 78). Since the Kirkegaard & Perry beads are no longer available, Dynal (Lake Success, N.Y.) M280 magnetic beads coated with sheep anti-mouse IgG were used in later experiments. Comparisons showed that subset purities were similar in assays using the beads from the two manufacturers. CD45RA- and CD69-positive and -negative subsets were obtained as follows. Magnetic beads were preincubated at 108 beads/ml in A-IMDM containing 1% BSA to prevent nonspecific binding to thymocytes and then coated with the CD45RA or CD69 monoclonal antibody (1.25 tests of the antibody as determined by the manufacturer/108 beads/ml) for at least 18 h at 4°C. Beads were washed once to remove excess unbound antibody immediately before use. For depletion of CD45RA+ cells, thymocytes were combined with CD45RA-coated beads at a bead-to-cell ratio of 1:2 and rotated at 4°C for 1 h. Cells bound to beads were removed with a magnet (Collaborative Research, Inc., Bedford, Mass.) and subjected to a second round of depletion. The CD45RA-depleted cells were then combined with the CD69-coated beads at a bead-to-cell ratio of 2:1 and rotated at 4°C for 1 h. The CD45RA-depleted cells bound to CD69-coated beads (CD69+ population) were magnetically removed, and unbound cells (CD69− population) were subjected to a second round of depletion. Following separation, the depleted subsets were immunophenotyped and analyzed by flow cytometry. Both positively and negatively immunoselected subsets were used for infection and culture experiments. Their viability, as determined by trypan blue dye exclusion, was >96%.
Immunofluorescent staining and flow cytometry.
Surface and cytoplasmic immunophenotyping of thymocytes with directly conjugated antibodies were done as previously described (57, 58). When unconjugated antibodies were used, cells were washed in phosphate-buffered saline (PBS) containing 1% BSA (PBS-BSA). After blocking with 50 μl of human AB serum to prevent nonspecific protein binding, thymocytes (1 × 105 to 5 × 105) were incubated with optimal amounts of unconjugated monoclonal antibody for 20 min at 4°C in a total volume of 100 μl and then washed with 3 ml of PBS-BSA. Goat anti-mouse IgG-FITC antibody was added for 20 min at 4°C in the presence of 50 μl of human AB serum. Cells were washed with 3 ml of PBS-BSA and incubated for 10 min at 4°C with 50 μl of mouse IgG (3 mg/ml) diluted 1:15 in PBS-BSA to prevent nonspecific protein binding before incubation with directly conjugated PE- or PerCP-labeled antibodies for 20 min at 4°C. To exclude dead cells, the thymocytes were incubated in a solution of 2 μg of 7-AAD per ml in PBS for 20 min at 4°C protected from the light. The cells were washed in PBS and incubated in 1% paraformaldehyde solution in PBS containing 4 μg of AD per ml (57, 59). The samples were subjected to flow cytometric analysis in the paraformaldehyde-AD solution.
A FACScan flow cytometer equipped with a standard filter setup (BDIS) was used in these experiments. A minimum of 10,000 events was acquired on each sample. Multiparameter data acquisition and analysis were performed with Cell Quest software (BDIS).
Quantitative DNA PCR.
At 16 to 20 h postinfection, 106 thymocytes were removed from the cultures, washed once in PBS, lysed in urea lysis buffer (4.7 M urea, 1.3% [wt/vol] sodium dodecyl sulfate, 0.23 M NaCl, 0.67 mM EDTA [pH 8.0], 6.7 mM Tris-HCl), and then subjected to multiple phenol-chloroform extractions and ethanol precipitation. Total nucleic acids obtained from thymocytes were subjected to quantitative DNA PCR as described previously (2, 81, 82). HIV DNA was detected by using the 32P-end-labeled M667-AA55 primer pair specific for the R/U5 region of the viral long terminal repeat (LTR) (81, 82). For detection of full-length reverse transcripts, the M667-M661 primer pair specific for the LTR/gag region was used (32, 82). Products obtained after 25 cycles of amplification were resolved on a 6% polyacrylamide gel. Standard curves for HIV-1 DNA were generated by using various dilutions of plasmid pYKJR-CSF linearized with EcoRI, which does not digest viral sequences. The dilutions were made into DNA from normal human PBMC (10 μg/ml). To normalize for cellular DNA, replicate samples were analyzed for human β-globin gene sequences (35, 81) by 25 cycles of amplification. Standard curves for human DNA were generated from two- and fivefold dilutions of PBMC DNA. Values were obtained by interpolation from the standard curves, using a radioanalytic imaging system (Ambis, San Diego, Calif.).
RESULTS
Cell surface expression of CCR5, CCR3, and CXCR4 on thymocytes from children.
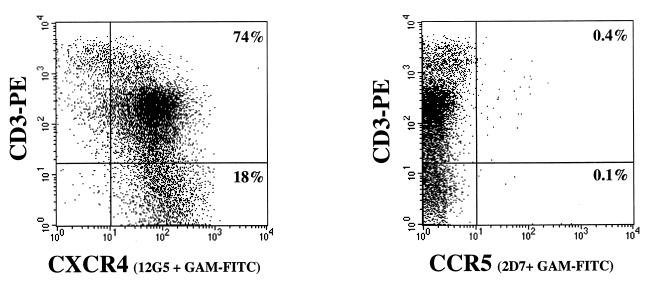
Postnatal thymus specimens obtained from 18 children (both sexes, 15 days to 4 years old) were used for these studies. Freshly isolated thymocytes were immunophenotyped with antibodies to CCR5 (2D7 and/or 3A9) and CXCR4 (12G5) to determine the thymic distribution of chemokine receptors that are reportedly the coreceptors for JR-CSF and NL4-3, respectively, in transfected CD4+ cells and PBMC (60, 80). In all specimens analyzed, more than 95% of postnatal thymocytes expressed CXCR4, while the percentages of CCR5+ cells ranged from 0.2 to 1% (mean ± standard deviation = 0.45% ± 0.22%). A representative experiment is shown in Fig. 1. The same coreceptor expression profile was found in thymocyte single-cell suspensions before and after nylon wool purification to enrich for T cells (data not shown). Figure 1 shows that high levels of CXCR4 expression were found in the immature CD3− and CD3+/low subsets, while the mature CD3+/high subset contained CXCR4+ and CXCR4− cells, as previously reported for fetal thymocytes (31). The determination of CCR5 expression on distinct thymocyte subsets by immunofluorescence methods was hampered by the low numbers of CCR5+ thymocytes. CCR3 surface expression was not detectable with antibody 7B11 in any of the eight thymocyte samples tested (not shown).
FIG. 1.
Distribution of chemokine receptor expression on freshly isolated human thymocytes. Thymocytes were isolated by nylon wool separation and phenotyped with CD3-PE and nonlabeled antibodies to the chemokine receptors CXCR-4 (antibody 12G5) and CCR5 (antibody 2D7), followed by goat anti-mouse IgG-FITC (GAM-FITC). Appropriate isotype control antibodies (IgG2a and IgG1) followed by goat anti-mouse IgG-FITC were used to set the cursors. The percentage of cells staining with the isotype control antibodies followed by goat anti-mouse IgG-FITC was 0%. Dead cells were excluded from the analysis by using 7-AAD.
Cytokines that favor HIV production by thymocytes upregulate CCR5 and CXCR4 surface expression.
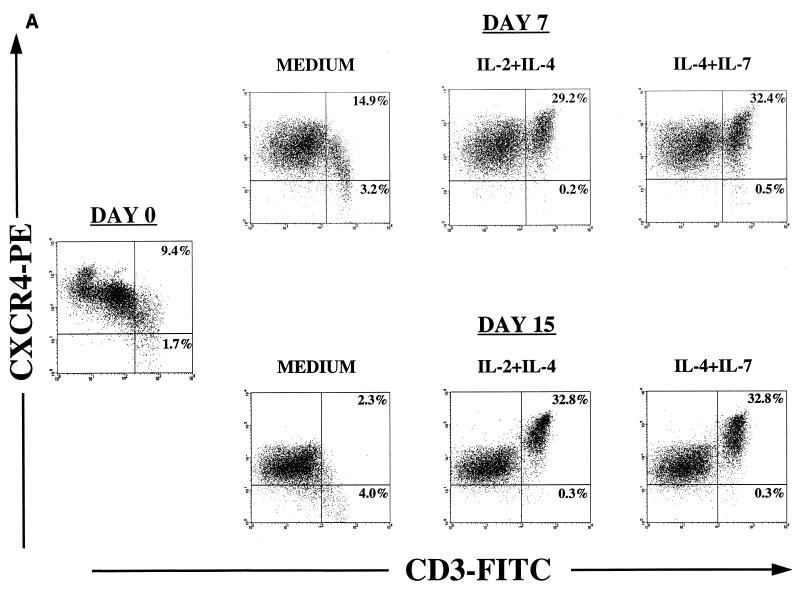
Expression of CCR5 and CXCR4 is tightly regulated on PBMC by stimulatory signals, mitogens, and cytokines such as IL-2 and IL-10 (6, 9, 38, 44, 45, 62, 80). We have previously shown that cytokines involved in thymocyte maturation distinctly regulate the expression of JR-CSF and NL4-3 in thymocyte subsets in vitro (72). To investigate the effect of these cytokines on chemokine receptor expression, cells were immunophenotyped at day 0 and cultured in serum-free medium in the presence of IL-2, IL-4, IL-7, IL-2 plus IL-4, or IL-4 plus IL-7 for 2 weeks. These cytokines affect proliferation and differentiation of different subpopulations, which results in different proportions of cells expressing high levels of CD3 (i.e., a CD3+/high population) (72, 73, 78). Cell surface phenotype was determined weekly, and the cursors were set in order to analyze coreceptor expression in the CD3+/high population (Fig. 2; Table 1).
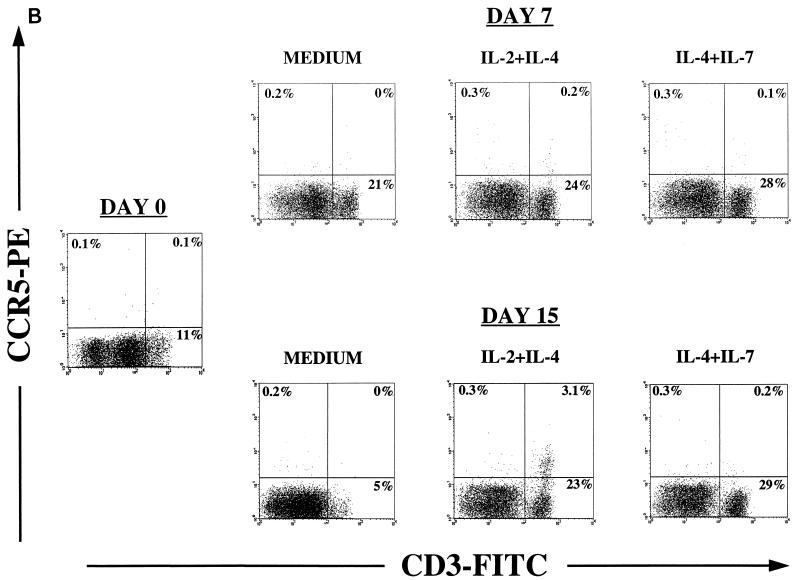
FIG. 2.
Effects of cytokines on chemokine receptor expression. Thymocytes were cultured for 2 weeks in serum-free medium alone or supplemented with IL-2 plus IL-4 or IL-4 plus IL-7. Before culture (day 0) and on days 7 and 15, cells were removed for immunophenotyping to examine expression of chemokine receptors by flow cytometry. Appropriate isotype control antibodies and single-color staining with CD3-FITC were used to set the cursors defining the CD3+/high population (A and B). Dead cells were excluded from the analysis by using 7-AAD. (A and B) CXCR4 and CCR5 expression on thymocyte subsets was determined by using the antibodies CD3-FITC, CXCR4-PE (12G5), and CCR5-PE (2D7). (C) In a different experiment, immunophenotyping was performed on day 12 of culture to identify the distribution of CCR5 on thymocyte subsets that respond to IL-2 plus IL-4. Thymocytes were stained with CD3-PE, CD4-PE, or CD1-PE in combination with nonlabeled antibody to CCR5 (2D7) and then with goat anti-mouse IgG-FITC (GAM-FITC). The percentage of cells staining with the IgG1 isotype control antibody for CCR5 followed by goat anti-mouse IgG-FITC was 0%.
TABLE 1.
Effects of cytokines on CXCR4 expression in thymocytesa
| Cytokine | Mean fluorescence intensity of CXCR4 on CD3+/high cells (% CD3+/high/CXCR4+ cells)b
|
|||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | |
| None | 47 (3) | 38 (2) | 37 (6) | 37 (5) |
| IL-2 | 60 (9) | 118 (21) | 139 (28) | ND |
| IL-4 | 444 (22) | 354 (24) | 359 (29) | 256 (15) |
| IL-7 | ND | 70 (25) | ND | 160 (24) |
| IL-2 + IL-4 | 431 (29) | 489 (33) | 361 (35) | ND |
| IL-4 + IL-7 | ND | 398 (33) | ND | 326 (17) |
Freshly isolated thymocytes were cultured for 2 weeks in serum-free medium in the presence or absence of cytokines. Surface expression of CXCR4 was determined by flow cytometry using directly conjugated antibodies specific for CD3 (FITC) and CXCR4 (PE), with 7-AAD used to exclude dead cells.
The geometric mean of the fluorescence intensity of CXCR4 in the CD3+/high population and the percentage of these cells in the gated population were calculated with the Cell Quest software. Cursors were set by using isotype controls for all cytokine conditions within an individual experiment. Single-color staining with CD3-FITC was used to identify the CD3+/high population. Note that the percentage of total CD3+/high cells depended on the cytokine used (Fig. 2A). ND, not determined.
Thymocytes cultured in the presence of IL-2 plus IL-4 or IL-4 plus IL-7 showed increased levels of CXCR4 expression as measured by fluorescence intensity and by the absence of CXCR4− cells (Fig. 2A). IL-4 alone was sufficient to increase the levels of CXCR4 expression (Table 1). Interestingly, IL-4 increased the expression of CXCR4 in the mature CD3+/high population that expresses low levels of CXCR4 in freshly isolated thymocytes. In thymocytes cultured with IL-2 alone, there was a threefold increase in the fluorescence intensity and in the percentage of CXCR4+/CD3+/high cells (Table 1), but the mature CD3+/high population that did not express CXCR4 was also expanded (data not shown). Thymocytes cultured with IL-7 showed this same profile (Table 1).
In contrast, the percentage of cells expressing CCR5 increased after 2 weeks of culture with IL-2 plus IL-4 but not in the presence of IL-4 plus IL-7 (Fig. 2B). In six of seven thymocyte culture experiments, IL-2 and IL-4 synergistically increased the percentages of CCR5-expressing cells from 0.2 to 1% on day 0 to 1 to 6% after 2 weeks of culture. No effect of IL-4 or IL-7 alone on CCR5 expression was seen, whereas upregulation of CCR5 expression in the CD3+/high by IL-2 alone was observed in only one of seven experiments. Further analysis of CCR5 distribution in thymocytes cultured in IL-2 plus IL-4 showed that CCR5 was expressed on the CD3+/high/CD4+/high/CD1− thymocyte subset, in which we have previously observed JR-CSF expression (Fig. 2C) (71).
NL4-3 and JR-CSF replication kinetics in thymocytes correlate with the expression levels of CXCR4 and CCR5.
The role of chemokine receptors in the different kinetics of replication of JR-CSF and NL4-3 in thymocytes was studied in vitro. Levels of CCR5 and CXCR4 expression were assessed before and after infection on freshly isolated thymocytes. Twenty-four hours after infection with JR-CSF (200 IU/104 cells) or NL4-3 (10 IU/104 cells), 106 cells were taken and analyzed by PCR for proviral DNA content as described previously (81). The level of proviral DNA in thymocytes infected with JR-CSF was significantly lower than the level of proviral DNA detected in thymocytes infected with NL4-3 (Fig. 3A), despite the 20-fold-higher MOI of JR-CSF, as determined in PHA-stimulated PBMC. This finding suggests that the difference observed between the replication kinetics of the two viruses is determined at the entry level. The copy number of NL4-3 proviral DNA in thymocytes (more than 50 copies/ng) correlated with the high numbers of cells expressing CD4 and CXCR4 in the thymus. The low level of JR-CSF proviral DNA in thymocytes (approximately 1 copy/ng) correlated with the low level of CCR5 surface expression (0.4%) on the specimen analyzed on day 0 (Fig. 1 and 3). Nevertheless, at 2 weeks postinfection p24 levels in the supernatant of JR-CSF infected cells reached 110 ng/ml. Notably, in all of eight infection experiments, thymus specimens containing at most 1% CCR5+ cells at the time of infection (day 0) were able to sustain JR-CSF production, as measured by p24 levels in the supernatant.
FIG. 3.
CXCR4 and CCR5 expression levels correlate with the amount of NL4-3 and JR-CSF proviral DNA after infection. Thymocytes were infected with JR-CSF (200 IU/104 cells) or NL-4-3 (10 IU/104 cells) and cultured with IL-2 plus IL-4. CCR5 expression on day 0 is shown in Fig. 1. (A) Twenty-four hours postinfection, 106 cells were removed and analyzed by using primers (R/U5) specific for the LTR region of HIV-1 to detect the presence of proviral DNA. To normalize for the amount of cellular DNA, PCR was performed in parallel for sequences in the β-globin gene. (B) JR-CSF-infected thymocytes were cultured with IL-2 plus IL-4. At 13 days postinfection, cells were subjected to surface staining with CCR5-PE/CD3-PerCP followed by intracellular staining with KC57-FITC.
To determine if JR-CSF was produced by the small subset of CCR5+ thymocytes, cell surface staining was combined with intracellular staining for HIV Gag proteins with the KC57 antibody (10, 40, 71). As observed for uninfected thymocytes, expression levels of both CXCR4 and CCR5 increased on mature thymocytes during culture of infected cells with IL-2 plus IL-4, although the percentage of CCR5+ positive cells detected remained below 10% (Fig. 3B). At 2 weeks after infection with JR-CSF, KC57+ cells were detected in both the CCR5+ and CCR5− populations (Fig. 3B). In subsequent experiments, at later time points, JR-CSF expression was detected only in the CCR5− population, in a manner reminiscent of the presence of HIV expression in the CD4− thymocyte subpopulation at late stages of infection (30).
Therefore, the slower replication of JR-CSF compared to that of NL4-3 in thymocytes correlated with lower levels of proviral DNA after infection. This observation could be attributed at least in part to the differences in the availability of cells expressing CD4 and the appropriate coreceptor, presumably CCR5 and CXCR4, at the time of infection.
Low levels of CCR5 support replication of JR-CSF in thymocytes.
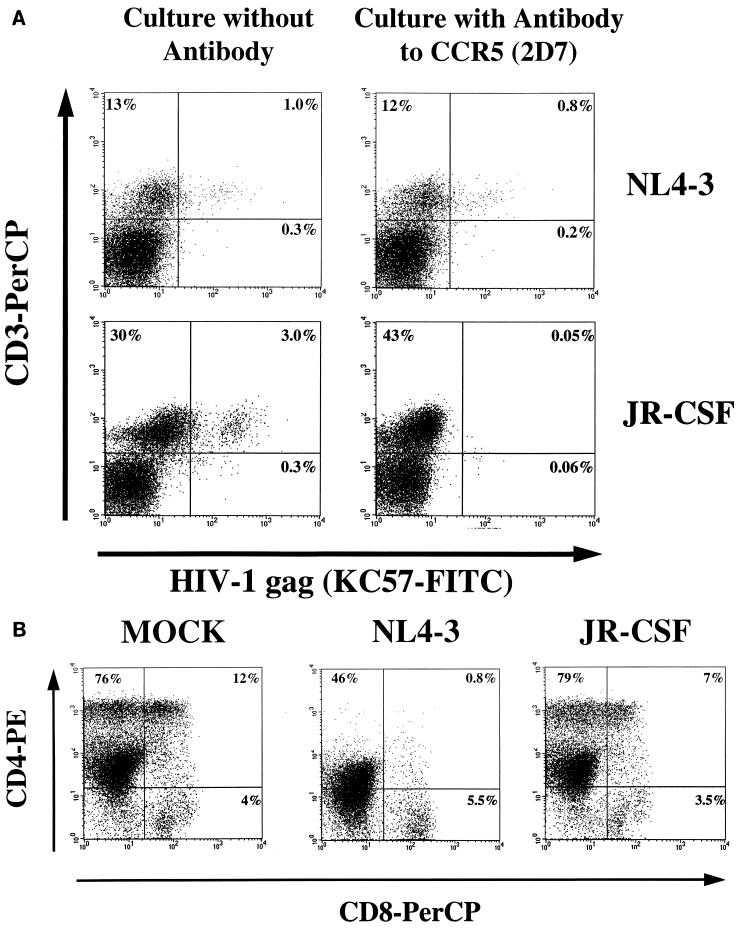
The presence of JR-CSF in the CCR5− population could indicate downregulation of CCR5 on JR-CSF-infected cells. However, JR-CSF adaptation to CXCR4 in culture and/or usage of an alternative coreceptor could not be excluded in the experiments described above. The antibody 2D7 was used to determine if JR-CSF replication in thymocytes could be prevented by blocking the coreceptor CCR5 (79). In all of four experiments, the p24 levels were reduced up to 50-fold in JR-CSF-infected cells cultured in the presence of 2D7, while in the presence of the 12G5 antibody to CXCR4 (16, 41) there was no reduction in p24 levels. As can be seen in Fig. 4, thymocytes infected with JR-CSF (30 IU/104 cells) produced high levels of p24 at 3 weeks postinfection. However, there was a delay in HIV expression in the presence of 1 μg of 2D7 per ml, while with 5 μg/ml the p24 levels were barely detectable up to 3 weeks postinfection. Pretreatment and culture of thymocytes in the presence of antibodies to both CXCR4 and CCR5 gave the same results as treatment with antibody to CCR5 alone, indicating poor, if any, usage of CXCR4 by JR-CSF in this system. Addition of 5 μg of 2D7 per ml 1 h after infection of thymocytes with JR-CSF decreased p24 peak levels to 16 ng/ml, compared to 354 ng/ml in thymocytes cultured in the absence of antibody.
FIG. 4.
The antibody (2D7) to CCR5 is able to block productive infection of thymocytes by JR-CSF. Thymocytes were preincubated in the presence or absence of antibody to CCR5 (2D7) or CXCR4 (12G5) for 2 h before infection with JR-CSF (30 IU/104 cells). The antibodies were present during the infection and throughout the culture with IL-2 plus IL-4. HIV replication was detected by measuring p24 antigen in the culture supernatants on days 8, 15, and 22 postinfection. Preincubation conditions: no antibody (gray bars), 1 μg of 2D7 (vertically striped bars), 5 μg of 2D7 (diagonally striped bars), 10 μg of 12G5 (horizontally striped bars), 1 μg of 2D7 plus 10 μg of 12G5 (checkered bars), 5 μg of 2D7 plus 10 μg of 12G5 (black bars), and 100 μg of CD4-IgG (white bars).
To determine if the effect of 2D7 on HIV replication on thymocytes was due to blocking of CCR5 coreceptor function and not to another effect of 2D7 on the cells which prevented them from producing virus, thymocytes were pretreated with 5 μg of 2D7 per ml and infected with either JR-CSF or NL4-3. Production of NL4-3 was not blocked by antibody to CCR5 but could be partially blocked by antibody to CXCR4 at 10 μg/ml (data not shown). The p24 data were confirmed by intracellular staining with the KC57 antibody 2 and 3 weeks after infection (Fig. 5). After infection with JR-CSF, KC57 expression was detected in 3% of the untreated thymocytes, as opposed to 0.05% of the cells in the presence of 5 μg of CCR5 antibody per ml. In thymocytes infected with NL4-3, percentages of KC57+ cells were similar in the nontreated thymocytes and in the thymocytes treated with antibody to 2D7 at 2 weeks postinfection (Fig. 5A). In addition, when 2D7 was present, a profound depletion of CD4+ thymocytes, a hallmark of NL4-3 infection, had already taken place (Fig. 5B). Cells infected with JR-CSF in the presence of 2D7 did not have a CD4/CD8 profile significantly different from that of mock infected cells cultured with 2D7 (Fig. 5B).
FIG. 5.
The antibody to CCR5 (2D7) specifically blocks expression of JR-CSF in thymocytes. Thymocytes were preincubated in the presence or absence of 5 μg of CCR5 antibody per ml for 2 h before infection with JR-CSF (30 IU/104 cells) or NL4-3 (1.5 IU/104 cells). The antibody was present during infection and throughout the culture with IL-2 plus IL-4. (A) At 2 weeks postinfection, cells were subjected to surface staining with CD3-PerCP followed by intracellular staining with KC57-FITC. (B) To determine the effect of CCR5 antibody on thymocytes, uninfected and infected cells were immunophenotyped with CD4-PE and CD8-PerCP and intracellularly stained with KC57-FITC 2 weeks postinfection. CD4-PE/CD8-PerCP expression is shown.
Taken together, these results suggest that JR-CSF uses CCR5 as a coreceptor in thymocytes. Furthermore, they indicate that very low levels of CCR5 surface expression can support replication of CCR5-tropic viruses in the thymus.
JR-CSF and NL4-3 production by different thymocyte subsets is determined at the postentry level.
We used the CD69 and CD45RA molecules as markers of thymocyte development to further characterize the thymocyte subsets susceptible to JR-CSF and NL4-3 productive infection. Thymocytes subsets at different stages of maturation were obtained by using antibody-coated magnetic beads to negatively and positively select specific subsets. As shown in Fig. 6A, thymocytes expressing CD69 are found in the mature CD3+/high subset, which includes CD4 and CD8 single-positive cells and 5 to 10% of the CD4+/CD8+ thymocytes as previously described (67, 76). Mature CD3+/high/CD45RA+ cells were removed before CD69 depletion to eliminate the most mature CD4 and CD8 single-positive thymocytes that are CD69− but express CD45RA (7, 52, 66, 76). In the experiment shown in Fig. 6, this procedure removed all but 0.8% of the CD69+ cells, which included single-positive CD4+ and CD8+ cells, but did not remove the cells expressing CCR5 or CXCR4 (Fig. 6A). In other experiments, CCR5 expression was very low or below the detection level in CD69− cells while present in low levels in the CD69+ population; therefore CCR5 expression could not be ascribed to specific thymocyte subsets defined by CD69 expression (data not shown). Given the low levels of CCR5 surface expression on thymocytes and the results obtained in the blocking experiments, we tried to determine which thymocyte subsets expressed CCR5 on the basis of their susceptibility to JR-CSF infection (see below).
FIG. 6.
Infection of total thymocytes and CD69+ and CD69− thymocyte subsets by JR-CSF and NL4-3. CD69+ and CD69− subsets were obtained by using antibody-coated immunomagnetic beads from the CD45RA− thymocytes and immunophenotyped to determine the purity of the isolation. The resulting CD45RA−/CD69+ cells bound to beads, and CD45RA−/CD69− cells were used for infection. (A) Immunophenotype of thymocytes before and after depletion of CD45RA- and CD69-expressing cells. (B) The total thymocyte population and the CD69+ and CD69− thymocyte subsets were infected with JR-CSF (100 IU/104 cells) or NL4-3 (10 IU/104 cells) and cultured for 2 weeks in the presence of IL-2 plus IL-4. Twenty-four hours postinfection 106 cells were removed and analyzed by using primers (R/U5) specific for the LTR region of HIV-1 to detect the presence of proviral DNA. To normalize for the amount of cellular DNA, PCR was performed in parallel for sequences in the β-globin gene. Heat-inactivated virus (−) were run in parallel with the live-virus-treated samples (+) as controls for DNA contamination from the inoculum. (C) PCR amplification of proviral DNA was performed on diluted DNA samples from the NL4-3-infected cells described above to detect the presence of fully reverse transcribed (RT) proviral DNA (LTR/gag) in parallel with the partially reverse transcribed (R/U5) proviral DNA. (D) HIV replication was detected by measuring p24 antigen in the culture supernatants of JR-CSF-infected CD69+ cells (vertically striped bars), JR-CSF-infected CD69− cells (white bars), NL4-3-infected CD69+ cells (black bars), and NL4-3-infected CD69− cells (gray bars) on days 1, 5, and 12 postinfection.
The total population and the immunoselected thymocyte subsets were infected with either JR-CSF (100 IU/104 cells) or NL4-3 (10 IU/104 cells), and virus production was monitored by measuring p24 levels in the culture supernatants for up to 3 weeks. Proviral DNA levels in the distinct thymocyte subsets were assessed by using PCR primers detecting partial and full-length reverse transcription. Figure 6B shows that the amount of proviral DNA in the total population infected by NL4-3 or JR-CSF correlated with the expression levels of the respective coreceptors as shown in Fig. 3A. JR-CSF and NL4-3 proviral DNA could be detected in both CD69− and CD69+ populations, suggesting that viral entry occurred in both subsets. However, NL4-3 copy number in each subset was at least 1,000-fold higher than JR-CSF copy number in the same subset. The copy number of NL4-3 DNA was slightly higher in the CD45RA−/CD69− cells than in the CD45RA−/CD69+ cells, while the low copy number of JR-CSF DNA did not permit a quantification of proviral levels in the different subsets. These results indirectly suggest that CCR5 was expressed in both populations, albeit at very low levels, confirming the phenotype determined by flow cytometry (Fig. 6A). The ability of the different subsets to complete reverse transcription after NL4-3 infection was assessed by amplifying the DNA samples with primers detecting the LTR/gag region (32, 81, 82) as shown in Fig. 6C. Full-length reverse transcripts were present in the total population and in both CD69+ and CD69− thymocyte subsets at relative levels (>50%) that indicate completion of reverse transcription in all subsets (Fig. 6C). Yet, in five of five experiments, the levels of p24 were higher in the supernatant of CD69+ cells than in the supernatant of CD69− cells after infection with JR-CSF or NL4-3 (Fig. 6D and data not shown). This difference in viral expression was observed in the presence of the appropriate coreceptors and of similar amounts of proviral DNA in both thymocyte subsets (Fig. 6A, B, and D).
These results suggest that postentry events determine the ability of HIV to preferentially replicate in the more mature CD69+ thymocyte subset. Furthermore, full reverse transcription and low levels of p24 expression were detected in CD69− cells infected with NL4-3, indicating that late events in the virus cycle are possibly involved in the differential tropism of HIV for different thymocyte subsets. The expression level of a given virus isolate (JR-CSF or NL4-3) in these different thymocyte subsets was not determined at the entry level, although the differences between expression of different virus isolates in a given subset (i.e., CD69-depleted cells) could be explained by availability of the respective coreceptors.
DISCUSSION
In this study, we have demonstrated that the distribution of CXCR4 and CCR5 on thymocytes is a major determinant for NL4-3 and JR-CSF tropism and determines the replication kinetics of these two isolates (71). The majority of freshly isolated postnatal thymocytes from uninfected children expressed moderate to high levels of CXCR4, in comparison to CCR5 expression, which was present at low levels on 0.1 to 2% of the thymocyte population. Although we have shown that expression of CXCR4 and CCR5 on thymocytes was necessary for viral entry, additional host factors were required for a highly productive infection in the CD69+ thymocyte subset. This was evident in studies demonstrating that both the CD69+ and CD69− cell populations allowed NL4-3 and JR-CSF entry, whereas only the CD69+ population was identified as highly susceptible to NL4-3 and JR-CSF productive infection.
CCR5 expression in fresh thymocytes, determined by both surface and intracellular staining, was detected on few cells. Underestimation of CCR5 expression could be occurring in our system due to downregulation of CCR5 in thymocytes by ligand occupation or virus binding. This is unlikely because low levels of CCR5 mRNA were also detected by reverse transcription-PCR (data not shown). In addition, Wu et al. reported that 2D7 recognizes the chemokine binding site and does not downregulate CCR5 expression (79). Furthermore, while low levels of CCR5 could be detected on thymocytes with 2D7, this antibody could block JR-CSF infection of thymocytes as previously reported for other cell types (60, 79, 80). JR-CSF usage of alternative coreceptors on thymocytes cannot be excluded by our studies (4, 54). However, an indirect effect of CCR5 blocking by 2D7 on such putative receptors affecting JR-CSF and not NL4-3 replication would be necessary to explain our data. For example, a link between mutations in CCR2 and the level of expression of CCR5 has been proposed (56). However, we favor the explanation that CCR5− cells expressing HIV originated as CCR5+ cells that have either internalized CCR5 due to virus binding or matured into CCR5− cells.
In the postnatal thymus, CXCR4 was present at high levels in immature CD1+/CD3+/low thymocytes and at lower levels in most but not all of the CD3+/high/CD69−/CD45RA+ thymocytes, cells that have the potential to leave the thymus (52, 76). Our results further suggest that there are fewer CCR5-expressing thymic emigrants than CXCR4-expressing thymic emigrants, which is consistent with reported studies demonstrating low numbers of CCR5-expressing cells in the cord blood (48). This finding is also in agreement with the fact that in adults, CXCR4 expression in circulating T cells is detected mainly in the naive CD26low/CD45RA+/CD45RO− population, while CCR5 is expressed mostly in the effector/memory CD26high/CD45RAlow/CD45R0+ population that has previously undergone activation (6, 80).
In PBMC, CXCR4 is upregulated within 72 h upon stimulation with PHA or anti-CD3, while increased CCR5 expression on stimulated T cells requires addition of IL-2 for 2 to 3 weeks (6, 80). These culture conditions form the basis of the slow/low versus rapid/high biological phenotype of CCR5 and CXCR4 tropic primary isolates in PBMC (5). In both PBMC and the SCID-hu mouse, the distribution of thymocyte coreceptors described in this study is a major determinant of the biological phenotype of NL4-3 and JR-CSF (27, 28, 64, 71, 74). The expression of CXCR4 on the immature CD3−/CD4+/low/CD8+ thymocytes may lead to a rapid productive infection and destruction of this actively proliferating cell population. We have found that cultures containing IL-4 increased the level of CXCR4 expression in the mature CD3+/high thymocyte subset, thereby increasing the number of NL4-3 targets. The high levels of CXCR4 expression in freshly isolated immature thymocytes, detected in all specimens analyzed, may be related to the presence of IL-4 in the subcortical area where immature thymocytes responding to IL-4 are found (22, 75). Consistent with this notion, Papiernik et al. reported that pathological abnormalities in fetuses aborted from HIV-1-seropositive women were present mainly in the cortex (47). Our observations further suggest that immature thymocyte subsets from children may be infected in vivo with CXCR4-tropic HIV isolates, as observed in the SCID-hu model (27). Confirmation of a similar effect of IL-4 on upregulation of CXCR4 expression in the periphery might signify that the proposed shift from a Th1 to Th2 pattern of cytokine synthesis could favor the propagation of CXCR4-tropic viruses in late stages of diseases (11). Furthermore, a Th2-like cytokine pattern has been observed in perinatally infected children progressing to AIDS (26).
Increased CCR5 expression in thymocytes was observed only in cultures containing IL-2 in combination with IL-4. As seen in stimulated PBMC, upregulation of CCR5 expression in thymocytes required the presence of IL-2 for at least 2 weeks (6, 80). The slower replication of JR-CSF in thymocytes was initially due to low availability of CCR5 and was reflected in the low levels of viral entry detected by PCR. The increase in JR-CSF production seen in IL-2 plus IL-4-supplemented cultures was presumably from upregulation of CCR5 on mature thymocytes and proliferation of these cells, thereby allowing viral spread. It is noteworthy that high levels of virus could be produced by very few infected cells, suggesting that a mature thymocyte population expressing CCR5 is highly permissive to JR-CSF replication.
We have found that both NL4-3 and JR-CSF replicate preferentially in the CD69+ thymocyte population. This population includes cells at various stages of maturation from the less mature CD1+/CD4+/CD8+ cells through the single-positive CD4+ or CD8+ populations (52, 76). Since JR-CSF is not produced in immature CD1+ cells (71), we conclude that the thymocyte subset producing high levels of JR-CSF is a mature subset that has downregulated CD1, but not yet CD69, and therefore is not ready to leave the thymus. In this CD1−/CD69+ subset, NL4-3 production is also highly favored, but the broad distribution of CXCR4 expression allows NL4-3 entry into the immature CD1+/CD69− populations, thereby accounting for the low level of NL4-3 production seen in the immature thymocyte subset. Detection of full-length proviral DNA in all populations confirms that while coreceptor expression is a major determinant of tropism, cellular factors expressed at specific stages of T-cell development affect postentry events and can determine HIV replication in the thymus. In this regard, it should be noted that in vivo, the CD69+ population consists of thymocytes that are activated during the process of positive selection (43, 76) and thus should be permissive for viral entry and replication.
We have previously proposed that pediatric isolates able to infect immature thymocytes might have a greater impact on disease progression (71). Here we show that a CXCR4-tropic isolate could produce this effect. We are now in the process of determining whether coreceptor use of isolates obtained from children with rapid and slow disease progression correlates with specific receptor use and subsequent loss of thymocytes. In this regard, the early acquisition of CXCR4 tropism in rapid progressors observed by Scarlatti et al. could be associated with CXCR4 targeting in the thymus (56).
It has been proposed that differences in the expression levels of CCR5 due to genetic factors can affect the rate of disease progression in adults and children, where heterozygosity for the CCR5Δ32 deletion substantially reduces disease progression (42, 61). It is clear that in our in vitro conditions, at a low MOI, the threshold of CCR5 expression required for replication in thymocytes is very low. Although it takes longer, CD4 depletion occurs in SCID-hu mice infected with JR-CSF (27). Since in our system the contribution of stromal elements (potentially CCR5 positive) could not be evaluated, we cannot determine the full contribution of CCR5 for HIV pathogenesis on the thymus. Stanley et al. have shown that JR-CSF causes a more pronounced disruption of stromal elements than a T-tropic virus (64). The usage of coreceptors other than CCR5 and CXCR4 by pediatric isolates in the thymus needs to be investigated.
In conclusion, our studies indicate that the ability of thymocyte subsets to support HIV productive infection is determined by the presence of the appropriate coreceptor and by cellular factors related to the state of maturation of the cells that affect postentry events in the virus replication cycle.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by grants from the National Institutes of Health (HD 29341, HD 29341-S1, AI 28697, and DK49886), by UARP SRF01, and by student awards to K.B.G. from the Elizabeth Glaser Pediatric AIDS Foundation and the UCLA AIDS Institute (Esther Hays Graduate Student Award).
We thank Hillel Laks and his colleagues and staff for providing the thymus specimens; Jerome Zack and Irvin Chen for use of biocontainment facilities; Esther Hays, Beth Jamieson, John Ferbas, and Deborah Anisman-Posner for helpful discussions and critical reviews of the manuscript; and Deborah Anisman-Posner, Silvia Neagos, Kris Conners, and Prista Charuworn for excellent technical assistance.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S Y, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Combardiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 4.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyo E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blue M, Levine H, Daley J F, Branton K R, Schlossman S F. Expression of CD1 and class I MHC antigens by human thymocytes. J Immunol. 1989;142:2714–2720. [PubMed] [Google Scholar]
- 8.Calabro M L, Zanotto C, Calderazzo F, Crivellaro C, Del Mistro A, De Rossi A, Chieco-Bianchi L. HIV-1 infection of the thymus: evidence for a cytopathic and thymotropic viral variant in vivo. AIDS Res Hum Retroviruses. 1995;11:11–19. doi: 10.1089/aid.1995.11.11. [DOI] [PubMed] [Google Scholar]
- 9.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein W, Weislow O S, Brown C R, Berger E A, June C H, St. Louis D C. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 10.Chassagne J, Verrelle P, Dionet C, Clavel F, Barre-Sinoussi F, Chermann J C, Montagnier L, Gluckmann J C, Klatzmann D. A monoclonal antibody against LAV gag precursor: use for viral protein analysis and antigenic expression in infected cells. J Immunol. 1986;136:1442–1445. [PubMed] [Google Scholar]
- 11.Clerici M, Shearer G M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 14.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR5. Nature. 1996;381:667–672. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis Turner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 18.Gaulton G N, Scobie J V, Rosenzweig M. HIV-1 and the thymus. AIDS. 1997;11:403–414. doi: 10.1097/00002030-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Godiska R, Chantry D, Raport C J, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray P W. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hays E F, Uittenbogaart C H, Vollger L W, Brewer J, Zack J A. In vitro studies of HIV-1 expression in thymocytes from infants and children. AIDS. 1992;6:265–272. doi: 10.1097/00002030-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 21.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodrovski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 22.He W, Zhang Y, Deng Y, Kabelitz D. Induction of TCR-γδ expression on triple-negative (CD3-CD4-CD8-) human thymocytes. J Immunol. 1995;154:3726–3731. [PubMed] [Google Scholar]
- 23.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath P D, Mackay C R. Chemokine receptor usage by eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Investig. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, Miura R, Opdenakker G, Van Damme J, Yoshie O, Nomiyama H. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1a/LD78a and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140–1149. [PubMed] [Google Scholar]
- 25.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 26.Hyjek E, Lischner H W, Hyslop T, Bartkowiak J, Kubin M, Trinchieri G, Kozbor D. Cytokine patterns during progression to AIDS in children with perinatal HIV infection. J Immunol. 1995;155:4060–4071. [PubMed] [Google Scholar]
- 27.Jamieson B D, Pang S, Aldrovandi G M, Zha J, Zack J A. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamieson B D, Uittenbogaart C H, Schmid I, Zack J A. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggests direct viral killing of thymocytes in vivo. J Virol. 1997;71:8245–8253. doi: 10.1128/jvi.71.11.8245-8253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi V V, Oleske J M, Saad S, Gadol C, Connor E, Bobila R, Minnefor A B. Thymus biopsy in children with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110:837–842. [PubMed] [Google Scholar]
- 30.Kitchen S G, Uittenbogaart C H, Zack J A. Mechanism of human immunodeficiency virus type 1 localization in CD4-negative thymocytes: differentiation from a CD4-positive precursor allows productive infection. J Virol. 1997;71:5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korin Y D, Zack J A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyanagi Y, Miles S, Mitsuyasu R T, Merill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 34.Kozak S L, Platt E J, Madani N, Ferro, Peden K, Kabat D. CD4, CXCR-4 and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawn R M, Efstratiadus A, O’Connell C, Maniatis T. The nucleotide sequence of the human β-globin gene. Cell. 1980;21:647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- 36.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 38.Loetscher P, Seitz M, Baggliolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackall C L, Fleisher T A, Brown M R, Andrich M P, Chen C C, Feuerstein I M, Horowitz M E, Magrath I T, Shad A T, Steinberg S M, Wexler L H, Gress R E. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 40.Martin S J, Matear P M, Vyakarnam A. HIV-1 infection of human CD4+ T cells in vitro. J Immunol. 1994;152:330–342. [PubMed] [Google Scholar]
- 41.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misrahi M, Teglas J, N’Go N, Burgard M, Mayaux M, Rouzioux C, Delfraissy J, Blanche S. CCR5 chemokine receptor variant in HIV-1 mother-to-child transmission and disease progression in children. JAMA. 1998;279:277–280. doi: 10.1001/jama.279.4.277. [DOI] [PubMed] [Google Scholar]
- 43.Moore N C, Girdlestone J, Anderson G, Owen J J T, Jenkinson E J. Stimulation of thymocytes before and after positive selection results in the induction of different NF-κB/Rel protein complexes. J Immunol. 1995;155:4653–4660. [PubMed] [Google Scholar]
- 44.Moriuchi H, Moriuchi M, Fauci A S. Cloning and analysis of the promoter region of CCR5, a coreceptor for HIV-1 entry. J Immunol. 1997;159:5441–5449. [PubMed] [Google Scholar]
- 45.Moriuchi M, Moriuchi H, Turner W, Fauci A S. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunology. 1997;159:4322–4329. [PubMed] [Google Scholar]
- 46.Napolitano M, Zingoni A, Bernardini G, Spinetti G, Nista A, Starlazzi C T, Rocchi M, Santoni A. Molecular cloning of TER-1, a chemokine receptor-like gene expressed by lymphoid tissues. J Immunol. 1996;157:2759–2763. [PubMed] [Google Scholar]
- 47.Papiernik M, Brossard Y, Milliez N, Roume J, Brechot C, Barin F, Goudeau A, Bach J-F, Griscelli C, Henrion R, Vazeux R. Thymic abnormalities in fetuses aborted from human immunodeficiency virus type 1 seropositive women. Pediatrics. 1992;89:297–301. [PubMed] [Google Scholar]
- 48.Peng-Yang L, Riley J L, Carroll R G, June C H, Hoxie J, Patterson B K, Ohshima Y, Hodes R J, Delespesse G. Productive infection of neonatal CD8+ T lymphocytes by HIV-1. J Exp Med. 1998;187:1139–1144. doi: 10.1084/jem.187.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power C A, Meyer A, Nemeth K, Bacon K B, Hoogewerf A J, Proudfoot A E, Wells T N C. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 50.Prevot S, Audouin J, Andre-Bougaran J, Griffais R, Le Tourneau A, Fournier J G, Diebold J. Thymic pseudotumorous enlargement due to follicular hyperplasia in a human immunodeficiency virus sero-positive patient. Am J Clin Pathol. 1992;97:420–425. doi: 10.1093/ajcp/97.3.420. [DOI] [PubMed] [Google Scholar]
- 51.Raport C J, Goslings J, Schweickart V L, Gray P W, Charo I F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β and MIP-1α. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 52.Res P, Blom B, Hori T, Weijer K, Spits H. Downregulation of CD1 marks acquisition of functional maturation of human thymocytes and defines a control point in late stages of human T cell development. J Exp Med. 1997;185:141–151. doi: 10.1084/jem.185.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 56.Scarlatti G, Tresoldi E, Bjorndal A, Frederiksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 57.Schmid I, Krall W J, Uittenbogaart C H, Braun J, Giorgi J V. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 58.Schmid I, Uittenbogaart C H, Giorgi J V. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12:279–285. doi: 10.1002/cyto.990120312. [DOI] [PubMed] [Google Scholar]
- 59.Schmid I, Uittenbogaart C H, Keld B, Giorgi J V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 60.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith M W, Dean M, Carrington M, Winkler C, Huttley G A, Lomb D A, Goedert J J, O’Brien T R, Jacobson L P, Kaslow R, Buchbinder S, Vittinghoff E, Vlahov D, Hoots K, Hilgartner M W. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 62.Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells T N C, Biswas P, Vicennzi E, Poli G, Mantovani A. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spina C A, Guatelli J C, Richman D D. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tedder T F, Clement L C, Cooper M D. Human lymphocyte differentiation antigens HB-10 and HB-11. I. Ontogeny of antigen expression. J Immunol. 1985;134:2983–2988. [PubMed] [Google Scholar]
- 67.Testi R, Phillips J H, Lanier L L. Constitutive expression of a phosphorylated activation antigen (Leu 23) by CD3bright human thymocytes. J Immunol. 1988;141:2557–2563. [PubMed] [Google Scholar]
- 68.Tiffany H L, Lautens L L, Gao J, Pease J, Locati M, Combadiere C, Modi W, Bonner T I, Murphy P M. Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J Exp Med. 1997;186:165–170. doi: 10.1084/jem.186.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueno Y, Boone T, Uittenbogaart C H. Selective stimulation of human thymocyte subpopulations by recombinant IL-4 and IL-3. Cell Immunol. 1989;118:382–393. doi: 10.1016/0008-8749(89)90386-9. [DOI] [PubMed] [Google Scholar]
- 70.Ueno Y, Hays E, Hultin L, Uittenbogaart C H. Human thymocytes do not respond to interleukin-2 after removal of mature “bright” CD5 positive cells. Cell Immunol. 1989;124:239–251. doi: 10.1016/0008-8749(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 71.Uittenbogaart C H, Anisman D J, Jamieson B D, Kitchen S, Schmid I, Zack J A, Hays E F. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS. 1996;10:F9–F16. doi: 10.1097/00002030-199606001-00001. [DOI] [PubMed] [Google Scholar]
- 72.Uittenbogaart C H, Anisman D J, Zack J A, Economides A, Schmid I, Hays E F. Effects of cytokines on HIV-1 production by thymocytes. Thymus. 1995;23:155–175. [PubMed] [Google Scholar]
- 73.Uittenbogaart C H, Higashitani S, Schmid I, Vollger L W, Boone T, Clement L T. Interleukin-4 induces expression of the CD45RA antigen on human thymocyte subpopulations. Int Immunol. 1990;2:1179–1187. doi: 10.1093/intimm/2.12.1179. [DOI] [PubMed] [Google Scholar]
- 73a.Uittenbogaart, C. H., et al. Unpublished data.
- 74.Valentin H, Nugeyre M-T, Vuillier F, Boumsell L, Schmid M, Barre-Sinoussi F, Pereira R A. Two subpopulations of human triple-negative thymic cells are susceptible to infection by human immunodeficiency virus type 1 in vitro. J Virol. 1994;68:3041–3050. doi: 10.1128/jvi.68.5.3041-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandekerckhove B A E, Barcena A, Schols D, Mohan-Peterson S, Spits H, Roncarolo M. In vivo cytokine expression in the thymus. J Immunol. 1994;152:1738–1743. [PubMed] [Google Scholar]
- 76.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–1872. [PubMed] [Google Scholar]
- 77.Vicari A P, Figueroa D J, Hedrick J A, Foster J S, Singh K P, Menon S, Copeland N G, Gilbert D J, Jenkins N A, Bacon K B, Zlotnik A. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 78.Vollger L W, Uittenbogaart C H. Interleukin-7 promotes the generation of phenotypically mature CD45RA positive human thymocytes in-vitro. Cytokine. 1993;5:157–168. doi: 10.1016/1043-4666(93)90055-a. [DOI] [PubMed] [Google Scholar]
- 79.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen N, Humblias J, Samson M, Parmentier M, Moore J P, Mackay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodrovski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zack J A, Arrigo S J, Weitman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 82.Zack J A, Haislip A M, Krogstad P, Chen I S Y. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]