Abstract
Male sex is a risk factor for colorectal cancer (CRC) with higher illness burden and earlier onset. Thus, we hypothesized that loss of chromosome Y (LOY) in the tumor micro-environment (TME) might be involved in oncogenesis. Previous studies show that LOY in circulating leukocytes of aging men was associated with shorter survival and non-hematological cancer, as well as higher LOY in CD4 + T-lymphocytes in men with prostate cancer vs. controls. However, nothing is known about LOY in leukocytes infiltrating TME and we address this aspect here. We studied frequency and functional effects of LOY in blood, TME and non-tumorous tissue. Regulatory T-lymphocytes (Tregs) in TME had the highest frequency of LOY (22%) in comparison to CD4 + T-lymphocytes and cytotoxic CD8 + T-lymphocytes. LOY score using scRNA-seq was also linked to higher expression of PDCD1, TIGIT and IKZF2 in Tregs. PDCD1 and TIGIT encode immune checkpoint receptors involved in the regulation of Tregs function. Our study sets the direction for further functional research regarding a probable role of LOY in intensifying features related to the suppressive phenotype of Tregs in TME and consequently a possible influence on immunotherapy response in CRC patients.
Subject terms: Tumour immunology, Cancer, Computational biology and bioinformatics, Genetics, Immunology
Introduction
Colorectal cancer (CRC) is one of the leading contributors to cancer-related morbidity and mortality worldwide1. The liver is a main site of metastases from primary tumors of CRCs, likely due to direct blood drainage through portal system. Consequently, about 50% of CRC patients develop synchronous or metachronous liver metastases (LM_CRC)2, which frequently leads to the death of the patient3. Observations of CRC cohorts conducted for over the past 25 years indicate that CRC shows pronounced sexual dimorphism4. Male sex is a risk factor for CRC, with higher illness burden, and earlier onset and metastatic disease, of which ~ 30% are synchronous with CRC diagnosis4. Mutations in numerous genes have been associated with CRC risk, development of recurrence and metastatic disease5. However, there is limited evidence that could explain the higher risk and worse prognosis of men with CRC compared to women, and this aspect attracted our attention because of possible role of the mosaic loss of chromosome Y (LOY) in the disease process6.
LOY is the most frequent human post-zygotic mutation and is most prevalent in the hematopoietic system of aging men. It is detectable in whole blood DNA from > 40% of men above the age of 70 years7, reaching 57% in the analysis of 93-year-old men8. Recent single-cell transcriptomic analyses of leukocytes from 29 aging men (median age 80 years) identified cells with LOY in all studied subjects9. LOY has also been observed in other tissues, but with lower frequencies8,10. The most recent example of LOY analysis in another organ than the hematopoietic system was performed in human brains11. Serial analysis of men showed that LOY is a dynamic process12,13. LOY presumably causes a clonal expansion of affected cells and may coexist with so called CHIP mutations14. Moreover, LOY affects different lineages of hematopoietic cells with varying frequencies and it plays a role in transcriptional dysregulation of autosomal genes in a pleiotropic, cell type specific manner9. Furthermore, dysregulation of immune genes was pronounced in cells with LOY, particularly related to immune checkpoint (IC) genes9,15,16. Major risk factors for LOY include age, smoking and germline predisposition7,8,17,18. LOY has been associated with increased risk for all-cause mortality and many common age-related diseases, predominantly chronic, but also acute, inside and outside of the hematopoietic system. Notably, LOY likely has an impact on susceptibility to cancer6,7, Alzheimer’s disease (AD)17, cardiovascular diseases19, and severity of COVID-1913. Previous studies suggested that LOY disrupts proper functions of the immune system, especially the immune-surveillance mechanisms in AD and cancer9,16. Moreover, patients with prostate cancer showed higher levels of LOY in granulocytes and CD4 + T lymphocytes, suggesting that these cells, when affected by LOY, might convey an increased risk of cancer, and this finding motivated the current study9.
Since 2014, LOY has been investigated in the blood of aging men, but essentially nothing is known about the status of this very common mutation in leukocytes that infiltrate the tumor micro-environment (TME). TME is a complex milieu that includes different types of immune cells, including tumor infiltrating lymphocytes (TILs) and myeloid cells, but also connective tissue cells, like fibroblasts, blood vessels and tumor cells. TME can be divided into two main subtypes: (i) ‘infiltrated-excluded’, known as poorly immunogenic, with CD8 + cytotoxic T lymphocytes (CTLs) located mainly along the tumor margin; and (ii) ‘infiltrated-inflamed’, with highly activated CTLs, expressing PD-1 IC receptor20. As reported previously for CRC patients, the TME is predominantly infiltrated with circulating regulatory T cells (Tregs)21,22 that inhibit the activity of CD4 + T helper cells (Th) and CTLs23,24. It was observed that increased number of Tregs in TME may show a dual outcome for CRC patients, either with improved25 or worsened26 prognosis. This could be related to differences in the expression level of IC receptors on TILs27, which under normal conditions, induce the inhibitory signals for effector T cells. Therefore, by blocking ICs with monoclonal antibodies, the antitumor immune response of T cells can be enhanced28,29. Unfortunately, not all patients benefit equally from the treatment30. As proposed by Kumagaj et al., 2020, the successful therapy using IC inhibitors with anti-PD-1 antibodies can be predicted by the balance between the PD-1 expression levels on CTLs and Tregs in TME31. In this context, revealing new factors influencing the normal function of Tregs and consequently treatment response, could improve the effectiveness of the targeted immunotherapy.
The current study addresses the gap in knowledge about the LOY status of immune cells that infiltrate TME. We show that leukocytes with LOY can be found in the primary tumors and non-tumorous uninvolved margin tissue (UM) of CRC patients as well as LM_CRCs. We performed an integrative computational and experimental study showing that the lymphocyte subset that is the most affected by LOY in tumor, UM as well as in blood are Tregs. Our meta-analysis of two single-cell RNA sequencing (scRNA-seq) datasets focused on the analysis of infiltrating leukocytes in TME. Since LOY causes dysregulation of immune genes9,13,15,16, we hypothesized that it may also affect expression level of markers in TILs related to the efficacy of immunotherapy treatment.
Results
Study design for CRC patients
Aiming to establish the presence and functional effects in LOY on leukocytes that infiltrate solid cancer tissue, we performed computational and experimental analysis of multiple tissues: primary or metastatic tumor, non-tumorous uninvolved margin tissue (UM) surrounding tumor in colon or liver as well as blood (Fig. 1). We re-analyzed two publicly available scRNA-seq datasets: (i) leukocytes obtained from different tissues of CRC patients32; and (ii) tumor-infiltrating immune cell atlas derived from various types of cancer33. We applied to both datasets a previously developed method for LOY-scoring based on the lack of expression of genes located in the male-specific region of chromosome Y (MSY), as the LOY-signature in a single cell9,11,13,34. Using these datasets, we also investigated the transcriptomic profile of selected genes in LOY vs. non-LOY-cells in TME.
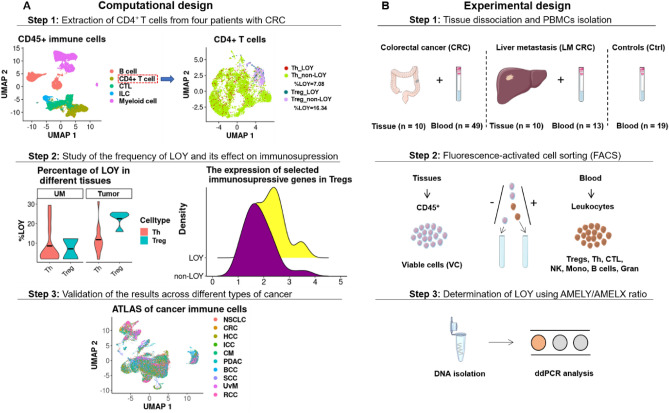
Figure 1.
Schematic workflow of computational (Panel A) and experimental (Panel B) design applied in our study. (A) Computational analysis of two scRNA-seq datasets; Zhang et al.32 and Nieto et al.33. In the first step, CD4 + T cells from the tumor microenvironment (TME), uninvolved margin (UM) and blood samples obtained from four CRC patients32 were re-clustered and categorized into Th and Tregs. Next, LOY-cells were defined based on the lack of expression of MSY-specific genes. Subsequently, the status of selected immunosuppressive genes was investigated in LOY and non-LOY Tregs located in the UM and tumor sites. The results obtained from the Zhang et al.32 dataset and comprising the scRNA-seq were validated in the Nieto et al.33 dataset, the latter covering an atlas of CD45 + immune cells from eight types of cancer, including CRC. (B) Experimental design for analysis of cells obtained from TME of human CRCs (n = 10) and LM_CRCs (n = 10) tissues and blood samples collected from CRC (n = 49), LM_CRC (n = 13) and Ctrl (n = 19) patients showed in three consecutive steps. Suspensions of single cells were sorted by FACS, followed by DNA isolation and ddPCR analysis using TaqMan-probes. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/). TME tumor microenvironment, UM non-tumorous uninvolved margin tissue, CRC colorectal cancer, LM_CRC liver metastasis of CRC, Ctrl control patients, Tregs regulatory T cells, Th helper T cells, CTL CD8 + cytotoxic T cells, ddPCR droplet digital PCR, scRNA-seq single-cell RNA-seq.
Moreover, we extended the analyses by performing tissue dissociations on tumors and UM fragments from two independent sets of 10 primary CRC- and 10 LM_CRC patients. Additionally, we implemented a protocol, which enabled the isolation of CD45 + immune cells obtained from both tumors and UMs as well as sorting of seven subpopulations of leukocytes from blood (Fig. 1). The analysis of LOY status was performed using previously described droplet-digital PCR (ddPCR) method12,13. Blood samples for sorting experiments were collected from 49 CRCs, 13 LM_CRCs and 19 control patients. All study subjects were qualified for our research according to established inclusion criteria (Materials and Methods, Table 1, Table S1). The variable level of leukocytes was confirmed in dedicated histopathological analysis of the hematoxylin/eosin-stained sections from tumor and UM (Table 1). Summary of clinical characteristics for all cancer patients and controls can be found in Table S1.
Table 1.
Summary of clinical data and experimental results from 10 CRC and 10 LM_CRC patients qualified for the dissociation experiments.
| Patient ID | Diagnosis | Age | Smoking | ICD10 | %Immune Infiltrates [Histpat H/E] | Blood [ddPCR %LOY] | UM [ddPCR %LOY] | Tumor [ddPCR %LOY] |
|---|---|---|---|---|---|---|---|---|
| 1 | CRC | 42 | Yes | C20 | 10 | 0 | 0 | 0 |
| 2 | CRC | 48 | No | C18.2 | 5 | 0 | 0 | 0 |
| 3 | CRC | 49 | No | C18.3 | 10 | 0 | 0 | 0 |
| 4 | CRC | 59 | Yes | C18.7 | 5 | 8 | 0 | 0 |
| 5 | CRC | 63 | Yes | C18.7 | 1 | 11 | 7 | 6 |
| 6 | CRC | 71 | Yes | C20 | 3 | 10 | 11 | 11 |
| 7 | CRC | 71 | Yes | C18.0 | 10 | 13 | 0 | 11 |
| 8 | CRC | 72 | Yes | C18.9 | 10 | 6 | 6 | 16 |
| 9 | CRC | 73 | Yes | C18.2 | 3 | 10 | 0 | 0 |
| 10 | CRC | 74 | Yes | C20 | 5 | 11 | 15 | 13 |
| 11 | LM CRC | 42 | Yes | C78.7 | 5 | 0 | 0 | 0 |
| 12 | LM CRC | 43 | No | C78.7 | 10 | 6 | 0 | 8 |
| 13 | LM CRC | 62 | Yes | C78.7 | 3 | 0 | 0 | 0 |
| 14 | LM CRC | 67 | Yes | C78.7 | 10 | 11 | 14 | 17 |
| 15 | LM CRC | 67 | Yes | C78.7 | 15 | 8 | 0 | 0 |
| 16 | LM CRC | 70 | Yes | C78.7 | 15 | 9 | 0 | 0 |
| 17 | LM CRC | 71 | No | C78.7 | 0 | 10 | 10 | 0 |
| 18 | LM CRC | 73 | No | C78.7 | 5 | 9 | 0 | 30 |
| 19 | LM CRC | 73 | No | C78.7 | 5 | 0 | 0 | 0 |
| 20 | LM CRC | 78 | Yes | C78.7 | 20 | 44 | 45 | 23 |
ICD10 international statistical classification of diseases and related health problems. Immune infiltrates were assessed by the pathologist during histopathological analysis in the hematoxylin/eosin-stained sections from tumor and surrounding normal tissue. UM uninvolved margin of normal tissues surrounding tumor. Diagnoses: CRC colorectal cancer, LM_CRC liver metastasis of CRC.
Tregs showed the highest percentage of LOY-cells among CD4 + T cells in primary CRCs
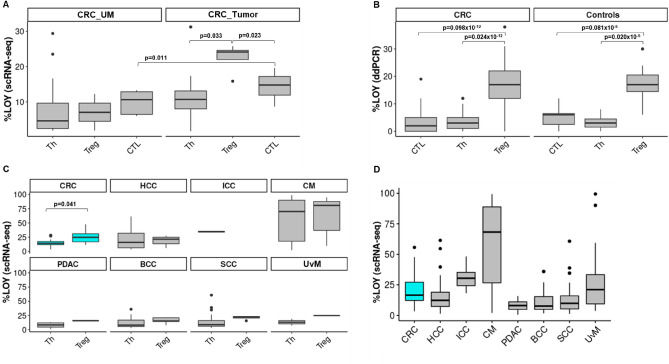
As previously reported, higher levels of LOY were found in the population of CD4 + T lymphocytes (CD4 + T cells) among all leukocytes from men with prostate cancer, versus controls9. Therefore, we performed the analysis of scRNA-seq from Zhang-dataset32, see “Materials and methods”) to determine the distribution of LOY-cells in the three most common subpopulations of leukocytes infiltrating the tumors, namely Tregs, Th and CTLs. We focused on four male patients from the Zhang-dataset32 and two groups of previously annotated cells, i.e. CD4 + T cells and CTLs. CD4 + T cells were reclustered and then categorized as Th and Tregs. Tregs were distinguished from the group of Th cells based on the expression of CTLA4 and IL23R markers (Table S2). All analyzed groups of cells were further divided into two groups based on the lack of expression of six genes located in the MSY and indicating LOY status (see Materials and Methods). As detailed in Table S2, Tregs were represented by a pooled dataset of 498 cells collected from four patients, whereas Th and CTLs were represented by 1460 cells from four CRC patients and 681 cells from three patients, respectively. Interestingly, we found that Tregs in the tumors had a higher level of LOY-cells, in comparison to Th, with the mean level of LOY of 22% for Tregs and 11% for Th cells (p = 0.033, Wilcoxon test with Bonferroni correction). In the case of CTLs, the mean LOY value was 14% and the difference between Tregs and CTLs showed similar statistical significance (p = 0.023, Fig. 2).
Figure 2.
Experimental and computational analysis of distribution of LOY in subpopulations of T lymphocytes. (A) Meta-analysis of the distribution of LOY in Regulatory T lymphocytes (Tregs) (n = 2, cells = 203), Helper T lymphocytes (Th) (n = 4, cells = 1818) and CD8 + cytotoxic T cells (CTL) (n = 3, cells = 1441) lymphocytes located in the uninvolved margin (UM) and Tregs (n = 4, cells = 498), Th (n = 4, cells = 1460) and CTL (n = 3, cells = 681) located in tumor of male patients with CRC. Only significant p-values are shown and n - number of patients. (B) Experimental analysis of the distribution of LOY in Tregs, Th and CTL in blood samples collected from 49 CRC patients and 19 healthy controls. CRC – colorectal cancer, Ctrl – age-matched control patients without prior diagnoses of cancer and AD. Only significant p-values are shown. (C) Meta-analysis of distribution of LOY in Tregs and Th in eight different cancer types: CRC colorectal cancer (n = 10, cells = 3349); HCC hepatocellular carcinoma (n = 9, cells = 8329); ICC intrahepatic cholangiocarcinoma (n = 1, cells = 222); CM cutaneous melanoma (n = 24, cells = 13,306); PDAC pancreatic ductal adenocarcinoma (n = 4, cells = 845); BCC basal cell carcinoma (n = 8, cells = 14,053); SCC squamous-cell carcinomas (n = 8, cells = 13,889); UvM uveal melanoma (n = 1, cells = 248). (D) Meta-analysis of distribution of %LOY in CD45 + immune cells located in TME of different cancers. CRC colorectal cancer (n = 10, cells = 11,165), HCC hepatocellular carcinoma (n = 16, cells = 21,604), ICC intrahepatic cholangiocarcinoma (n = 5, cells = 1497), CM cutaneous melanoma (n = 33, cells = 41,087), PDAC pancreatic ductal adenocarcinoma (n = 11, cells = 4749), BCC basal cell carcinoma (n = 8, cells = 35,359), SCC squamous-cell carcinomas (n = 4, cells = 25,924), UvM uveal melanoma (n = 4, cells = 5018).
Similarly, we studied the UMs of CRC patients. We did not observe similar differences in the distribution of LOY in Tregs, Th or CTLs in UMs, as in the tumors (Figs. 2 and 3). In general, we found a low level of LOY in all analyzed cell types of the UMs, where Tregs were represented by a pooled dataset of 203 cells. However, this result is based on only two patients. Th- and CTL-cells from UMs were represented by 1818 pooled cells from four CRC patients and 1441 pooled cells from three patients, respectively.
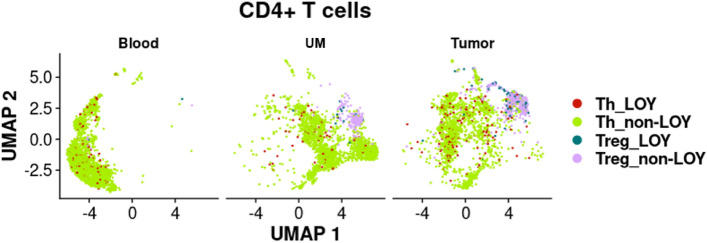
Figure 3.
Computational analysis of distribution of LOY CD4 + T lymphocytes in different tissues of CRC patients. CD4 + T cells were extracted from the tumor, uninvolved margin (UM) and blood samples obtained from four CRC patients (Zhang et al.32) and categorized into Th and Tregs. Next, LOY-cells were defined based on the lack of expression of MSY-specific genes.
Tissue dissociations confirm the presence of LOY among CD45 + immune cells from primary tumors and liver metastases of CRC
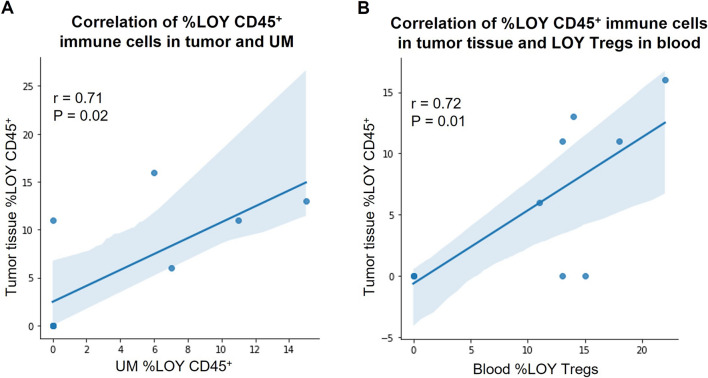
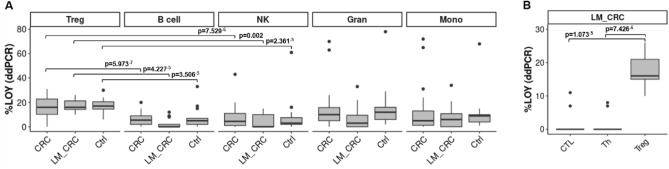
As mentioned above, LOY has been associated with different types of cancers, but the presence of LOY in leukocytes infiltrating solid tumors was not studied. We aimed here to validate the computational analysis of public scRNA-seq datasets by examination of LOY in CD45 + immune cells from primary tumors, UMs and whole blood as well as in sorted leukocytes isolated from matched peripheral blood of CRC patients. Since CRC patients have ~ 50% risk of liver metastases, we also enrolled patients with diagnosed LMs (Table 1). Out of 20 patients, five with CRCs and four with LM_CRCs showed detectable LOY in CD45 + immune cells infiltrating the tumors using ddPCR, with the mean percentage of LOY (%LOY) in the range of 6–30% (Table 1). However, the remaining 11 cases did not reveal LOY above the general detection threshold of 5% measured with ddPCR, suggesting a heterogeneity of CRCs/LM_CRCs, when %LOY-cells that infiltrate the solid tumors is considered. Corresponding analysis of CD45 + immune cells infiltrating UMs from the same set of patients showed seven cases with detectable LOY. The level of LOY-cells in UMs was generally lower (6–15%) than in tumors, with the notable exception of UM from patient ID 20 (45% of LOY-cells) (Table 1). Analysis of bulk DNA from whole blood of the same patients also showed seven patients with LOY, and there were no differences in the %LOY between blood DNA and CD45 + immune cells infiltrating the tumors. As could be expected from the above results, there were positive correlations between LOY in CD45 + immune cell infiltrates of the tumors and UMs (n = 10, r = 0.71, p = 0.02) as well as between immune cell infiltrates in the tumor and blood DNA (Fig. 4). It should be stressed here that we have performed measurement of %LOY in blood-derived CD4 + Th cells depleted of Tregs from patients with primary CRC shown in Fig. 4 and the LOY could not be detected in these cells.
Figure 4.
Analysis of association between the level of %LOY and cell types located in different types of tissues. (A) Association between the level of %LOY detected in tumor and uninvolved margin (UM) obtained from men diagnosed with primary tumors of CRC and described in Table 1. (B) Association between the level of %LOY detected in tumor and Tregs obtained from men diagnosed with CRC. Shaded areas indicate 95% confidence intervals. The correlation between %LOY CD45 + cells in tumor and LOY Th cells (depleted of Tregs) in blood is not shown because detected %LOY for all subjects was at 0 (zero) in these cells.
We supported the above results by additional comprehensive analysis of LOY in leukocytes of CRC patients and controls (free of cancer and AD) aiming at analysis of sorted Tregs along with other cellular subsets. We analyzed the distribution of LOY in the peripheral blood of 49 CRC patients and 19 healthy individuals in seven sorted subsets of cells, as well as in the bulk DNA from blood. The LOY analysis using ddPCR revealed that Tregs had significantly higher %LOY in comparison to Th- and CTL-cells in both CRC patients and controls (p = 0.024 × 10–12 and p = 0.02 × 10–5, respectively) (Fig. 2B). The %LOY for Tregs in CRC patients was 17% and 4% for the Th-cells. Similar comparison in case of controls showed the level of LOY for Tregs was 17% and 3% for Th-cell fraction. In another comparison Tregs with CTLs, the mean level of LOY for CRC patients was 4% and for controls 5% (p = 0.098 × 10–12 and p = 0.081 × 10–5, respectively) (Fig. 2B). Tregs showed an overall higher %LOY in blood in comparison to all other sorted cell subpopulations. In a similar fashion, we investigated blood samples derived from 13 LM_CRC patients (Fig. 5). In conclusion, our experimental data suggest that Tregs have a significantly higher %LOY-cells in blood in comparison to Th and CTLs in CRC and LM_CRC as well as control patients. The similar levels of LOY in Tregs from CRC patients and controls is, however, a surprising finding.
Figure 5.
Experimental detection of the distribution of LOY in sorted blood cells from patients with primary CRC, liver metastases of CRC and controls. (A) Distribution of LOY in five subpopulations of CD45 + blood leukocytes. LOY was analyzed in sorted B cells, Gran, Mono, NK, Tregs obtained from 49 male CRC patients, 13 LM_CRC patients and 19 healthy controls. CRC colorectal cancer, LM_CRC liver metastasis of CRC, Ctrl control patients. Pairwise comparison (Wilcoxon test with Bonferroni correction) is shown only for Tregs vs. B cells and Tregs vs. NK cells. These subsets (B cells and NK cells) displayed the highest statistical significance upon comparison with Tregs. (B) Distribution of LOY in FACS sorted subpopulations of T lymphocytes. Analysis of the distribution of LOY in Tregs, Th and CTLs in blood samples collected from 13 LM_CRC patients.
Tregs with LOY show higher expression of immunosuppressive genes in TME than non-LOY Tregs in CRC patients
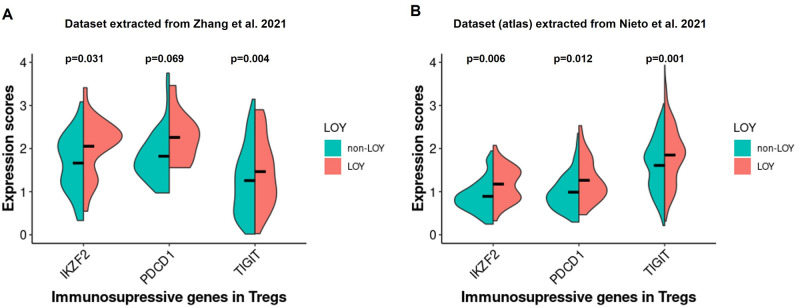
To identify the functional effect of LOY on Tregs in the TME, we re-analyzed the dataset of Zhang et al.32 to reveal changes between the expression levels of genes related to LOY Tregs vs. non-LOY Tregs (see “Materials and methods”). First, we separated 101 LOY Tregs from 397 non-LOY Tregs in four male CRC patients. We focused in particular on genes related to the suppressive features of Tregs, among others, genes encoding IC receptors (CTLA4, PDCD1, HAVCR2, LAG3, TIGIT and ICOS), genes connected with manipulation of antigens presenting cells (ENTPD1, NT5E, CD80) and migration abilities (CCR1, CCR4, CCR5, CCR7, CCR8). We also studied the expression level of IKZF2 gene, the member of Ikaros family of zinc-finger proteins, which encodes a transcription factor named Helios. Based on the gene expression score (“Materials and methods”), we focused on the three genes, PDCD1, TIGIT and IKZF2, encoding PD-1 and TIGIT surface markers and Helios transcription factor, respectively. TIGIT and IKZF2 showed a significantly higher expression in LOY Tregs than non-LOY Tregs (p = 0.004 and p = 0.031 respectively) (Fig. 6). For the PDCD1 we observed a similar trend but our results were not significant (p = 0.069). PD-1 and TIGIT are known IC receptors characteristic for suppressive phenotype of Tregs in TME. Also, co-expression of PD-1 with Helioshigh molecules is characteristic for suppressive features of Tregs in TME.
Figure 6.
Estimation of the level of immunosuppressive gene expression in LOY vs non-LOY Tregs located in TME of CRC patients. Horizontal lines in violin plots show mean values. (A) Representation of the data extracted from Zhang et al., 2020 in the tumors of four CRC patients (LOY cells = 101; non-LOY cells = 397). (B) Validation of the results with the data obtained from Nieto et al., 2021, representing data extracted from the tumors of 11 CRC patients (LOY cells = 396; non-LOY cells = 887).
A corresponding analysis of the scRNA-seq dataset extracted from Nieto et al.33 confirmed the above observations about significantly higher expression of PDCD1, TIGIT and IKZF2 in LOY Tregs located in TME (Fig. 6). In this atlas dataset, we validated our results on a group of 11 CRC patients, with 396 LOY Tregs and 887 non-LOY Tregs. Our study also revealed four additional genes (IL32, CD27, MAGEH1 and LAYN) that were the top-ranked genes correlated with the expression of TIGIT in Tregs located in the TME (Table S3). These genes were reported previously to be associated with the regulation of Tregs function and induction of their suppressive phenotype followed by a poor prognosis of CRC patients35. In conclusion, the results suggest that LOY may intensify features related to the suppressive phenotype of Tregs in TME of CRC patients.
Tregs in TME from CRC and melanoma display highest levels of LOY
We further explored the dataset from the tumor immune cell atlas33 for the presence of LOY in CD45 + immune cells, and Tregs in particular, within TME of CRC and seven other types of cancer (Fig. 2C). These other diagnoses were hepatocellular carcinoma (HCC); intrahepatic cholangiocarcinoma (ICC); cutaneous melanoma (CM); pancreatic ductal adenocarcinoma (PDAC); basal cell carcinoma (BCC); squamous-cell carcinoma (SCC); and uveal melanoma (UvM). LOY was scored as described above in 146,403 of CD45 + immune cells. With this analysis, we verified the presence of LOY in immune cells within TME in eight cancer diagnoses (Fig. 2C). The CRC diagnosis was represented by a pooled dataset of 3349 cells from 10 patients (Table S4). In agreement with our approach described above, we tested whether Tregs had a significantly higher percentage of cells with LOY in comparison to Th also in eight types of cancer. Interestingly, a significant difference between the percentage of cells with LOY in Tregs and Th was found only in the CRC diagnosis (p = 0.041) (Fig. 2C). Combined with other data, the results suggest that Tregs with a significantly higher percentage of cells with LOY in comparison to Th-subset might be a part of characteristic signature of CRC diagnosis.
Discussion
The findings of LOY in Tregs from peripheral blood and TME of CRC patients considerably expand our previous results concerning the higher levels of LOY in the CD4 + T cells in men with prostate cancer vs. controls9. Moreover, recent work by Mattisson et al. supports our data that indeed Tregs in blood show the highest frequency of cells with LOY36. We might at least partially explain here why LOY in CD4 + T cells is associated with increased risk of cancer, although CD4 + T cells in these patients are affected by LOY at relatively low frequency, compared to other subsets of leukocytes9. Tregs represent 2–10% of CD4 + T cells in blood of healthy subjects37, but in cancer patients, the frequency of circulating Tregs increases38,39. Tregs that have infiltrated into human TME account for 10–50% of CD4 + T cells40, which is in line with our results of the identification of Treg cells in the UM and tumor samples shown in Fig. 3. Furthermore, increased levels of Tregs in TME has been positively correlated with poor prognosis and low survival rates in various cancers37,41–43. However, these studies were performed in cohorts combining men and women and LOY was not examined. Hence this aspect requires further investigation.
The importance of TME in the improvement of CRC treatment is emerging and Tregs are an attractive therapeutic target in cancer immunotherapy35,37. Depending on the interaction with factors in the local environment, Tregs in TME can also differentiate into distinct subpopulations of naïve, effector and memory cells. Tumor-associated Tregs are known to be predominantly composed of effector cells, which upregulate surface molecules associated with enhanced immune-suppressive activity. This can lead to a worse immunological response followed by facilitation of tumor growth44. On the other hand, effector Tregs are also known for IL-10 secretion in TME of CRC patients, resulting in the inhibition of the Th17 cells, which are responsible for the inflammation and tumor immunity45. It should be stressed that in our analysis CRCs had a higher number of Tregs carrying LOY in TME, compared to the rest of the CD4 + T lymphocytes.
The blockade of PD-1 IC receptor with monoclonal antibodies can lead to enhanced anti-tumor immune response28,29 and therefore immunotherapy became an attractive alternative treatment method. Unfortunately, many patients (both males and females) do not respond properly to immunotherapy and the reason(s) for this is not clear, but in the case of CRC, it may be related to the phenotype of infiltrating Tregs28. We showed here that LOY affects expression levels of PDCD1, TIGIT and IKZF2 genes in Tregs. Two of these genes (PDCD1 and TIGIT), which are IC receptors and together with transcription factor IKZF2 are involved in the formation of suppressive phenotype of Tregs in TME and this presumable effect of LOY might be related to the efficacy of immunotherapy.
In our analysis, the 19 control subjects that were free from diagnoses of cancer and AD have essentially the same LOY-levels in circulating Tregs as cancer patients, which is a surprising finding (Fig. 2B). Multiple explanations could be proposed here: (i) The strongest factor associated with increasing levels of LOY in blood is age and the mean age of our controls is higher than cancer patients cohort in our study (see Table S1). However, it is unlikely that it is the sole explanation for this result; (ii) The size of our cohort is relatively small and this issue should be followed up in a considerably larger number of of CRC patients and controls; (iii) The relative number of cells in each category of tumor infiltrating cells (Tregs, Ths, CTLs) should also be taken into account, as it has been suggested that cancer patients have higher number of Tregs, compared to healthy subjects (see above); (iv) Tumor immune-surveillance is a defensive mechanism against cancer (and not driving the tumor development), which might not function properly in some healthy subjects, but in the absence of the processes that drive the tumorigenesis, there might be no negative consequences (i.e. tumors) for such a subject; (v) Tregs might not be the only failing component of tumor immune-surveillance as a result of LOY in immune cells. The recent paper by Abdel-Hafiz et al.46 suggests that CD8 + cytotoxic T lymphocytes are driven to dysfunction (or exhaustion) in the tumor microenvironment (TME) of mice modeled with induced hematopoietic LOY. Importantly, the latter finding agrees well with our observation that CD8 + cytotoxic T lymphocytes in TME of CRC patients have increased levels of LOY (Fig. 2A) compared to UM (p = 0.011). CTLs are one of the critical components of tumor immune-surveillance within TME and CTLs are, upon stimulation with an antigen, undergoing clonal expansion and this might be related to their elevated level of LOY, since LOY is presumed to occur as a result of an error during mitotic division. In parallel, LOY might compromise some of the normal functions of CTLs within TME and this aspect deserves future studies using scRNA-seq exploring T-cell receptor (TCR) sequencing combined with global transcriptome analysis. We have shown previously that circulating CTLs in blood had very low levels of LOY in prostate cancer patients, AD patients and age-matched healthy controls9, but the situation might be different within TME.
Loss of chromosome Y has also been shown as a very frequent mutation in tumor cells from numerous cancers and LOY correlates with an overall worse prognosis47,48. This agrees with the concept that LOY provides proliferative advantage to cells affected by this aneuploidy49. This is also in line with the fact that men have a higher incidence and/or mortality from most sex-unspecific cancers, which is unexplained by known risk factors50,51. This sex disparity is an understudied aspect, which might be related to LOY occurring both in the tumor cells and in cells responsible for the defensive mechanism of tumor immune-surveillance within TME. Thus, future studies should pay attention to the analysis of LOY in tumor cells and in the normal immune cells infiltrating TME. It is interesting in this context that our meta-analysis of Nieto et al. dataset of multiple cancers33 showed that, in addition to CRC, melanoma displays high levels of LOY, which should stimulate future analyses. It is noteworthy that cutaneous malignant melanoma displays sex bias. By the age of 65 and by the age 80, men are twice and three times more likely to get melanoma, respectively, when compared to women. Moreover, males of any age have also a greater risk of death from this disease52.
We performed an integrative analysis of LOY-cells in tumor, normal tissue (unchanged large bowel mucosa) surrounding tumor and blood, using experimental tissue dissociation and publicly available scRNA-seq datasets, aiming to identify and characterize LOY-affected leukocytes. Experiments using scRNA-seq methods has recently revolutionized the medical field53 and carry a promise of extending the research into personalized diagnostics34. scRNA-seq allows to overcome limitations regarding the investigation of the LOY frequency and LOY-associated transcriptomic effects, especially when the number of cells is not sufficient for the bulk analysis, as is often the case of immune cells isolated from solid tissues, or when the data from homogenous cell populations is required13. On the other hand, the advantage of using ddPCR is its simplicity and a low input of DNA, allowing detection of LOY in DNA from sorted cells, starting at about 10,000 cells13. Results presented here and during the past few years regarding chromosome Y showed that it should no longer be considered as a “genetic wasteland”9.
In conclusion, our study sets the direction for further research regarding a probable functional role of LOY in intensifying features related to the suppressive phenotype of Tregs in TME and, as a consequence, a possible influence on immunotherapy response in CRC patients44. The presence of LOY has previously been associated with a considerable dysregulation of autosomal gene expression in leukocytes, in particular responsible for the immune-surveillance functions of circulating cells9,15,16. Here, we extend these findings to TME of CRC patients and this aspect warrants further studies.
Materials and methods
Human specimens
For the tissue dissociation, 20 male patients were enrolled including 10 patients with a primary CRCs recruited in the Oncology Center in Bydgoszcz and 10 patients with metachronous (seven patients) and synchronous (three patients) liver metastases of CRCs (LM_CRC) recruited in the University Clinical Center in Gdańsk. Seven metachronous LM_CRC patients were treated with an adjuvant chemotherapy before the surgery; four LM_CRC individuals were diagnosed with rectum adenocarcinoma and six with colon adenocarcinoma. The inclusion criteria for the recruitment of CRC was tumor size of at least 3 cm in diameter. The neoadjuvant therapy was considered as an exclusion criterion for primary CRCs. The age of patients with primary CRC ranged from 42 to 74, with a median age of 62, and from 42 to 78, with a median age of 65 for the LM_CRC group. Detailed clinical information is shown in Tables 1 and S1. Additionally, blood samples were collected from 49 CRC, 13 LM_CRC and 19 control (Ctrl) patients recruited in the University Clinical Center in Gdańsk. Ctrl patients were collected from the general male population of Gdańsk and their age ranged from 62 to 74, with a median age of 68, as described54. Written informed consent was obtained from all the patients prior to material collection. Our study followed principles of the Declaration of Helsinki and was approved by the Independent Bioethics Committee for Research at the Medical University of Gdańsk (approval number NKBBN/564/2018 with multiple amendments).
For tissue dissociation we used two fragments excised from the same patients: UMs located at least 3–5 cm away (edge to edge distance) from the tumor and a fragment of primary tumor. Each collected sample was divided into two parts: one for tissue dissociation, one for FFPE block. The splitting surface underwent histological assessment of the actual tissue content and immune cells infiltration. This approach provides the correct histopathological assessment of the actual content of our material, which was carried out by experienced pathologists. The absence of tumor cells was confirmed in all UM fragments. Samples for dissociation were placed directly in the MACS Tissue Storage Solution (Miltenyi Biotec), transported on ice and, according to the manufacturer’s instructions, processed within 24 h.
Tissue dissociation
Samples for tissue dissociation studies were washed with phosphate-buffered solution (PBS), minced into one mm fragments on ice and mechanically dissociated using gentleMACS Dissociator (Miltenyi Biotec). Enzymatic digestion was performed using the Tissue Dissociation Kit (Miltenyi Biotec) at 37 °C with agitation. Digestion process was stopped after two 30 min incubations in RPMI-1640 (Sigma Aldrich) with 10% FBS (Sigma Aldrich). The cell suspension was filtered with 70 µM nylon cell strainer (BioLogix) and centrifuged at 300 × g in 4 °C for 7 min. Red blood cells were lysed using one ml of lysing solution (BD Biosciences).
FACS sorting of tissue infiltrating leukocytes
Cell suspensions obtained from tumor and UM fragments after dissociation were incubated with a Fixable Viability Dye eFluortm 780 (Invitrogen, ThermoFisher Scientific) to label dead cells and with anti-CD45 + antibodies (BD Biosciences) to distinguish leukocyte populations. Cells were incubated with the dye and antibody for 30 min at 4 °C, washed and sorted to PBS with 0.04% BSA (Sigma Aldrich), centrifuged at 3200×g in 4 °C for 10 min and stored in − 80 °C for DNA extraction.
Immunomagnetic enrichment and FACS sorting of peripheral blood leukocytes
Granulocytes and peripheral blood mononuclear cells (PBMCs) were collected from 6 and 32 ml blood into EDTA-K2 tubes (BD Biosciences) and BD Vacutainer® CPT™ Mononuclear Cell Preparation Tubes (BD Biosciences), respectively. A sample of 1.5 ml whole blood (EDTA-K2 tubes) was taken for DNA extraction. The number of PBMCs obtained by density gradient centrifugation method was estimated using EVE Automatic Cell Counter and immediately processed for FACS. The number of 0.5 × 106 PBMCs were centrifuged at 3200×g in 4 °C for 10 min and stored in − 80 °C for DNA extraction. The population of CD4 + T cells was enriched using negative immunomagnetic separation with CD4 + T Cell Isolation Kit, human (Miltenyi Biotec) and subsequently incubated for 30 min at 4 °C with Human Regulatory T Cell Cocktail (BD Biosciences). Gating strategy used for FACS of selected populations of leukocytes from peripheral blood is shown in Fig. S1. Positively separated cells were labeled with fluorophore-conjugated antibodies for Monocytes (Mono); Natural killers (NK); CD19 + B lymphocytes (B cells); and CD8 + cytotoxic T lymphocytes (CTL) (BD Biosciences). Labeled cells were washed and resuspended in one ml of PBS + 0.04% BSA for the FACS sorting procedure. CD4 + T cells were sorted into Treg (CD4 + , CD25 + , CD127low) and Th (CD4 + , CD25 + , CD127 +) cell populations using BD FACSAriaTM III Cell Sorter (Becton Dickinson). The remaining PBMCs were sorted into Mono (CD45 + , CD3–, CD14 +), B cells (CD45 + , CD3–, CD19 +), CTL (CD45 + , CD3 + , CD8 +) and NKs (CD45 + , CD3–, CD56 + , CD16 +). Granulocytes were sorted according to SSC/FSC parameters and CD45 + antigen expression (BD Bioscience). Cells were analyzed and sorted using BD FACSDiva™ Software ver. 8.0.1. (Becton Dickinson).
Measurement of LOY using ddPCR
Quantification of the level of LOY in analyzed samples was performed using previously described procedure12. Briefly, DNA extracted from sorted fractions of cells was digested with HindIII enzyme (#FD0504, ThermoFisher Scientific) for 15 min in 37 °C. Next, 50 ng of digested and diluted DNA was thoroughly mixed with ddPCR supermix for probes no dUTP (Bio-Rad), PCR primers and probes targeting 6 bp difference between the AMELX and AMELY gene assay (C_990000001_10, ThermoFisher Scientific). For the processing of samples and fluorescent measurement of the signal in channels FAM and VIC, we used the QX200 Droplet Digital PCR System (Bio-Rad). For the generation and analysis of the data we used the dedicated software QuantaSoft (version 1.7.4.0917).
scRNA-seq data collection and analysis of LOY
For our meta-analysis we selected two scRNA-seq datasets, which were assessed for their quality as described in Fig. S2. The first dataset was obtained from Zhang et al.32 generated by the 10 × Genomics platform. The gene expression profiles made by 10 × Genomics platform contained the information for 18 colorectal cancer patients, including 14 females and four males. In our study we focused only on male patients, and two groups of previously annotated cells i.e. CD4 + T and CTL cells. Further on, these two groups of cells were distinguished based on the patients, tissue of origin i.e. tumor, UM, blood and cell subpopulations. Later, groups with the sum of LOY and non-LOY-cells lower than 15 were removed from our study.
The second dataset that was used for the validation of our results was obtained from Nieto et al.33. It contained the gene expression profiles of 500,000 CD45 + immune cells acquired from 217 patients and 13 cancer types. Patients were separated to male, female and unknown genders. To distinguish the unknown group to male and female, we calculated the percentage of gene expression for the set of chromosome Y specific genes i.e. RPS4Y1; ZFY; USP9Y; DDX3Y; KDM5D; and EIF1AY using the default parameters of the ‘PercentageFeatureSet’ function of Seurat R package (version 4.2.0)55. Patients with and without expression of these six genes were defined as male and female, respectively. After removal of female patients and cancers with less than four patients, we extracted information from 98 male patients and eight cancers. According to the percentage of gene expression defined by the ‘PercentageFeatureSet’ function of Seurat R package in both datasets, the cells showing zero expression for all six chromosome Y specific genes were classified as LOY and the rest were defined as non-LOY. In this dataset, cells were distinguished based on patients, cell subpopulations and cancer types. Since the second dataset consisted of a higher number of patients and cells in comparison with the first dataset, we used stricter threshold for data filtering. Hence, groups with the sum of LOY and non-LOY-cells below 50 were removed from our study.
Immunosuppressive gene expression scores in Tregs with/without LOY
Tregs located in the tumors were distinguished from the group of CD4 + T cells based on the expression of CTLA-4 and IL23R markers for both datasets used for the meta-analysis32,33. Next, Tregs were divided into two groups based on the LOY status and their expression profiles were compared, as described above. We computed the gene expression scores per cell for IKZF2, PDCD1 and TIGIT genes using the ‘AddModuleScore’ function of Seurat R package by default parameters. Expression scores were the average expression levels of desired genes in each cluster (i.e., LOY and non-LOY Tregs) subtracted by the aggregated expression of randomly selected control feature sets.
Statistical analysis
We compared the percentage of LOY between Tregs and other cells in Figs. 2 and 4 in addition to gene expression scores for immunosuppressive genes between LOY and non-LOY Tregs in Fig. 6. The significance in all pairwise comparisons was computed using the Wilcoxon test and p-values were adjusted using the Bonferroni correction method. For this purpose, the ‘wilcox.test’ function of R with default parameters was used.
Supplementary Information
Acknowledgements
We thank the anonymous patients and volunteer healthy controls for sample contribution and information provided in the questionnaire. We are thankful to Dr. Daniil Sarkisyan for critical review of the manuscript. We also thank physicians and nurses involved in the patient recruitment process, collaborating technicians, diagnosticians and pathologists from Oncology Center-Prof. Franciszek Łukaszczyk Memorial Hospital in Bydgoszcz (Jowita Nowaczewska, Katarzyna Krzysiak, Anetta Słupicka, Mateusz Matusiak) and University Clinical Centre in Gdańsk (Grażyna Stęplewska, Elżbieta Wierszyło, Elżbieta Pietruszka, Grażyna Dombrowska, Justyna Pietruszewska, Irena Pellowska, Michał Kunc, Aleksandra Korwat, Ewa Miłoszewska).
Author contributions
M.W., U.J., K.D-C., K.C. conducted laboratory experiments; J.M., J.P.D. conceived of the idea; E.M., M.W., U.J. analyzed the data; J.P.D. obtained funding; M.W., U.J., J.M., N.F., J.P.D. supervised the project; M.W., U.J., E.M., J.M., J.P.D. contributed to interpretation of the results; M.W., U.J., E.M., E.R-B., B.B-O., H.D., K.C., P.O., N.F., J.P.D. drafted the manuscript; M.W., U.J., K.D-C., E.R-B., M.B., M.J., O.R., A.H., R.P., J.K., M.Z., J.K., W.Z., W.B., Ł.S., P.R., N.F. contributed to selection and collection of samples from patients; All co-authors read and approved the submission.
Funding
This study was supported by grants from the Foundation for Polish Science under the International Research Agendas Program (grant number MAB/2018/6; co-financed by the European Union under the European Regional Development Fund), Swedish Heart–Lung Foundation (grant number 20210051), Swedish Research Council (grant number 2020-02010), Swedish Cancer Society, Hjärnfonden, and Alzheimerfonden to J.P.D.
Data availability
The data that support the findings of this study are available on request from the corresponding authors.
Competing interests
J.P.D. is a cofounder and shareholder in Cray Innovation AB. J.M. is a cofounder and shareholder in Genegoggle Ltd. The remaining authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Magdalena Wójcik, Ulana Juhas and Elyas Mohammadi.
These authors jointly supervised this work: Natalia Filipowicz and Jan P. Dumanski.
Contributor Information
Natalia Filipowicz, Email: natalia.filipowicz@gumed.edu.pl.
Jan P. Dumanski, Email: jan.dumanski@igp.uu.se
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60049-y.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Price TJ, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121:830–835. doi: 10.1002/cncr.29129. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transd. Target. Ther. 2022;7:70. doi: 10.1038/s41392-022-00922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abancens M, Bustos V, Harvey H, McBryan J, Harvey BJ. Sexual dimorphism in colon cancer. Front. Oncol. 2020 doi: 10.3389/fonc.2020.607909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huyghe JR, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat. Genet. 2019;51:76–87. doi: 10.1038/s41588-018-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsberg LA, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat. Genet. 2014;46:624–628. doi: 10.1038/ng.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson D, et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature. 2019;575:652–657. doi: 10.1038/s41586-019-1765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsberg L, et al. Mosaic loss of chromosome Y (LOY) in leukocytes matters. Nat. Genet. 2019;51:4–7. doi: 10.1038/s41588-018-0267-9. [DOI] [PubMed] [Google Scholar]
- 9.Dumanski J, et al. Immune cells lacking Y chromosome show dysregulation of autosomal gene expression. Cell. Mol. Life Sci. 2021;78:4019–4033. doi: 10.1007/s00018-021-03822-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haitjema S, et al. Loss of Y chromosome in blood is associated with major cardiovascular events during follow-up in men after carotid endarterectomy. Circ. Cardiovasc. Genet. 2017;10:e001544. doi: 10.1161/CIRCGENETICS.116.001544. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen MC, Pearse R, Young-Pearse T, Mostafavi S. Mosaic loss of chromosome Y in aged human microglia. Genome Res. 2022;32:1795–1807. doi: 10.1101/gr.276409.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danielsson M, et al. Longitudinal changes in the frequency of mosaic chromosome Y loss in peripheral blood cells of aging men varies profoundly between individuals. Eur. J. Hum. Genet. 2020;28:349–357. doi: 10.1038/s41431-019-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruhn-Olszewska B, et al. Loss of Y in leukocytes as a risk factor for critical COVID-19 in men. Genome Med. 2022;14:139. doi: 10.1186/s13073-022-01144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungstrom V, et al. Loss of Y and clonal hematopoiesis in blood-two sides of the same coin? Leukemia. 2022;36:889–891. doi: 10.1038/s41375-021-01456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattisson J, et al. Leukocytes with chromosome Y loss have reduced abundance of the cell surface immunoprotein CD99. Sci. Rep. 2021;11:15160. doi: 10.1038/s41598-021-94588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakalski M, et al. Loss of Y is associated with multi-omic changes in immune cells from Alzheimer's disease patients. medRxiv. 2023 doi: 10.1101/2023.02.07.23285520. [DOI] [Google Scholar]
- 17.Dumanski JP, et al. Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am. J. Hum. Genet. 2016;98:1208–1219. doi: 10.1016/j.ajhg.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumanski JP, et al. Smoking is associated with mosaic loss of chromosome Y. Science. 2015;347:81–83. doi: 10.1126/science.1262092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sano S, et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science. 2022;377:292–297. doi: 10.1126/science.abn3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binnewies M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loddenkemper C, et al. In situ analysis of FOXP3+ regulatory T cells in human colorectal cancer. J. Transl. Med. 2006;4:52. doi: 10.1186/1479-5876-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toor SM, et al. Immune checkpoints in circulating and tumor-infiltrating CD4+ T cell subsets in colorectal cancer patients. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.02936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed Khaja AS, Toor SM, El Salhat H, Ali BR, Elkord E. Intratumoral FoxP3+helios+ regulatory T cells upregulating immunosuppressive molecules are expanded in human colorectal cancer. Front. Immunol. 2017 doi: 10.3389/fimmu.2017.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer. 2020;19:116. doi: 10.1186/s12943-020-01234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frey DM, et al. High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int. J. Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 26.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: The paradox of colorectal cancer. Cancer Immunol. Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito T, et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 28.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 29.Makaremi S, et al. Immune checkpoint inhibitors in colorectal cancer: Challenges and future prospects. Biomedicines. 2021;9:1075. doi: 10.3390/biomedicines9091075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019;25:389–402. doi: 10.1038/s41591-019-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumagai S, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020;181:442–459.e429. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Nieto P, et al. A single-cell tumor immune atlas for precision oncology. Genome Res. 2021;31:1913–1926. doi: 10.1101/gr.273300.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koczkowska M, et al. Personalized health risk assessment based on single cell RNA sequencing analysis of a male with 45, X/48, XYYY karyotype. Sci. Rep. 2022;12:20854. doi: 10.1038/s41598-022-25308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aristin Revilla S, Kranenburg O, Coffer PJ. Colorectal cancer-infiltrating regulatory T Cells: Functional heterogeneity, metabolic adaptation, and therapeutic targeting. Front. Immunol. 2022;13:903564. doi: 10.3389/fimmu.2022.903564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattisson J, et al. Loss of chromosome Y in regulatory T cells. BMC Genom. 2024 doi: 10.1186/s12864-024-10168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleh R, Elkord E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–185. doi: 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Ahmadzadeh M, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotsakis A, et al. Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci. Rep. 2016 doi: 10.1038/srep39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Kim BS, Lee SK. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. 2020;20:e4. doi: 10.4110/in.2020.20.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell Biol. 2018;96:21–33. doi: 10.1111/imcb.1003. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines. 2016;4:28. doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015 doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward-Hartstonge KA, Kemp RA. Regulatory T-cell heterogeneity and the cancer immune response. Clin. Transl. Immunol. 2017;6:e154. doi: 10.1038/cti.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, et al. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci. Rep. 2016;6:24249. doi: 10.1038/srep24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel-Hafiz HA, et al. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature. 2023;619:624–631. doi: 10.1038/s41586-023-06234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duijf PH, Schultz N, Benezra R. Cancer cells preferentially lose small chromosomes. Int. J. Cancer. 2013;132:2316–2326. doi: 10.1002/ijc.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller P, et al. Why loss of Y? A pan-cancer genome analysis of tumors with loss of Y chromosome. Comput. Struct. Biotechnol. J. 2023;21:1573–1583. doi: 10.1016/j.csbj.2023.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell E, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606:343–350. doi: 10.1038/s41586-022-04786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomark. Prev. 2011;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgren G, Liang L, Adami HO, Chang ET. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 2012;27:187–196. doi: 10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- 52.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 53.Pohl ST, Prada ML, Espinet E, Jurkowska R. Practical considerations for complex tissue dissociation for single-cell transcriptomics. Methods Mol. Biol. 2023;2584:371–387. doi: 10.1007/978-1-0716-2756-3_19. [DOI] [PubMed] [Google Scholar]
- 54.Filipowicz N, et al. Comprehensive cancer-oriented biobanking resource of human samples for studies of post-zygotic genetic variation involved in cancer predisposition. PLoS One. 2022;17:e0266111. doi: 10.1101/2021.09.03.458865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587e3529. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors.