Abstract
We have sequenced the envelope genes from each of the five members of the gibbon ape leukemia virus (GALV) family of type C retroviruses. Four of the GALVs, including GALV strain SEATO (GALV-S), were originally isolated from gibbon apes, whereas the fifth member of this family, simian sarcoma-associated virus (SSAV), was isolated from a woolly monkey and shares 78% amino acid identity with GALV-S. To determine whether these viruses have identical host ranges, we evaluated the susceptibility of several cell lines to either GALV-S or SSAV infection. GALV-S and SSAV have the same host range with the exception of Chinese hamster lung E36 cells, which are susceptible to GALV-S but not SSAV. We used retroviral vectors that differ only in their envelope composition (e.g., they contain either SSAV or GALV-S envelope protein) to show that the envelope of SSAV restricts entry into E36 cells. Although unable to infect E36 cells, SSAV infects GALV-resistant murine cells expressing the E36-derived viral receptor, HaPit2. These results suggest that the receptors present on E36 cells function for SSAV. We have constructed several vectors containing GALV-S/SSAV chimeric envelope proteins to map the region of the SSAV envelope that blocks infection of E36 cells. Vectors bearing chimeric envelopes comprised of the N-terminal region of the GALV-S SU protein and the C-terminal region of SSAV infect E36 cells, whereas vectors containing the N-terminal portion of the SSAV SU protein and C-terminal portion of GALV-S fail to infect E36 cells. This finding indicates that the region of the SSAV envelope protein responsible for restricting SSAV infection of E36 cells lies within its amino-terminal region.
The first step in retroviral infection requires binding of the viral envelope protein to a cell surface receptor (29). Binding is followed by fusion of the viral envelope with the plasma membrane, delivering the viral nucleocapsid to the cell cytosol. Blocks to either of these steps will render a cell resistant to viral infection. Although the presence of an appropriate receptor is the predominant requirement for cellular susceptibility to retroviral infection, other cellular factors accessory to the viral receptor play a role. For example, murine cells which express CD4, the receptor for human immunodeficiency virus type 1 (HIV-1), remain resistant to infection by HIV (16, 20). HIV-1 binds to all cells that express CD4, but a second factor is required for entry. Several chemokine receptors have been found to function as entry cofactors (2), which are required for HIV-1 to undergo fusion. CXCR4 is a coreceptor for T-cell-tropic HIV-1 (19), and CCR5 is a coreceptor for macrophagetropic HIV-1 (28, 35).
The gibbon ape leukemia virus (GALV) family comprises four strains of exogenous type C retroviruses isolated from nonhuman primates in various states of disease—SEATO (S), SF, Brain (Br), and Hall’s Island (H)—as well as simian sarcoma-associated virus (SSAV) (32). All members of the GALV family use the same receptor, Pit1, a multimembrane-spanning class III phosphate transporter (18, 22), to infect human cells (27, 32). The in vitro host range of GALV is similar to that of xenotropic murine leukemia viruses (MuLVs) in that GALV can infect most mammalian (e.g., human, bat, rat, and cow) cells, while murine and hamster (CHO) cells are resistant to GALV-S infection (10, 32). Murine cells are resistant to GALV because they lack functional GALV receptors (21). The cellular components which restrict GALV infection of hamster cells have not been determined. E36 cells, derived from Chinese hamster lung tissue, differ from other hamster cells in their ability to be readily infected by GALV-S. Because of this unusual property of E36 cells, we used them to evaluate whether any host range differences among the GALV isolates could be detected. We found that SSAV differs from the other GALV strains; it is unable to infect E36 cells.
We sought to resolve the molecular mechanism underlying the block to SSAV infection of E36 cells. Our results show that SSAV cannot infect E36 cells, despite the presence of functional GALV receptors, and this appears to be due to inherent differences between the SSAV and GALV-S envelope proteins. GALV-S and SSAV chimeric envelope studies have enabled us to map the region of the SSAV envelope responsible for the block to infection of E36 cells. The N-terminal region of SSAV envelope including variable regions A and B (VRA and VRB) restricts SSAV infection of E36 cells at the entry stage.
MATERIALS AND METHODS
Cells and viruses.
The following cell lines were used in this study: NIH 3T3 murine fibroblasts (ATCC CRL 1658), Mus dunni tail fibroblasts (MDTF) (obtained from Olivier Danos, Institut Pasteur, France; also available as ATCC CRL 2017), Rat2 embryo fibroblasts (ATCC CRL 1764), M. musculus molossinus MMK cells (ATCC CRL 6439), mink lung fibroblast cells (ATCC CL64), and E36 Chinese hamster lung cells (provided by Christine Kozak, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, Md.). 293T cells were obtained from Cell Genesys Inc., Foster City, Calif. All cells were maintained in Dulbecco’s modified essential medium (DMEM; Whittaker Bioproducts, Inc., Walkersville, Md.) supplemented with 5% fetal bovine serum, 100 U of penicillin and 100 μg of streptomycin per ml, and 4 mM glutamine. Wild-type GALV strains, S, Br, H, and SF were obtained from the supernatant of mink cells, and SSAV was obtained from marmoset 71-AP-1 cells.
Production of retroviral vectors.
293T cells were seeded at a density of 106 cells/10-cm-diameter dish 2 days before transfection. The following three plasmids were transfected into 293T cells by the calcium phosphate precipitation method (Promega): (i) 10 μg of pRT43.2TnIsbgal (12), a Moloney MuLV (MoMuLV)-based packageable genome containing the packaging signal and the β-galactosidase gene coding sequence; (ii) 2.5 μg of MoMuLV gag-pol-expressing plasmid (12); and (iii) 5 μg of pCI-neo (Promega) plasmid with GALV-S or SSAV envelope coding region. GALV-S or SSAV enveloped retroviral vectors were harvested from supernatant of transfected 293T cells 60 to 72 h after transfection.
Viral infections and vector transduction.
E36, Rat2, MMK, and NIH 3T3 target cells were seeded at a density of 3.0 × 104 cells/well in a 12-well plate 1 day prior to virus infection. Cell medium from virus producer cells (mink or marmoset 71-AP-1 cells) was passed through a 0.45-μm-pore-size filter and adjusted to a final Polybrene concentration of 10 μg/ml. Target cells were exposed to the harvested supernatant for 24 h, trypsinized, and reseeded at 1/10 density. Supernatant of cells exposed to wild-type virus was analyzed for reverse transcriptase activity by measuring the counts per minute of incorporated [3H]TTP 8 days after exposure to virus (34). For GALV-S and SSAV enveloped retroviral vector infections, 1.5 × 104 target cells/well were seeded in a 24-well plate 1 day prior to exposure to retroviral vector-containing supernatant. After 48 to 72 h, cells were analyzed for expression of β-galactosidase by histochemical staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as described previously (31).
Isolation and hybridization of unintegrated viral DNA.
E36 and Rat2 cells were seeded at a density of 5 × 106 target cells 1 day prior to infection. The next day, cells were exposed to wild-type virus and incubated for 24 h. Unintegrated, low-molecular-weight DNA was isolated from cells by the method of Hirt (17). The extrachromosomal Hirt DNA preparation was digested with a restriction enzyme (EcoRI or XhoI) and run on a 0.8% agarose gel. The DNA was transferred to a nitrocellulose membrane and prehybridized for 2 h at 65°C in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.01 M EDTA–5× Denhardt’s solution–0.5% sodium dodecyl sulfate, 0.1 mg of salmon sperm DNA per ml and then hybridized in the same solution containing the labeled probe (2 × 106 cpm/ml) overnight. The nick-translated 32P-labeled BamHI-PstI DNA fragment from the MOV-GAS env plasmid (30), encoding the GALV-S envelope glycoproteins, was used as the probe. The hybridized membrane was washed in 2× SSC twice at room temperature and then twice at 65°C. The autoradiogram was developed after exposure of the hybridized membrane to Kodax XAR-2 film with two screens at −70°C.
Cloning and sequencing of envelope glycoproteins.
Virus was harvested from mink cells chronically infected with GALV-Br or GALV-H, and mRNA was prepared by using a FastTrack 2.0 kit (Invitrogen). cDNA was prepared in a reverse transcription reaction using avian myeloblastosis virus reverse transcriptase (Promega) and random hexamer primers. Several overlapping fragments were produced by PCR using primers based on the nucleotide sequence of GALV-S envelope (6). Products were subcloned into the TA vector pCRII (Invitrogen) and sequenced by dideoxy sequencing, using GALV-S-based primers. A consensus sequence was obtained, and the 5′ end was sequenced by using a sense primer derived from the pol region of GALV-S and an antisense primer derived from a previously sequenced region, 64 nucleotides downstream of the putative envelope start codon. For the 3′ end, we designed a sense primer based on a sequenced region of GALV-H and an antisense primer based on the nucleotide sequence from the U3 region of GALV-S, bases 7772 to 7749. pGV-3 (26) was used as a template to sequence the GALV-SF envelope. Plasmid pGAS-2 (14) was used as a sequence template for GALV-S, and SSAV envelope was sequenced from the pB11 clone (13), using Sequenase version 2.0 (U.S. Biochemicals, Cleveland, Ohio).
Construction of GALV-S and SSAV envelope chimeras.
Chimeric SSAV envelope constructs containing both GALV-S VRA and VRB were made by exchanging the region between XbaI and XmaI sites of SSAV with the corresponding region of GALV-S. The same strategies were used to construct chimeric GALV-S envelopes containing the SSAV VRA and VRB.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the envelope sequences of the five members of the GALV family are as follows: GALV-S, AF055060; GALV-H, AF055061; GALV-Br, AF055062; GALV-SF, AF055063; and SSAV, AF055064.
RESULTS
E36 cells are resistant to infection by SSAV but not GALV-S.
We have previously reported that the four GALV strains and SSAV can use Pit1 as a receptor (27, 32). To determine whether there are any differences in cell tropism between these viruses, we evaluated the susceptibility of four cell lines to individual strains of GALV and monitored the viral infection by measuring the reverse transcriptase activity from culture supernatant (Table 1). All four strains of GALV and SSAV efficiently infected Rat2 cells (positive control) and failed to infect NIH 3T3 cells (negative control). We examined the susceptibility to GALVs infection of MMK and E36 cells because of their unusual receptor properties previously reported (32, 33). We found that MMK cells were permissive for infection of all GALV strains and SSAV, while E36 cells can be efficiently infected by all four GALV strains but not SSAV.
TABLE 1.
Host range of GALV strains and SSAV
| Strain or virus | Susceptibility to wild-type virusa
|
|||
|---|---|---|---|---|
| NIH 3T3 | Rat2 | MMK | E36 | |
| GALV strain | ||||
| S | − | + | + | + |
| H | − | + | + | + |
| Br | − | + | + | + |
| SF | − | + | + | + |
| SSAV | − | + | + | − |
Determined by measuring [3H]TTP incorporation in a reverse transcriptase assay performed on cell supernatant 8 days after exposure to virus as described in Materials and Methods.
SSAV DNA is not detected in extrachromosomal DNA from E36 cells exposed to SSAV.
To determine the stage at which SSAV infection of E36 cells was blocked, we first analyzed DNA purified from E36 cells exposed to SSAV for the presence of unintegrated viral DNA. Twenty-four hours after exposure to SSAV, extrachromosomal, low-molecular-weight DNA was isolated by the method of Hirt (17) and examined by Southern blot analysis (Fig. 1). DNA recovered from cells exposed to SSAV or GALV-S was analyzed by restriction enzyme digestion. EcoRI cleaves SSAV DNA at a single site within the pol coding region; XhoI cleaves GALV-S DNA at a single site within the gag coding region. A 9.0-kb DNA fragment corresponding to the expected size of linear viral DNA was detected in DNA isolated from both E36 and Rat2 fibroblasts exposed to GALV-S by hybridization to a nick-translated 32P-labeled GALV-S probe. The additional DNA fragments hybridizing to this probe are presumably derived from defective provirus or incompletely digested DNA fragments. DNA from Rat2 cells exposed to SSAV bound to the GALV probe, indicating the presence of extrachromosomal viral DNA in Rat2 cells. The observed absence of a hybridizing DNA fragment in E36 cells exposed to SSAV under identical assay conditions suggests that the block to SSAV infection of E36 cells occurs early in the viral infection-replication process, before reverse transcription of viral RNA into double-stranded DNA.
FIG. 1.
Analysis of extrachromosomal Hirt preparation DNA from cells exposed to virus. Cells were exposed to either SSAV or GALV-S for 18 h prior to isolation of low-molecular-weight DNA (see Materials and Methods). Digested and undigested DNAs were hybridized to a 32P-labeled BamHI-to-PstI DNA fragment derived from the GALV-S envelope coding region. Extrachromosomal DNA was digested with enzymes predicted to produce a 9.0-kb linear viral DNA band in infected cells.
SSAV infection of E36 cells is restricted at the level of virus envelope-receptor interaction.
To determine the viral component which is responsible for the inability of SSAV to infect E36 cells, we used retroviral vectors differing only in their envelope glycoproteins, either from GALV-S or SSAV. These retroviral vectors contain identical MoMuLV core proteins and a MoMuLV-based packageable genome containing β-galactosidase gene coding sequence. Retroviral vectors bearing GALV-S envelope efficiently infected E36 cells, whereas those bearing the SSAV envelope did not (Table 2). These results demonstrate that the SSAV envelope is the viral component which restricts SSAV infection of E36 cells.
TABLE 2.
GALV-S and SSAV enveloped retroviral vector infection of E36 cells and MDTF cells expressing Pit1 or HaPit2 receptor
| Cell line | Mean no. of β-galactosidase-expressing cells ± SDa
|
|
|---|---|---|
| SSAV | GALV-S | |
| E36 | 0 | (3.6 ± 0.6) × 106 |
| MDTF | 0 | 0 |
| MDTFPit1 | (2.0 ± 0.4) × 105 | (9.0 ± 1.1) × 106 |
| MDTFHaPit2 | (1.1 ± 0.2) × 102 | (4.6 ± 0.6) × 104 |
Average of at least three independent experiments.
We have previously shown that E36 cells express two distinct cell surface proteins, HaPit1 and HaPit2, which both function as receptors for GALV-S infection (33). MDTF cells, normally resistant to SSAV, are susceptible to infection by SSAV-enveloped vectors when they express HaPit2 (Table 2). This finding demonstrates that E36 cells express receptors that are functional for SSAV infection, even though SSAV cannot infect these cells.
Envelope gene sequences from four GALV strains and SSAV.
We sought to elucidate the molecular basis for the observed differences in the host range of SSAV by comparing the envelope sequences of the GALV family. The GALV-Br and GALV-H envelope genes were cloned (see Materials and Methods), and these as well as previously cloned GALV-S (14), GALV-SF (26), and SSAV (13) envelope genes were sequenced. Alignment of the amino acid sequences deduced from the nucleotide sequences of the four strains of GALV and SSAV is shown in Fig. 3. We found that the predicted transmembrane (TM) portion of the envelope of GALV-S is longer than previously reported (6), extending 18 amino acids at the carboxyl terminus. Both GALV-Br and GALV-H are highly related to GALV-S, showing 91 and 93% amino acid identity, respectively, whereas SSAV and SF show 78 and 81% identity, respectively. VRA and VRB of MuLVs have been defined and shown to influence receptor specificity (1). These regions were determined for GALV-S and SSAV by alignment of their deduced envelope sequences with sequences from MuLVs (Fig. 3).
FIG. 3.
Alignment of the deduced amino acid sequences in the envelope regions of five members of GALV family. The underlined segments correspond to the putative VRA and VRB. Gaps in the alignment are indicated by dashes, with the consensus sequence indicated on the bottom line.
The N-terminal half of the SSAV envelope restricts the ability of SSAV to infect E36 cells.
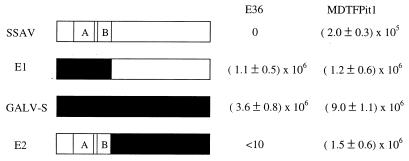
SSAV and GALV-S envelope plasmids were used as templates to construct GALV-S/SSAV chimeric envelope cDNAs to map the region of the SSAV envelope which restricts infection of E36 cells. We exchanged the regions encoding the amino-terminal portion, including VRA and VRB, between GALV-S and SSAV envelope genes and tested the susceptibility of E36 cells to infection by retroviral vectors bearing these envelope chimeras (Fig. 2). E1 chimeric envelope was constructed by replacing the portion encoding the first 219 amino acids of the SSAV envelope gene with the corresponding GALV-S region; E2 was constructed in a similar manner, where the portion encoding the first 226 amino acids of GALV-S envelope was replaced with the corresponding region of SSAV. E36 cells are resistant to vectors containing the full-length SSAV envelope. However, vectors bearing the E1 chimeric envelope infected E36 cells, and the titer of these vectors in E36 cells was similar to that of vectors bearing the full-length GALV-S envelope. Conversely, the vectors bearing the E2 chimeric envelope proteins failed to infect E36 cells. These results demonstrate that vectors bearing the N-terminal portion of the SSAV envelope cannot infect E36 cells, in correlation with the inability of SSAV to infect E36 cells (Table 2).
FIG. 2.
Schematic representation of chimeric envelopes, and results of infection with vectors bearing various SSAV, GALV-S, and SSAV/GALV-S chimeric envelopes in E36 and MDTFPit1 cells. For each construct, sequences from the SSAV envelope are in white, and those from GALV-S envelope are in black. A, VRA; B, VRB. Plasmids with various envelope chimeras were cotransfected with plasmids encoding MoMuLV core proteins and packageable genome into 293T cells. The supernatant was harvested and used to infect E36 and MDTFPit1 cells as described in Materials and Methods. The titers were averaged from at least three independent experiments and are expressed as mean number of β-galactosidase-expressing cells ± standard deviation of the mean.
DISCUSSION
E36 cells can be infected by the four strains of GALV but not by the closely related retrovirus SSAV. Blocks to viral infection can occur at any one of several stages in the infection process: (i) binding of the virus to its receptor; (ii) fusion; (iii) after entry but before reverse transcription of the viral RNA into DNA; (iv) after reverse transcription but before integration of the double-stranded DNA intermediate; or (v) after integration (including viral transcription and/or viral assembly). We show here that SSAV replication in E36 cells is blocked prior to reverse transcription of the viral RNA into double-stranded DNA, suggesting that the block occurs at an early stage of infection. The vectors with SSAV envelope are unable to infect E36 cells, suggesting that this block is envelope mediated. An E36-derived receptor, HaPit2, can function as a receptor for SSAV when expressed in murine cells, even though this receptor when expressed in E36 cells fails to facilitate SSAV entry. Together these data show that the block to SSAV infection of E36 cells occurs at the stage of entry.
There are several possible explanations for the ability of SSAV to infect MDTF cells expressing the E36 Pit 2 homolog, HaPit2, but not E36 cells. The block to SSAV infection of E36 cells may be due to cell-specific posttranslational modification of the receptor. For example, the HaPit2 protein, unlike the human homolog, has a consensus sequence for N-linked glycosylation present in the second extracellular domain (33). We have previously shown that differences in glycosylation can affect ecotropic MuLV (E-MuLV) receptor function in murine and hamster cells (11, 31). The E-MuLV receptor expressed in MDTF cells functions for all E-MuLVs except MoMuLV (11). Inhibition of N-linked glycosylation in these cells or site-specific mutagenesis of one of two potential N-linked glycosylation sites in the MDTF E-MuLV receptor renders the receptor functional for MoMuLV (11). However, pretreatment of E36 hamster cells with tunicamycin, an inhibitor of N-linked glycosylation, does not allow SSAV to infect E36 cells (data not shown), suggesting that N-linked glycosylation by itself does not account for the loss of SSAV receptor function for the endogenous receptor in E36 cells. Alternatively, cellular factors in addition to the viral receptor may influence the ability of SSAV to use the E36 GALV receptors. At least 10 coreceptors (CCR5, CXCR4, CCR3, CCR2b, STRL33, GPR15, GPR1, V28, CCR8, and US28) have been identified to be used by HIV and simian immunodeficiency virus (2, 7) and participate in the postbinding stage of entry. Furthermore, the requirement for cellular factors has been shown to be both cell type and viral strain dependent in a manner similar to what we have observed for GALV-S and SSAV. E36 cells may either express specific factors which inhibit SSAV entry or lack accessory proteins, present in murine MDTF cells, which are required for HaPit1/HaPiT2-mediated SSAV entry.
We have sequenced the SU (surface) and TM regions of the envelope gene from GALV-S, -H, -Br, and -SF, as well as SSAV, to determine what region(s) of SSAV envelope correlate with its inability to infect E36 cells. The GALV-S envelope open reading frame extends an additional 54 bp from that originally proposed by Delassus et al. (6). The protein expressed by the open reading frame predicted from the previously published sequence is highly fusogenic when expressed in mammalian cells (11a). In contrast, the full-length GALV-S envelope does not induce cell-cell fusion when expressed in murine cells or in human 293T cells. This finding is consistent with a previous report that cleavage of the corresponding C-terminal 16 residues of the E-MuLV envelope activates membrane fusion (24, 25) and suggests that the fusogenic properties that result from the removal of the terminal 16 residues of the E-MuLV TM also occurs in other members of the mammalian type C family of retroviruses.
The 10A1 MuLV, feline leukemia virus subgroup B (FeLV-B), and each of the GALV strains have been demonstrated to use Pit1 as a receptor to infect human cells (27, 32, 33). Two distinct regions, VRA and VRB (1), within the SU of MuLV envelopes are involved in receptor utilization. Interestingly, comparison of the FeLV-B (3), 10A1 (23), SSAV (13), and GALV envelopes (6) in the regions corresponding to VRA and VRB reveals considerably divergent amino acid sequences (Fig. 4), despite their common receptor utilization. VRA of FeLV-B contains 37 residues, compared to 42 residues in 10A1 and 68 in SSAV and GALV-S. VRB is much longer for both FeLV-B and 10A1 (30 residues) than for GALV-S or SSAV (12 residues). The sequences comprising VRA and VRB for FeLV-B, 10A1, and SSAV/GALV-S are not closely related, indicating that Pit1 receptor recognition does not impose substantial conservation of SU envelope sequence or that other regions are involved in receptor recognition for this group of retroviruses. Amphotropic-MuLV enveloped vectors do not utilize Pit1 as a receptor but can be modified to have 10A1 host range properties by replacing as few as three residues in their SU regions, two within VRA and one within VRB, with the corresponding 10A1 envelope residues (15). FeLV-A can be modified to utilize Pit1 as a receptor by substituting 37 residues corresponding to VRA of FeLV-B (4).
FIG. 4.
Protein sequence alignment of the GALV-S, SSAV, 10A1 MuLV, and FeLV-B envelope VRA and VRB. Gaps in the alignment are indicated by dashes, with the consensus sequence indicated on the bottom line. Residue numbers correspond to those in the mature envelope protein after removal of the signal peptide.
In this report, we have shown that the N-terminal half of GALV-S envelope, encompassing VRA and VRB, can be substituted for the corresponding residues within the SSAV SU, and vectors bearing these chimeric envelopes, in contrast to vectors bearing SSAV envelopes, are able to infect hamster E36 cells. There are 12 amino acid residues that differ between the 68-residue VRA sequences of GALV-S and SSAV, and 9 of the 12 residues in their VRB sequences differ. Further analysis of the SSAV and GALV-S envelopes may distinguish more specifically the regions or residues which are important for E36 receptor recognition and fusion.
REFERENCES
- 1.Battini J-L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A):S3–S16. [PubMed]
- 3.Boomer S, Gasper P, Whalen L R, Overbaugh J. Isolation of a novel subgroup B feline leukemia virus from a cat infected with FeLV-A. Virology. 1994;204:805–810. doi: 10.1006/viro.1994.1597. [DOI] [PubMed] [Google Scholar]
- 4.Boomer S, Eiden M V, Burns C C, Overbaugh J. Three distinct envelope domains, variably present in subgroup B feline leukemia virus recombinants, mediate Pit1 and Pit2 receptor recognition. J Virol. 1997;71:8116–8123. doi: 10.1128/jvi.71.11.8116-8123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapham P R, Blanc D, Weiss R A. Specific cell surface requirements for the infection of CD4-positive cells by human immunodeficiency virus type 1 and 2 and by simian immunodeficiency virus. Virology. 1991;181:703–715. doi: 10.1016/0042-6822(91)90904-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delassus S, Sonigo P, Wain-Hobson S. Genetic organization of gibbon ape leukemia virus. Virology. 1989;173:205–213. doi: 10.1016/0042-6822(89)90236-5. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov D S. How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity. Cell. 1997;91:721–730. doi: 10.1016/s0092-8674(00)80460-2. [DOI] [PubMed] [Google Scholar]
- 8.Dragic T, Charneau P, Clavel F, Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992;66:4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragic T, Alizon M. Different requirements for membrane fusion mediated by the envelopes of human immunodeficiency virus type 1 and 2. J Virol. 1993;67:2355–2359. doi: 10.1128/jvi.67.4.2355-2359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eglitis M A, Eiden M V, Wilson C A. Gibbon ape leukemia virus and the amphotropic murine leukemia virus 4070A exhibit an unusual interference pattern on E36 Chinese hamster cells. J Virol. 1993;67:5472–5477. doi: 10.1128/jvi.67.9.5472-5477.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eiden M V, Farrell K B, Wilson C A. Glycosylation-dependent inactivation of the ecotropic murine leukemia virus receptor. J Virol. 1994;68:626–631. doi: 10.1128/jvi.68.2.626-631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Finer, M. Personal communication.
- 12.Finer M H, Dull T J, Qin L, Farson D, Roberts M R. Kat: a high efficiency retroviral transduction system for primary human T lymphocytes. Blood. 1994;83:43–50. [PubMed] [Google Scholar]
- 13.Gelmann E P, Wong-Staal F, Kramer R, Gallo R C. Molecular cloning and comparative analysis of the genomes of simian sarcoma virus and its associated helper virus. Proc Natl Acad Sci USA. 1981;78:3373–3377. doi: 10.1073/pnas.78.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelmann E P, Trainor C D, Wong-Staal F, Reitz M S. Molecular cloning of circular unintegrated DNA of two types of the SEATO strain of gibbon ape leukemia virus. J Virol. 1982;44:269–275. doi: 10.1128/jvi.44.1.269-275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J-Y, Cannon P M, Lai K-M, Zhao Y, Eiden M V, Anderson W F. Identification of envelope protein residues required for the expanded host range of 10A1 murine leukemia virus. J Virol. 1997;71:8103–8108. doi: 10.1128/jvi.71.11.8103-8108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington R D, Geballe A P. Cofactor requirement for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 18.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell surface receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Colding H. Evidence for cell-surface association between fusion and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 20.Maddon P J, Dalgleish A G, McDougal J S, Weiss P R, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 21.O’Hara B, Johann S V, Klinger H P, Blair D G, Rubinson H, Dunne K J, Sass P, Vitek S M, Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Diff. 1990;1:119–127. [PubMed] [Google Scholar]
- 22.Olah Z, Lehel C, Anderson W B, Eiden M V, Wilson C A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity phosphate transporter. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 23.Ott D, Friedrich R, Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990;64:757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p2E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rein A, Mirro J, Gordon J A, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott M L, McKereghan K, Kaplan H S, Fry K E. Molecular cloning and partial characterization of unintegrated linear DNA from gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1981;78:4213–4217. doi: 10.1073/pnas.78.7.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi Y, Vile R G, Simpson G, O’Hara B, Collins M K L, Weiss R A. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992;66:1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Mayer C C, Robinson J, Maddon P J, Moore J P. CD4-dependent antibody-sensitive interactions between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 29.Weiss R A, Tailor C S. Retrovirus receptors. Cell. 1995;82:531–533. doi: 10.1016/0092-8674(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 30.Wilson C A, Reitz M S, Okayama H, Eiden M V. Formation of infectious hybrid virions with gibbon ape leukemia virus and human T-cell leukemia virus retroviral envelope glycoproteins and the Gag and Pol proteins of Moloney murine leukemia virus. J Virol. 1989;63:2374–2378. doi: 10.1128/jvi.63.5.2374-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson C A, Eiden M V. Viral and cellular factors governing hamster cell infection by murine and gibbon ape leukemia viruses. J Virol. 1991;65:5975–5982. doi: 10.1128/jvi.65.11.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson C A, Farrell K B, Eiden M V. Comparison of cDNAs encoding the gibbon ape leukemia virus receptor from susceptible and non-susceptible murine cells. J Gen Virol. 1994;75:1901–1908. doi: 10.1099/0022-1317-75-8-1901. [DOI] [PubMed] [Google Scholar]
- 33.Wilson C A, Farrell K B, Eiden M V. Properties of a unique form of the murine amphotropic leukemia virus receptor expressed on hamster cells. J Virol. 1994;68:7697–7703. doi: 10.1128/jvi.68.12.7697-7703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson C A, Eiden M V, Marsh J W. Quantitative micro P30 and reverse transcriptase assays for Moloney murine leukemia virus. J Virol Methods. 1994;48:109–118. doi: 10.1016/0166-0934(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD-4 induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor for CCR5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]