Highlights
-
•
Severe asthma exacerbation (SAE) is a life-threatening condition and there is a lack of data regarding patients admitted in ICU.
-
•
The proportion of SAE patients admitted in ICU decreases over time.
-
•
Patients with SAE in ICU are older and more severe, however the use of mechanical ventilation remains uncommon, and catecholamine use decreases.
-
•
ICU and hospital mortality decreases over time.
Keywords: Epidemiology, Intensive care unit, Mechanical ventilation, Severe asthma exacerbation
Abstract
Background
Despite advances in asthma treatments, severe asthma exacerbation (SAE) remains a life-threatening condition in adults, and there is a lack of data derived from adult patients admitted to intensive care units (ICUs) for SAE. The current study investigated changes in adult patient characteristics, management, and outcomes of SAE over a 20-year period in 40 ICUs in the greater Paris area.
Methods
In this retrospective observational study, admissions to 40 ICUs in the greater Paris area for SAE from January 1, 1997, to December 31, 2016 were analyzed. The primary outcome was the proportion of ICU admissions for SAE during 5-year periods. Secondary outcomes were ICU and hospital mortality, and the use of mechanical ventilation and catecholamine. Multivariate analysis was performed to assess factors associated with ICU mortality.
Results
A total of 7049 admissions for SAE were recorded. For each 5-year period, the proportion decreased over time, with SAE accounting for 2.84% of total ICU admissions (n=2841) between 1997 and 2001, 1.76% (n=1717) between 2002 and 2006, 1.05% (n=965) between 2007 and 2011, and 1.05% (n=1526) between 2012 and 2016. The median age was 46 years (interquartile range [IQR]: 32–59 years), 55.41% were female, the median Simplified Acute Physiology Score II was 20 (IQR: 13–28), and 19.76% had mechanical ventilation. The use of mechanical ventilation remained infrequent throughout the 20-year period, whereas the use of catecholamine decreased. ICU and hospital mortality rates decreased. Factors associated with ICU mortality were renal replacement therapy, catecholamine, cardiac arrest, pneumothorax, acute respiratory distress syndrome, sepsis, and invasive mechanical ventilation (IMV). Non-survivors were older, had more severe symptoms, and were more likely to have received IMV.
Conclusion
ICU admission for SAE remains uncommon, and the proportion of cases decreased over time. Despite a slight increase in symptom severity during a 20-year period, ICU and hospital mortality decreased. Patients requiring IMV had a higher mortality rate.
Introduction
In recent decades, asthma prevalence and severity have increased worldwide. Asthma tends to be a lifelong condition associated with high morbidity and an economic burden on patients, their families, and society.[1] Asthma can involve acute exacerbations leading to acute respiratory failure requiring admission to an intensive care unit (ICU) for monitoring and/or mechanical ventilation. The latest French surveys indicate a prevalence of current asthma of 6–7% in adults [2] and a decline in asthma mortality since 1986.[3] Asthma causes approximately 250,000 deaths a year worldwide, and despite advances in the field, it is still an underlying cause of death in France. While severe asthma exacerbation (SAE) is a life-threatening condition in adults,[4] evidence is sparse regarding management and outcomes in ICUs, where it may account for up to 1% of mechanically ventilated patients.[5]
Recent studies from England and Wales investigated the demographics, outcomes, and trends of admission in patients with SAE in critical care units between 2002 and 2011, and demonstrated that it represents a modest burden of work with a high survival rate in ICUs.[6] Another cohort in Australia, spanning from 1996 to 2003, showed a reduction in the proportion of ICU admissions for SAE, and reduced mortality.[7,8] Non-invasive mechanical ventilation (NIV) use for SAE seems to be increasing, and few data exist regarding the use of IMV in this population.[5,9] The aim of the current retrospective study was to investigate patient characteristics, ICU management, and outcomes of adult SAE admissions in 40 ICUs in the greater Paris area from the Collège des Utilisateurs de Bases de données en Réanimation (CUB-REA) network [10] over a 20-year period.
Methods
Study and patients
The CUB-REA network was used, which prospectively collects data from all patients admitted to 40 ICUs in the greater Paris area, as described previously.[11] Participating centers are listed in the supplementary material online (Appendix S1). All adult admissions for SAE from January 1, 1997, to December 31, 2016, were included. Only patients with a primary or secondary diagnosis of SAE were included. Admissions with an associated chronic obstructive pulmonary disease (COPD) diagnosis were excluded.
Definitions
SEA was defined as all hospital stays with a primary diagnosis of SAE or status asthmaticus, or with SAE as a secondary diagnosis associated with cardiac arrest, acute respiratory failure, acute respiratory distress syndrome (ARDS), pulmonary infection (viral or bacterial), bronchitis, influenza or pleural effusion, sepsis, systemic inflammatory response syndrome, hemoptysis, or pneumothorax. The exclusion criterion was any diagnosis of COPD.
Data collection
Demographic characteristics including age, sex, and Charlson Comorbidity Index (CCI) were collected, as were life support treatments including catecholamine, NIV, invasive mechanical ventilation (IMV), renal replacement therapy (RRT), and extracorporeal life support (ECLS). Severity was assessed using the Simplified Acute Physiology Score II (SAPS II).
Outcomes
The primary outcome was the proportion of ICU admissions for SAE. Secondary outcomes were ICU and hospital mortality, diagnoses associated with SAE, and the use of NIV, IMV, catecholamine, RRT, and ECLS.
Statistical analysis
Quantitative data were expressed as medians and interquartile ranges (IQRs) and compared via analysis of variance. Categorical data were expressed as numbers and percentages, and compared via the χ2 test. The primary outcome was the proportion of ICU admissions for SAE, divided into four 5-year time periods (1997–2001, 2002–2006, 2007–2011, 2012–2016). Multivariable analyses of ICU mortality were performed using a generalized linear model including all variables with a P-value below 10 % in univariate analysis. Model selection was then performed based on a backward stepwise procedure in order to retain the best model according to the Akaike Information Criterion.[12] Patients with missing data on hospital mortality were considered dead. All analyses were two-sided, and P <0.10 was considered statistically significant. Statistical analysis was performed using R studio software (Integrated Development for R. RStudio, Inc., Boston, MA, 2019).
Results
Population characteristics
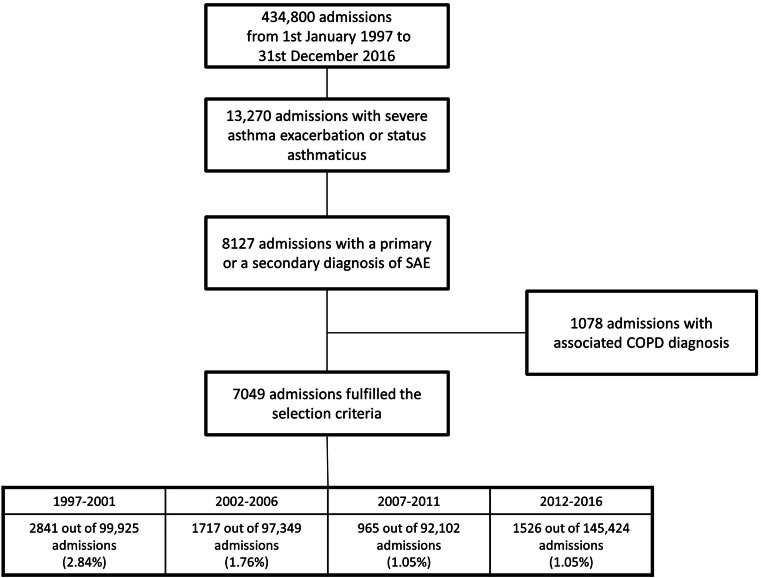
During the study period, 434,800 consecutive ICU admissions were recorded at the 40 participating ICUs of Cub-Réa. A total of 13,270 admissions with an SAE code were reviewed (3.05%). Of these, 7049 admissions for SAE without an associated diagnosis of COPD were included in the analysis (Figure 1). The median age was 46 years (IQR: 32–59 years), 3906 (55.41 %) were female, the median SAPS II was 20 (IQR: 13–28), and the median CCI was 0 (IQR: 0–1). Among the 7049 admissions, 15.19% (n=1071) received IMV, 6.10% (n=430) received NIV, and 1.53% (n=108) received both NIV and IMV. Catecholamines were used in 17.69% (n=1247) of admissions and RRT was used in 0.60% (n=42). The ICU mortality rate was 2.41% (n=170) and the hospital mortality rate was 3.90% (n=275, Table 1).
Figure 1.
Flowchart representing the current population-based study investigating admissions for SAE or status asthmaticus without associated COPD diagnosis in the 40 CUB-Réa ICUs, divided into four 5-year periods.
COPD: Chronic obstructive pulmonary disease; ICUs: Intensive care units; SAE: Severe asthma exacerbation.
Table 1.
Trends in demographic characteristics, management, and outcomes in adult SAE patients admitted to the ICU over the study period.
| Patients' characteristics and outcomes | Missing data | Total period (n=7049) | 1997–2001 (n=2841) | 2002–2006 (n=1717) | 2007–2011 (n=965) | 2012–2016 (n=1526) | P-value |
|---|---|---|---|---|---|---|---|
| Patients admitted in ICU | NA | 434,800 | 99,925 | 97,349 | 92,102 | 145,424 | |
| Number of patients with SAE | NA | 7049 (1.62) | 2841 (2.84) | 1717 (1.76) | 965 (1.05) | 1526 (1.05) | <0.001 |
| Age (years) | 0 | 46 (32–59) | 44 (32–58) | 46 (32–58) | 49 (36–61) | 46 (32–60) | <0.001 |
| Female | 4 | 3906 (55.41) | 1598 (56.24) | 931 (54.22) | 519 (53.78) | 858 (56.22) | 0.335 |
| SAPS II | 48 | 20 (13–28) | 18 (13–26) | 20 (13–28) | 22 (15–30) | 22 (15–30) | <0.001 |
| CCI | 0 | 0 (0–1) | 0 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) | <0.001 |
| IMV | 0 | 1071 (15.19) | 373 (13.13) | 265 (15.43) | 201 (20.82) | 232 (15.20) | <0.001 |
| NIV | 0 | 430 (6.10) | 0 (0) | 96 (5.59) | 128 (13.26) | 206 (13.50) | <0.001 |
| NIV and IMV | 0 | 108 (1.53) | 0 (0) | 21 (1.22) | 35 (3.63) | 52 (3.41) | <0.001 |
| Catecholamine use | 0 | 1247 (17.69) | 1035 (36.43) | 209 (12.17) | 1 (0.10) | 2 (0.03) | <0.001 |
| ECMO | 0 | 8 | 0 | 1 | 1 | 6 | |
| RRT | 0 | 42 (0.60) | 10 (0.35) | 12 (0.70) | 6 (0.62) | 14 (0.92) | 0.120 |
| ICU length of stay (days) | 0 | 3 (1–5) | 2 (1–4) | 3 (2–4) | 3 (2–5) | 6 (3–11) | <0.001 |
| Hospital length of stay (days) | 62 | 6 (3–10) | 6 (3–11) | 6 (2–10) | 6 (3–10) | 6 (3–11) | 0.885 |
| ICU mortality | 0 | 170 (2.41) | 68 (2.39) | 55 (3.20) | 26 (2.69) | 21 (1.38) | 0.008 |
| Hospital mortality | 78 | 275 (3.90) | 131 (4.61) | 86 (5.00) | 32 (3.32) | 26(1.70) | 0.002 |
Data are expressed as n (%) or median (Interquartile range).
CCI: Charlson Comorbidity Index; ECMO: Extracorporeal membrane oxygenation; ICU: Intensive care unit; IMV: Invasive mechanical ventilation; NIV: Non-invasive mechanical ventilation; RRT: Renal replacement therapy; SAE: Severe asthma exacerbation; SAPS II: Simplified Acute Physiology Score II.
Proportion of ICU admissions for SAE
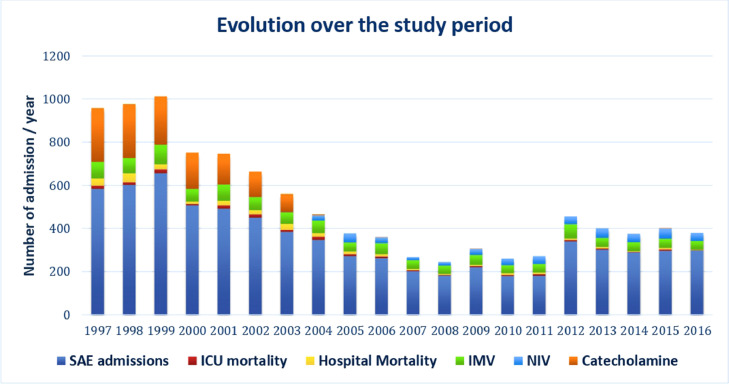
ICU admissions for SAE decreased over time (P <0.001). They accounted for 2.84% of total ICU admissions (n=2841) between 1997 and 2001, 1.67% (n=1717) between 2002 and 2006, 1.05% (n=965) between 2007 and 2011, and 1.05% (n=1526) between 2012 and 2016 (Table 1 and Figure 2). The number of admissions for SAE increased in 2012 in conjunction with an increase in the total number of ICU admissions.
Figure 2.
Changes in SAE admissions, ICU mortality, and hospital mortality, and the use of IMV, NIV, and catecholamine over the study period. The proportion of admissions to the ICU for SAE decreased over time, as did ICU mortality and hospital mortality. The use of catecholamine almost disappeared from 2005. The use of mechanical ventilation remained infrequent. ICU: Intensive care unit; IMV: Invasive mechanical ventilation; NIV: Non-invasive mechanical ventilation; SAE: Severe asthma exacerbation.
Changes over time
Median age increased over the study period, and even though patients admitted for SAE tended to be relatively young with few comorbidities, SAPS II and the CCI increased (Table 1). The use of NIV increased slightly over time but remained infrequent, whereas the use of catecholamine decreased (Table 1 and Figure 2).
Outcomes
The median ICU stay lengths were 3 days (IQR: 1–5 days) until 2011, and 6 days (IQR: 3–11 days) from 2012 to 2016 (P <0.001). The median hospital stay remained unchanged from 1997 to 2016 (6 days). ICU mortality decreased significantly over the four time periods, and it was 2.39% in the first, 3.20% in the second, 2.69% in the third, and 1.38% in the fourth (P=0.008). Hospital mortality exhibited the same trend, with respective rates of 4.61%, 5.00%, 3.32%, and 1.70% (P=0.002) (Table 1 and Figure 2).
SAE and associated diagnoses
Among the 7049 admissions for SAE throughout the study period, 534 had at least one associated diagnosis. A total of 132 admissions (1.87%) were registered with an associated cardiac arrest (Table 2).
Table 2.
Changes in associated diagnoses over the study period*.
| Associated diagnoses | Total period (n = 7049) |
1997–2001 (n = 2841) |
2002–2006 (n = 1717) |
2007–2011 (n = 965) |
2012–2016 (n = 1526) |
P value |
|---|---|---|---|---|---|---|
| Cardiac arrest | 132 (1.87) | 48 (1.69) | 35 (2.03) | 21 (2.18) | 28 (1.83) | <0.001 |
| Pneumothorax | 80 (1.13) | 22 (0.77) | 19 (1.10) | 8 (0.83) | 31 (2.03) | |
| ARDS | 64 (0.90) | 26 (0.92) | 24 (1.40) | 2 (0.21) | 12 (0.79) | |
| Sepsis | 27 (0.38) | 8 (0.28) | 8 (0.47) | 5 (0.52) | 6 (0.39) | |
| Pulmonary infection | 231 (3.28) | 52 (1.83) | 33 (1.92) | 38 (3.94) | 108 (7.08) | <0.001 |
| Bacterial pneumonia | 135 (58.44) | 18 (34.62) | 32 (96.97) | 26 (68.42) | 59 (54.63) | |
| S. pneumoniae | 13 (5.62) | 1 (1.92) | 1 (3.03) | 1 (2.63) | 10 (9.26) | |
| H. influenzae | 4 (1.73) | 0 (0) | 0 (0) | 0 (0) | 4 (3.70) | |
| Viral pneumonia | 8 (3.46) | 0 (0) | 0 (0) | 1 (2.63) | 7 (6.48) | |
| Influenzae | 41 (17.75) | 3 (5.77) | 0 (0) | 10 (26.32) | 28 (25.93) |
Data were presented as n (%).
3206 admissions had associated diagnoses, including 231 diagnoses of pulmonary infection.
ARDS: Acute respiratory distress syndrome; S. pneumoniae: Streptococcus pneumoniae; H. influenzae: Haemophilus influenzae.
SAE and respiratory infections
Pulmonary infections associated with SAE were reported in 231 of the 7049 admissions (3.28%). There was an increase in pulmonary infections over the four time periods (P <0.001, Table 2).
Factors associated with mortality
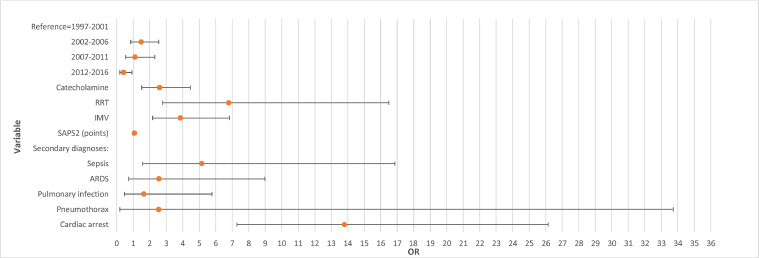
ICU mortality was 2.41% (170/7049). Patients who died tended to be older than those who did not, and the respective median ages were 64 years (IQR: 48–75 years) and 45 years (IQR: 32–58 years) (P <0.001). Their illness also tended to be more severe and they had more comorbidities. Their median SAPS II was 61 (IQR: 43–75) and their median CCI was 1 (IQR: 0–1), vs. respective values of 19 (IQR: 13–27) (P <0.001) and 0 (IQR: 0–1) in those who did not die. ICU mortality was higher in patients treated with IMV; 14.25% (150/1053) vs. 0.78% (47/5996) (P <0.001). Multivariate analysis of factors associated with mortality is shown in Figure 3. Briefly, SAPS II (odds ratio [OR]=1.06, 95% CI: 1.05 to 1.07), RRT (OR=6.78, 95% CI: 2.79 to 16.49), IMV (OR=3.86, 95% CI: 2.18 to 6.84), and catecholamine (OR=2.6, 95% CI: 1.5 to 4.5) were significantly associated with ICU mortality. Among the secondary diagnoses associated with SAE, cardiac arrest (OR=13.8, 95% CI: 7.3 to 26.1), sepsis (OR=5.15, 95% CI: 1.57 to 16.86), pneumothorax (OR=2.55, 95% CI: 0.19 to 33.73), and ARDS (OR=2.56, 95% CI: 0.73 to 8.98) were associated with ICU mortality (P <0.001). Factors associated with hospital mortality were the same (Supplementary Table S1). Comorbidities were associated with ICU mortality in multivariate analysis.
Figure 3.
Forest plot of results of multivariate analysis of factors associated with mortality.
ARDS: Acute respiratory distress syndrome; IMV: Invasive mechanical ventilation; OR: Odds ratio; RRT: Renal replacement therapy; SAPS II: Simplified Acute Physiology Score II.
Discussion
In this study, there was a decrease in the proportion of ICU admissions for SAE from 1997 to 2016. ICU and hospital mortality decreased despite increases in patient severity and age. The first report of the Global Initiative for Asthma published in 1995 provided the foundation for asthma guidelines, which have undergone a major paradigm shift; a change in the late 1990s from an opinion-based to an evidence-based approach for the management of asthma severity.[13] The notable decrease in SAE admissions during the study period may imply better control and a clearer understanding of this inflammatory disease over the years,[14] and a possible decrease in ambient air pollution. These same factors may have contributed to the observation that only older and more severely ill patients required ICU admission.
SAE is characterized by a major increase in airway resistance and a dramatic reduction in expiratory flow, with resultant major dynamic hyperinflation.[15] Studies have shown that patients with severe airflow obstruction receiving mechanical ventilation are at risk of inadvertent pulmonary hyperinflation with morbidity and mortality caused by pneumothorax and circulatory depression. Given the high mortality risk in severe intubated patients, avoiding IMV is an essential goal.[[16], [17], [18], [19], [20], [21]]
The evidence regarding the role of NIV in asthma is weak and no recommendation is offered in the latest guidelines.[22] A few uncontrolled studies have compared NIV to routine care in cases of acute respiratory failure due to SAE, and reported physiological improvements in some patients.[23,24] In several observational studies NIV improved ventilation/perfusion mismatch, decreased the work of breathing, and had a bronchodilator effect,[20,21] and it was used in patients with SAE as a means to obviate the need for intubation and IMV and associated detrimental effects.[[25], [26], [27]] A review of the effects of NIV in SAE concluded that its benefits include reducing the burden on respiratory muscles, diminishing airway resistance, and improving gas exchange.[28] Nonetheless, its use should not delay intubation when needed.[29] Notably, a meta-analysis reported that the use of NIV in SAE did not improve outcomes.[30] Given the unclear effects of NIV in SAE patients on mortality, intubation, and length of ICU stay, the European Respiratory Society/American Thoracic Society Guidelines Committee was unable to advocate any recommendation on the use of NIV for acute respiratory failure due to asthma.[31] In the present study, there were no substantial changes in IMV or NIV because their overall use was infrequent. In a large retrospective American cohort study, Althoff et al.[5] analyzed data derived from 53,653 patients from 2010 to 2017 at 682 centers in the United States. In that cohort, 25% had NIV, 27% had IMV, and 2.4 % died. In the current study, the corresponding proportions were lower. The absence of guidelines and strong recommendations may explain the differential use of NIV in the two countries.
We speculate that even though the use of mechanical ventilation remained uncommon during the study period, its management has improved over the last decade because of strategies aimed at preventing or reducing ventilator-induced lung injury and mortality.[32,33] Even if the need for IMV was associated with mortality, the need for catecholamine decreased and almost disappeared starting 2005, implying that improvement in mechanical ventilation for SAE may have reduced heart-lung interaction with the lowest dynamic hyperinflation.[34] If mortality associated with SAE remains stable despite more severe illness, it is imperative that clinicians treating such patients have a clear understanding of how gas trapping can occur, and of how it may be recognized, measured, and limited.
The major strength of the present study is the large size of the population included in the CUB-Réa network, with good reliability of data pertaining to stays, illness severity, and workload that facilitated detailed evaluation of changes over two decades.[10] Notably, however, a lack of reproducibility of diagnosis coding in the CUB-Réa network, the retrospective design of this study, and the restraints of the database constitute study limitations.
The parameters of mechanical ventilation were not recorded, and neither were details on the timing of ventilation, the sequence of ventilation modalities, or the use of medical therapy. Pre-admission data such as maintenance therapy, parameters of gas exchange, body mass index, and smoker status were also not mentioned in the database. Throughout the two-decade study period, the need for catecholamine decreased – almost disappearing from 2005 – and outcomes of severe asthma improved, with fewer deaths, suggesting better management of hyperinflation. Notably, however, a lack of tracking negated clear identification of a possible association between progress in mechanical ventilation techniques and improved hyperinflation management, and mortality rates related to other interventions such as ECLS were not recorded.
ECLS was seldom used during the 20-year study period, and the type of extracorporeal membrane oxygenation, whether it was veno-venous or veno-arterial, was not specified. There may also be bias in the coding of associated diagnoses. Even though there was an increase in the incidence of infections the numbers remained low, probably due to a lack of registration in the database. Whether infections were nosocomial or community-acquired was not specified in the database.
Conclusions
The proportion of ICU admissions for SAE decreased over time, and patient age, severity of illness, and CCI increased. The use of mechanical ventilation was uncommon, but patients requiring IMV had a higher mortality rate. Catecholamine use and mortality rates significantly decreased over time. This likely resulted from better management of SAE in ICUs.
Author Contributions
Conceptualization: Romy Younan, Jean Loup Augy, and Nadia Aissaoui. Acquisition, analysis, or interpretation of the data: Romy Younan, Jean Loup Augy, Ana Novara. Statistical analysis: Jean Loup Augy and Bertrand Hermann. Drafting of the manuscript and editing: Romy Younan, Jean Loup Augy, Bertrand Hermann, Bertrand Guidet, Philippe Aegerter,Nicolas Peron, Emmanuel Guerot, Ana Novara, Caroline Hauw-Berlemont, Amer Hamdan, Clotilde Bailleul, Francesca Santi, Jean-Luc Diehl, Nadia Aissaoui.
Acknowledgments
The authors thank all the participating members of the CUB-Réa Database.
Funding
CUB-Réa was initially funded by the Paris public hospital system, Assistance Publique-Hôpitaux de Paris.
Ethics Statement
In line with the French regulations for the ethical use of personal data, the CUB-Réa project was approved by the Commission National Informatique et Liberté (French Data-protection Watchdog agreement #564407). The present study was also approved by the ethics committee of the Société de Réanimation de Langue Française (French Society of Intensive Care Medicine) with a waiver of informed consent.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Managing Editor: Jingling Bao/ Zhiyu Wang
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jointm.2023.08.008.
Appendix. Supplementary materials
References
- 1.Nunes C., Pereira A.M., Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1):1. doi: 10.1186/s40733-016-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuhrman C., Delmas M.C., pour le groupe épidémiologie et recherche clinique de la SPLF Epidemiology of chronic obstructive pulmonary disease in France. Rev Mal Respir. 2010;27(2):160–168. doi: 10.1016/j.rmr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Tual S., Godard P., Bousquet J., Annesi-Maesano I. The decrease in asthma-related mortality in France. Rev Mal Respir. 2010;27(7):e1–e5. doi: 10.1016/j.rmr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Engelkes M., de Ridder M.A., Svensson E., Berencsi K., Prieto-Alhambra D., Lapi F., et al. Multinational cohort study of mortality in patients with asthma and severe asthma. Respir Med. 2020;165 doi: 10.1016/j.rmed.2020.105919. [DOI] [PubMed] [Google Scholar]
- 5.Althoff M.D., Holguin F., Yang F., Grunwald G.K., Moss M., Vandivier R.W., et al. Noninvasive ventilation use in critically ill patients with acute asthma exacerbations. Am J Respir Crit Care Med. 2020;202(11):1520–1530. doi: 10.1164/rccm.201910-2021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbison B., Griggs K., Mukherjee M., Sheikh A. Ten years of asthma admissions to adult critical care units in England and Wales. BMJ Open. 2013;3(9) doi: 10.1136/bmjopen-2013-003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stow P.J., Pilcher D., Wilson J., George C., Bailey M., Higlett T., et al. Improved outcomes from acute severe asthma in Australian intensive care units (1996 2003) Thorax. 2007;62(10):842–847. doi: 10.1136/thx.2006.075317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghaddas F., Smith C., Pilcher D., O'Hehir R., Hew M., Dabscheck E. Need for intensive care in patients admitted for asthma: red flags from the social history. Respirol. 2016;21(7):1251–1254. doi: 10.1111/resp.12831. [DOI] [PubMed] [Google Scholar]
- 9.Abdelkarim H., Durie M., Bellomo R., Bergmeir C., Badawi O., El-Khawas K., et al. A comparison of characteristics and outcomes of patients admitted to the ICU with asthma in Australia and New Zealand and United States. J Asthma. 2020;57(4):398–404. doi: 10.1080/02770903.2019.1571082. [DOI] [PubMed] [Google Scholar]
- 10.Aegerter P., Auvert B., Buonamico G., Sznajder M., Beauchet A., Guidet B., et al. Organization and quality control of a clinical database on intensive care medicine in central and suburban Paris. Rev Epidemiol Sante Publique. 1998;46(3):226–237. [PubMed] [Google Scholar]
- 11.Puymirat E., Fagon J.Y., Aegerter P., Diehl J.L., Monnier A., Hauw-Berlemont C., et al. Cardiogenic shock in intensive care units: evolution of prevalence, patient profile, management and outcomes, 1997-2012. Eur J Heart Fail. 2017;19(2):192–200. doi: 10.1002/ejhf.646. [DOI] [PubMed] [Google Scholar]
- 12.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 13.Kroegel C. Global initiative for asthma (GINA) guidelines: 15 years of application. Expert Rev Clin Immunol. 2009;5(3):239–249. doi: 10.1586/eci.09.1. [DOI] [PubMed] [Google Scholar]
- 14.Ramsahai J.M., Hansbro P.M., Wark P.A.B. Mechanisms and management of asthma exacerbations. Am J Respir Crit Care Med. 2019;199(4):423–432. doi: 10.1164/rccm.201810-1931CI. [DOI] [PubMed] [Google Scholar]
- 15.Oddo M., Feihl F., Schaller M.D., Perret C. Management of mechanical ventilation in acute severe asthma: practical aspects. Intensive Care Med. 2006;32(4):501–510. doi: 10.1007/s00134-005-0045-x. [DOI] [PubMed] [Google Scholar]
- 16.Tuxen D.V., Lane S. The effects of ventilatory pattern on hyperinflation, airway pressures, and circulation in mechanical ventilation of patients with severe air-flow obstruction. Am Rev Respir Dis. 1987;136(4):872–879. doi: 10.1164/ajrccm/136.4.872. [DOI] [PubMed] [Google Scholar]
- 17.Brenner B., Corbridge T., Kazzi A. Intubation and mechanical ventilation of the asthmatic patient in respiratory failure. J Allergy Clin Immunol. 2009;124(2 Suppl):S19–S28. doi: 10.1016/j.jaci.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Gupta D., Keogh B., Chung K.F., Ayres J.G., Harrison D.A., Goldfrad C., et al. Characteristics and outcome for admissions to adult, general critical care units with acute severe asthma: a secondary analysis of the ICNARC case mix programme database. Crit Care. 2004;8(2):R112–R121. doi: 10.1186/cc2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro J.M. Intensive care management of status asthmaticus. Chest. 2001;120(5):1439–1441. doi: 10.1378/chest.120.5.1439. [DOI] [PubMed] [Google Scholar]
- 20.Soroksky A., Klinowski E., Ilgyev E., Mizrachi A., Miller A., Ben Yehuda T.M., et al. Noninvasive positive pressure ventilation in acute asthmatic attack. Eur Respir Rev. 2010;19(115):39–45. doi: 10.1183/09059180.00006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández M.M., Villagrá A., Blanch L., Fernández R. Non-invasive mechanical ventilation in status asthmaticus. Intensive Care Med. 2001;27(3):486–492. doi: 10.1007/s001340100853. [DOI] [PubMed] [Google Scholar]
- 22.Levy M.L., Bacharier L.B., Bateman E., Boulet L.P., Brightling C., Buhl R., et al. Key recommendations for primary care from the 2022 global initiative for asthma (GINA) update. NPJ Prim Care Respir Med. 2023;33(1):7. doi: 10.1038/s41533-023-00330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murase K., Tomii K., Chin K., Tsuboi T., Sakurai A., Tachikawa R., et al. The use of non-invasive ventilation for life-threatening asthma attacks: changes in the need for intubation. Respirology. 2010;15(4):714–720. doi: 10.1111/j.1440-1843.2010.01766.x. [DOI] [PubMed] [Google Scholar]
- 24.Ganesh A., Shenoy S., Doshi V., Rishi M., Molnar J. Use of noninvasive ventilation in adult patients with acute asthma exacerbation. Am J Ther. 2015;22(6):431–434. doi: 10.1097/MJT.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 25.Hill N.S., Brennan J., Garpestad E., Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35(10):2402–2407. doi: 10.1097/01.CCM.0000284587.36541.7F. [DOI] [PubMed] [Google Scholar]
- 26.Kostakou E., Kaniaris E., Filiou E., Vasileiadis I., Katsaounou P., Tzortzaki E., et al. Acute severe asthma in adolescent and adult patients: current perspectives on assessment and management. J Clin Med. 2019;8(9):1283. doi: 10.3390/jcm8091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallin M., Naughton M.T. Noninvasive ventilation in acute asthma. J Crit Care. 2014;29(4):586–593. doi: 10.1016/j.jcrc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Diehl J.L., Guérot E. Non-invasive ventilation in severe asthma attacks. Minerva Anestesiol. 2013;79(8):926–933. [PubMed] [Google Scholar]
- 29.Stefan M.S., Nathanson B.H., Lagu T., Priya A., Pekow P.S., Steingrub J.S., et al. Outcomes of noninvasive and invasive ventilation in patients hospitalized with asthma exacerbation. Ann Am Thorac Soc. 2016;13(7):1096–1104. doi: 10.1513/AnnalsATS.201510-701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim W.J., Mohammed Akram R., Carson K.V., Mysore S., Labiszewski N.A., Wedzicha J.A., et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD004360.pub4. [DOI] [PubMed] [Google Scholar]
- 31.Rochwerg B., Brochard L., Elliott M.W., Hess D., Hill N.S., Nava S., et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 32.Peñuelas O., Muriel A., Abraira V., Frutos-Vivar F., Mancebo J., Raymondos K., et al. Inter-country variability over time in the mortality of mechanically ventilated patients. Intensive Care Med. 2020;46(3):444–453. doi: 10.1007/s00134-019-05867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leatherman J. Mechanical ventilation for severe asthma. Chest. 2015;147(6):1671–1680. doi: 10.1378/chest.14-1733. [DOI] [PubMed] [Google Scholar]
- 34.Stather D.R., Stewart T.E. Clinical review: mechanical ventilation in severe asthma. Crit Care. 2005;9(6):581–587. doi: 10.1186/cc3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.