Abstract
Background
Tranexamic acid (TXA) is an antifibrinolytic agent that reduces bleeding in a multitude of clinical settings from postpartum hemorrhage to trauma. TXA may have clinical effects unrelated to bleeding; plasminogen, the target of TXA, alters immune responses, and TXA appears to decrease the risk of infection in patients undergoing cardiac surgery, as well as joint arthroplasty.
Objectives
To address whether TXA alters rates of infection and inflammatory outcomes in patients with hematologic malignancies.
Methods
We performed a post hoc analysis of outcomes of patients randomized to receive either TXA or placebo in the double-blinded, multicenter American Trial to Evaluate Tranexamic Acid Therapy in Thrombocytopenia (Clinicaltrials.gov identifier: NCT02578901).
Results
TXA did not change the overall rate of infections, but the rate of severe infections (Common Toxicology Criteria for Adverse Events grade 3+) was lower in patients who received TXA compared with the placebo group. Patients who experienced grade 3+ infections had higher rates of World Health Organization grade 2+ bleeding and red blood cell transfusion requirements than patients who did not experience a grade 3+ infection, irrespective of treatment group. TXA did not impact other inflammatory outcomes such as mucositis, rash, or graft vs host disease.
Conclusion
Patients with hematologic malignancies who received TXA had less severe infections than those who received placebo with no difference in overall rate of infection or other inflammatory outcomes. Further investigation is needed on the impact of TXA on infections in this population.
Keywords: fibrinolytic agents, hematologic malignancies, infection, thrombocytopenia, tranexamic acid
Essentials
-
•
Tranexamic acid has been shown to reduce the risk of some surgical site infections.
-
•
Patients with hematologic malignancies received either tranexamic acid or a placebo.
-
•
There were less severe (grade 3+) infections in recipients of tranexamic acid vs placebo.
-
•
There was no difference in overall rate of infections or other inflammatory outcomes.
1. Introduction
Tranexamic acid (TXA) is an antifibrinolytic agent used in a wide range of clinical situations, from menorrhagia to trauma. TXA blocks lysine binding sites on plasminogen, thereby inhibiting plasmin formation and fibrinolysis. Most studies of TXA have focused on bleeding outcomes, but its target, plasminogen, has biological effects beyond fibrinolysis. Plasminogen shifts inflammatory responses by altering interactions with complement, immune cell migration, and cytokine responses [1,2]. TXA has been shown to reduce inflammatory markers and endothelial injury in animal and in vitro models [[3], [4], [5], [6]]. Clinical data on TXA and inflammation are limited to the surgical setting in patients without hematologic malignancies. TXA has been shown to decrease circulating levels of D-dimer, plasminogen activator inhibitor 1, and creatinine kinase in patients undergoing coronary artery bypass, which correlated with a decrease in vasoplegic shock, use of norepinephrine, and duration of mechanical ventilation [7]. However, another surgical study suggested a paradoxical increase in systemic inflammatory markers such as interleukin-6 but did not evaluate clinical outcomes associated with inflammation [8]. TXA may also mitigate infection risk. Perioperative TXA reduces the rate of surgical site infections in some surgical settings. Cardiac surgery patients who received perioperative TXA had altered immune marker expression on immune cell subsets and reduced levels of proinflammatory cytokines, which correlated with a lower rate of postoperative infection [9]. The use of TXA in patients undergoing primary or revision total joint arthroplasty for aseptic failure has been associated with reduced risks of subsequent acute periprosthetic joint infection [[10], [11], [12], [13]]. However, the effect of TXA on infection rates in patients undergoing shoulder arthroplasty was nonestimable due to lack of infection events in a recent meta-analysis [14]. Although the data on trauma are limited, a study of military personnel treated with TXA for life-threatening injuries showed no impact on infections [15]. In nonsurgical populations, it is not known whether TXA alters inflammatory or infectious outcomes.
We investigated the use of TXA in patients with hypoproliferative thrombocytopenia associated with treatment for hematologic malignancy in a multicenter, double-blind, randomized controlled trial, the American Trial to Evaluate Tranexamic Acid Therapy in Thrombocytopenia (A-TREAT) [16]. Unlike surgical and general patient populations, patients with hematological malignancies are at higher risk for infections due to treatment, often combined with a disease-related immunocompromised state. While TXA did not improve bleeding outcomes in A-TREAT, the study offers a unique patient population to analyze the effects of TXA on infectious and/or inflammatory outcomes such as fever, rash, and mucositis. In A-TREAT, a subgroup of patients underwent allogeneic hematopoietic stem cell transplantation, which may be associated with life-threatening inflammatory complications, including graft vs host disease, sinusoidal obstruction syndrome, and diffuse alveolar hemorrhage. The high incidence and variety of inflammatory clinical outcomes make this an ideal population in which to evaluate the effect of TXA on inflammation and infection.
2. Methods
This is a post hoc analysis of the A-TREAT clinical trial data. The A-TREAT protocol has been published, and the trial was registered with Clinicaltrials.gov (NCT02578901) [16]. Patients aged >18 years were eligible if they had a hematologic malignancy or aplasia and were undergoing chemotherapy, immunotherapy, or hematopoietic stem cell transplant and anticipated to have hypoproliferative thrombocytopenia with a platelet count of ≤10,000/μL for a minimum of 5 days. Each institutional review board approved the study. A subset of patients consented to a separate laboratory study evaluating fibrinolysis and cytokine responses [17]. The last patient was enrolled in February 2020. Once the platelet count fell <30,000/μL, the TXA arm received TXA 1300 mg orally or 1 g intravenously every 8 hours. Dosing was renally adjusted when serum creatinine was >1.36 mg/dL. The study drug was discontinued once the platelet count was >30,000/μL for 46 hours without transfusion or until 30 days after treatment activation, whichever came first.
The primary bleeding outcome was measured during the 30 days after starting the study drug, and adverse events were measured from consent to 30 days after the study drug was discontinued. Patients, treating physicians, and study staff abstracting the data were blinded to the treatment group. Adverse event outcomes were graded using the Common Toxicology Criteria for Adverse Events (CTCAE) version 4.03, dated June 14, 2010. Infections were considered to be grade 1 when patients were asymptomatic or had mild symptoms that could be observed; grade 2 infections required treatment with oral anti-infective medications and/or local intervention; grade 3 infections required intravenous anti-infective medications and/or invasive intervention; grade 4 infections were life-threatening; and grade 5 infections were fatal.
Cytokine data were analyzed from a subset of patients who also consented to a separate laboratory study evaluating markers of fibrinolysis and cytokine responses (“Fibrinolysis Evaluation in A-TREAT” [FEAT] study). Detailed methods of this substudy have been previously described [17]. Briefly, a “trough” sample was drawn when TXA was expected to be at a steady state level following study drug initiation and within 2 hours before the next scheduled dose administration (mean [SD], 5 [1.2] days following study drug initiation). Trough plasma samples collected for the ancillary to A-TREAT study were used to quantify 27 cytokines, chemokines, and growth factors by Bio-Plex Pro Human Cytokine 27-plex Assay (#M500KCAF0Y, Bio-Rad Laboratories) according to the methodology provided by the manufacturer. The assay characteristics, as well as the complete list of cytokines, chemokines, and growth factors detected by the Bio-Rad multiplex microbead assays, can be found on the manufacturer’s website (https://www.bio-rad.com/en-ch/sku/m500kcaf0y-bio-plex-pro-human-cytokine-27-plex-assay?ID=m500kcaf0y). Data were collected using a Bio-Rad BioPlex 200 instrument equipped with Bio-Plex Manager software version 6.0 (Bio-Rad Laboratories) and analyzed using MS Excel and GraphPad Prism version 7.05 (GraphPad Software Inc).
To explore the potential relationship between treatment with TXA and inflammatory outcomes, we compared the number and proportion of patients experiencing rashes, other skin adverse events, mucositis, fever, infection, transfusion reactions, and graft vs host disease between treatment arms while participants were on the study drug. We calculated the Pearson correlation coefficient of cytokines with global fibrinolysis assays and TXA levels. In a post hoc analysis, the incidence of grade 3 or higher infections while on study treatment was compared across treatment arms using a chi-squared test of the equality of the proportions. An adjusted relative risk regression of the difference across treatment arms in the proportions of patients with a grade 3 or higher infection was performed as a sensitivity analysis; the estimate was adjusted for study site, therapeutic group, biological sex, and age. Plots of the cumulative incidence of grade 3+ infection were produced using the relative risk regression with death treated as a competing risk; study participants were censored when they stopped taking the study drug [18]. The cumulative risk regression was repeated, adjusting for the covariates listed above. All CIs were performed at the 95% level. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc) and R 4.1.2 (R Foundation for Statistical Computing).
3. Results
The 326 patients who received study medication (163 TXA; 163 placebo) were included in the analysis, including 190 males (58%) and 136 females (42%) with a mean age of 53.7 (SD, 13.1) years (Table 1). One hundred twenty-eight (39%) patients were treated with chemotherapy alone, 70 (21%) underwent autologous stem cell transplant, and 128 (39%) underwent allogeneic stem cell transplant. Patients received study drugs for a mean of 15.2 days (SD, 8.5) in the placebo group and a mean of 15.1 days (SD, 8.1) in the TXA group. There was no difference in the incidence or severity of neutropenia between the placebo and TXA arms, while on the study drug, patients had severe neutropenia (absolute neutrophil count < 500/mm3) for a mean of 13.6 days (SD, 7.7) in the placebo arm and 13.4 days (SD, 6.8) in the TXA arm.
Table 1.
Demographics in the American Trial to Evaluate Tranexamic Acid Therapy in Thrombocytopenia safety population.
| Demographic | Placebo |
TXA |
Total |
|---|---|---|---|
| (n = 163) | (n = 163) | (N = 326) | |
| Age (y), mean [19] | 53.8 (13.2) | 53.7 (13.1) | 53.7 (13.1) |
| Male sex, n (%) | 99 (61) | 91 (56) | 190 (58) |
| Race, n/N (%) | |||
| American Indian/Alaska Native | 4/160 (3) | 2/159 (1) | 6/319 (2) |
| Asian | 9/160 (6) | 3/159 (2) | 12/319 (4) |
| Black or African American | 12/160 (8) | 11/159 (7) | 23/319 (7) |
| Native Hawaiian/Pacific Islander | 3/160 (2) | 2/159 (1) | 5/319 (2) |
| White | 127/160 (79) | 140/159 (88) | 267/319 (84) |
| More than one race | 5/160 (3) | 1/159 (1) | 6/319 (2) |
| Hispanic ethnicity, n/N (%) | 5/130 (4) | 8/138 (6) | 13/268 (5) |
| Therapeutic group, n (%) | |||
| Allogeneic transplant | 65 (40) | 63 (39) | 128 (39) |
| Autologous transplant | 36 (22) | 34 (21) | 70 (21) |
| Chemo/immuno therapy | 62 (38) | 66 (40) | 128 (39) |
TXA, tranexamic acid.
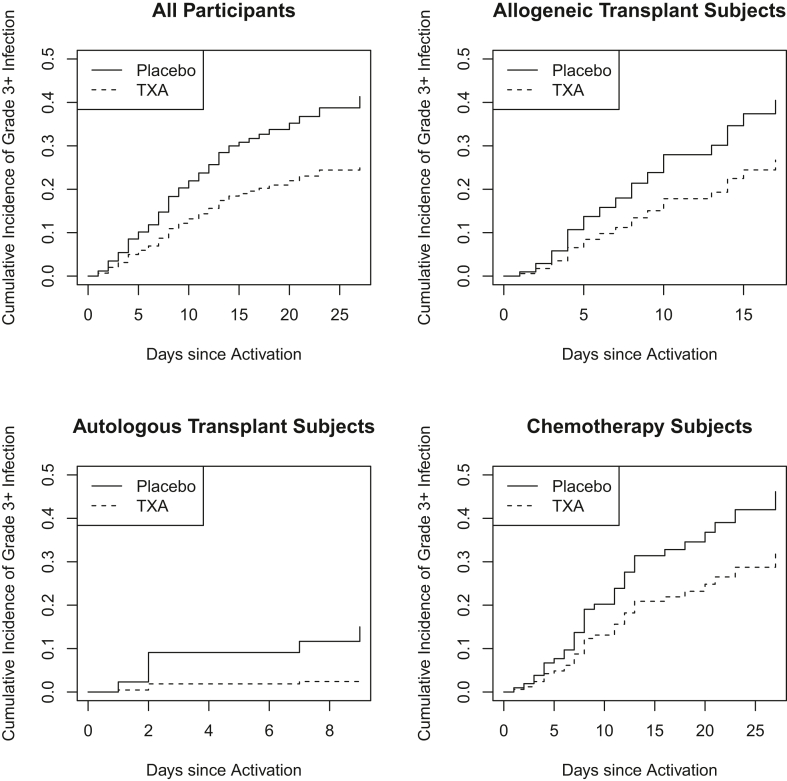
There was no statistically significant difference in the overall incidence of infections (grades 1-5) between the TXA and placebo groups (42.3% vs 48.5%) during the treatment period. However, there were fewer grade 3+ infections in patients on TXA while on the study drug (Figure 1; subhazard ratio, 0.57; 95% CI, 0.36, 0.91; adjusted subhazard ratio 0.44; 95% CI, 0.26, 0.75). This reduction was seen in the overall study population and when patients were analyzed according to whether they were treated with chemotherapy alone, autologous stem cell transplant, or allogeneic stem cell transplant (Figure 1). Grade 3+ infections while on the study drug occurred in 17.8% of the TXA group vs 28.2% in the placebo group (risk difference, −10.4%; 95% CI, −20.1%, −0.7%; adjusted difference, −17.3%; 95% CI, −29.4%, −5.1%). Similar causes of grade 3+ infections were diagnosed in both study arms (Supplementary Table). There was no difference in the rate or severity of infections between males and females. There were 3 fatal (grade 5) infections that began while the patients were on the study drug (2 patients on TXA and 1 patient on placebo). Patients who experienced grade 3+ infections had higher rates of World Health Organization grade 2+ bleeding and increased transfusion requirements compared with patients who did not develop grade 3+ infections, regardless of the treatment arm (Table 2). Additionally, patients with grade 3+ infections had longer durations of neutropenia, time to platelet recovery, and time on the study drug (Table 2).
Figure 1.
Cumulative incidence of grade 3+ infections while on study drug (tranexamic acid [TXA] or placebo). Outcomes of patients were censored when discontinuing the study drug; death was treated as a competing risk.
Table 2.
Association of grade 3+ infections while on study drug and bleeding outcomes.
| Clinical outcomes | No grade 3+ infection |
Grade 3+ infection |
||||
|---|---|---|---|---|---|---|
| Placebo |
TXA |
Total |
Placebo |
TXA |
Total |
|
| (n = 117) | (n = 134) | (N = 251) | (n = 46) | (n = 29) | (N = 75) | |
| Highest grade bleed, mean [19] | 1.3 (0.7) | 1.4 (0.7) | 1.3 (0.7) | 1.8 (0.9) | 1.8 (0.9) | 1.8 (0.9) |
| Any grade 2+ bleed, n (%) | 47 (40.2) | 53 (39.6) | 100 (39.8) | 30 (65.2) | 19 (65.5) | 49 (65.3) |
| Grade 2+ bleeding before infection, n (%) | -- | -- | -- | 29 (63.0) | 19 (65.5) | 48 (64.0) |
| RBC transfusions, mean [19] | 4.7 (3.9) | 5.1 (3.9) | 4.9 (3.9) | 8.5 (4.4) | 8.9 (4.8) | 8.6 (4.5) |
| Platelet transfusions, mean [19] | 5.0 (4.7) | 6.0 (6.0) | 5.6 (5.5) | 10.7 (7.2) | 11.3 (7.7) | 10.9 (7.4) |
| Time on study drug, mean [19] | 13.5 (7.6) | 14.8 (8.0) | 14.2 (7.8) | 20.1 (8.6) | 18.4 (8.0) | 19.4 (8.4) |
| Time to platelet recovery, mean [19] | 13.8 (6.6) | 16.8 (8.3) | 15.4 (7.7) | 22.0 (10.3) | 21.0 (7.6) | 21.6 (9.4) |
| Duration of severe neutropenia | 14.1 (8.4) | 15.8 (8.5) | 15.0 (8.5) | 19.3 (7.9) | 19.9 (7.5) | 19.5 (7.7) |
The Common Toxicology Criteria for Adverse Events version 4.03 grading system was used to grade infections; the World Health Organization bleeding scale was used to grade bleeds. Severe neutropenia was defined as <500/μL; duration truncated at 30 days.
RBC, red blood cell; TXA, tranexamic acid.
There were no differences in the incidence of other inflammatory outcomes between the TXA and placebo groups, including neutropenic fever (without documented infection), rash, mucositis, graft vs host disease, veno-occlusive disease/sinusoidal obstruction syndrome, or transfusion reactions (Table 3).
Table 3.
Post hoc analysis of inflammatory adverse events and American Trial to Evaluate Tranexamic Acid Therapy in Thrombocytopenia outcomes.
| Inflammatory adverse event | Allo |
Auto |
Chemo |
Total |
Total |
||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo |
TXA |
Placebo |
TXA |
Placebo |
TXA |
Placebo |
TXA |
||
| (n = 65), n (%) | (n = 63), n (%) | (n = 36), n (%) | (n = 34), n (%) | (n = 62), n (%) | (n = 66), n (%) | (n = 163), n (%) | (n = 163), n (%) | (N = 326), n (%) | |
| All rashes during study treatment | 28 (43.1) | 30 (47.6) | 5 (13.9) | 7 (20.6) | 16 (25.8) | 24 (36.4) | 49 (30.1) | 61 (37.4) | 110 (33.7) |
| Grade 3+ mucositis during study treatment | 24 (36.9) | 31 (49.2) | 6 (16.7) | 10 (29.4) | 2 (3.2) | 0 (0.0) | 32 (19.6) | 41 (25.2) | 73 (22.4) |
| Febrile neutropenia during study treatment | 33 (50.8) | 28 (44.4) | 18 (50.0) | 22 (64.7) | 43 (69.4) | 51 (77.3) | 94 (57.7) | 101 (62.0) | 195 (59.8) |
| Fever during study treatment | 11 (16.9) | 5 (7.9) | 2 (5.6) | 4 (11.8) | 4 (6.5) | 8 (12.1) | 17 (10.4) | 17 (10.4) | 34 (10.4) |
| Any infection during study treatment | 34 (52.3) | 29 (46.0) | 11 (30.6) | 10 (29.4) | 34 (54.8) | 30 (45.5) | 79 (48.5) | 69 (42.3) | 148 (45.4) |
| Grade 3+ infections during study treatment | 19 (29.2) | 12 (19.0) | 5 (13.9) | 1 (2.9) | 22 (35.5) | 16 (24.2) | 46 (28.2) | 29 (17.8) | 75 (23.0) |
| GVHD during study treatment | 4 (6.2) | 2 (3.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (2.5) | 2 (1.2) | 6 (1.8) |
| VOD/SOS | 2 (3.1) | 3 (4.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.2) | 3 (1.8) | 5 (1.5) |
| WHO grade 2+ bleeding | 38 (58.5) | 36 (57.1) | 8 (22.2) | 5 (14.7) | 31 (50.0) | 31 (47.0) | 77 (47.2) | 72 (44.2) | 149 (45.7) |
| Platelet transfusions | 7.1 (6.2) | 7.9 (8.3) | 2.8 (2.0) | 3.6 (3.6) | 8.3 (6.5) | 7.9 (5.6) | 6.6 (6.1) | 7.0 (6.7) | 6.7 (6.3) |
| RBC transfusions | 6.0 (4.1) | 6.3 (4.6) | 1.9 (2.2) | 2.1 (2.0) | 7.8 (4.1) | 7.1 (3.9) | 5.8 (4.4) | 5.7 (4.3) | 5.7 (4.4) |
Outcomes by therapeutic group (allogeneic stem cell transplant [allo], autologous stem cell transplant [auto], and chemotherapy [chemo] only) and the total study population. The Common Toxicology Criteria for Adverse Events version 4.03 grading system was used for all outcomes except bleeding, which used the WHO grading scale.
GVHD, graft vs host disease; RBC, red blood cell; SOS, sinusoidal obstruction syndrome; TXA, tranexamic acid; VOD, veno-occlusive disease; WHO, World Health Organization.
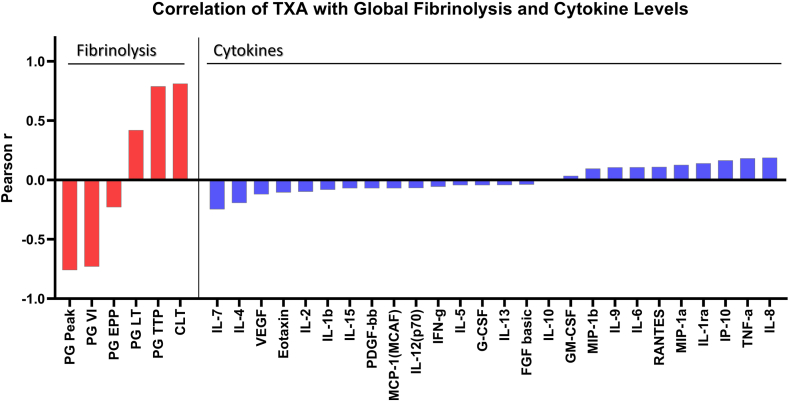
Cytokine data were also compared for the 92 patients with trough samples who were enrolled in the FEAT study [17]. The FEAT participants had a higher mean body mass index than non-FEAT A-TREAT subjects, as well as different primary diagnoses and treatment strata (Supplementary Table). Notably, however, the proportion of FEAT vs non-FEAT participants who experienced grade 2+ bleeding (45% vs 46%, respectively) was identical. Similarly, the frequency of the primary endpoint in FEAT subjects who were randomized to placebo vs TXA did not differ. Specifically, 25 of 58 subjects (43.1%) in the placebo group and 26 out of 57 subjects (45.6%) in the TXA group experienced a grade 2+ bleed. The correlation of the cytokine data with TXA and its impact on fibrinolysis are presented in Figure 2. While global fibrinolysis, as measured by euglobulin clot lysis time, plasmin generation, and tissue plasminogen activator challenged clot lysis time, was strongly associated with TXA, none of the measured cytokine levels appear to be associated with drug presence or plasma levels of TXA.
Figure 2.
Correlation of tranexamic acid (TXA) with global fibrinolysis and cytokine levels. Correlation between TXA concentration, global fibrinolysis assays (left section), and human cytokine concentrations (right section). All parameters were measured in plasma at a steady state level of the trial drug. Data are presented as Pearson r correlation coefficients. The Pearson correlation coefficient quantifies the strength of a linear relationship between 2 variables. In this context, values above .5 indicate a strong positive correlation between cytokines and global fibrinolysis assays or TXA levels, while values below -.5 suggest a strong negative correlation. The Pearson correlation coefficients are represented by bars. The red bars correspond to fibrinolysis parameters, and the blue bars represent cytokine levels. The color coding helps in distinguishing the 2 different types of analytes being correlated. TXA concentration strongly correlates with global fibrinolysis assay parameters, in contrast to a weak (or absent) correlation with human cytokine levels. This implies that TXA, in our study, based on the measured cytokine levels, did not show a significant and direct impact on inflammation. CLT, tPA-challenged clot lysis time; EPP, endogenous plasmin potential; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IP, interferon gamma-induced protein; LT, lag time, MCP, monocyte chemoattractant protein; MCAF, monocyte chemotactic and activating factor; MIP, macrophage inflammatory protein; PDGF, platelet-derived growth factor; PG, plasmin generation; TNF, tumor necrosis factor; TTP, time to peak; VEGF, vascular endothelial growth factor; VI, velocity index.
4. Discussion
To our knowledge, this is the first study to evaluate the effect of TXA on inflammatory outcomes and infections in patients with hematologic malignancies. There was no difference in inflammatory outcomes such as fever, rash, and mucositis. Additionally, the overall rate of infections was the same; however, patients who received TXA had fewer grade 3+ infections compared with those who received placebo.
While TXA did not alter the overall incidence of infections in this study, this is a challenging outcome to demonstrate due to the CTCAE grading and practice pattern in this patient population. The patients enrolled in A-TREAT were all neutropenic while on the study drug and were started on empiric intravenous antibiotics at the onset of a fever. This practice, as well as the use of intravenous antibiotics for other forms of infection, means that most infections were considered a grade 3 or higher based on the CTCAE scale. Additionally, the use of prophylactic antibiotics while neutropenic may have also reduced the rate of grades 1 to 2 infections. Our results differ from work on surgical site infections where TXA appears to reduce the overall incidence of infection and likely reflects the differences in antibiotic use, as well as the unique predisposition toward infections in patients with hematologic malignancies who experience severe neutropenia while undergoing myelosuppressive treatment.
Although prophylactic use of TXA did not impact overall bleeding outcomes in A-TREAT [16], patients who experienced grade 3+ infections had higher rates of grade 2+ bleeding and higher transfusion requirements compared with those who did not develop a grade 3+ infection (Table 3). Bleeding occurred before the onset of infection in all but one of the patients with a grade 3+ infection who experienced bleeding. The relationship between bleeding and infection is unclear, and the role of TXA warrants further investigation.
It has been hypothesized that TXA reduces surgical site infections by reducing surgical site bleeding and subsequent biofilm formation, which may be especially critical in implant-associated infections [20]. Central venous access devices can also serve as a nidus for biofilm formation and infection [21], which may play a role in patients with hematologic malignancy, given widespread use of central venous catheters. We did not identify a difference in the incidence of catheter-related infections between the placebo and TXA arms, possibly due to the challenge of confirming these infections in the study population.
TXA may attenuate cytokine responses to infection and reduce severity by blocking the interaction between plasmin and/or plasminogen with immune cells. However, the cytokine data in this study do not support this hypothesis (Figure 2). The trough samples showed a strong correlation between TXA concentration and changes in fibrinolytic parameters, but there was no correlation between TXA and cytokine levels, suggesting that altered cytokine expression alone does not explain the impact of TXA on infection severity. However, it is possible that alterations in cytokine expression would have been seen in the TXA group if plasma samples had been obtained during an infection rather than at preset time points that predated the onset of infection in most patients. Cytokine data are also difficult to interpret without quantifying circulating lymphocyte subsets; these patients experienced profound pancytopenia, and subtle changes in cytokine expression may have been below the limit of detection. Studies in certain surgical populations have suggested that TXA may impact cytokine expression, and evaluation of circulating immune cell subsets is required to explain any effect of TXA on immune cells. In a planned subset analysis of the Aspirin and Tranexamic Acid for Coronary Artery Surgery trial, patients on TXA had reduced proinflammatory cytokine production and altered expression of immune markers on peripheral blood myeloid and lymphoid cell subsets, which correlated with a reduced rate of surgical site infection (14.3% vs 20.6%, P = .04) [9]. Samples drawn at the onset of infection, including assessment of immune cell subsets, may help to clarify whether TXA reduces the severity of infection by altered cytokine expression in patients with hematologic malignancies.
It has also been proposed that TXA may have a direct bactericidal effect, potentially from interfering with lysine analogs essential for bacterial growth. Recent in vitro work has shown reduced growth of Cutibacterium acnes and Staphylococcus aureus when cultured with TXA 10 mg/mL [22]. TXA also reduces the minimal inhibitory concentration required for vancomycin and gentamicin alone and in combination to reduce in vitro growth of several clinical strains of staphylococci [23]. It is unclear if TXA has an antimicrobial effect on a broader range of bacteria or if this effect occurs in vivo.
This study is a secondary analysis intended to generate a hypothesis and was not included in the prespecified statistical analysis plan of A-TREAT. The rate and severity of infections were tracked as adverse events rather than as prespecified outcomes in A-TREAT; it is possible that the incidence and severity of infections may be higher if these were tracked as prespecified outcomes. Limitations include lack of ongoing cytokine data among all patients, including at the onset of infection. The study included a heterogeneous population of patients with different hematologic malignancies and was underpowered to detect a particular subtype of hematologic malignancy for which TXA would be particularly beneficial. However, known and unknown confounders were controlled for due to randomization (when comparing placebo vs TXA), and the reduction in grade 3+ infections with TXA was seen regardless of whether patients received chemotherapy alone vs autologous or allogeneic stem cell transplant.
No impact was seen on other inflammatory outcomes; however, many inflammatory outcomes in this patient population occur beyond the period that patients were monitored in A-TREAT. Patients received the study drug for an average of 15 days, which may not have been a sufficient time frame to see an impact on inflammatory outcomes such as acute and chronic graft vs host disease. Some inflammatory outcomes, such as sinusoidal obstruction syndrome, were rare events, and the study was underpowered to detect any impact from TXA on rare events. Grade 3+ infections in this study population included infections requiring intravenous antibiotics. Some infection grades may have been increased due to a subject’s inability to tolerate oral antibiotics or at the initial onset of neutropenic fever. Notably, there was no difference in the incidence of neutropenic fever between the TXA and placebo arms or in the patients who did or did not experience grade 3+ infections. Alternative strategies to evaluate infection severity may be required, and a prospective trial of TXA that includes the incidence and severity of infections as a prespecified outcome is needed.
TXA may have physiological effects beyond the initially studied mechanism of action that will only be discovered by analyzing broader clinical outcomes. Evaluating additional effects could lead to new indications for TXA as well as improve the understanding of inflammation itself. Further work is needed to understand the impact of TXA on infections and inflammatory outcomes outside the surgical setting. The high rate of inflammatory events and infections in patients with hematologic malignancy makes this an important area for future study.
Acknowledgments
The authors wish to acknowledge the patients who graciously agreed to participate in this study, as well as the clinical coordinators at the 3 study sites. The authors would like to thank Carlton Anderson and the University of North Carolina Advanced Analytics Core (Center for Gastrointestinal Biology and Disease, P30 DK034987) for their assistance with the Bio-Plex Pro Human Cytokine 27-plex bead assays used in this study.
Funding
This study was supported by National Institute of Health (NIH) grants HL146226, HL122894, HL122272, and HL143403. The content of this work is solely the responsibility of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the funder (National Heart, Lung, and Blood Institute).
Author contributions
The investigators designed and conducted the study; gathered, analyzed, and interpreted the data; wrote the manuscript draft; and made the decision to submit it for publication. S.P.B. and S.M. had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis. A.I. and N.K. performed the cytokine and fibrinolysis assays. J.N.P. drafted the first version of the manuscript.
Relationship disclosure
J.N.P. reports consultancy with TeraImmune. C.E.J. has received research funding from ASCO/Conquer Cancer Foundation and AbbVie. D.J.T. reports consultancy with Realta and Fresenius Kabi. N.S.K. reports consultancy for BioMarin, Uniqure, Genentech, and Novo Nordisk and has received research funding from Grifols and the National Heart, Lung, and Blood Institute (NHLBI). T.B.G. reports consultancy for Amgen, Alpine Immune Science, Argenx, BioProducts Laboratories, Cellphire, Dova, Sanofi, and Palisade Bio and has received research funding from the NHLBI, Dova and Sanofi. S.M. reports funding for research from the NHLBI. None of the other authors have conflicts of interest to report regarding the subject of this manuscript.
Data availability
De-identified individual participant data is available at the National Heart, Lung, and Blood Institute BioLINCC repository, along with other study documentation such as the protocol, data collection forms, data dictionary, and manual of operations.
Footnotes
Handling Editor: Dr Michelle Sholzberg
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102358
Supplementary material
References
- 1.Draxler D.F., Sashindranath M., Medcalf R.L. Plasmin: A Modulator of Immune Function. Semin Thromb Hemost. 2017;43:143–153. doi: 10.1055/s-0036-1586227. [DOI] [PubMed] [Google Scholar]
- 2.Sugimoto M.A., Ribeiro A.L.C., Costa B.R.C., Vago J.P., Lima K.M., Carneiro F.S., Ortiz M.M.O., Lima G.L.N., Carmo A.A.F., Rocha R.M., Perez D.A., Reis A.C., Pinho V., Miles L.A., Garcia C.C., Teixeira M.M., Sousa L.P. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood. 2017;129:2896–2907. doi: 10.1182/blood-2016-09-742825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draxler D.F., Daglas M., Fernando A., Hanafi G., McCutcheon F., Ho H., Galle A., Gregory J., Larsson P., Keragala C., Wright D.K., Tavancheh E., Au A.E., Niego B., Wilson K., Plebanski M., Sashindranath M., Medcalf R.L. Tranexamic acid modulates the cellular immune profile after traumatic brain injury in mice without hyperfibrinolysis. J Thromb Haemost. 2019;17:2174–2187. doi: 10.1111/jth.14603. [DOI] [PubMed] [Google Scholar]
- 4.Diebel L.N., Martin J.V., Liberati D.M. Early tranexamic acid administration ameliorates the endotheliopathy of trauma and shock in an in vitro model. J Trauma Acute Care Surg. 2017;82:1080–1086. doi: 10.1097/TA.0000000000001445. [DOI] [PubMed] [Google Scholar]
- 5.Peng Z., Ban K., LeBlanc A., Kozar R.A. Intraluminal tranexamic acid inhibits intestinal sheddases and mitigates gut and lung injury and inflammation in a rodent model of hemorrhagic shock. J Trauma Acute Care Surg. 2016;81:358–365. doi: 10.1097/TA.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X., Dubick M.A., Schwacha M.G., Cap A.P., Darlington D.N. Tranexamic Acid Attenuates The Loss of Lung Barrier Function in a Rat Model of Polytrauma And Hemorrhage With Resuscitation. Shock. 2017;47:500–505. doi: 10.1097/SHK.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez J.J., Iribarren J.L., Lorente L., Rodriguez J.M., Hernandez D., Nassar I., Perez R., Brouard M., Milena A., Martinez R., Mora M.L. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11:R117. doi: 10.1186/cc6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant A.L., Letson H.L., Morris J.L., McEwen P., Hazratwala K., Wilkinson M., Dobson G.P. Tranexamic acid is associated with selective increase in inflammatory markers following total knee arthroplasty (TKA): a pilot study. J Orthop Surg Res. 2018;13:149. doi: 10.1186/s13018-018-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draxler D.F., Yep K., Hanafi G., Winton A., Daglas M., Ho H., Sashindranath M., Wutzlhofer L.M., Forbes A., Goncalves I., Tran H.A., Wallace S., Plebanski M., Myles P.S., Medcalf R.L. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. 2019;3:1598–1609. doi: 10.1182/bloodadvances.2019000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klement M.R., Padua F.G., Li W.T., Detweiler M., Parvizi J. Tranexamic Acid Reduces the Rate of Periprosthetic Joint Infection After Aseptic Revision Arthroplasty. J Bone Joint Surg Am. 2020;102:1344–1350. doi: 10.2106/jbjs.19.00925. [DOI] [PubMed] [Google Scholar]
- 11.Lacko M., Jarcuska P., Schreierova D., Lackova A., Gharaibeh A. Tranexamic acid decreases the risk of revision for acute and delayed periprosthetic joint infection after total knee replacement. Jt Dis Relat Surg. 2020;31:8–13. doi: 10.5606/ehc.2020.72061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yazdi H., Klement M.R., Hammad M., Inoue D., Xu C., Goswami K., Parvizi J. Tranexamic Acid Is Associated With Reduced Periprosthetic Joint Infection After Primary Total Joint Arthroplasty. J Arthroplasty. 2020;35:840–844. doi: 10.1016/j.arth.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Hong G.J., Wilson L.A., Liu J., Memtsoudis S.G. Tranexamic Acid Administration is Associated With a Decreased Odds of Prosthetic Joint Infection Following Primary Total Hip and Primary Total Knee Arthroplasty: A National Database Analysis. J Arthroplasty. 2021;36:1109–1113. doi: 10.1016/j.arth.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Rojas J., Srikumaran U., McFarland E.G. Inconclusive evidence for the efficacy of tranexamic acid in reducing transfusions, postoperative infection or hematoma formation after primary shoulder arthroplasty: A meta-analysis with trial sequential analysis. Shoulder Elbow. 2021;13:38–50. doi: 10.1177/1758573219896794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis C.J., Li P., Stewart L., Weintrob A.C., Carson M.L., Murray C.K., Tribble D.R., Ross J.D. Tranexamic acid in life-threatening military injury and the associated risk of infective complications. Br J Surg. 2016;103:366–373. doi: 10.1002/bjs.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gernsheimer T.B., Brown S.P., Triulzi D.J., Key N.S., El Kassar N., Herren H., Poston J.N., Boyiadzis M., Reeves B.N., Selukar S., Pagano M.B., Emerson S.S., May S. Prophylactic tranexamic acid in patients with hematologic malignancy: a placebo controlled, randomized clinical trial. Blood. 2022 doi: 10.1182/blood.2022016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilich A., Gernsheimer T.B., Triulzi D.J., Herren H., Brown S.P., Holle L.A., Lucas A.T., de Laat B., El Kassar N., Wolberg A.S., May S., Key N.S. Absence of hyperfibrinolysis may explain lack of efficacy of tranexamic acid in hypoproliferative thrombocytopenia. Blood Adv. 2022 doi: 10.1182/bloodadvances.2022008255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine J.P., Gray R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. doi: 10.2307/2670170. [DOI] [Google Scholar]
- 19.Montague S.J., Delierneux C., Lecut C., Layios N., Dinsdale R.J., Lee C.S., Poulter N.S., Andrews R.K., Hampson P., Wearn C.M., Maes N., Bishop J., Bamford A., Gardiner C., Lee W.M., Iqbal T., Moiemen N., Watson S.P., Oury C., Harrison P., Gardiner E.E. Soluble GPVI is elevated in injured patients: shedding is mediated by fibrin activation of GPVI. Blood Adv. 2018;2:240–251. doi: 10.1182/bloodadvances.2017011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Zhang Z., Li J., Huang B., Jiang Z., Pan Y., He T., Hu Y., Wang L. Tranexamic acid protects against implant-associated infection by reducing biofilm formation. Sci Rep. 2022;12:4840. doi: 10.1038/s41598-022-08948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousif A., Jamal M.A., Raad I. Biofilm-based central line-associated bloodstream infections. Adv Exp Med Biol. 2015;830:157–179. doi: 10.1007/978-3-319-11038-7_10. [DOI] [PubMed] [Google Scholar]
- 22.Benjumea A., Díaz-Navarro M., Hafian R., Sánchez-Somolinos M., Vaquero J., Chana F., Muñoz P., Guembe M. Effect of Tranexamic Acid against Staphylococcus spp. and Cutibacterium acnes Associated with Peri-Implant Infection: Results from an In Vitro Study. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.01612-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjumea A., Diaz-Navarro M., Hafian R., Cercenado E., Sanchez-Somolinos M., Vaquero J., Chana F., Munoz P., Guembe M. Tranexamic Acid in Combination With Vancomycin or Gentamicin Has a Synergistic Effect Against Staphylococci. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.935646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data is available at the National Heart, Lung, and Blood Institute BioLINCC repository, along with other study documentation such as the protocol, data collection forms, data dictionary, and manual of operations.