Abstract
Background
The most prevalent probiotic bacterium employed in the food industry is Lactobacillus because it can produce metabolites with antibacterial capabilities and exhibits hostility towards infections and microorganisms that cause spoilage.
Aim
This study set out to identify naturally occurring Lactobacillus and plantaricin (pln EF) coding genes in raw cow milk and to assess the antibacterial potency of isolated Lactobacillus isolates.
Methods
Following enrichment in De Man, Rogosa and Sharpe (MRS) broth, single colonies were isolated, and pure colonies were obtained by streaking on MRS agar. The 16S rRNA gene was amplified using polymerase chain reaction (PCR) to confirm the cultural positivity of all isolates. Additionally, the presence of plantaricin was verified by targeting the pln EF gene through PCR.
Outcome
Out of the 166 raw milk specimens acquired from cows, 153 (91.17%; CI: 86.98–95.76) were identified as positive for Lactobacillus through both culture and biochemical screening. Subsequently, 121 (72.89%; CI: 65.46–79.49) of the isolates were affirmed to harbour Lactobacillus through PCR analysis. Within this subset, 6 isolates (4.96%; CI: 1.84–10.48) were found to possess the plnEF gene. When exposed to Lactobacillus isolates, Salmonella Typhimurium and Salmonella enterica displayed an average maximum zone of inhibition with a diameter measuring 24 mm. In contrast, Escherichia coli exhibited an average minimum zone of inhibition, featuring a diameter of 11 mm. Additionally, the Lactobacillus isolates demonstrated inhibitory zones against Staphylococcus aureus, Klebsiella pneumoniae and Klebsiella oxytoca, measuring 14, 22 and 19 mm, respectively.
Clinical significance
Lactic acid bacteria, particularly Lactobacilli, are plentiful in cow milk and possess broad‐spectrum antibacterial properties.
Keywords: antibacterial, lactic acid bacteria, Lactobacillus, polymerase chain reaction
Lactic acid bacteria specifically Lactobacillus, abundantly present in cow milk, have demonstrated significant broad‐spectrum antibacterial efficacy against multidrug‐resistant bacteria, particularly through the expression of plantaricin (pln EF) coding genes. The occurrence of lactobacillus spp. was 72.89%, with the plnEF coding gene detected in 4.96%. Lactobacillus isolates carrying the plnEF gene exhibited significant inhibition zones against Salmonella Typhimurium (24 mm) and Salmonella enterica (24 mm), Escherichia coli (11 mm), Staphylococcus aureus (14 mm), Klebsiella pneumoniae (22 mm) and Klebsiella oxytoca (19 mm).
1. INTRODUCTION
Disease has always posed a significant challenge in the livestock sector, adversely affecting animal health and welfare. The emergence of antimicrobial resistance among pathogenic bacteria in both humans and animals has become a pressing concern in recent times. Historically, chemotherapeutic drugs and antibiotics supplemented in animal feed as growth promoters have been used to combat and prevent diseases. However, to counteract the development of antibiotic resistance, several countries have implemented restrictions or bans on the use of antibiotics in animal feed. Consequently, the livestock industry is now compelled to seek alternatives to antibiotics for growth promotion and prophylaxis (Manyi‐Loh et al., 2018). One area gaining increasing attention is the development of novel probiotic‐based foods. Probiotic foods are anticipated to comprise around 60%–70% of the functional food industry (Ashaolu, 2021; Kołozyn‐Krajewska & Dolatowski, 2012). Lactic acid bacteria (LAB) is considered the most significant group of probiotic bacteria used in processed dairy products, particularly in milk, their natural habitat (Ahansaz et al., 2023; Delavenne et al., 2012; Wouters et al., 2002).
Lactobacilli, being one of the most important and robust probiotic bacteria, produce antimicrobial peptides and volatile organic acids, rendering them inherently resistant to most antibiotics (Saadatzadeh et al., 2013). Bacteriocin, an antimicrobial peptide produced by LAB, holds the potential to eliminate phylogenetically related strains and prevent spoilage without heat treatment (Simons et al., 2020). In the face of antimicrobial resistance posing a significant threat to animals and human alike, bacteriocins may serve as a natural alternative to antibiotics (Gradisteanu Pircalabioru et al., 2021). Moreover, there has been growing interest in using direct‐fed microbes in animal feed as potential substitutes for antibiotics and growth promoters (McAllister et al., 2011).
In monogastric animals, LAB probiotics are employed to stabilize gut flora, whereas in ruminants, they are used to stabilize the ruminal environment and for biological therapies under Generally Recognized as Safe guidelines (Bhogoju & Nahashon, 2022). Lactobacillus, found in milk and other dairy products, has demonstrated its ability to enhance nutrient bioavailability, and its metabolites can serve as preservatives (Ayivi et al., 2020). Over the past few decades, numerous Lactobacillus species have been incorporated into a wide range of food products to enhance their nutritional value for consumption by both human and animals (Giraffa et al., 2010). Furthermore, the identification of Lactobacillus strains are crucial due to the strain‐specific characteristics of probiotics, the need for quality control of certified strains to prevent health risks and misleading claims, and the requirement to describe new strains (Markiewicz et al., 2010). Molecular techniques, such as DNA‐DNA hybridization, DNA sequence analysis and polymerase chain reaction (PCR) tests, have been developed to accurately identify lactobacilli (Huang et al., 2018). Phylogenetic analysis using 16S rRNA gene sequences, along with genotypic and phenotypic comparisons with strains stored in databases, is widely employed for species‐level identification of Lactobacillus (Patel et al., 2012).
In Bangladesh, researchers have characterized and evaluated LAB from dahi, milk, cheese and yogurt for their potential probiotic properties in districts, such as Dhaka, Chattogram and Jashore (Afrin et al., 2021; Reuben et al., 2020; Shahriar et al., 2019). However, the molecular detection of Lactobacillus or probiotics present in raw cow milk, along with their antimicrobial performance, has not been extensively studied in Bangladesh. Therefore, the objective of the present study was to investigate the molecular detection of the Lactobacillus genus, natural probiotic presence and pln EF (plantaricin) coding genes in raw milk from cows. Additionally, the study aimed to evaluate the antibacterial activity of Lactobacillus isolates.
2. MATERIALS AND METHODS
2.1. Study area and sampling
The study was conducted in various government and private dairy farms in Sylhet, located at 24°36′–25°11′ north latitudes and 91°38′–92°30′ east longitudes, covering an area of 3452.07 km2 (Figure 1). A total of 166 milk samples were collected from local and cross‐breed dairy cattle in different dairy farms in Sylhet, selected through simple random sampling. Data collection during sampling was performed using a well‐structured questionnaire. The study was carried out between November 2021 and April 2022.
FIGURE 1.
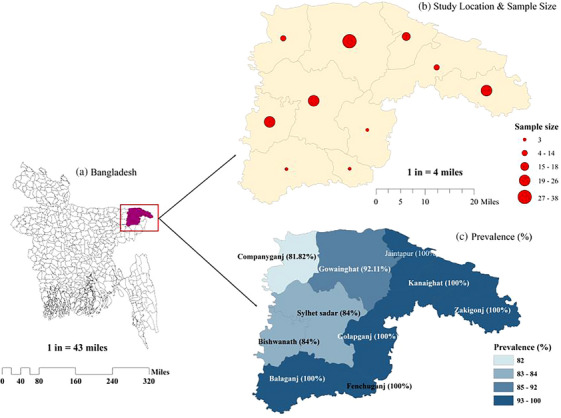
Geo‐spatial mapping of study area showing specific study location, sample size and location‐based prevalence. (a) Geographical area of Bangladesh (b) Selected study area
2.2. Bacteria isolation and biochemical characterization
In a test tube, 9 mL of MRS (De Man, Rogosa and Sharpe) broth (Hi‐Media) was combined with 1 mL of milk sample and incubated at 37°C for 24 h to observe the bacterial culture. Primary culture colonies were sub‐cultured on MRS agar (Hi‐Media) and incubated at 37°C for 24 h under strict microaerophilic conditions. Large white colonies from the MRS agar were sub‐cultured into new MRS agar plates. The cultures were streaked on MRS agar twice to obtain pure cultures. Finally, desired bacterial colonies from the pure culture were collected for morphological characterization, biochemical tests and DNA extraction. The remaining colonies were stored in BHI (brain heart infusion) broth and 15% glycerin for future use. The chemical nature of the bacterial isolates was assessed through biochemical tests, including the catalase test, methyl red test, citrate utilization test and carbohydrate fermentation tests, following standard protocols.
2.3. Salt (NaCl) tolerance test
Lactobacillus isolates were tested for their salt tolerance (Soni et al., 2021) in MRS broth containing different concentrations of NaCl (2%, 4% and 6.5%). Fresh cultures were inoculated into the salt‐containing MRS broth and incubated for 48 h at 37°C. Un‐inoculated MRS broth was used as a control. The presence of turbidity indicated tolerance to salt.
2.4. Molecular detection of genus Lactobacillus and PlnEF gene
The genomic DNA of the entire genome was extracted using the Add Prep Genomic DNA Extraction kit, following the manufacturer's instructions (AddBio Inc. Ltd.). For the amplification of the 16S rRNA gene of Lactobacillus species, universal primers 27F (5‐AGAGTTTGATCCTGGCTCAG‐3′) and 1392R (5‐GGTTACCTTGTTACGACTT‐3′) were used, resulting in a product size of 1350 bp (Saeed et al., 2020; Vasudha et al., 2023). Conventional PCR was performed using DreamTaq Green PCR Master Mix (2X) (Thermo Fisher Scientific), following the manufacturer's instructions. The PCR reaction mixture (20 μL) contained 1 μL (10 pmol/μL) of each forward and reverse primer, 5 μL of the DreamTaq Green PCR Master Mix (2X) and 8 μL of nuclease‐free water. Finally, 5 μL of the DNA template was added to each reaction tube. The amplification conditions included an initial denaturation (94°C for 3 min), followed by denaturation, annealing and extension (35 cycles at 94°C for 45 s, 56°C for 45 s and 72°C for 45 s), and a final extension at 72°C for 7 min.
Similarly, for the Pln EF gene amplification, the primers F (5′‐GGCATAGTTAAAATTCCCCCC ‐3′) and R (5′‐CAGGTTGCCGCAAAAAAAG‐3′) were used, resulting in a 428‐bp fragment (Refay et al., 2020). The PCR reaction mixture (25 μL) contained 0.5 μL (10 pmol/μL) of each forward and reverse primer, 12.5 μL of GoTaq Promega Green Master Mix (2X) (Promega Corporation, 2800 Woods Hollow Road Madison) and 8.5 μL of nuclease‐free water. Finally, 3 μL of the DNA template was added to each reaction tube. The amplified PCR products were subjected to electrophoresis in a 1.5% agarose gel (Sambrook et al., 1989). A 100‐bp DNA ladder (KAPA Universal DNA Ladder, cat # KK6302) was used as a molecular weight marker, and the gels were stained with safe gel stain dye, examined, and photographed under a UV transilluminator (Vilber‐Lourmater UV light EEC/France). Positive amplification for the universal 16S rRNA gene and Pln EF gene was confirmed by observing fragment sizes of approximately 1350 and 428 bp, respectively.
2.5. Antimicrobial performance of Lactobacillus isolates against food‐borne ESBL producing organisms
The antibacterial potential of Lactobacillus isolates was evaluated against multidrug‐resistant and extended spectrum beta‐lactamase (ESBL) producing strains of Klebsiella pneumoniae, Klebsiella oxytoca, Escherichia coli, Staphylococcus aureus, Salmonella Typhimurium and Salmonella enterica using an agar well diffusion assay. After preparing Mueller Hinton Agar plates, PCR‐confirmed Lactobacillus isolates were inoculated into MRS broth and incubated at 37°C for 48 h. The grown culture was then preserved as Lactobacillus cell culture in an Eppendorf tube. For testing, four or five isolated colonies of the target organisms were suspended in 2 mL of sterile salt solution using a sterile swab. The tube was vortexed to ensure uniform consistency of the suspension. The test organisms were streaked onto the Mueller Hinton Agar plate using the carpet culture technique, ensuring complete coverage of the plate's surface. After allowing the plate to air dry for 5 min, 6 mm diameter wells were created in the agar, and each well was filled with 100 μL of Lactobacillus cell culture, whereas the control well contained sterile MRS broth. The plates were then inverted and incubated for 24 h at 37°C. After the incubation period, the zone of inhibition was measured using a metric ruler, considering the diameter of the well in the calculation.
2.6. Statistical analysis
Data from the animal sources and laboratory work were entered into a Microsoft Excel spreadsheet. Prevalence was analysed using the following formula:
Prevalence of Lactobacillus = (total number of Lactobacillus‐positive samples/total number of milk samples) × 100
Prevalence of Pln EF gene = (total number of Pln EF‐positive samples/total number of Lactobacillus‐positive samples) × 100.
Conducted a univariate analysis utilizing the Chi‐square test to assess the associations among various explanatory variables. In cases where the expected count in a cell was less than 5 and occurred in at least 20% of the cells, Fisher's exact test was applied. Confidence intervals were calculated using the Binomial exact test, and a significance level of less than 0.05 was chosen for determining statistical significance. The data analysis was carried out using SPSS version 28 (IBM, 2021).
2.6.1. Geo‐spatial mapping and plot
The study area mapping was generated using ArcMap 10.7 (Vaisi‐Raygani et al., 2021), utilizing a shapefile extracted from (www.diva‐gis.org). This data was employed to create both choropleth and dot maps, effectively visualizing the prevalence of some explanatory variables as well as the corresponding sample sizes. Additionally, to illustrate the antimicrobial properties of Lactobacillus isolates, we employed OriginPro (www.originlab.com) (Seifert, 2014) and utilized the Polar Heat Map file exchange format. This allowed to us create informative polar heat maps and mean plots, offering a comprehensive view of the data.
3. RESULTS
3.1. Results of bacterial isolation and biochemical characterization
3.1.1. Primary isolation of positive samples by culture media
One hundred sixty‐six raw milk samples from apparently healthy cows were analysed for detection of Lactobacillus where 153 samples were found positive in primary isolation using culture media. The overall prevalence of Lactobacillus was found 91.17% (Table 1). Lactobacillus on MRS agar produced white or creamy yellow single colonies with round edges and smooth surfaces with diameters ranging from 0.5 to 3 mm (Figure S1A–C).
TABLE 1.
Prevalence of Lactobacillus and Pln EF gene on milk sample of cattle from Sylhet district.
| Category | Explanatory variable | Test (+ve) | Total tested | Prevalence% (95% CI) | p‐Value |
|---|---|---|---|---|---|
| Diagnostic test | <0.0001 | ||||
| Culture and biochemical | 153 | 166 | 92.17 (86.98–95.76) | ||
| PCR | 121 | 166 | 72.89 (65.46–79.49) | ||
| PCR findings | <0.0001 | ||||
| Lactobacillus | 121 | 166 | 72.89 (65.46–79.49) | ||
| Pln EF gene | 6 | 121 | 4.96 (1.84–10.48) | ||
| Location | 0.195 b | ||||
| Balaganj | 3 | 3 | 100.00 (29.24–100.00) a | ||
| Bishwanath | 21 | 25 | 84.00 (63.92–95.46) | ||
| Companyganj | 9 | 11 | 81.82 (48.22–97.72) | ||
| Fenchuganj | 3 | 3 | 100.00 (29.24–100.00) a | ||
| Golapganj | 3 | 3 | 100.00 (29.24–100.00) a | ||
| Gowainghut | 35 | 38 | 92.11 (78.62–98.34) | ||
| Kanaighat | 14 | 14 | 100.00 (76.84–100.00) a | ||
| Jaintapur | 18 | 18 | 100.00 (81.47–100.00) a | ||
| Sylhet sadar | 21 | 25 | 84.00 (63.92–95.46) | ||
| Zakigonj | 26 | 26 | 100.00 (86.77–100.00) a | ||
| Lactation | 0.099 | ||||
| First lactation | 25 | 28 | 89.29 (71.77–97.73) | ||
| Second lactation | 31 | 37 | 83.78 (67.99–93.81) | ||
| Third lactation | 65 | 67 | 97.01 (89.63–99.64) | ||
| Fourth lactation | 32 | 34 | 94.12 (80.32–99.28) |
Abbreviations: CI, confidence interval; PCR, polymerase chain reaction.
One‐sided 97.5% confidence interval.
Fisher's exact test where minimum 20% cell have expected count less than 5.
3.1.2. Results of morphological examination
The bacteria were stained blue and purple, and rod‐like bacilli or sphere‐shaped cocci without spores were visible under the microscope (Figure S1A–C). Gram‐positive microorganisms were presumptively identified as Lactobacillus.
3.1.3. Results of biochemical tests
3.1.3.1. Results of catalase test
The absence of bubble indicated that the isolated bacteria lack catalase and consequently in capable of mediating the decomposition of hydrogen per oxide into oxygen (Figure S2A). The catalase test is used to assess whether or not bacteria have the enzyme catalase, which is involved in the conversion of hydrogen peroxide into water and oxygen.
3.1.3.2. Results of carbohydrate fermentation test
All of the isolates produced acid by fermenting the three basic sugars (Lactose, Glucose and Sucrose). The change in colour from reddish to yellow in both slant and butt indicated a drop in the pH because of acid production without the formation of gas or hydrogen sulphide (Figure S2B).
3.1.3.3. Results of citrate utilization test
The isolates were unable to utilize citrate as a solitary source of carbon and were not able to generate sodium bi carbonate or ammonia. Thus, there was no alteration in the colour of media (Figure S2C). All of the isolates were tested negative for citrate.
3.1.3.4. Results of methyl‐red test
The appearance of red or pink colour on the surface medium after adding five drops of methyl red suggested acidity and indicated that the isolates were MR test positive (Figure S2D).
3.2. Results of salt (NaCl) tolerance test
In MRS broth containing 2%, 4% and 6.5% NaCl, all of the isolates were able to grow (Table S1).
3.3. Overall prevalence of genus Lactobacillus and Pln EF gene in cow raw milk
The amplified PCR products using appropriate primers were visualized by UV trans‐illuminator. Fragment sizes of approximately 1350 bp for the universal 16S rRNA gene (Figure 2A) and 428 bp for the plnEF gene were confirmed as positive (Figure 2B).
FIGURE 2.
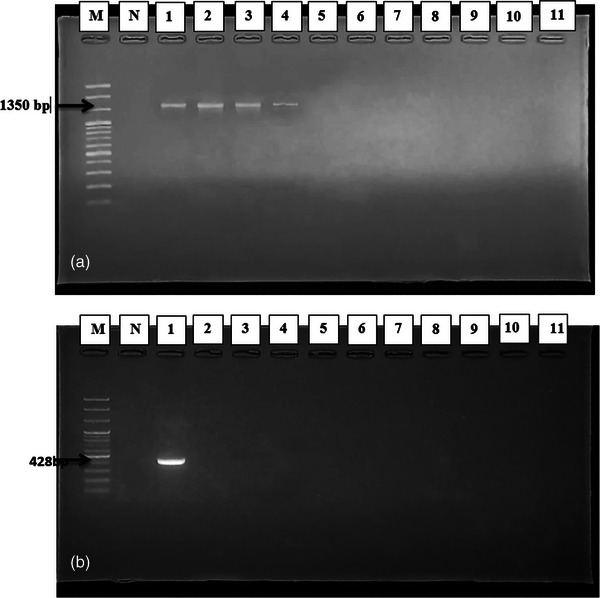
(a and b) Electrophoresis on 1.5% agarose gel showing specific amplified band of 16S rRNA gene and Pln EF gene amplification by polymerase chain reaction (PCR); Lane M: 100 bp Marker DNA; Lane N: Control (−ve); Lane (1–4) reaction specific (+ve) for 16S rRNA gene (1350 bp) of Lactobacillus (a); Lane 1 reaction specific (+ve) for Pln EF gene (428 bp) of isolated Lactobacillus (b).
The prevalence was determined on different category based on diagnostic test, PCR findings, location and lactation number, as shown in Table 1. Out of 166 raw milk samples from cow, 153 (91.17%) were found positive for Lactobacillus by cultural and biochemical examination (Table 1). Prevalence of Lactobacillus in cow raw milk was (72.89%; CI: 65.46–79.49) by PCR assay. From the 121 isolates were confirmed as Lactobacillus, 6 tested positive for plnEF genes by PCR. The prevalence of bacteriocin protein plnEF gene was (4.96%; CI: 1.84–10.48) by PCR assay. Most of the sub‐districts of Sylhet showed 100% positive for Lactobacillus, whereas the Companiganj showed the lowest prevalence (81.82%; CI: 48.22–97.72) in Sylhet district. In case of lactation number, the highest prevalence (97.01%; CI: 89.63–99.64) of Lactobacillus was observed at third lactation, whereas the lowest prevalence (83.78%; CI: 67.99–93.81) was found on second lactation (Table 1).
3.4. Antimicrobial performance
The agar‐well diffusion method was used to assess the antibacterial activity of Lactobacillus isolates against E. coli, S. aureus, K. pneumoniae, K. oxytoca, S. Typhimurium and S. enterica, which are important pathogens for both humans and animals. The zone of inhibition revealed that Lactobacillus isolates possess antibacterial properties against the examined pathogens (Figure 3). The isolated Lactobacillus exhibited its most significant antibacterial activity with an average inhibition zone of 24 mm against S. Typhimurium and 22 mm against K. pneumoniae, whereas its lowest average inhibition, measuring 11 mm, was observed against E. coli (Figure 4).
FIGURE 3.
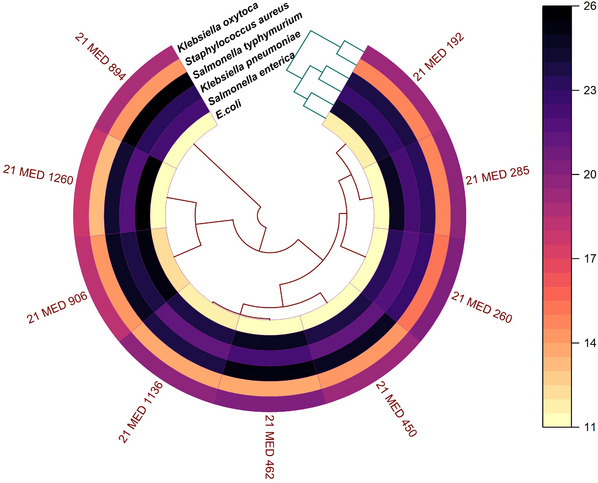
Polar heatmap showing the antimicrobial potency of Lactobacillus isolates against selected multi‐drug resistant bacteria.
FIGURE 4.
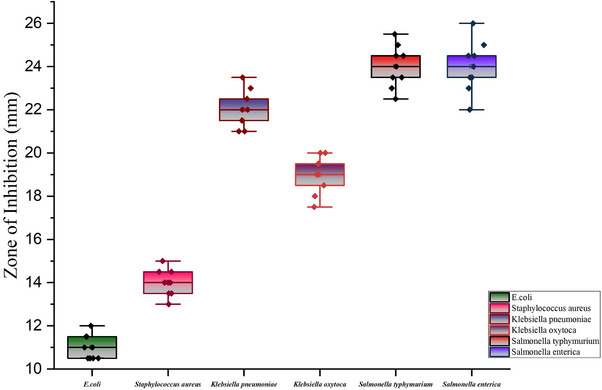
Mean plot showing the antimicrobial activity (average zone of inhibition) against Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Klebsiella oxytoca, Salmonella Typhimurium and Salmonella enterica.
4. DISCUSSION
According to the results of this study, Lactobacillus colonies produced a diameter of 0.5–2 mm with a white to yellowish white colour and round edges on MRS agar when incubated at 37°C, which is consistent with the findings of a previous study where milky white round colonies were also observed on MRS agar (Jose et al., 2015). In a separate study (Hoque et al., 2010), Lactobacillus from yogurt samples was examined for morphology, as well as various biochemical and physiological properties. Under the microscope, gram‐positive rods or sphere‐shaped bacteria were observed. The isolated bacteria were catalase negative, citrate negative and MR positive. Fermentation of lactose, glucose and sucrose was also observed in Lactobacillus isolates, which aligns with the findings of Hoque et al. (2010). Similar morphological and biochemical characteristics of LAB isolated from dahi samples were reported in the study by Harun‐Ur‐Rashid et al. (2007). The use of MRS medium for initial identification of the Lactobacillus genus was chosen due to the inability of other species to grow in this medium, which is in agreement with another report suggesting the prevalence of the Lactobacillus genus in MRS medium (López‐Díaz et al., 2000; Vasudha & Gayathri, 2023).
In the present investigation, the prevalence of Lactobacillus isolates in raw cow milk was assessed using both cultural and biochemical methods, revealing a rate of 91.07%. However, when employing PCR, the prevalence was slightly lower at 72.02%. These results align with those reported by Abdullah and Osman, who conducted a study on the prevalence of the Lactobacillus genus in Sudanese fermented milk (rob), raw milk and white cheese. In their research, the prevalence, determined through cultural, physiological and biochemical tests, was reported to be 69.23% (Abdullah & Osman, 2010). In a separate investigation (Saeed et al., 2020), the prevalence of Lactobacillus in raw goat milk was determined to be 15% through PCR analysis. This suggests a higher incidence of probiotic Lactobacillus strains in cow milk when compared to goat milk. Furthermore, when examining the prevalence of the Lactobacillus genus in raw milk, cheese and yogurt using cultural, physiological and biochemical tests, the reported rate was 24.38% (Taye et al., 2021). It is noteworthy that this prevalence is comparatively lower than the findings observed in the current study. Moreover, the prevalence of the bacteriocin protein plnEF gene in this research was determined to be 4.96%, which stands in stark contrast to a prior study in Egypt (Refay et al., 2020). In their investigation, the prevalence of the plnEF gene was reported to be substantially higher at 32.35% (22/68).
Antibacterial activity is crucial for Lactobacilli colonization in the intestinal mucosa as it acts as a barrier and provides protection against pathogens (Dempsey & Corr, 2022). Lactobacillus produces antimicrobial components, such as organic acids, hydrogen peroxide, diacetyl and bacteriocins – low molecular weight antimicrobial compounds – that exhibit inhibitory effects against pathogens (Santos et al., 2003). Due to concerns such as the emergence of resistant bacteria and the presence of residual antibiotics in livestock products, the usage of antibiotics in animal feed needs to be controlled, and organic approaches for livestock rearing have been recommended (Vanderhaeghen & Dewulf, 2017). In this study, the Lactobacillus isolates demonstrated antibacterial activity and inhibited the growth of the tested pathogens. These findings are consistent with other studies that suggest Lactobacillus species as a common probiotic bacteria used as an alternative measure to prevent Salmonella‐related diseases (Kowalska et al., 2020). Similarly, Djadouni and Kihal (2012) reported that LAB produce antibacterial compounds that inhibit the growth of indicator organisms, resulting in inhibition zones of 10–14 mm in diameter against E. coli, S. aureus and S. Typhimurium using the agar spot test. The antagonistic activity of LAB isolates against Salmonella typhi, K. pneumoniae and S. aureus observed in this study was similar to the findings of (Prabhurajeshwar & Chandrakanth, 2017). The antibacterial capability of the isolates in this study was comparable, and the fact that Lactobacillus isolates exhibited antibacterial activity against both gram‐positive and gram‐negative organisms demonstrates their broad‐spectrum activity.
5. CONCLUSION
Lactobacillus is a probiotic with various applications for both human and animal health. In this study, the prevalence of Lactobacillus in raw cow milk was found to be 72.89%, whereas the prevalence of the pln EF gene was determined to be 4.96% using PCR assay. The LAB isolates exhibited significant inhibition zones against S. Typhimurium and S. enterica, with an average maximum diameter of 24 mm, and against E. coli, with an average minimum diameter of 11 mm. Lactobacillus isolates also demonstrated zone of inhibition against S. aureus, K. pneumoniae and K. oxytoca, with diameters of 14, 22 and 19 mm, respectively. These findings indicate the antibacterial capability of the LAB isolates.
Due to the emergence of antibiotic resistance, finding alternatives to antibiotics for growth promotion and disease prevention is crucial in the livestock industry. When considering Lactobacillus isolates, additional requirements must be met beyond antibacterial traits, as Lactobacillus has the potential to transfer antibiotic resistance genes to other species. Further focused research, including in vitro and in vivo investigations, animal model studies and human trials, is necessary to ensure the safety and efficacy of clinical applications involving Lactobacillus. Additionally, the discovery and characterization of new Lactobacillus species strains that offer potential benefits for human and animal health require further study. Lactobacillus and bacteriocin molecules may play even more intriguing roles in the future, such as antiquorum sensing and targeted drug delivery.
AUTHOR CONTRIBUTIONS
Data curation; formal analysis; investigation; methodology; resources; software; validation; visualization; writing – original draft: Mashuka Nahida Asha, Md. Anisur Rahman, Ahsan Al Emon, Fatema Yeasmin Tanni and Md. Rafiqul Islam. Data curation; formal analysis; investigation; methodology; resources; software; validation; visualization; writing – original draft; writing – review and editing: Md. Shahidur Rahman Chowdhury, Hemayet Hossain and Md. Mukter Hossain. Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; software; validation; visualization; writing – original draft; writing – review and editing: Md. Mahfujur Rahman.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
Funding none.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1463.
ETHICS STATEMENT
In order to ensure the wellbeing of the animal and to limit animal use on meritorious research the ‘Animal Experimentation and Ethics Committee (AEEC)’, Sylhet Agricultural University, Bangladesh assessed and approved this experiment. This experiment holds an approved Animal Use Protocol [#AUP2022038] for conducting the experiment. The memo no: [SAU/Ethical committee/AUP/22/38].
Supporting information
Supporting Information
Asha, M. N. , Chowdhury, M. S. R. , Hossain, H. , Rahman, M. A. , Emon, A. A. , Tanni, F. Y. , Islam, M. R. , Hossain, M. M. , & Rahman, M. M. (2024). Antibacterial potential of lactic acid bacteria isolated from raw cow milk in Sylhet district, Bangladesh: A molecular approach. Veterinary Medicine and Science, 10, e1463. 10.1002/vms3.1463
DATA AVAILABILITY STATEMENT
Data available on request from the corresponding author.
REFERENCES
- Abdullah, S. A. , & Osman, M. M. (2010). Isolation and identification of lactic acid bacteria from raw cow milk, white cheese and Rob in Sudan. Pakistan Journal of Nutrition, 9(12), 1203–1206. 10.3923/pjn.2010.1203.1206 [DOI] [Google Scholar]
- Afrin, S. , Hoque, M. A. , Sarker, A. K. , Satter, M. A. , & Bhuiyan, M. N. I. (2021). Characterization and profiling of bacteriocin‐like substances produced by lactic acid bacteria from cheese samples. Access Microbiology, 3(6), 000234. 10.1099/acmi.0.000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahansaz, N. , Tarrah, A. , Pakroo, S. , Corich, V. , & Giacomini, A. (2023). Lactic acid bacteria in dairy foods: Prime sources of antimicrobial compounds. Fermentation, 9(11), 1–21. 10.3390/fermentation9110964 [DOI] [Google Scholar]
- Ashaolu, T. J. (2021). Probiotics and prebiotics as functional foods. In Functional foods (pp. 391–417). Wiley. [Google Scholar]
- Ayivi, R. D. , Gyawali, R. , Krastanov, A. , Aljaloud, S. O. , Worku, M. , Tahergorabi, R. , da Silva, R. C. , & Ibrahim, S. A. (2020). Lactic acid bacteria: Food safety and human health applications. Dairy, 1(3), 202–232. 10.3390/dairy1030015 [DOI] [Google Scholar]
- Bhogoju, S. , & Nahashon, S. (2022). Recent advances in probiotic application in animal health and nutrition: A review. Agriculture (Switzerland), 12(2), 1791. [Google Scholar]
- Delavenne, E. , Mounier, J. , Déniel, F. , Barbier, G. , & Blay, G. Le (2012). Biodiversity of antifungal lactic acid bacteria isolated from raw milk samples from cow, ewe and goat over One‐year period. International Journal of Food Microbiology, 155(3), 185–190. 10.1016/j.ijfoodmicro.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Dempsey, E. , & Corr, S. C. (2022). Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Frontiers in Immunology, 13, 840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djadouni, F. , & Kihal, M. (2012). Antimicrobial activity of lactic acid bacteria and the spectrum of their biopeptides against spoiling germs in foods. Brazilian Archives of Biology and Technology, 55(3), 435–444. 10.1590/S1516-89132012000300015 [DOI] [Google Scholar]
- Giraffa, G. , Chanishvili, N. , & Widyastuti, Y. (2010). Importance of lactobacilli in food and feed biotechnology. Research in Microbiology, 161(6), 480–487. 10.1016/j.resmic.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Gradisteanu Pircalabioru, G. , Popa, L. I. , Marutescu, L. , Gheorghe, I. , Popa, M. , Barbu, I. C. , Cristescu, R. , & Chifiriuc, M. C. (2021). Bacteriocins in the Era of antibiotic resistance: Rising to the challenge. Pharmaceutics, 13(2), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun‐Ur‐Rashid, M. , Togo, K. , Ueda, M. , & Miyamoto, T. (2007). Identification and characterization of dominant lactic acid bacteria isolated from traditional fermented milk Dahi in Bangladesh. World Journal of Microbiology and Biotechnology, 23(1), 125–133. 10.1007/s11274-006-9201-x [DOI] [Google Scholar]
- Hoque, M. Z. , Akter, F. , Hossain, K. M. , Rahman, M. S. M. , Billah, M. M. , & Islam, K. M. D. (2010). Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World Journal of Dairy & Food Sciences, 5(1), 39–46. [Google Scholar]
- Huang, C. H. , Li, S. W. , Huang, L. , & Watanabe, K. (2018). Identification and classification for the Lactobacillus casei group. Frontiers in Microbiology, 9, 1974. 10.3389/fmicb.2018.01974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp . Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.
- Jose, N. M. , Bunt, C. R. , & Hussain, M. A. (2015). Comparison of microbiological and probiotic characteristics of Lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms, 3(2), 198–212. 10.3390/microorganisms3020198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołozyn‐Krajewska, D. , & Dolatowski, Z. J. (2012). Probiotic meat products and human nutrition. Process Biochemistry, 47(12), 1761–1772. [Google Scholar]
- Kowalska, J. D. , Nowak, A. , Śliżewska, K. , Stańczyk, M. , Łukasiak, M. , & Dastych, J. (2020). Anti‐salmonella potential of new Lactobacillus strains with the application in the poultry industry. Polish Journal of Microbiology, 69(1), 5–18. 10.33073/pjm-2020-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Díaz, T. M. , Alonso, C. , Román, C. , García‐López, M. L. , & Moreno, B. (2000). Lactic acid bacteria isolated from a hand‐made blue cheese. Food Microbiology, 17(1), 23–32. 10.1006/fmic.1999.0289 [DOI] [Google Scholar]
- Manyi‐Loh, C. , Mamphweli, S. , Meyer, E. , & Okoh, A. (2018). Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules, 23(4), 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz, L. H. , Biedrzycka, E. , Wasilewska, E. , & Bielecka, M. (2010). Rapid molecular identification and characteristics of Lactobacillus strains. Folia Microbiologica, 55(5), 481–488. 10.1007/s12223-010-0080-z [DOI] [PubMed] [Google Scholar]
- McAllister, T. A. , Beauchemin, K. A. , Alazzeh, A. Y. , Baah, J. , Teather, R. M. , & Stanford, K. (2011). Review: The use of direct fed microbials to mitigate pathogens and enhance production in cattle. Canadian Journal of Animal Science, 91(2), 193–211. [Google Scholar]
- Patel, A. , Prajapati, J. B. , & Nair, B. M. (2012). Methods for isolation, characterization and identification of probiotic bacteria to be used in functional foods. International Journal of Fermented Foods, 1(1), 1–13. [Google Scholar]
- Prabhurajeshwar, C. , & Chandrakanth, R. K. (2017). Probiotic potential of lactobacilli with antagonistic activity against pathogenic strains: An in vitro validation for the production of inhibitory substances. Biomedical Journal, 40(5), 270–283. 10.1016/j.bj.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refay, R. M. , Abushady, H. M. , Amer, S. A. , & Mailam, M. A. (2020). Determination of bacteriocin‐encoding genes of lactic acid bacteria isolated from traditional dairy products of Luxor Province, Egypt. Future Journal of Pharmaceutical Sciences, 6(1), 1–14 10.1186/s43094-020-00031-3 [DOI] [Google Scholar]
- Reuben, R. C. , Roy, P. C. , Sarkar, S. L. , Rubayet Ul Alam, A. S. M. , & Jahid, I. K. (2020). Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. Journal of Dairy Science, 103(2), 1223–1237. 10.3168/jds.2019-17092 [DOI] [PubMed] [Google Scholar]
- Saadatzadeh, A. , Fazeli, M. R. , Jamalifar, H. , & Dinarvand, R. (2013). Probiotic properties of lyophilized cell free extract of Lactobacillus casei . Jundishapur Journal of Natural Pharmaceutical Products, 8(3), 131–137. 10.17795/jjnpp-8564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed, Z. K. , Abbas, B. A. , & Othman, R. M. (2020). Molecular identification and phylogenetic analysis of lactic acid bacteria isolated from goat raw milk. Iraqi Journal of Veterinary Sciences, 34(2), 259–263. 10.33899/ijvs.2019.125896.1176 [DOI] [Google Scholar]
- Sambrook, J. , Fritsch, E. F. , & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed., Vols. 1, 2 and 3). Cold Spring Harbor Laboratory Press. [Google Scholar]
- Santos, A. , San Mauro, M. , Sanchez, A. , Torres, J. M. , & Marquina, D. (2003). The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Systematic and Applied Microbiology, 26(3), 434–437. 10.1078/072320203322497464 [DOI] [PubMed] [Google Scholar]
- Seifert, E. (2014). OriginPro 9.1: Scientific data analysis and graphing software—Software review. Journal of Chemical Information and Modeling, 54(5), 1552. [DOI] [PubMed] [Google Scholar]
- Shahriar, A. , Alo, M. , Hossain, M. F. , Emran, T. B. , Uddin, M. Z. , & Paul, A. (2019). Prevalence of multi‐drug resistance traits in probiotic bacterial species from fermented milk products in Bangladesh. Microbiology Research Journal International, 29(2), 1–10. 10.9734/mrji/2019/v29i230161 34169184 [DOI] [Google Scholar]
- Simons, A. , Alhanout, K. , & Duval, R. E. (2020). Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug‐resistant bacteria. Microorganisms, 8(5), 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni, M. , Shah, H. R. , & Patel, S. M. (2021). Isolation, identification and analysis of probiotic characteristics of Lactobacillus spp. from regional yoghurts from Surendranagar District, Gujarat. Asian Journal of Dairy and Food Research, 40(3), 267–272. 10.18805/ajdfr.DR-1631 [DOI] [Google Scholar]
- Taye, Y. , Degu, T. , Fesseha, H. , & Mathewos, M. (2021). Isolation and identification of lactic acid bacteria from cow milk and milk products. Scientific World Journal, 2021, 4697445. 10.1155/2021/4697445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisi‐Raygani, A. , Mohammadi, M. , Jalali, R. , Salari, N. , & Hosseinian‐Far, M. (2021). Prevalence of cystic echinococcosis in slaughtered livestock in Iran: A systematic review and meta‐analysis. BMC Infectious Diseases, 21(1), 429. 10.1186/s12879-021-06127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen, W. , & Dewulf, J. (2017). Antimicrobial use and resistance in animals and human beings. The Lancet Planetary Health, 1(8), e307–e308. 10.1016/S2542-5196(17)30142-0 [DOI] [PubMed] [Google Scholar]
- Vasudha, M. , & Gayathri, D. (2023). Metabolism and functional heterogeneity of fermented milk origin lactic acid bacteria for lactose intolerance. Journal of Microbiology, Biotechnology and Food Sciences, 13(3), e9654. 10.55251/jmbfs.9654 [DOI] [Google Scholar]
- Vasudha, M. , Prashantkumar, C. S. , Bellurkar, M. , Kaveeshwar, V. , & Gayathri, D. (2023). Probiotic potential of β‐galactosidase‐producing lactic acid bacteria from fermented milk and their molecular characterization. Biomedical Reports, 18(3), 1–12. 10.3892/br.2023.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters, J. T. M. , Ayad, E. H. E. , Hugenholtz, J. , & Smit, G. (2002). Microbes from raw milk for fermented dairy products. International Dairy Journal, 12, 91–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data available on request from the corresponding author.


