Abstract
This article provides a summary of the clinical spectrum of no obstructive coronary arteries. We describe the pathologies, invasive and noninvasive assessment, and management strategies.
Keywords: Ischemia, Epicardial coronary arteries, Functional testing, Intracoronary imaging, Cardiac imaging
1. Background
Angina or ischemia with non-obstructive coronary artery disease (INOCA) has become an increasingly recognized diagnosis. Up to 50 % of patients who present with stable angina undergoing coronary angiography have no significant epicardial coronary artery obstruction, with a greater prevalence of 65 % in women versus 32 % in men [1]. INOCA are defined as angina or ischemia with <50 % epicardial coronary artery stenosis on angiography [2]. Along the same spectrum of disease is myocardial infarction with no obstructive coronary arteries (MINOCA), defined as an acute myocardial infarction where there is detection of a rise or fall of cardiac troponin >99th percentile of the upper reference level on serial assessment plus clinical evidence of infarction with <50 % epicardial coronary stenosis on angiography. There is a 3 % per year rate for major adverse cardiac events (MACE) in patients with MINOCA, while mortality in patients with MINOCA approaches 5 % at 1 year [3].
A high prevalence of INOCA in women was demonstrated in the Women's Ischemia Syndrome Evaluation (WISE) study, which found that among a cohort of women referred for coronary angiography for evaluation of suspected ischemic heart disease, 62 % had no obstructive coronary disease (≥ 50 % diameter reduction). The study demonstrated that the 10-year rate of cardiovascular mortality or non-fatal MI in women with INOCA was 12.8 %, indicating that INOCA is not a benign disease [4]. Timely diagnosis and personalized management of patients with INOCA is critical to improving patient symptoms, quality of life, and treatment satisfaction [5].
When invasive coronary reactivity testing is performed in patients with INOCA, 75–90 % of these patients will receive a diagnosis of microvascular spasm, epicardial coronary spasm, coronary microvascular dysfunction (CMD), and/or myocardial bridging as a cause of their symptoms and presentation [[5], [6], [7], [8]]. The CorMicA (coronary microvascular angina) trial was a randomized, controlled, blinded clinical trial that studied the efficacy of a stratified medical management versus usual care of patients presenting with angina without obstructive epicardial coronary disease. The median age of study participants was 61 years, and the majority were women (73.5 %). Invasive coronary functional testing was performed with thermodilution and acetylcholine reactivity testing, and then patients were divided into 4 diagnostic groups: noncardiac, microvascular angina, vasospastic angina, or both microvascular and vasospastic angina. When medical management was informed by invasive coronary functional testing, there was significant improvement in quality-of-life and angina at 6 months compared with usual care without knowledge of coronary reactivity testing (P = 0.001). There was no difference in MACE at 6 months (P = 0.8) [5].
These startling findings suggest that the overwhelming majority of patients undergoing invasive coronary angiography (but who do not undergo reactivity testing) are incompletely evaluated, remain undiagnosed, and are undertreated or untreated as a result. Often, because the diagnosis is overlooked, the patient may have medications withdrawn, as the physician inaccurately deduces that the patient does not have a cardiac source of symptoms. Despite the availability of both invasive and noninvasive diagnostic modalities, complete evaluation of patients with ANOCA, INOCA, or MINOCA remains inconsistent [9].
The 2019 European Society of Cardiology Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes and the 2021 Guideline for the Evaluation and Diagnosis of Chest Pain were the first clinical practice guidelines published incorporating these patients into a clinical pathway with a systematic diagnostic evaluation [2,10]. Evidence from both WISE and CorMicA provided support for a class IIA recommendation for invasive coronary functional testing in patients with suspected INOCA [2].
This review article provides a summary of the clinical spectrum of no obstructive coronary arteries including ANOCA, INOCA, and MINOCA. We describe the varying pathologies of the disease, illustrate both invasive and noninvasive modalities to further evaluate these patients, and summarize management and prevention strategies. Lastly, we present two clinical case examples.
2. Range of pathologies
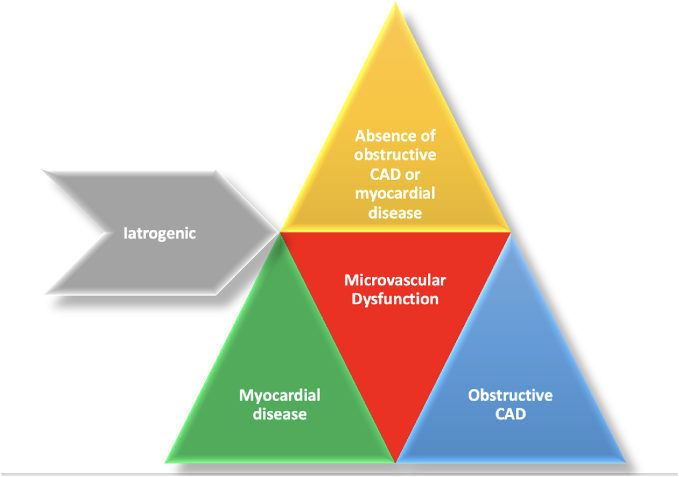
The epicardial coronary arteries serve as conduits to deliver oxygenated blood to the myocardium. Much of the focus of the ischemic evaluation is on determining the presence and extent of epicardial coronary artery obstruction. When non-obstructive disease is demonstrated on coronary angiography in the presence of documented ischemia, it is critical to evaluate the microvasculature. Microvascular dysfunction is a hallmark of ischemia with non-obstructive coronary arteries (INOCA). The microvascular circulation is a large territory of the vascular system consisting of pre-arterioles, arterioles, capillaries, and venules. This network regulates local blood perfusion, determines resistance to flow and conducts blood–tissue exchange of oxygen. Coronary microvascular disease (CMD) is generally categorized as microvascular dysfunction in the absence of obstructive coronary artery disease (CAD) or myocardial diseases, occurring in the presence of myocardial disease, occurring in the presence of obstructive CAD, or iatrogenic such as post percutaneous revascularization (Fig. 1) [11].
Fig. 1.
Causes of microvascular dysfunction.
CAD: Coronary artery disease.
Other disorders including heart failure with preserved ejection fraction (HFpEF) who often have abnormal coronary flow reserve (CFR). This subset of patients can have microvascular inflammation and fibrosis contributing to the abnormal CFR. Other pathologies not often considered when evaluating microvascular dysfunction include cardiomyopathies. Here the mechanism of dysfunction is marked by a combination of capillary rarefaction, extrinsic compression and abnormal vascular remodeling including interstitial fibrosis [12,13].
It is important to note that 20–25 % of individuals undergoing percutaneous revascularization experience angina due to microvascular dysfunction [14,15]. In addition, distal embolization, reperfusion injury, myocardial edema and capillary compression along with pre-existing microvascular dysfunction have all been reported as mechanism after balloon angioplasty and stenting [12,13]. Finally, disorders such as Takotsubo syndrome may have associated microvascular spasm, edema and compression or pre-existing microvascular dysfunction as well [16].
It is important to note that isolated and pre-existing microvascular dysfunction predominantly affects women and frequently occurs without traditional cardiovascular risk factors. Yet the most commonly associated risk factor is diabetes mellitus [17,18].
3. Diagnosis of INOCA
The Coronary Microvascular Angina (CorMicA) study indicated that coronary angiography often fails to identify patients' microvascular angina (and vasospastic angina) and stratified medical therapy improves symptoms in such patients [19]. Yet despite the growing evidence highlighting the prognostic significance of coronary microvascular dysfunction (CMD), the 2019 European Society of Cardiology (ESC) guidelines provide a class IIa recommendation for invasive testing and a class IIb recommendation for non-invasive testing [20]. The most recent 2021 American guidelines afforded a IIA recommendation for stress positron emission testing (PET) and stress cardiac magnetic resonance (CMR) and a IIB recommendation for stress echocardiography [2].
3.1. Non-invasive diagnosis
Non-invasive methods assess the vasodilator capacity of the coronary microvasculature and estimate myocardial blood flow during hyperemia compared with flow at rest. Insights from the ISCHEMIA trial note that the prevalence of INOCA was 13 % and the severity of ischemia was not associated with severity of nonobstructive atherosclerosis. Exercise electrocardiography (ECG), stress echocardiography, and myocardial perfusion imaging (MPI) using single photon emission computed tomography (SPECT) are all non-invasive modalities to aid in assessment of coronary microvascular blood flow. With all modalities, the proportion of patients with INOCA was higher when there was moderate rather than severe ischemia which was observed most with stress nuclear testing [21].
Transthoracic Doppler Echocardiography (TTDE) can identify the maximal diastolic flow in the mid-distal left anterior descending artery (LAD) at rest and during adenosine/dipyridamole stress to estimate coronary flow velocity reserve (CFVR) by means of the pulse wave Doppler technique. Reduced CFVR (≤2–2.5) is a marker of coronary microvascular dysfunction. Schroder et al. demonstrated that the CFVR value has a prognostic value. Despite being low-cost, radiation-free, and available tests, it lacks accuracy and requires extensive training [22].
Myocardial Contrast Echocardiography is a technology in which an ultrasonic contrast agent and microbubble with RBC size (<7 μm) is injected. This contrast reflects an echo signal that correlates with capillary blood volume. The myocardial blood flow is measured by the product of peak contrast intensity (dp) and myocardial flow velocity (db/s) in each myocardial segment. Coronary Flow Reserve is the ratio of MBF at peak hyperemia compared to rest. Due to the considerable variation of results among operators, the quantitative and qualitative measurement of myocardial perfusion from myocardial contrast echo needs further validation [23,24].
PET is a well-validated and reliable test for the evaluation of coronary microcirculation. It uses positron-emitting radiotracers such as 15O-water, 82Rubidium, 13N-ammonia, and 18F-labeled agents for assessment of the myocardial blood flow (MBF), myocardial perfusion reserve (MPR), and myocardial flow reserve (MFR). The MPR is the MBF at maximum stress, whereas MFR is the ratio of MBF at maximal coronary vasodilation and MBF at rest. A MFR < 1.5 indicates coronary microvascular dysfunction [25]. Low MFR is associated with poor prognosis, and a preserved MFR has a high negative predictive value (97 %) for excluding ischemia [9,25,26]. The use of PET is limited by its availability, cost, and radiation exposure.
CMR has a higher spatial resolution, no radiation exposure, and provides accurate information regarding myocardial edema and fibrosis. Stress perfusion CMR using gadolinium contrast offers higher accuracy than exercise ECG and SPECT MPI. Perfusion is directly proportional to T1 signal intensity given by the diffusion from the microcirculation into the interstitial space. Myocardial perfusion reserve index (MPRI) is a semi-quantitative method used to estimate the coronary blood flow reserve and correlates with invasive testing. The CFR evaluation and myocardial perfusion reserve index have been demonstrated to predict the rate of MACE with high prognostic power beyond late gadolinium enhancement and ischemia [27,28]. Data from WISE-CVD showed that an MPRI of ≤1.84 predicted invasive CFR abnormality with a sensitivity of 73 % and specificity of 74 % using a 1.5 T magnet and CAAS MRV 3.4 as post-processing software [29,30].
Perfusion Coronary Computed Tomography (CT) is a technology that consists of a dynamic first-pass vasodilator stress segment that induced by adenosine and rest perfusion imaging segment that allows perfusion quantification [31,32]. It provides greater spatial and temporal resolution compared with cardiac PET. However, it requires repeated image acquisition, which exposes the patient to higher radiation and iodinated contrast increasing the risk of contrast induced nephropathy in those with underlying renal dysfunction. In addition, it has not been well-validated or widely adopted in practice. Table 1 summarizes the different noninvasive testing modalities.
Table 1.
Summary of the non-invasive diagnostic modalities for the evaluation of coronary microcirculation.
| Imaging modality | Technique | Material | Threshold | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Transthoracic Doppler echocardiography | Pulsed wave Doppler of the LAD | Vasodilators | CFRV < 2 |
|
|
| Myocardial contrast echocardiography | Microbubble velocity & myocardial blood volume | Microbubble contrast agent | MBF < 2 |
|
|
| Positron emission tomography | Rest & stress perfusion imaging | Radioisotope agent & vasodilators | MPR < 2 |
|
|
| Cardiac magnetic resonance | Rest & stress perfusion imaging | Gadolinium contrast & vasodilators | MPRI < 2 |
|
|
| Contrast enhanced cardiac computed tomography | Rest & stress perfusion imaging | Iodine contrast & vasodilators | MPR < 2 |
|
|
LAD: Left anterior descending artery; CFRV: Coronary flow reserve velocity; MBF: Myocardial blood flow; CMD: Coronary microvascular dysfunction; MPR: Myocardial perfusion reserve; MPRI: Myocardial perfusion reserve index. The most validated imaging modalities are Positron Emission Tomography and Cardiac Magnetic Resonance. Other non-invasive tests have limited data on the exact percentage of accuracy, sensitivity and specificity compared to invasive testing.
3.2. Invasive diagnosis
Invasive Diagnosis of INOCA requires initial assessment of microvascular function via measurement of CFR and IMR followed by acetylcholine provocation test to rule out vasospasm.
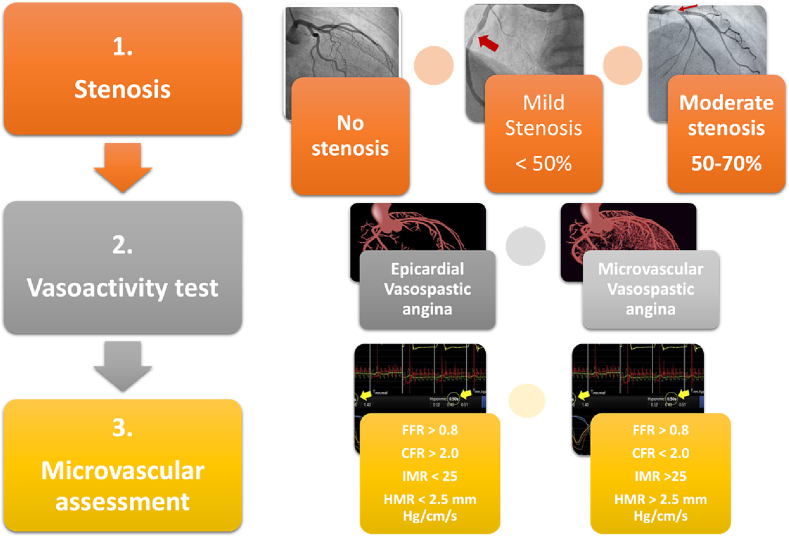
Microvascular dysfunction is assessed using Coronary flow reserve (CFR) or the index of microcirculatory resistance (IMR). CFR measures the blood flow in the epicardial arteries and microvasculature, IMR measures the blood flow in the microvasculature. Microvascular spasm is evaluated by the induction of epicardial and microvascular spasm after intracoronary acetylcholine administration, which can identify a microvascular hypercontractive response (Fig. 2).
Fig. 2.
Central illustration.
FFR: Fractional flow reserve; CFR: Coronary flow reserve; IMR: Index of microcirculatory resistance; HMR: Hyperemic microvascular resistance.
Two methods may be used to measure coronary flow reserve: intracoronary Doppler or intracoronary thermodilution which measure transit time. The thermodilution method is the most widely available and allows the evaluation of fractional flow reserve (FFR), CFR, and IMR [33]. IMR can be calculated by the formula IMR = Pd × Tmn, where Pd = mean distal coronary pressure, and Tmn = mean hyperemic transit time.
Invasive functional testing is considered a low-risk procedure when performed by a trained interventional cardiologist. In the WISE study of 293 women who underwent invasive functional testing, there were no deaths due to functional testing; two serious adverse events occurred in 2 women (0.7 %): one coronary artery dissection and one myocardial infarction associated with coronary spasm [34].
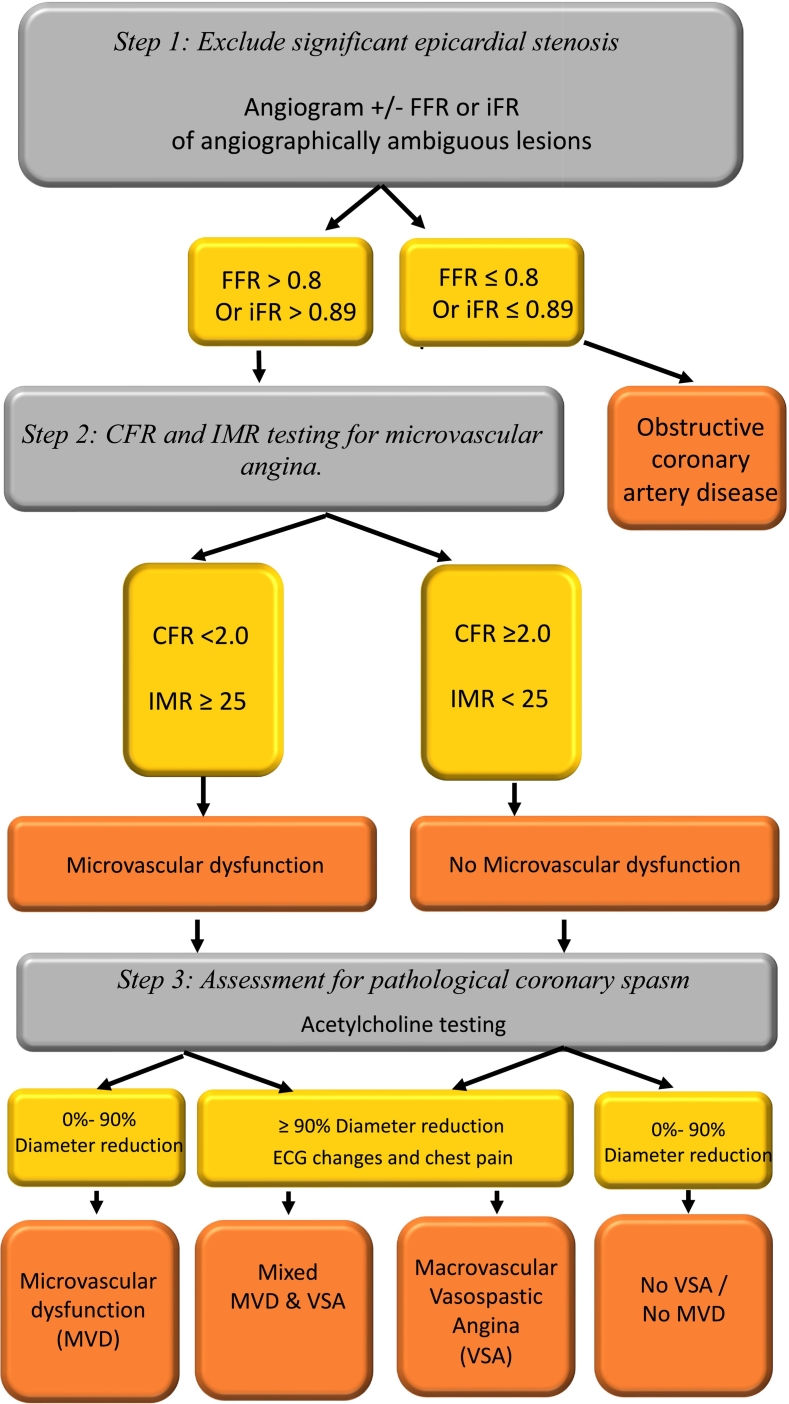
Workflow for the invasive assessment of INOCA should be performed in three steps [35] which are summarized in Fig. 3.
Fig. 3.
Invasive diagnosis of INOCA.
3.2.1. Step 1: exclude significant epicardial stenosis
The first step in invasive coronary functional testing during invasive coronary angiography is ruling out significant epicardial coronary artery stenosis as the cause of myocardial ischemia with angiography ± physiology assessment of flow with fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) as required.
Detailed explanation of Physiological assessment of coronary flow is well described elsewhere but can be summarized as follows. FFR is the ratio of mean distal coronary pressure to aortic pressure during hyperemia, or non-hyperemic pressure ratio (NHPR). Resting full cycle ratio (RFR), calculates the minimal distal pressure with reference to the aortic pressure during five entire cardiac cycles. iFR is calculated by measuring the resting pressure gradient across a coronary lesion during the portion of diastole when microvascular resistance is low and stable. The cut-off value of 0.80 in FFR and 0.89 in iFR diagnoses functionally significant coronary artery disease.
Adenosine is used in FFR and IMR assessment and is the most common agent used to achieve maximum hyperemia, it can be administered as an IV infusion (140–210 μg/kg/min) or intracoronary bolus (50–200 μg), and has endothelium dependent and non-endothelium dependent effects [36]. Regadenoson, a selective A2a receptor agonist, can replace adenosine.
3.2.2. Step 2: CFR and IMR testing for microvascular angina
To test for microvascular angina physiology assessment is required using pressure/temperature wires and adenosine. A guidewire designed for physiological assessment with distal pressure or temperature sensors is typically placed into the distal left anterior descending artery. To measure CFR and IMR, resting mean transient time (Tmn) is measured by injecting 3 ml of room temperature saline (thermodilution) (this measurement is taken a minimum of three times) to achieve to ensure similar reproducible values are recorded. The aortic pressure at the guiding catheter (Pa) and distal coronary pressure at the tip of the guidewire (Pd) are simultaneously recorded. The same measurements are then also recorded after inducing hyperemia with adenosine.
CFR is the ratio of basal to maximum hyperemic to coronary mean transit time. It is calculated as the average resting transient time divided by the average hyperemic transient time, averaging the three consecutive measurements under each condition.
The index of microvascular resistance (IMR) is the product of the distal coronary artery mean pressure and the mean transit time during maximum hyperemia.
Coronary microvascular dysfunction is defined as a CFR <2.0 and/or an IMR >25.
3.2.3. Step 3: Assessment for pathological coronary spasm
Diagnosing coronary spasm (epicardial and microvascular) requires an intracoronary provocation test with acetylcholine or ergonovine. Acetylcholine is a vasoactive substance that provokes vasospasm via cholinergic receptors on vascular smooth muscle cells. Normal endothelium responds by vasodilatation through the release of nitric oxide. In the case of endothelial dysfunction, vasoconstriction occurs in response to acetylcholine. Incrementally increasing doses of 20, 50 and 100 μg of acetylcholine are infused over 1–3 min into left anterior descending artery (LAD) under continuous heart rate, blood pressure, and 12 lead ECG monitoring [37]. A coronary angiogram should be repeated after every infusion dose to determine any changes in coronary diameter.
Macrovascular spasm is diagnosed when anginal chest pain and ischemic ECG changes are present in addition to coronary diameter reduction >90 % either focally or diffusely. Microvascular spasm is diagnosed when the patient experiences typical ischemic chest pain, and ECG changes with no evidence of angiographically evident epicardial coronary vasoconstriction. If coronary spasm occurs, intracoronary nitroglycerin should be administered.
The pharmacological provocation test is considered a safe procedure with a severe complication risk of <1 %, as reported by the large retrospective from Japan of 21,512 patients [38,39]. Predictors for complications during acetylcholine provocation testing included a history of paroxysmal AF, moderate-to-severe left ventricular diastolic dysfunction, and higher QT dispersion at baseline ECG. A recent prospective analysis by Montone et al. demonstrated that these complications are not associated with a worse prognosis at a medium to long-term follow-up [40].
4. Management
Patient and physician education, lifestyle modification, and medications are central tools for the management of INOCA [41]. The management and prevention of INOCA varies based on endotype, epicardial vasospastic angina, microvascular angina, or mixed angina. However, many principles of management and prevention are shared across these groups.
Management approaches include addressing lifestyle factors, risk factors, and medications to address microvascular dysfunction and vasospasm. These strategies are endorsed by the EAPCI and Microvascular Network consensus documents on the management of INOCA [37,40,42,45]. Similar recommendations are found in guidelines [2]. ESC guidelines recommend in patients with abnormal CFR and a negative acetylcholine provocation test, beta-blockers, ACE inhibitors, and statins, along with lifestyle changes and weight loss [43,46]. These guidelines also state in patients with epicardial or microcirculatory vasomotor disorders, including coronary spasm calcium channel blockers and long-acting nitrates constitute the treatment of choice, in addition to the control of cardiovascular risk factors and lifestyle changes [44,45,47,48].
4.1. Pharmacological management
Beta-blockers have been shown to reduce symptoms and improve exercise tolerance in angina secondary to microvascular dysfunction, but should be avoided in proven vasospastic angina [45,48]. ACE inhibitors have demonstrated improvement in exercise test parameters, and frequency of angina. A combination of statins and ACE inhibitors have also been shown to improve quality of life and exercise duration at 6 month follow up [45,48]. Two randomized control trials (RCTs) found that patients with microvascular angina randomized to an ACE-inhibitor had a significant improvement in CFR compared to those treated with placebo [46,47,49,50]. Calcium channel blockers have been shown to improve symptoms and exercise test parameters in several but not all studies. Amlodipine has been shown in animal studies to improve remodeling in coronary microvascular dysfunction, and for this reason is often recommended as a first line agent in patients with microvascular spasm [48,51,45,48]. Statins are recommended based on their pleotropic effects (LDL reducing and reduction of vascular inflammation). Two RCTs of statin vs. placebo in patients with microvascular dysfunction found a significant improvement in brachial artery flow mediated dilation with statins when compared to placebo [49,50,52,53].
In patients with refractory symptoms despite treatment with these traditional anti-anginal drugs, novel or non-traditional antianginal drugs such as ranolazine, nicorandil, or ivabradine are recommended, as are drugs potentially effective for heightened nociception, such as xanthines and tricyclics antidepressants [48]. Anti-anginal medications can be used for INOCA, but RCT data and evidence for benefit is acknowledged to be limited [43,46,51,54]. Anti-anginal drugs are reported to be effective in only half of patients with microvascular angina [51,54], likely due to variable underlying pathophysiology. In microvascular angina, calcium channel blockers, beta-blockers, nicorandil, ranolazine, ivabradine and trimetazidine may all be considered [52,55]. In vasospastic angina, calcium channel blockers, long-acting nitrates, and nicorandil may all be used [52,55]. Statins and ranolazine have been evaluated in smaller trials for the different phenotypes [43,46,51,54]. Angiotensin receptor blockers are still being tested in ongoing studies. Table 2 provides a summary of these pharmacological options.
Table 2.
Summary of pharmacological therapies for the treatment of microvascular disease.
| Medication class | Examples | Mechanism of action | Special considerations |
|---|---|---|---|
| First-line therapies | |||
| Beta-blockers | Propranolol Atenolol Carvedilol Nebivolol |
Lowers heart rate, contractility, blood pressure, and oxygen consumption. | Avoid in proven vasospastic angina |
| ACEI/ARBs | Enalapril Quinapril Irbesartan |
Vasoconstriction by increasing superoxide production. Stimulates nitric oxide production by lowering bradykinin degradation. |
Combined with statins shown to improve quality of life and exercise duration at 6 months |
| Calcium-channel blockers | Amlodipine Nifedipine Verapamil Diltiazem |
Negative inotropic and vasodilatory effects, thereby reducing afterload. Protects endothelium against free radial injury. Increases nitrate. |
Amlodipine is first-line for microvascular spasm |
| Statins | Pravastatin Fluvastatin |
Improves endothelial function by increasing nitric oxide bioavailability. | Combined with ACEI shown to improve quality of life and exercise duration at 6 months |
| Antiplatelet | Aspirin | Inhibits thromboxane A2. | |
| Second-line therapies | |||
| Nitric oxide modulators | L-arginine Sildenafil Cilostazol Tetrahydrobiopterin |
Vasodilation induced by nitrates through activation of guanylyl cyclase signaling pathway. | |
| Novel drugs | Ivabradine Fasudil |
Lowers heart rate and reduces myocardial oxygen demand. Mediates vascular smooth muscle and endothelial cell function. |
|
| Miscellaneous | Nicorandil Imipramine |
Vasodilation by stimulating guanylyl cyclase and increasing cGMP levels. Elevates pain threshold. |
Nicorandil is not approved for use in the United States |
| Last-line/least effective therapies | |||
| Nitric oxide modulators | Nitrates | Vasodilation by stimulating guanylyl cyclase and increasing cGMP levels. | |
| Novel drugs | Ranolazine | Inhibits late sodium current in cardiomyocytes. Improves endothelial function. |
Showed no benefit except in the subgroup with reduced coronary flow reserve per the R-WISE study |
| Miscellaneous | Trimetazidine Xanthine derivatives |
Inhibits the long-chain of 3-ketoacyl coenzyme A thiolase, which inhibits beta-oxidation of free fatty acids, thus decreasing oxygen consumption. Vasodilation. |
|
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; cGMP: cyclic guanosine monophosphate; R-WISE:
4.2. Lifestyle and risk factors modification
Several therapeutic lifestyle factor targets for the treatment of and prevention of INOCA have been proven effective. Smoking is a risk factor for vasospastic angina and coronary microvascular dysfunction and should be addressed by encouraging smoking cessation [43,46]. Hypertension, and in particular, recurrent spikes of high blood pressure, can trigger epicardial coronary spasm. Therefore, consistent blood pressure control is an important target for treatment and may prevent progression of microvascular changes [43,46,52,56]. Diabetes management with medications, exercise, and weight loss are all important targets for treatment, as are lipid control, and addressing and managing stress and stressors. The EAPCI expert consensus document suggests behavioural interventions supported by nurse practitioners, experts in nutrition, psychologists, exercise physiotherapists, and sports medicine, although, these resource-intensive interventions may not be easily achievable in some healthcare settings [43,46].
4.3. CorMicA trial treatment strategies
Evidence-based tailored treatment of INOCA is limited. The best evidence for invasive investigation followed by treatment stratifies based on these investigations comes from the CorMicA trial [46]. It is important to note that the CorMicA trial has found a benefit for treatment following invasive investigation of INOCA in terms of symptoms, but to date no RCT evidence for prognostic benefit in terms of hard clinical endpoints such as death and MI have been established [46].
The CorMicA trial enrolled 391 patients that had established risk factors for ischemic heart disease (IHD), and many were already taking cardiac medications (67 % were taking beta-blockers, 47 % long-acting nitrates, and 34 % took calcium-channel blockers). Patients without obstructive disease 39 % (151/391) were randomized to either intervention with stratified medical therapy based on the findings of invasive CFR, RFR, FFR and acetylcholine test results, or to a sham procedure followed by standard care.
The intervention included non-pharmacological and pharmacotherapy. Prescription recommendations were as follows: baseline of aspirin, statin, and ACE-I for all patients, with as needed sublingual nitroglycerin. For microvascular angina (MVA), first line treatment was a beta-blocker use (e.g., carvedilol), second line was substitution of a non-dihydropyridine calcium channel blocker (NDHP-CCB) (such as diltiazem or verapamil), and third line was the addition of amlodipine (if on beta-blockers), nicorandil or ranolazine. Of note, nicorandil is not approved for use in the United States and the RWISE study showed no benefit for ranolazine, except in the subgroup with reduced CFRs [53,57]. For vasospastic angina (VSA), first line treatment was a NDHP-CCB, second line included addition of a nitrate such as isosorbide mononitrate, and third line was to replace nitrate with nicorandil. For those with mixed MVA and VSA, first line treatment was NDHP-CCB and second line treatment was nicorandil (58/54). This management strategy provides an evidence-based treatment strategy including all recommendations above.
4.4. Future directions and potential therapeutic options
Weight management with newer antidiabetic drugs, such as semaglutide (an injectable glucagon like peptide-1 receptor agonist) are yet to be fully investigated with respect to their impact on INOCA but may hold important future therapeutic options for treatment. These medications have been shown to significantly reduce cardiovascular events; however, the mechanism of benefit is still unknown. Similarly, the role of ticagrelor, a cyclopentyl-triazolo-pyrimidine (CPTP) which acts by blocking the P2Y12 receptor, with adenosine-like properties which theoretically could improve flow in the microcirculation may be potentially beneficial. Cyclopentyltriazolopyrimidine are unstudied with respect to INOCA. Animal studies have shown that ticagrelor augments the adenosine-induced increase in coronary blood flow, which is responsible for the reactive hyperaemic response, which could have potential to reduce macrovascular dysfunction (59–60/55–56). Ticagrelor pre-treatment improves coronary microvascular function in non–ST-segment–elevation acute coronary syndrome patients but is yet to be tested in the setting of INOCA or MINOCA (61/57).
5. Case examples
Typically, an IMR 25 or greater and a CFR of 2.0 or lower indicates the presence of microvascular dysfunction. However, occasionally, discordant results may occur.
5.1. Case presentation 1
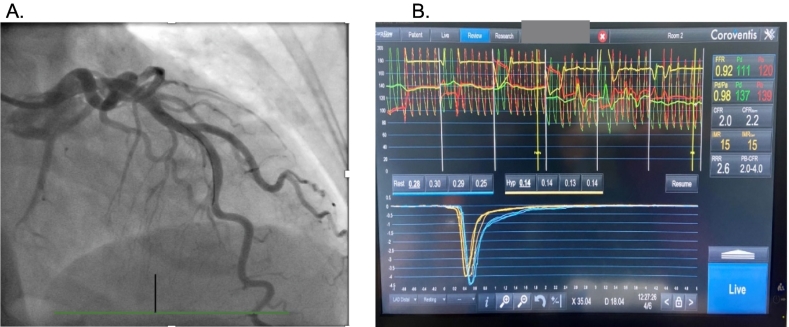
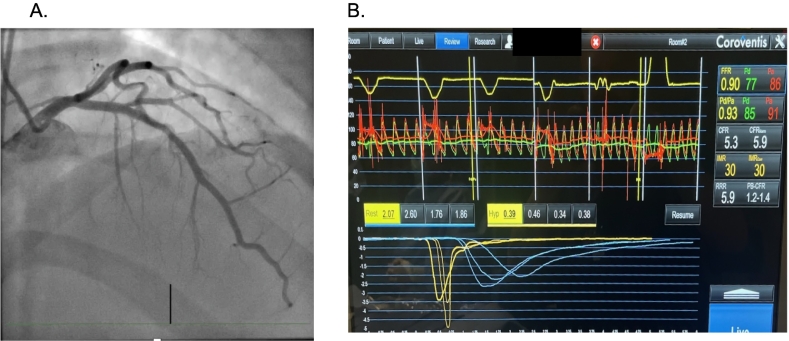
A 56-year-old female presents with exertional chest pain and a stress echocardiogram demonstrating equivocal multi-territory ischemia. The patient declines a coronary CT scan, instead opting for an invasive coronary angiogram with coronary functional testing if the angiogram reveals no obstructive coronary artery disease (Panel A). The patient is found to have non-obstructive coronary artery disease. Coronary functional testing included coronary acetylcholine administration, with no evidence of spasm. Microvascular functional testing was performed (Panel B). The coronary flow reserve was 2.0 and the index of microvascular resistance, 15. Would this patient be diagnosed as having microvascular dysfunction?
In this case, the coronary flow reserve could be low for several possible reasons. If the wire was not sufficiently into the LAD (with the radio-opaque transition zone advanced 6 cm or 2/3 or more into the vessel), that could lower the transit time at rest and with hyperemia, making it challenging to interpret the results. Alternatively, patients with high baseline coronary blood flow may not be able to double their coronary flow and have a low coronary flow reserve [1]. Based on the short transit times in this example, the patient most likely does not have coronary microvascular dysfunction, even though the coronary flow reserve is 2.0. This is an area of controversy and requires further investigation since patients with low CFR do have a higher risk of adverse clinical outcomes.
A coronary angiogram reveals no obstructive coronary artery disease (Panel A). The patient is found to have non-obstructive coronary artery disease. Coronary functional testing included coronary acetylcholine administration, with no evidence of spasm. Microvascular functional testing was performed (Panel B). The coronary flow reserve was 2.0 and the index of microvascular resistance, 15.
Nardone M, McCarthy M, Ardern CI, Nield LE, Toleva O, Cantor WJ, Miner SES. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. 2022 Mar; 15(3): e011323. https://doi.org/10.1161/CIRCINTERVENTIONS.121.011323. Epub 2022 Feb 9. PMID: 35135301.
5.2. Case presentation 2
A 72 year old man with a history of PCI to the LAD has ongoing exertional dyspnea and chest discomfort symptoms after PCI. He returns for coronary angiography to rule out new obstructive coronary artery disease. No new obstructive coronary artery disease is present (Panel A), and his left ventricular filling pressure is normal. Coronary functional testing including microvascular dysfunction testing is performed (Panel B). His baseline coronary flow is slow and increases significantly after hyperemia is induced with adenosine. His CFR is >2, but his index of microvascular resistance is still elevated, 30. This patient was diagnosed with microvascular dysfunction even though his coronary flow reserve is over 2. His symptoms improved with switching his beta-blocker to carvedilol and the addition of a calcium channel blocker.
No new obstructive coronary artery disease is present (Panel A), and his left ventricular filling pressure is normal. Coronary functional testing including microvascular dysfunction testing is performed (Panel B). His baseline coronary flow is slow and increases significantly after hyperemia is induced with adenosine. His CFR is greater than 2, but his index of microvascular resistance is still elevated, 30.
CRediT authorship contribution statement
Shereen AlShaikh: Writing – review & editing, Writing – original draft. Charlene L. Rohm: Writing – review & editing, Writing – original draft. Nadia R. Sutton: Writing – review & editing, Writing – original draft. Sonya N. Burgess: Writing – review & editing, Writing – original draft. Mirvat Alasnag: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: N.R.S. is a consultant for Abbott and Philips and has received honoraria for speaking from Abbott, Philips, and Zoll. None of the other authors have any relevant disclosures.
References
- 1.Jespersen L., Hvelplund A., Abildstrom S.Z., Pedersen F., Galatius S., Madsen J.K., Jorgensen E., Kelbaek H., Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., Blankstein R., Boyd J., Bullock-Palmer R.P., Conejo T., Diercks D.B., Gentile F., Greenwood J.P., Hess E.P., Hollenberg S.M., Jaber W.A., Jneid H., Joglar J.A., Morrow D.A., O’Connor R.E., Ross M.A., Shaw L.J. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. (Epub 2021 Oct 28. Erratum in: Circulation. 2021 Nov 30; 144(22): e455) [DOI] [PubMed] [Google Scholar]
- 3.Lindahl B., Baron T., Erlinge D., Hadziosmanovic N., Nordenskjöld A., Gard A., Jernberg T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017 Apr 18;135(16):1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336. [DOI] [PubMed] [Google Scholar]
- 4.Sharaf B., Wood T., Shaw L., Johnson B.D., Kelsey S., Anderson R.D., Pepine C.J., Bairey Merz C.N. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am. Heart J. 2013 Jul;166(1):134–141. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford T.J., Stanley B., Sidik N., Good R., Rocchiccioli P., McEntegart M., Watkins S., Eteiba H., Shaukat A., Lindsay M., et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA) JACC Cardiovasc. Interv. 2020;13:33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford T.J., Yii E., Sidik N., Good R., Rocchiccioli P., McEntegart M., Watkins S., Eteiba H., Shaukat A., Lindsay M., Robertson K., Hood S., McGeoch R., McDade R., McCartney P., Corcoran D., Collison D., Rush C., Stanley B., McConnachie A., Sattar N., Touyz R.M., Oldroyd K.G., Berry C. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ. Cardiovasc. Interv. 2019 Dec;12(12) doi: 10.1161/CIRCINTERVENTIONS.119.008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee B.K., Lim H.S., Fearon W.F., Yong A.S., Yamada R., Tanaka S., Lee D.P., Yeung A.C., Tremmel J.A. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015 Mar 24;131(12):1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sara J.D., Widmer R.J., Matsuzawa Y., Lennon R.J., Lerman L.O., Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc. Interv. 2015 Sep;8(11):1445–1453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Schindler T.H., Fearon W.F., Pelletier-Galarneau M., Ambrosio G., Sechtem U., Ruddy T.D., Patel K.K., Bhatt D.L., Bateman T.M., Gewirtz H., Shirani J., Knuuti J., Gropler R.J., Chareonthaitawee P., Slart R.H.J.A., Windecker S., Kaufmann P.A., Abraham M.R., Taqueti V.R., Ford T.J., Camici P.G., Schelbert H.R., Dilsizian V. Myocardial perfusion PET for the detection and reporting of coronary microvascular dysfunction: a JACC: cardiovascular imaging expert panel statement. JACC Cardiovasc. Imaging. 2023 Apr;16(4):536–548. doi: 10.1016/j.jcmg.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Douglas P.S., Hoffmann U., Patel M.R., Mark D.B., Al-Khalidi H.R., Cavanaugh B., Cole J., Dolor R.J., Fordyce C.B., Huang M., Khan M.A., Kosinski A.S., Krucoff M.W., Malhotra V., Picard M.H., Udelson J.E., Velazquez E.J., Yow E., Cooper L.S., Lee K.L. PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruthi S., Siddiqui E., Smilowitz N.R. Beyond coronary artery disease: assessing the microcirculation. Interv. Cardiol. Clin. 2023 Jan;12(1):119–129. doi: 10.1016/j.iccl.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivaratharajah K., Coutinho T., deKemp R., Liu P., Haddad H., Stadnick E., Davies R.A., Chih S., Dwivedi G., Guo A., Wells G.A., Bernick J., Beanlands R., Mielniczuk L.M. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ. Heart Fail. 2016 Jul;9(7) doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 13.Ashokprabhu N.D., Quesada O., Alvarez Y.R., Henry T.D. INOCA/ANOCA: mechanisms and novel treatments. Am. Heart J. Plus. 2023 Jun;30 doi: 10.1016/j.ahjo.2023.100302. (Epub 2023 May 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fearon W.F., Low A.F., Yong A.S., McGeoch R., Berry C., Shah M.G., Ho M.Y., Kim H.S., Loh J.P., Oldroyd K.G. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013 Jun 18;127(24):2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. (Epub 2013 May 16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izzo P., Macchi A., De Gennaro L., Gaglione A., Di Biase M., Brunetti N.D. Recurrent angina after coronary angioplasty: mechanisms, diagnostic and therapeutic options. Eur. Heart J. Acute Cardiovasc. Care. 2012 Jun;1(2):158–169. doi: 10.1177/2048872612449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Buono M.G., Montone R.A., Camilli M., Carbone S., Narula J., Lavie C.J., Niccoli G., Crea F. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021 Sep 28;78(13):1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taqueti V.R., Di Carli M.F. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018 Nov 27;72(21):2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kothawade K., Bairey Merz C.N. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr. Probl. Cardiol. 2011 Aug;36(8):291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford T.J., Stanley B., Good R., Rocchiccioli P., McEntegart M., Watkins S., Eteiba H., Shaukat A., Lindsay M., Robertson K., Hood S., McGeoch R., McDade R., Yii E., Sidik N., McCartney P., Corcoran D., Collison D., Rush C., McConnachie A., Touyz R.M., Oldroyd K.G., Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J. Am. Coll. Cardiol. 2018 Dec 11;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur. Heart J. 2019;41:407–477. doi: 10.15829/1560-4071-2020-2-3757. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds H.R., Diaz A., Cyr D.D., Shaw L.J., Mancini G.B.J., Leipsic J., Budoff M.J., Min J.K., Hague C.J., Berman D.S., Chaitman B.R., Picard M.H., Hayes S.W., Scherrer-Crosbie M., Kwong R.Y., Lopes R.D., Senior R., Dwivedi S.K., Miller T.D., Chow B.J.W., de Silva R., Stone G.W., Boden W.E., Bangalore S., O’Brien S.M., Hochman J.S., Maron D.J., ISCHEMIA Research Group Ischemia with nonobstructive coronary arteries: insights from the ISCHEMIA trial. JACC Cardiovasc. Imaging. 2023 Jan;16(1):63–74. doi: 10.1016/j.jcmg.2022.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder J., Michelsen M.M., Mygind N.D., Suhrs H.E., Bove K.B., Bechsgaard D.F., Aziz A., Gustafsson I., Kastrup J., Prescott E. Coronary flow velocity reserve predicts adverse prognosis in women with angina and no obstructive coronary artery disease: results from the iPOWER study. Eur. Heart J. 2021;42:228–239. doi: 10.1093/eurheartj/ehaa944. [DOI] [PubMed] [Google Scholar]
- 23.Vogel R., Indermühle A., Reinhardt J., Meier P., Siegrist P., Namdar M., et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J. Am. Coll. Cardiol. 2005;45:754–762. doi: 10.1016/j.jacc.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 24.Karogiannis N., Senior R. Contrast echocardiography for detection of myocardial perfusion abnormalities: a clinical perspective. Herz. 2017;42:287–294. doi: 10.1007/s00059-017-4536-7. [DOI] [PubMed] [Google Scholar]
- 25.Campisi R., Marengo F.D. Coronary microvascular dysfunction in women with nonobstructive ischemic heart disease as assessed by positron emission tomography. Cardiovasc. Diagn. Ther. 2017;7:196–205. doi: 10.21037/cdt.2017.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dilsizian V., Bacharach S.L., Beanlands R.S., Bergmann S.R., Delbeke D., Dorbala S., et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J. Nucl. Cardiol. 2016;23:1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 27.Taqueti V.R., Shaw L.J., Cook N.R., Murthy V.L., Shah N.R., Foster C.R., et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135:566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Indorkar R., Kwong R.Y., Romano S., White B.E., Chia R.C., Trybula M., Evans K., Shenoy C., Farzaneh-Far A. Global coronary flow reserve measured during stress cardiac magnetic resonance imaging is an independent predictor of adverse cardiovascular events. JACC Cardiovasc. Imaging. 2019;12(Pt 2):1686–1695. doi: 10.1016/j.jcmg.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Ayub M.T., Kalra D. Coronary microvascular dysfunction and the role of noninvasive cardiovascular imaging. Diagnostics. 2020;10:679. doi: 10.3390/diagnostics10090679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson L.E.J., Wei J., Agarwal M., et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. Circ. Cardiovasc. Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002481. (PMID: 25801710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindler T.H., Dilsizian V. Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc. Imaging. 2020;13:140–155. doi: 10.1016/j.jcmg.2018.11.036. (PMID: 30982670) [DOI] [PubMed] [Google Scholar]
- 32.Mathew R.C., Bourque J.M., Salerno M., Kramer C.M. Cardiovascular imaging techniques to assess microvascular dysfunction. J. Am. Coll. Cardiol. Img. 2020;13:1577–1590. doi: 10.1016/j.jcmg.2019.09.006. (PMID: 31607665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams R.P., de Waard G.A., De Silva K., Lumley M., Asrress K., Arri S., et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am. J. Cardiol. 2018;121:1–8. doi: 10.1016/J.AMJCARD.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J., Mehta P.K., Johnson B.D., et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc. Interv. 2012;5(6):646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunadian V., Chieffo A., Camici P.G., Berry C., Escaned J., Maas A., Prescott E., Karam N., Appelman Y., Fraccaro C., Buchanan G., Manzo-Silberman S., Al-Lamee R., Regar E., Lansky A., Mehran R., Capodanno D., Baumbach A. An EAPCI expert consensus document on ischemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020;41:3504–3520. doi: 10.1093/euroheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escaned J., Berry C., De Bruyne B., Shabbir A., Collet C., Lee J.M., Appelman Y., Barbato E., Biscaglia S., Buszman P.P., Campo G., Chieffo A., Colleran R., Collison D., Davies J., Giacoppo D., Holm N.R., Jeremias A., Paradies V., Piróth Z., Raposo L., Roguin A., Rudolph T., Sarno G., Sen S., Toth G.G., Van Belle E., Zimmermann F.M., Dudek D., Stefanini G., Tarantini G. Applied coronary physiology for planning and guidance of percutaneous coronary interventions. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology. EuroIntervention. 2023 Aug 21;19(6):464–481. doi: 10.4244/EIJ-D-23-00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuels B.A., Shah S.M., Widmer R.J., Kobayashi Y., Miner S.E.S., Taqueti V.R., Jeremias A., Albadri A., Blair J.A., Kearney K.E., Wei J., Park K., Barseghian El-Farra A., Holoshitz N., Janaszek K.B., Kesarwani M., Lerman A., Prasad M., Quesada O., Reynolds H.R., Savage M.P., Smilowitz N.R., Sutton N.R., Sweeny J.M., Toleva O., Henry T.D., Moses J.W., Fearon W.F., Tremmel J.A., Microvascular Network (MVN) Comprehensive management of ANOCA, part 1-definition, patient population, and diagnosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2023 Sep 19;82(12):1245–1263. doi: 10.1016/j.jacc.2023.06.043. (PMID: 37704315) [DOI] [PubMed] [Google Scholar]
- 38.Isogai T., Yasunaga H., Matsui H., Tanaka H., Ueda T., Horiguchi H., et al. Serious cardiac complications in coronary spasm provocation tests using acetylcholine or ergonovine: analysis of 21 512 patients from the diagnosis procedure combination database in Japan. Clin. Cardiol. 2015;38:171–177. doi: 10.1002/clc.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi T., Samuels B.A., Li W., Parikh M.A., Wei J., Moses J.W., Fearon W.F., Henry T.D., Tremmel J.A., Kobayashi Y., Microvascular Network Safety of provocative testing with intracoronary acetylcholine and implications for standard protocols. J. Am. Coll. Cardiol. 2022 Jun 21;79(24):2367–2378. doi: 10.1016/j.jacc.2022.03.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montone R., Rinaldi R., Del Buono M., Gurgoglione F., La Vecchia G., Russo M., et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. Eurointervention. 2022;18:e666–e676. doi: 10.4244/EIJ-D-2100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smilowitz N.R., Prasad M., Widmer R.J., Toleva O., Quesada O., Sutton N.R., Lerman A., Reynolds H.R., Kesarwani M., Savage M.P., Sweeny J.M., Janaszek K.B., Barseghian El-Farra A., Holoshitz N., Park K., Albadri A., Blair J.A., Jeremias A., Kearney K.E., Kobayashi Y., Miner S.E.S., Samuels B.A., Shah S.M., Taqueti V.R., Wei J., Fearon W.F., Moses J.W., Henry T.D., Tremmel J.A., Microvascular Network (MVN) Comprehensive management of ANOCA, part 2-program development, treatment, and research initiatives: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2023 Sep 19;82(12):1264–1279. doi: 10.1016/j.jacc.2023.06.044. (PMID: 37704316) [DOI] [PubMed] [Google Scholar]
- 42.Ford T.J., Berry C. Angina: contemporary diagnosis and management. Heart. 2020;106:387–398. doi: 10.1136/heartjnl-2018-314661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jespersen L., Hvelplund A., Abildstrom S.Z., Pedersen F., Galatius S., Madsen J.K., Jorgensen E., Kelbaek H., Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 44.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 45.Ong P., Athanasiadis A., Sechtem U. Pharmacotherapy for coronary microvascular dysfunction. Eur. Heart J. Cardiovasc. Pharmacother. 2015;1:65–71. doi: 10.1093/ehjcvp/pvu020. [DOI] [PubMed] [Google Scholar]
- 46.Chen J.-W., Hsu N.-W., Wu T.-C., Lin S.-J., Chang M.-S. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am. J. Cardiol. 2002;90:974–982. doi: 10.1016/S0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 47.Pauly D.F., Johnson B.D., Anderson R.D., Handberg E.M., Smith K.M., Cooper-DeHoff R.M., et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE) Am. Heart J. 2011;162:678–684. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon R.O., Watson R.M., Rosing D.R., Epstein S.E. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am. J. Cardiol. 1985;56:242–246. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 49.Kayikcioglu M. Benefits of statin treatment in cardiac syndrome-X. Eur. Heart J. 2003;24:1999–2005. doi: 10.1016/S0195-668X(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 50.Fábián E., Varga A., Picano E., Vajo Z., Rónaszéki A., Csanády M. Effect of simvastatin on endothelial function in cardiac syndrome X patients. Am. J. Cardiol. 2004;94:652–655. doi: 10.1016/j.amjcard.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 51.Soleymani M., Masoudkabir F., Shabani M., Vasheghani-Farahani A., Behnoush A.H., Khalaji A. Updates on pharmacologic management of microvascular angina. Cardiovasc. Ther. 2022 Oct 25;2022 doi: 10.1155/2022/6080258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 53.Bairey Merz C.N., Handberg E.M., Shufelt C.L., Mehta P.K., Minissian M.B., Wei J., Thomson L.E., Berman D.S., Shaw L.J., Petersen J.W., Brown G.H., Anderson R.D., Shuster J.J., Cook-Wiens G., Rogatko A., Pepine C.J. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016 May 14;37(19):1504–1513. doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford T.J., Stanley B., Good R., Rocchiccioli P., McEntegart M., Watkins S., et al. Stratified medical therapy using invasive coronary function testing in angina. J. Am. Coll. Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 55.van Giezen J.J.J., Sidaway J., Glaves P., Kirk I., Björkman J.-A. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J. Cardiovasc. Pharmacol. Ther. 2012;17:164–172. doi: 10.1177/1074248411410883. [DOI] [PubMed] [Google Scholar]
- 56.Burgess S.N., Mallard T.A., Juergens C.P. Review of ticagrelor in the management of acute coronary syndromes. Expert Opin. Drug Metab. Toxicol. 2012;8:1315–1325. doi: 10.1517/17425255.2012.717931. [DOI] [PubMed] [Google Scholar]
- 57.Xu J., Lo S., Mussap C.J., French J.K., Rajaratnam R., Kadappu K., et al. Impact of ticagrelor versus clopidogrel on coronary microvascular function after non–ST-segment–elevation acute coronary syndrome. Circ. Cardiovasc. Interv. 2022:15. doi: 10.1161/CIRCINTERVENTIONS.121.011419. [DOI] [PubMed] [Google Scholar]