Abstract
Pressure recovery downstream of the aortic valve constitutes an important factor affecting the calculation of pressure gradient (PG) across the valve and therefore the accuracy of the calculated aortic valve area. Some clinical studies hypothesized that stent and valve cusps design contribute to flow acceleration and Doppler-measured valve gradients across the balloon-expandable transcatheter aortic valve. This study aims at elucidating the physical mechanisms behind pressure recovery variations between Edwards SAPIEN 3 and Medtronic Evolut TAVs through the measurements of sensitive and precise axial pressure profiles. A 23 mm Edwards SAPIEN3 and a 26 mm Medtronic Evolut were deployed in a pulse duplicator. A Millar catheter was used to record 50 cycles of pressure data along the centerline of the valve chamber upstream and downstream of the valve. The peak PG obtained with SAPIEN at vena contracta (VC) is 18.83 ± 0.75 mmHg and after recovery, 9.56 ± 0.78 mmHg. For Evolut at VC, peak PG is 18.25 ± 0.63 mmHg and after recovery, 10.3 ± 0.57 mmHg. The differences in peak PG at VC and at the recovery were statistically significant (p < 0.001). With SAPIEN 3 at VC, the mean PG obtained is 10.11 ± 0.63 mmHg and after recovery 7.06 ± 0.46 mmHg. For Evolut, mean PG at VC is 10.45 ± 0.67 mmHg and after recovery 7.99 ± 0.61 mmHg. The differences between the mean PG between the two valves was not statistically significant at VC (p = 0.71) but significant post-recovery (p < 0.00001). While gradients at the VC are higher with the SAPIEN 3, the net gradient after pressure recovery is significantly lower compared to Evolut TAV. Efficiency of pressure recovery significantly depends on valve type due to stent interference with the recovering blood flow.
Keywords: Transcatheter aortic valve, Pressure recovery, Doppler, Catheterization, Turbulence, Pressure gradient
INTRODUCTION
Transcatheter aortic valve (TAV) replacement (TAVR) has emerged as an alternative to surgical aortic valve replacement.5 Briefly, TAVR consists of deploying a valve percutaneously to replace a diseased aortic valve. While TAVR has been advancing tremendously for the past decade, the accuracy of hemodynamic measurements such as transvalvular pressure gradients (PG) may still be dependent on the assessment modality.15 In particular, accurate assessment of net PG experienced by the ventricle is critical due to its implication on both valve function and durability.
Now, TAV devices differ in their mechanism for anchoring with the balloon expandable valves structurally distinct from self-expandable valves. In particular, self-expandable valves such as the Medtronic Evolut valve consist of stabilizing stent structures that engage with the ascending aorta. They also have a longer conduit upstream of the leaflets compared to balloon expandable valves owing to their more supraannular leaflet configurations. From a hemodynamics standpoint, these structural differences appeared to have independently contributed to increased turbulence distal to the valve for the case of self-expandable valves.11 It is therefore hypothesized that the increased turbulence in self-expandable valves dampen pressure recovery in these valves resulting in overall increased net PG. In fact, this has been hypothesized to be the mechanism at least in part related to discordance in echo and cath-based gradients.9,15
Several studies presented detailed comparative works between echo-based gradients and cath-based gradients. Generally, and as part of a standard procedure, the assessment of TAV function post-implantation is routinely performed using standard Doppler echocardiography,9,21 a method that became standard due to its non-invasive nature. Another method of measurement of transvalvular pressure gradient (PG) is the cardiac catheterization,17 which requires catheters to be inserted percutaneously. Both measurement techniques yield different pressure gradient results with the Doppler measuring the gradient at the vena contracta (VC) immediately downstream of the aortic valve opening, whereas catheterization typically measures pressure differences between the left ventricle and the fully recovered static pressure in the aorta. Therefore, cardiac catheterization takes into consideration the pressure recovery post-TAVR as well as true losses that are not reflected in the modified or simplified Bernoulli equation. Although both these gradients exist in vivo, the recovered pressure represents the work load seen by the left ventricle and may be the most hemodynamically relevant measure.14 Pressure recovery downstream of the aortic valve also constitutes an important factor affecting the calculation of PG across the valve and therefore the aortic valve area.3
Recent reports of the expected normal mean gradients and aortic valve area of the balloon-expandable and the self-expanding TAVs suggest differences may exist between these valve types1,9 however, another study15 showed that there was no significant difference between balloon-expandable and self-expandable valves in regards to their cath or echo gradients. Given these discordant reports, the objective of this study is to independently determine in a controlled manner whether pressure recovery distal to the self-expanding Medtronic Evolut valve is inferior to that distal to the balloon-expandable Edwards SAPIEN3 valve. This study aims to address this objective through a highly controlled physiological and pulsatile condition in vitro with high-precision pressure measurements as a function of time and axial position along the test chamber. As pressure recovery is what determines the net pressure drop which in turn is an indication of valve performance, this study aims to provide new insight into how valve designs may influence the spatial evolution of pressure gradient thereby helping future valve engineering efforts to consider pressure recovery characteristics in addition to just orifice characteristics. The objective of this study is to determine whether pressure recovery of the self-expandable TAV (Evolut) is inferior to that of the balloon-expandable TAV (SAPIEN).
METHODS
A 23 mm Edwards SAPIEN 3 and a 26 mm Medtronic Evolut TAVs were hemodynamically assessed in a pulse duplicator left heart simulator flow loop under physiological pressure and flow conditions (cardiac output = 5 L/min; heart rate = 60 beats per minutes; systolic to diastolic pressures = about 120/80 mmHg). The pulse duplicator briefly comprises a reservoir to store the fluid, a bioprosthetic mitral valve that separates the reservoir and the pump, a bladder pump that is controlled by an in-house LabVIEW program, an aortic chamber, a compliance chamber and a valve to control the flow.10,12
Both valves were placed in the same annulus of the same aortic root as described in previous studies.11 From a design standpoint, clear differences characterize each valve as shown in Fig. 1. The SAPIEN 3 is characterized by a frame height of 18 mm and a valve diameter of 23 mm.20 The outer skirt length is 9.3 mm. Whereas the Medtronic Evolut is characterized by a frame height of 45 mm, an inflow diameter of 26 mm and a skirt height of 13 mm.20
FIGURE 1.
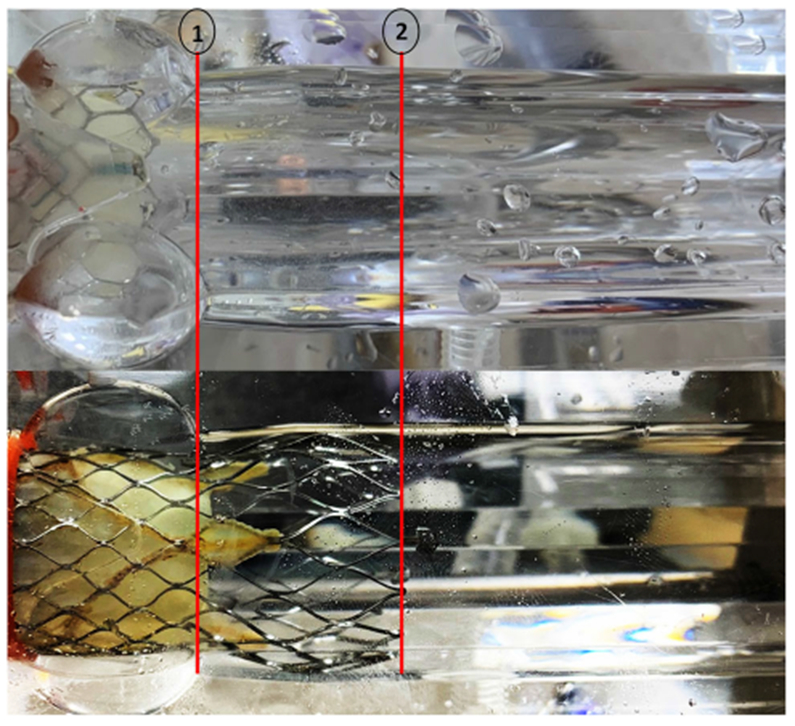
Figure of the (a) SAPIEN 3 and (b) Evolut TAVs placed in the aortic root chambers of the experimental setup for hemodynamic assessment. Annotation 1 denotes the sinotubular junction limit at 4 cm and 2 the limit of Evolut stent at 6.5 cm.
The working fluid in this study was a mixture of water-glycerin producing a density of 1060 kg/m3 and a kinematic viscosity of 3.5 cSt similar to blood properties. The temperature of the fluid used was around 25 °C. Flow data were acquired using ultrasonic flow probes (HXL, Transonic Inc., Ithaca, NY, USA), and pressures at all the measurement locations were measured with a Millar catheter (ADInstruments Inc., 2205 Executive Circle, Colorado Springs CO). The Millar catheter was inserted longitudinally along the centerline of the aortic valve chamber in order to record the pressure at every axial location with intervals of 0.5 cm downstream of the valves and 0.1 cm inside the valves. Position 0 cm corresponds to the most upstream measurement (ventricular), and position 11.7 cm corresponds to the last measurement point in the measurement region of the chamber. A picture of the experimental setup where some axial points were highlighted with axial locations is shown in Fig. 1. Fifty consecutive cardiac cycles of pressure and flow rate data were recorded at a sampling rate of 100 Hz. Peak and mean pressure measurements were performed at each distance along the axial flow line and peak flow time was determined by the time point where flow is maximum from the generated curves. Transvalvular pressure gradient (PG) is the drop in pressure across the aortic valve during the time when the valve is open permitting flow from the ventricle into the aorta. Mean and Peak PG may be defined based on the instantaneous variations in PG as the flow accelerates to a peak and then decelerates until the valve closes. In addition, the pressure gradient at peak flow was also determined as this was observed to be less than the true peak pressure gradient, which consistently occurred slightly before the flow peak occurred.
Mann–Whitney test was performed to assess the statistical significance of the pressure gradient values obtained at vena contracta and at the recovery zone between the 2 different valves after confirming nonnormality of the data using the Kolmogorov–Smirnov test with p < 0.15.
RESULTS
The pressure measurements (averaged over 50 cycles) of the SAPIEN 3 at different locations in the aortic valve chamber at different time points during systole over 50 different cardiac cycles are plotted in Fig. 2a. The peak flow time point is at 0.52 s. The results are plotted from the ventricular side upstream of the valve to the downstream side as shown in the Fig. 1. As the flow enters the valve, the pressure decreases from the left ventricular outflow tract (LVOT) to the annulus (0 to 1.7 cm). The pressure keeps decreasing and reaches its minimal value at the vena contracta at about 4.4 cm. After crossing the VC, the pressure starts increasing gradually indicating the onset of recovery. During peak flow, at 0 cm position, the pressure was measured to be 123.01 ± 0.47 mmHg and at 11.7 cm downstream of the valve at peak flow time point, the pressure reaches 116.72 ± 0.98 mmHg. The pressure curves demonstrate a slope indicating that they will continue to “recover” beyond the 11.7 cm limit of the measurement zone.
FIGURE 2.
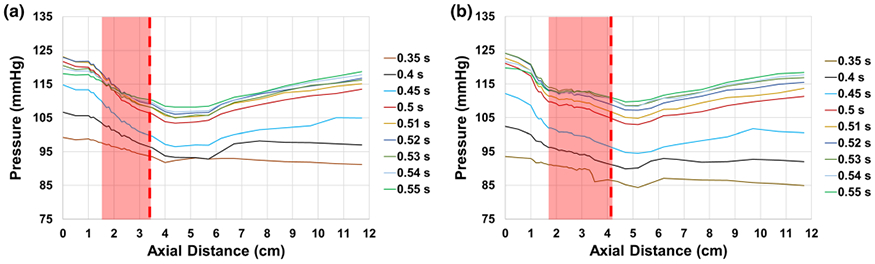
Variations of pressure as a function of axial distance at selected time points during systole with (a) SAPIEN 3 and (b) Evolut TAVs. The edge of the fully open leaflet is at (a) around 3.5 cm with SAPIEN and (b) around 4.2 cm with Evolut as highlighted with the dotted line. The shaded red area represent the location of the valve with respect to the measurement region.
The pressure measurements (averaged over 50 cycles) of the Evolut at different locations in the aortic valve chamber at different time points during systole over 50 different cardiac cycles are shown in Fig. 2b. The peak flow time point is at 0.52 s. The results are plotted from the ventricular side upstream of the valve to the downstream side as shown in Fig. 1. Similar to the SAPIEN valve there is high variability before and after peak flow. During peak flow time, pressure continues to drop before the valve is reached. Inside the valve, the pressure relatively stabilizes or levels off before decreasing once the leaflet portion is reached. During peak flow, at 0 cm position, the pressure was measured to be 124.17 ± 0.56 mmHg. The pressure at the end of the measurement zone (recovery zone) at 11.7 cm almost plateaued and relatively stabilizing at 116.87 ± 1.34 mmHg at peak flow time.
The variations of pressure gradient with SAPIEN 3 at different locations in the aortic valve chamber at different time points during systole are shown in Fig. 3a. The pressure gradient in this figure was obtained after subtracting the initial upstream ventricular pressure from the rest of the instantaneous measurements. The peak pressure gradient is about 16.99 ± 1.72 mmHg at peak systole time (0.52 s) at the VC. Again, the curve does not reach a constant value at 11.7 cm instead it is characterized by a positive slope and the net PG is 6.29 ± 0.98 mmHg. The pressure gradient reaches an absolute maximum of 18.30 ± 0.59 mmHg at the VC, before peak flow occurs as the flow is still accelerating (between 0.45 and 0.5 s).
FIGURE 3.

Variations of pressure gradient (obtained from subtracting the initial upstream pressure measurement from the other measurements) with (a) SAPIEN 3 and (b) Evolut at different locations in the aortic valve chamber at different time points during systole. The edge of the fully open leaflet is at (a) 3.5 cm with SAPIEN and (b) 4.2 cm with Evolut as highlighted with the dotted line. The shaded red area represent the location of the valve with respect to the measurement region.
The variations of pressure gradient with the Evolut at different locations in the aortic valve chamber at different time points during systole are shown in Fig. 3b. The pressure gradient is about 16.89 ± 0.55 mmHg at peak systole time (0.52 s) at the vena contracta. At 11.7 cm, the pressure gradient reaches 8.57 ± 0.93 mmHg. Again, the curve is characterized by a near zero slope portion at the end of the measurement region of 8.57 mmHg. The pressure gradient reaches an absolute maximum of 16.90 ± 0.56 mmHg at the VC, before peak flow occurs as the flow is still accelerating (between 0.45 and 0.5 s).
The standard deviations of the pressure gradients with the SAPIEN 3 and Evolut respectively at different locations in the aortic valve chamber at different time points during systole are shown in Figs. 4a and 4b. The fluctuations and their magnitudes are lower for the SAPIEN 3 (not exceeding 1.84 mmHg) compared to the Evolut (exceeding 2 mmHg) for each time point. Specifically with the Evolut, the highest fluctuations are noted after the stent limit is crossed occurring earlier during systole.
FIGURE 4.
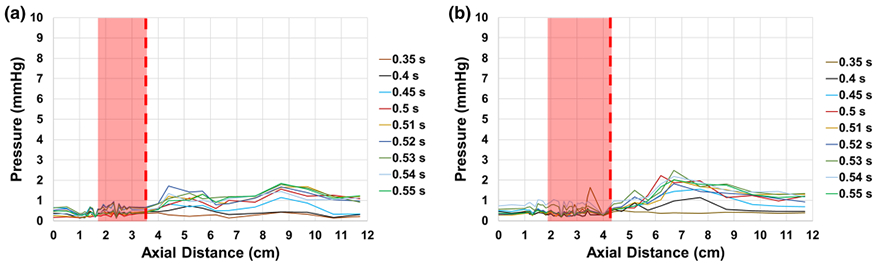
Variations of pressure standard deviations as a function of axial distance at selected time points during systole with (a) SAPIEN 3 and (b) Evolut TAVs. The edge of the fully open leaflet is at (a) 3.5 cm with SAPIEN and (b) 4.2 cm with Evolut as highlighted with the dotted line. The shaded red area represent the location of the valve with respect to the measurement region.
The variations of the peak and mean pressure gradients and the pressure gradient at peak flow as a function of axial distance with SAPIEN 3 and Evolut are shown in Fig. 5. It is important to note that the pressure gradient at peak flow and the peak pressure gradient are different with the peak PG occurring earlier than the peak flow PG. The variations of mean PG, peak flow PG and peak PG follow those observed in Fig. 2a for SAPIEN, with the vena contracta located at around 4.4 cm. At this location, the mean pressure gradient obtained is 10.11 ± 0.63 mmHg meanwhile that obtained at 11.7 cm is 7.06 ± 0.46 mmHg (see Fig. 6). For Evolut, similar to Fig. 2b, the vena contracta location is at around 5.2 cm and the corresponding mean PG is 10.45 ± 0.67 mmHg. Whereas the mean PG at 11.7 cm is found to be 7.99 ± 0.61 mmHg. The differences between the mean PG between the two valves was not statistically significant at VC (p = 0.71) but significant in the recovery zone (p < 0.00001) (Fig. 6).
FIGURE 5.
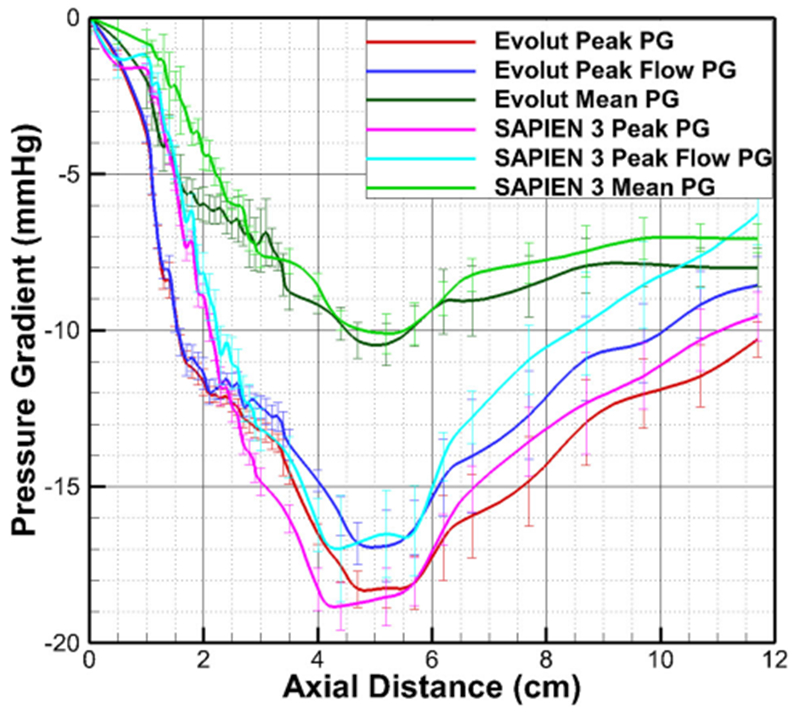
Peak PG, PG at peak flow and mean pressure gradient variations as a function of axial distance with SAPIEN 3 and Evolut. Error bars represent standard deviations.
FIGURE 6.
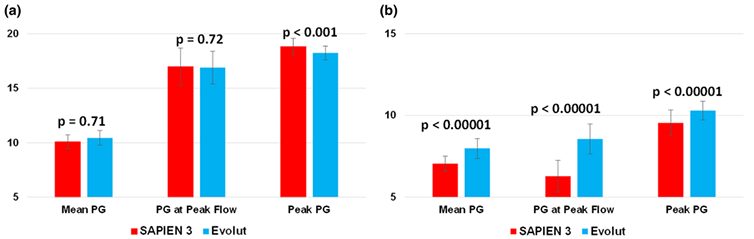
Bar chart summarizing the mean pressure gradient, pressure gradient at peak flow and peak pressure gradient with SAPIEN 3 and Evolut at (a) the vena contracta (VC) and (b) in the recovery zone. Error bars represent standard deviations.
At peak flow, for SAPIEN 3, the pressure gradient obtained is 16.99 ± 1.7 mmHg meanwhile that obtained at 11.7 cm is 6.29 ± 0.97 mmHg. For Evolut, the PG at VC is 16.90 ± 1.15 mmHg. Whereas the PG at 11.7 cm is found to be 8.57 ± 0.92 mmHg. The comparison between the two valves is not statistically significant at VC (p = 0.72) but significant post-recovery (p < 0.001) (Fig. 6).
The peak PG obtained with SAPIEN at VC is 18.83 ± 0.75 and at 11.7 cm, 9.56 ± 0.78 mmHg. For Evolut at VC, peak PG is 18.25 ± 0.63 mmHg and at 11.7 cm, 10.3 ± 0.57 mmHg. The differences at the VC and at the recovery were statistically significant (p < 0.00001) (Fig. 6).
The derivative peak flow PG variations as a function of axial distance with SAPIEN 3 and Evolut downstream of SAPIEN 3 vena contracta (4.4 cm) are plotted in Fig. 7. This figure aims at emphasizing the rate at which pressure is being recovered. The more positive derivative of the pressure gradient means the faster the pressure recovery is locally. It is shown that the peak rate of pressure recovery occurs for the SAPIEN 3 at a rate of 3.67 mmHg/cm and that with Evolut occurs at a rate of 2.48 mmHg/cm. At the end of the measurement zone, the pressure recovery is continuing for the SAPIEN at 1.34 mmHg/cm the pressure recovery for the Evolut is at 1.15 mmHg/ cm with the rate in a decreasing trend.
FIGURE 7.
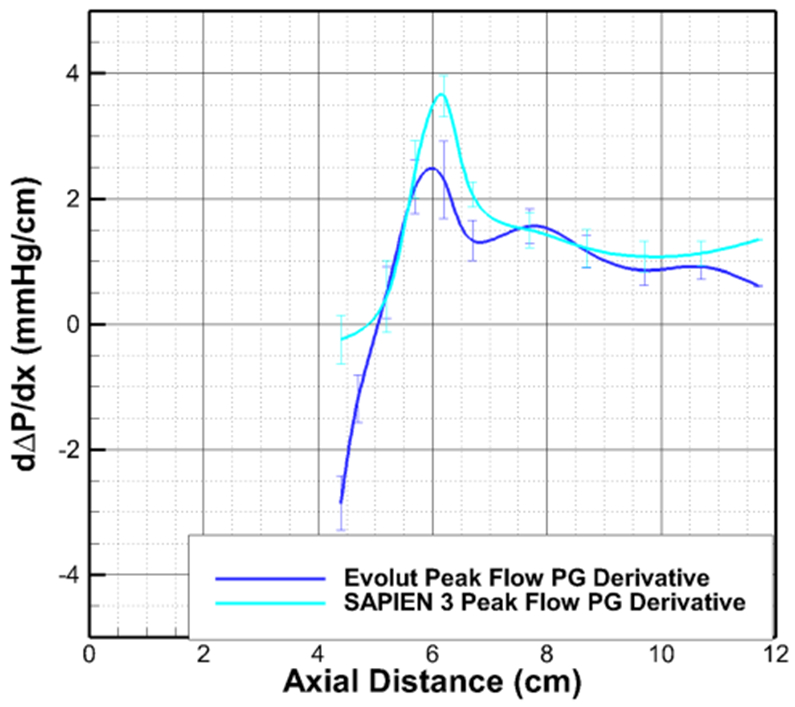
Variations of the derivative of pressure gradient at peak flow as a function of axial distance with SAPIEN 3 and Evolut downstream of SAPIEN 3 vena contracta. Error bars represent standard deviations.
DISCUSSION
Pressure recovery downstream of the aortic valve constitutes an important factor affecting the calculation of PG across the valve and therefore the aortic valve area.3 As the jet expands, its velocity decreases and pressure is recovered in amounts that are dictated by several factors such as turbulence,2 velocity of blood at the VC and the geometry of the aorta.6 This recovery is a form of energy conversion from kinetic back to potential energy. The flow converges towards the VC resulting in reduction of pressure and in conversion of potential energy to kinetic energy. Once the VC is crossed, a reconversion of energy to potential energy occurs. However, not all the static pressure drop across the aortic valve is available for recovery.6,13 Because Doppler-based echocardiography detects the peak flow velocity that takes place at the VC, the PG across the valve is overestimated, compared to that measured simultaneously in the aortic root distal to the VC.
In patients undergoing TAVR, prosthesis gradients are employed for surveillance of valve function and durability. The reliability of these gradients is therefore critically important as they often drive clinical decision-making. In a study by Mando et al.15 comparing pressure recovery after balloon-expandable and selfexpandable TAVs, it was found that Echo mean gradients were significantly higher than cath mean gradients post-TAVR. In addition, they found that this discrepancy occurs to a higher degree with smaller balloon-expandable valves and likely due to pressure recovery. However, there was no difference in cath and echo mean gradients between large and small self-expandable TAVR.
This study presented a highly controlled, comprehensive and high-resolution experiment that reinforced the clinical differences in pressure recovery between SAPIEN TAVs and Evolut TAVs. The current study is first to evaluate direct pressure measurements across the balloon-expandable and self-expanding valves to determine the role of pressure recovery on the clinically-identified differences in echocardiographic peak and mean pressure gradients. We found that there is a greater amount of pressure recovery for the SAPIEN 3 [3.05 mmHg in mean gradient, 9.27 mmHg in peak gradient and 10.7 mmHg in peak flow PG (Fig. 5)] compared to the Evolut TAV [2.46 mmHg in mean gradient, 7.97 mmHg in peak gradient and 8.33 mmHg in peak flow PG (Fig. 5)]. Lower peak and mean recovered pressure gradients not only would affect the calculation of aortic valve area, but also result in a lower net pressure seen by the left ventricle. The significantly higher pressure recovery for the SAPIEN 3 valve despite higher PG at the VC for the first time demonstrates valve dependence of pressure recovery. While these differences may not be clinically significant (up to 2 mmHg), they were statistically significant.
A previous study on the fluid dynamic characterization of different TAVs using particle image velocimetry as well as direct pressure measurements in a pulse duplicator was recently performed in the same aortic chamber and physiological conditions adopted in this study.11 When measuring the in vitro recovered pressure gradients, the mean transvalvular PG was shown to be lowest for SAPIEN 3 (7.76 mmHg), followed by Evolut (10.5 mmHg). The extent of pressure recovery for the SAPIEN valve may require further study. The positive slope identified in the SAPIEN 3 curves (Figs. 2, 3 and 5) suggests that the recovery was not complete and may be even greater than currently measured.
The current study has significant implications for the evaluation of the TAV following TAVR. Current methods for assessing post-TAVR valve area do not take into account the flow characteristics of the stented valve, using echocardiographic (and thus VC) gradients which underestimates the “true” aortic valve area of the balloon-expandable valve in the setting of pressure recovery. Current methods to correct for pressure recovery focus on the size of the ascending aorta4,7,14 and would not capture the intrinsic properties of the stented valves that also define this phenomenon. Thus, the findings of this study have significant implications for our assessment of valve function since it may be possible to define the expected pressure recovery and adjust our calculations accordingly.
In a previous study11 assessing the turbulence induced after implantation of SAPIEN 3 and Evolut in the same aortic root as the one used in this study, it was shown that turbulence characteristics were valvedependent. The SAPIEN yielded lower Reynolds Shear Stresses (RSS)—that account of the turbulent fluctuations of the blood velocity8,11,16,18—compared with the Evolut. In that study, it was highlighted that the difference between the more observed turbulence downstream of the Evolut might be attributed to the distal meshed stent frame of the Evolut that protrudes along the sinotubular junction. Several turbulence studies have emphasized the effect of the presence of grids on enhancing turbulence, promoting rapid decay and diffusion axially, and increasing the skewness of the velocity fluctuation.11,19 In addition, it was shown that the more significant and obvious leaflet flutter observed with Evolut comes in agreement with these lateral fluctuations thus enhancing the generated turbulence.11 As turbulence and Reynolds stresses make an important factor that increase conversion of kinetic energy to heat through viscous dissipation, these arguments explain the lower pressure recovery obtained with Evolut compared with the SAPIEN. These fluctuations can be partly seen in Figs. 4, 7 and Video 1 (Supplementary material). The standard deviations obtained downstream of the Evolut were significantly larger than those obtained with the SAPIEN.
With transvalvular pressure gradient being perhaps the most important indicator of valve performance clinically, accurately assessing its variations is deemed not only necessary but also mostly crucial. Pressure recovery in the context of valve hemodynamics is an indicator of an increase in pressure taking place after the vena contracta (where maximum jet velocity occurs). From a physics standpoint, pressure recovery represents the conversion of kinetic energy back to pressure as the jet re-attaches into the ascending aorta, however the conversion process is never 100% efficient as turbulence leading to viscous dissipation is a limiting factor that dictates that percentage. With turbulence downstream of the valve being dictated by the unique valve type and design, pressure recovery must vary accordingly. From a clinical perspective, the pressure gradient measured at the vena contracta (from peak jet velocity) by means of echocardiography does not account for pressure recovery leading to an overestimated net pressure gradient. It is currently clinically assumed that two different valves yield the same amount of pressure recovery if evaluated in the same aortic environment should they exhibit the same peak velocity. As we have shown in this study that this is not true because, this assumption does not take into consideration that valve design significantly changes flow physics downstream of the vena-contract. The clinical importance of this study stems from the fact that pressure gradients constitute the main indicators of valve performance that drives clinical decisionmaking (e.g., diagnosing patient prosthesis mismatch or assessing durability of the valve), in addition to the fact that different diagnostic modalities lead to not only different but also dissimilar pressure gradient estimation trends.
LIMITATIONS
The strength of the current study is the evaluation of these two different valve types within the same structural flow model. However, the flow characteristics of these valves in different anatomies with different deployment characteristics (non-circular, high or low implant) require further study. The current study has significant implications for the evaluation of the TAV following TAVR and methods to measure or correct for intrinsic pressure recovery should be studied. It is worth noting that the results of this study are also dependent on the length of the test chamber downstream of the valve.
CONCLUSION
In this study, it was shown that valve construct significantly affects the measurement of gradients across the TAV. Comparing both valves, the self-expandable one has a lower pressure recovery compared to the balloon-expandable one. The self-expanding valve has a lower VC gradient but higher turbulence downstream to the valve, resulting in less pressure recovery. The balloon-expandable valve has higher pre-leaflet and VC gradients, greater pressure recovery further downstream to the valve and a resulting net mean gradient that is lower than the self-expanding valve. The reliability of these measurements drive decision-making on a clinical level. Although theoretical limitations of Doppler and invasively obtained gradients have been demonstrated, this study provides a direct comparison of these gradients post-TAVR based on a device difference.
Supplementary Material
FUNDING
The research done was partly supported by National Institutes of Health (NIH) under Award Number R01HL119824 and the American Heart Association (AHA) under Award Number 19POST34380804.
ABBREVIATIONS
- PG
Pressure gradient
- TAVR
Transcatheter aortic valve replacement
- VC
Vena contracta
Footnotes
ELECTRONIC SUPPLEMENTARY MATERIAL
The online version of this article (https://doi.org/10.1007/s10439-019-02425-8) contains supplementary material, which is available to authorized users.
DISCLOSURES
Dr. Dasi reports having patent applications filed on novel polymeric valves, vortex generators and superhydrophobic/omniphobic surfaces. Dr. Hahn is a consultant for Edwards Lifescience and Medtronic, and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. No other conflicts were reported.
REFERENCES
- 1.Alston M., et al. 600.19 Invasive versus Doppler-derived gradients after TAVR. JACC Cardiovasc Interv 12(4):S48, 2019. [Google Scholar]
- 2.Bach DS Echo/Doppler evaluation of hemodynamics after aortic valve replacement: principles of interrogation and evaluation of high gradients. JACC Cardiovasc Imaging 3(3):296–304, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bahlmann E., et al. Impact of pressure recovery on echocardiographic assessment of asymptomatic aortic stenosis: a SEAS substudy. JACC Cardiovasc Imaging 3(6):555–562, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bahlmann E., et al. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 127(10):1149–1156, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Falk V, Bax JJ, De Bonis M, et al. ESC/EACTS Guidelines for the management of valvular heart disease: the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017(38):2739–2791, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Chambers J. Is pressure recovery an important cause of “Doppler aortic stenosis” with no gradient at cardiac catheterisation? Heart 76(5):381, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia D., et al. Discrepancies between catheter and Doppler estimates of valve effective orifice area can be predicted from the pressure recovery phenomenon: practical implications with regard to quantification of aortic stenosis severity. J Am Coll Cardiol 41(3):435–442, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Giersiepen M., et al. Estimation of shear stress-related blood damage in heart valve prostheses-in vitro comparison of 25 aortic valves. Int J Artif Organs 13(5):300–306, 1990. [PubMed] [Google Scholar]
- 9.Hahn RT, et al. Comprehensive echocardiographic assessment of normal transcatheter valve function. JACC Cardiovasc Imaging 12(1):25–34, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Hatoum H, and Dasi LP. Spatiotemporal complexity of the aortic sinus vortex as a function of leaflet calcification. Ann Biomed Eng 47(4):1116–1128, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatoum H, Heim F, and Dasi LP. Stented valve dynamic behavior induced by polyester fiber leaflet material in transcatheter aortic valve devices. J Mech Behav Biomed Mater 86:232–239, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatoum H, Moore BL, and Dasi LP. On the significance of systolic flow waveform on aortic valve energy loss. Ann Biomed Eng 46(12):2102–2111, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatoum H., et al. Modeling of the instantaneous transvalvular pressure gradient in aortic stenosis. Ann Biomed Eng 48:1748–1763, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancellotti P, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17(6):589–590, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Mando R., et al. Echo overestimates trans-aortic gradients immediately post TAVR: a pressure recovery phenomenon in a simultaneous CATH and ECHO study. J Am Coll Cardiol 73(9):1251, 2019. [Google Scholar]
- 16.Nygaard H., et al. Two-dimensional color-mapping of turbulent shear stress distribution downstream of two aortic bioprosthetic valves in vitro. J Biomech 25(4):429–440, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Otto CM Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol 47(11):2141–2151, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Schoephoerster RT, and Chandran KB. Velocity and turbulence measurements past mitrial valve prostheses in a model left ventricle. J Biomech 24(7):549–562, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Yang SK and Chung MK. Turbulent flow through mixing spacer grids in rod bundles. In: 1995 National Heat Transfer Conference: Proceedings, Volume 14: Thermal Hydraulics of Advanced Nuclear Reactors, Containment Thermal-Hydraulics, HTD Volume 316, 1995. [Google Scholar]
- 20.Yudi MB, et al. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol 71(12):1360–1378, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Zoghbi WA, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Angiography And Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 32(4):431–475, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


