Abstract
Pumpkin, nutritious vegetable, is renowned for its extended shelf life. In this study, seven pumpkin cultivars from Cucurbita moschata and Cucurbita maxima were comparatively characterized for 25 physiochemical quality factors, starch granule structures, antioxidant activity, and correlations at 0–60 days of postharvest (dop). The findings revealed that sucrose and carotenoid contents increased in C. moschata, while they initially increased and then decreased in C. maxima. Additionally, acidity, primarily driven by malic acid, decreased in C. maxima but increased in C. maxima. The starch content of C. moschata and C. maxima reached its maximum value at 30 dop and 20 dop, respectively. The DPPH radical scavenging activity correlated with the carotenoid content in both pumpkin species. Conclusively, C. moschata demonstrated improved nutritional and quality at 20–30 dop, while C. maxima exhibited higher commercial suitability at 10–20 dop. The findings suggested that pumpkin storage was crucial for quality improvement.
Keywords: Cucurbita moschata, Cucurbita maxima, Postharvest, Fruit quality, DPPH
Highlights
-
•
Sucrose and carotenoid contents increased in C. moschata, but initially increased and then decreased in C. maxima.
-
•
The primary organic acid affecting acidity, specifically malic acid, decreased in C. moschata but increased in C. maxima.
-
•
Soluble solids content was correlated with dry matter, starch, and sucrose in both C. maxima and C. moschata.
-
•
C. moschata had the best taste and nutrition 20–30 dop, whereas C. maxima was at 10–20 dop.
1. Introduction
Pumpkin is highly regarded as a wholesome food replete with nutritional benefits, commonly utilized in both culinary practices and food processing. Cucurbita moschata Duch. (C. moschata) and Cucurbita maxima Duch. (C. maxima) are two pumpkin species predominantly cultivated in China and consumed as mature squash (Karaağaç & Balkaya, 2013; Li et al., 2020; Rosul et al., 2022). These species are economically significant vegetables, adaptable to grow in diverse climates, from temperate to tropical zones (Sanjur, Piperno, Andres, & Wessel-Beaver, 2002). Their mature fruits are laden with essential nutrients such as carotenoids, polysaccharides, fibers, carbohydrates, and phenolic compounds (Akwaowo, Ndon, & Etuk, 2000; Chen & Huang, 2018; Karaağaç & Balkaya, 2013; Zhao et al., 2017). The bioactive components of pumpkins have been used to treat diseases due to their range of health-promoting effects, which include anti-diabetic, anti-hypertensive, anti-cancer, antioxidant, immune-modulatory, anti-inflammatory, and anti-allergic properties (Duan et al., 2008; Jacobo-Valenzuela, Maróstica-Junior, Zazueta-Morales, & Gallegos-Infante, 2011; Nawirska-Olszańska, Biesiada, Sokół-Łętowska, & Kucharska, 2014). The multifaceted benefits of pumpkins have not only captivated the agricultural sector but also garnered significant attention from the pharmaceutical and food industries in recent years. These sectors spotlight pumpkins and their derivatives as valuable contributors to nutritional well-being and health enhancement.
The fruit quality is a key factor determining its commodity and economic benefits. Dry matter (DM) is indicative of a fruit's texture, crude fiber, water, and starch content (Corrigan, Hurst, & Potter, 2001). The accumulation and distribution of DM are critical in determining the economic yield of crops and their processing characteristics (Wyatt, Strickler, Mueller, & Mazourek, 2016). The soluble solids content (SSC) is a comprehensive indicator of fruit quality, significantly influencing taste and flavor perception (Yuan, Ye, Chen, Li, & Zhao, 2022). Organic acids and sugars are key flavor chemicals that substantially affect consumer preference for specific fruits (Meng et al., 2022). In banana fruits, starch metabolism is linked with qualities like softness, aroma, sweetness, and stickiness (Tieman et al., 2017). Carotenoids not only give horticultural plants vibrant red, orange, and yellow colors, as well as aroma and flavor, but are also key metabolites for human nutrition and health (Meng et al., 2022; Nakkanong, Yang, & Zhang, 2012). Pumpkin polysaccharides are known to enhance cytokine activity and improve the immune system (Huang et al., 2021). Observations during the development of C. moschata fruit indicate an increase in DM, SSC, sugars, and carotenoids, a decrease in organic acids, and fluctuations in starch content over time (Abbas et al., 2020). Meanwhile, the levels of sugars, starch, and carotenoids tended to increase with fruit maturation in C. maxima (Huang et al., 2019). Postharvest, fruits begin a gradual nutrient decline while simultaneously undergoing critical physiological processes such as respiration and metabolism (Chavan et al., 2023). These ongoing activities significantly influence the fruit's quality throughout the storage period. Analyzing postharvest quality is essential for extending shelf life, meeting consumer expectations regarding quality, and maximizing nutrient retention in horticultural crops (Guo et al., 2020).
Compared with other vegetables, pumpkin fruits are suitable for long-term storage for consumption or processing. The DM content of C. maxima and Cucurbita pepo Duch. (C. pepo) decreased when stored at 10 °C for 3 months compared with 0 months (Nawirska-Olszańska et al., 2014). During the postharvest period of 15–120 days under ambient room conditions [27 °C–31 °C and 75%–90% relative humidity (RH)], the β-carotene and ascorbic acid contents in fully mature BARI Pumpkin-1 and BARI Pumpkin-2 (Cucurbita moschata poir) decreased throughout storage (Rahman, Miaruddin, Khan, Masud, & Begum, 2013). The contents of carotenoids, including lutein and violaxanthin, significantly decreased in the purees of C. moschata “Menina Brasileira” and C. maxima “Exposicao” kept at around 23 °C and 70% RH for 180 days (Provesi, Dias, & Amante, 2011). Previous studies on the postharvest quality of pumpkins mainly focused on a limited number of pumpkin varieties and harvest periods. However, data on the changes in quality, nutrient composition, and antioxidant activities of different types or cultivars of pumpkins at various postharvest points are lacking.
This study aimed to elucidate the changes in nutritional and quality profiles during storage and determine the optimal eating and processing periods for different types of pumpkins. Seven pumpkin cultivars belonging to two species, representing four main types of pumpkins consumed in China, were selected to compare the changes in the fruit quality profile and investigate the changing pattern and correlation of fruit quality factors after harvest. The findings of this study might provide valuable insights into the storage capacity, changes in nutritional quality during storage, and optimal eating and processing times for various pumpkin cultivars.
2. Materials and methods
2.1. Pumpkin fruit material and sample preparation
Fully mature fruits from seven pumpkin cultivars, representing two different species, were harvested from the experimental fields of the Vegetable Research Institute at the Guangdong Academy of Agricultural Sciences in Guangzhou, China (23°N, 113°E). These included large-fruited Miben types (Cmo-gm and Cmo-bs) and small-fruited types (Cmo-53 and Cmo-54), and C. maxima with green-skinned types (Cma-76 and Cma-78) and red-skinned types (Cma-81) (Fig. S1). The large-fruited types C. moschata, Cmo-bs and Cmo-gm, also known as Miben-type C. moschata, are widely cultivated in China (Abbas et al., 2022). Fruit samples of C. maxima and C. moschata were collected 35 and 45 days after pollination, respectively, when they were fully ripened. At least 15 pumpkin fruits, characterized by uniform size, shape, and color, were selected from each cultivar and stored at 22 °C with an RH of 75%. Three decay-free fruits from each cultivar were selected, and their pulp from the same part was collected directly into liquid nitrogen and stored at −80 °C for experiments at 0, 10, 20, 30, and 60 dop. Further, the fruit pulp was dried using a vacuum freeze-dryer (LaboGene, Denmark) and then finely ground into a powder for preservation at −80 °C for physicochemical analysis.
2.2. Measurement of dry matter and soluble solids contents
Weight of fresh pulp from 0, 10, 20, 30, and 60 dop pumpkin fruits was considered as fresh weight (FW), and then fully dried at 70 °C to get the constant dry weight (DW). The final dry weight relative to the initial fresh weight was used to determine the percent dry matter (DM). Similarly, fresh pumpkin pulp was blended into homogenized slurry with a blender and filtered with nylon mesh. The filtrate was placed on ATAGO PAL-2 refractometer to measure the soluble solids contents (SSC). Three biological and technical repeats were used for the analysis.
2.3. Determination of polysaccharide content
Quantitative analysis of pumpkin polysaccharides was performed according to the previous method (Huang et al., 2021). Water-soluble polysaccharides were obtained by extracting the 0.25 g freeze-dried pumpkin powder with 150 ml of alcohol,and then refluxed for >2 h at 80 °C, and finally cooled at room temperature. Precipitates were collected with 100 ml of ddH2O for final extraction at 100 °C for 2 h and finally supernatant was collected in flask and marked up to 250 ml with ddH2O. Finally, polysaccharide contents were determined by phenol-vitriol colorimetry (Jiao et al., 2023).
2.4. Measurement of the starch content
The freeze-dried samples were ground into a fine powder for extracting starch according to a previously described protocol (Abbas, Huang, Yang, et al., 2020). In brief, 1 g powder was soaked in 0.05% NaOH solution and incubated at room temperature for 24 h. After 24 h, the solution was discarded and washing was performed with ddH2O. The starch residues were washed with 10% toluene in 0.1 M NaCl after centrifugation, following washing with ddH2O and ethanol separately. Starch was recovered with Whatman No. 4 filter paper filtration and then dried at 35 °C. The amylose and amylopectin contents were determined by a dual-wavelength iodine-binding colorimetry method described in a previous study (Wang, Li, Tian, Xu, & Jin, 2010). Wavelengths of 615 and 458 nm were used for the amylose content, whereas 546 and 732 nm were used for determining the amylopectin content. The amylose and amylopectin contents were calculated using the relative standard curve and then converted into amylose/amylopectin.
2.5. Observation of starch granule morphology
The starch granule morphology was observed using a scanning electron microscope (HITACHI S-3400 N) at different magnifications according to a previously described protocol (Hurkman & Wood, 2011). In detail, fresh pumpkin pulp, approximately 1 to 2 mm thick, was initially pre-fixed with a solution containing 2.5% glutaraldehyde and 4% paraformaldehyde for 3 h at 4 °C. Subsequently, the samples were rinsed three times with a cacodylate buffer solution (0.05 M, pH 7.2, containing 2% glutaraldehyde) for 15 min each. Afterward, they were post-fixed in a well-ventilated area overnight using 1% osmium tetroxide. Finally, the samples were washed thrice with the cacodylate buffer solution, 15 min per wash. Then, they were dehydrated in a gradient using 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, 100%, 100%, and 100% alcohol solution for 20 min each time. They were dried in a critical point drier and then glued to the sample table for gold spraying. Finally, the samples were observed and photographed under different magnifications.
2.6. Measurement of sugar, organic acid, and carotenoid contents
The determination of soluble sugar content was carried out following a previously described protocol (Abbas et al., 2020), with slight modifications. Briefly, the 20 mg lyophilized powder was extracted with 50% acetonitrile water, separated on a medium-polarity NH2 column (Waters Xbridge BEH Amide-4.6 × 250 mm, 5 μm particle size) using acetonitrile/deionized water (80:20, v/v) as the mobile phase at a flow rate of 1 ml/min, and the concentration of soluble sugars was determined by High-Performance Liquid Chromatography (HPLC) coupled with a Refractive index (RI) detector. Glucose, fructose and sucrose were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as calibration standards.
Fumaric acid, citric acid, malic acid, formic acid, tartaric acid, and oxalic acid were detected according to the protocol proposed by Nawirska-Olszańska et al. (Nawirska-Olszańska et al., 2014) with minor modifications. Organic acids were determined using a Waters 2998 photodiode array detector (HPLC-PDA) equipped with a high-performance liquid chromatography system (Alliance e2695 HPLC system, Waters, Milford, MA, USA). Then, 20 mg of freeze-dried powder samples were extracted with distilled deionized water. The organic acid content was determined using a reverse-phase C18 chromatographic column (Waters Atlantis T3 C18 column, 250 mm × 4.6 mm i.d., 5 μm particle size) at a column temperature of 25 °C. The mobile phase consisted of a 5 g/l (NH4)2HPO4 solution adjusted to pH 2.5, mixed with methanol at a volume ratio of 97:3. The flow rate was set at 0.6 ml/min. Eluted compounds were detected at a wavelength of 214 nm using ultraviolet absorbance detection. Quantification was carried out using an external linear calibration method. Calibration standards of fumaric acid, citric acid, malic acid, formic acid, tartaric acid, and oxalic acid were acquired from Sigma-Aldrich (MO, USA).
The carotenoid contents were determined by referring to previous literature reports (Zhong et al., 2011). Briefly, HPLC-PDA equipped with a C18 column (Waters Spherisorb® ODS2 (4. 6 × 250 mm, 5 μm) was carried out to detect carotenoids. A sample of 20 mg lyophilized powder was extracted with acetone. The flow rate of the mobile phase was 1.0 ml/min, with a linear gradient from 100% solvent A (acetonitrile/methanol/0.1 M Tris-HCl (pH 8.0), 84:2:14, v/v/v) to 100% solvent B (methanol/ethyl acetate, 68:32, v/v) over a 15 min period, followed by 10 min of 100% solvent B. Different types of carotenoids were identified on the basis of their absorption spectra and retention times relative to standard compounds. Carotenoids were quantified by integrating peak areas and its concentrations were converted by comparison with authentic standards. Lutein, violaxanthin, neoxanthin, antheraxanthin, zeaxanthin, α-carotene, and β-carotene were purchased from Sigma (St Louis, MO, USA).
2.7. Analysis of antioxidant activity
The scavenging ability of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals in pumpkin powder was evaluated using the DPPH free radical scavenging assay kit (A153–1-1) provided by Jiancheng Bioengineering Research Institute, Nanjing, China. The DPPH content was measured following the kit instructions and the methodology described by Ren et al. (2021). Absorbance readings were taken at 517 nm using ethanol as the blank. The lighter the color, the lower the absorbance value, indicating a higher DPPH scavenging activity. The ability to scavenge DPPH radicals in the sample was then quantitatively assessed. A Trolox standard solution with concentrations of 0, 5, 10, 15, 20, and 25 μg/ml was tested under the same conditions. The results were reported as micrograms of Trolox equivalent antioxidant capacity per milliliter sample. All assays were conducted in triplicate.
2.8. Statistical analysis
Results are presented as the averages from three individual experiments ± standard deviation (SD). Statistical differences were calculated by one-way analysis of variance (ANOVA). Different letters above each bar indicate statistically significant differences as determined by Tukey's multiple testing methods (P ≤ 0.05). Figures were generated using GraphPad Prism software (Version 8.0) and R software.
3. Results and discussion
3.1. Variations in DM, soluble solids, and polysaccharide contents during storage of different pumpkin cultivars
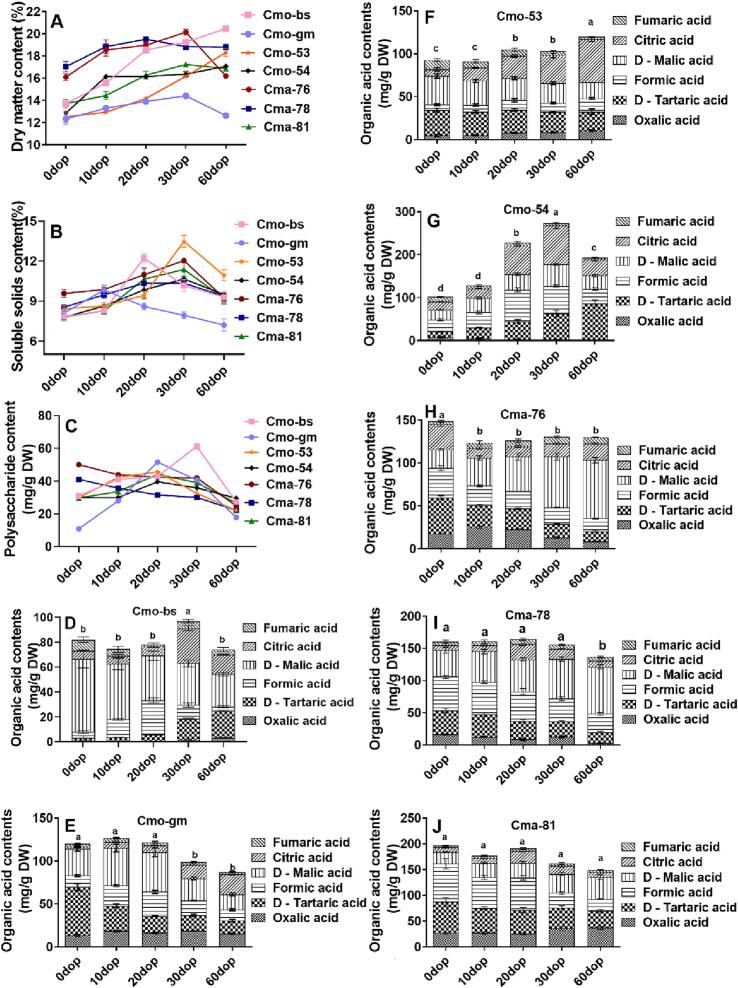
All C. maxima cultivars exhibited an initial higher and then gradually lower DM contents (Fig. 1A). Green-skinned C. maxima cultivars, Cma-76 and Cma-78, had higher DM than other cultivars, with an initial DM of 16.1% and 17.03%, respectively. From 0 to 60 dop, the DM contents increased steadily in C. moschata cultivars Cmo-bs, Cmo-53, and Cmo-54. In particular, the DM of Cmo-bs showed a significant increase from 13.7% to 20.46%, which recorded the highest DM among the seven cultivars at 60 dop. The SSC showed an increasing and then decreasing trend in all C. moschata and C. maxima cultivars from 0 to 60 dop. The result was consistent with previous findings that the SSC of C. moschata increased and then decreased after harvest. The SSC of pumpkin cultivars was maximum at 30 dop, except for the large-fruited types C. moschata Cmo-bs and Cmo-gm. At 0–30 dop, the SSC of Cmo-53 increased from 8.4% to 13.4%, representing a 1.6-fold increase compared with the initial level (Fig. 1B).
Fig. 1.
Changes in dry matter (DM), soluble solids content (SSC), polysaccharide components and organic acid contents in different cultivars of C. moschata and C. maxima at varying postharvest stages. (A, B, and C) The DM, SSC, and polysaccharide contents in different cultivars of C. moschata and C. maxima, respectively.The organic acid contents in (D) Cmo-bs, (E) Cmo-gm, (F) Cmo-53, (G) Cmo-54, (H) Cma-76, (I) Cma-78, and (J) Cma-81 culitvars. Results were expressed as mean ± standard deviation.Means with different letters were significantly different from each other (one-way analysis of variance, P ≤ 0.05).
Pumpkins are extensively used for polysaccharide extraction and health function engineering. However, studies available to assist stakeholders in selecting the appropriate cultivar and stage for extraction purposes are limited. The polysaccharide contents were significantly different in the seven cultivars based on the storage intervals. They initially increased and then decreased in C. moschata and red-skinned C. maxima, but consistently decreased throughout all stages in green-skinned C. maxima. Specifically, at 20 dop, C. moschata cultivars (Cmo-gm, Cmo-53, and Cmo-54) and red-skinned C. maxima (Cma-81) exhibited higher polysaccharide contents. Additionally, the green-skinned C. maxima showed the highest polysaccharide content at harvest (0 dop). Among all the cultivars, the large-fruited cultivar Cmo-bs had the highest polysaccharide content (61.22 mg/g DW) at 30 dop, indicating it to be the ideal time for polysaccharide extraction (Fig. 1C). The polysaccharide contents in Cmo-gm showed the maximum variation with 3.74-fold increase from 10.9 mg/g DW to 51.65 mg/g DW at 0–20 dop (Fig. 1C). This finding suggested that the optimal extraction time for polysaccharides was between 20 and 30 dop. Furthermore, large-fruited Miben-type cultivars of C. moschata were considered suitable for extraction due to their cost-effectiveness and high polysaccharide contents.
3.2. Analysis of the composition and contents of organic acids in pumpkin cultivars during storage
The trends and composition of different compounds in C. moschata and C. maxima varied in different postharvest stages. At harvest (0 dop), the main organic acids in Cmo-bs and Cmo-gm were D-malic acid and D-tartaric acid, accounting for about 72% and 47% of the total organic acid contents, respectively (Fig. 1D and E). The total organic acid content of C. maxima (149.05–203.72 mg/g DW) was higher than that of C. moschata (81.91–119.75 mg/g DW) at 0 dop. Malic acid was found to be the main organic acid at 0 and 60 dop, respectively, in the large-fruited C. moschata Cmo-bs and the green-skinned C. maxima (Fig. 1D and H). The total organic acid content in the large-fruited C. moschata Cmo-bs was lower than that in other pumpkin cultivars in each postharvest interval. C. maxima, particularly the red-skinned types, had higher total organic acid content compared with other C. moschata cultivars during the postharvest period, except for the small-fruited C. moschata Cmo-54.
From 0 to 60 dop, the total content of citric acid and D-malic acid constantly showed a decreasing trend in C. moschata (Cmo-bs, Cmo-gm, and Cmo-53) but an increasing trend in C. maxima (Cma-76, Cma-78, and Cma-81) (Fig. 1 D–J). Citric acid and malic acid were the important fruit quality indicators directly determining the acidity of fruits; further, malic acid exhibited higher acidity compared with citric acid (Hussain et al., 2022; Li et al., 2020). The difference in the trends of D-malic acid in large-fruited C. moschata and C. maxima suggested that the acidity of C. moschata decreased, whereas that of C. maxima increased with an increasing postharvest time. These results were consistent with the sour taste of the cultivars. Especially, the green-skinned C. maxima exhibited a significant increase in the main acids responsible for its sour taste, including malic acid and citric acid, with levels rising from 49.46 ng/g DW at 0 dop to 82.34 ng/g DW at 60 dop, indicating a 1.7-fold increase compared with the initial level (Fig. 1I). This indicated that the green-skinned C. maxima should be consumed within the first 20 days after harvesting.
3.3. Changes in sugar composition and contents in pumpkin fruits during storage
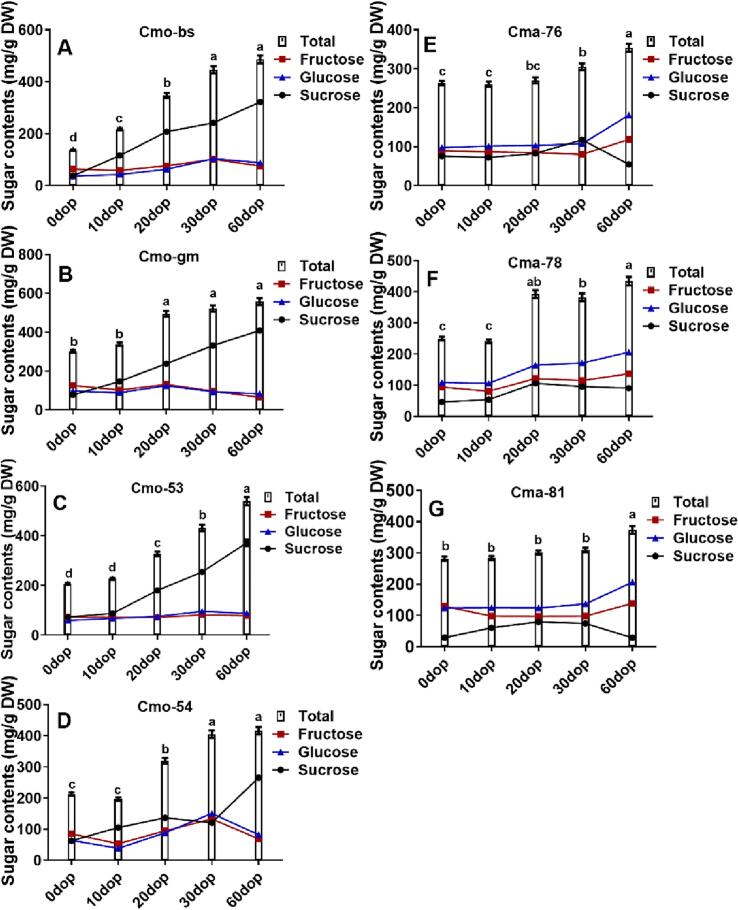
Previous studies indicated that sucrose predominated in C. moschata whereas glucose was the principal sugar in C. maxima during the maturation of pumpkin fruits (Abbas, Huang, Wang, et al., 2020). This also applied to the main soluble sugars in C. moschata and C. maxima during storage. Meanwhile, sucrose and total sugar exhibited an overall increasing trend, whereas glucose showed no significant changes in C. moschata with the increase in the postharvest time. In particular, the concentration of Cmo-bs exhibited a significant increase, rising from 37.56 mg/g DW at 0 dop to 322.19 mg/g DW at 60 dop, which represented an almost eightfold increase. Furthermore, the total sugar contents increased from 137.13 to 486.36 mg/g DW, indicating an almost threefold increase (Fig. 2A). For the cultivars of C. moschata, the content changing trends of sucrose and total sugars were similar, indicating that the changes in sucrose content determined the changes in the total sugar content (Fig. 2A–D). However, the sucrose contents in C. maxima tended to increase during the early postharvest (0–20 dop) and then decreased (30–60 dop), whereas glucose and total sugar contents showed an overall increasing trend during the postharvest period (Fig. 2). Minimum variations in the contents of fructose in the cultivars of both species were observed during all postharvest stages. At 60 dop, the glucose content of C. maxima (206.29 mg/g DW) was higher than that of C. moschata (83.94 mg/g DW) but the total sugar content of C. moschata (558.56 mg/g DW) was significantly higher compared with C. maxima (353.94 mg/g DW), especially the sucrose contents (Fig. 2).
Fig. 2.
Sugar contents of different cultivars of C. moschata and C. maxima at various stages after harvesting. (A) Cmo-bs, (B) Cmo-gm, (C) Cmo-53, (D) Cmo-54, (E) Cma-76, (F) Cma-78, and (G) Cma-81. Results were expressed as mean ± standard deviation. Means represented by different letters were significantly distinct from each other (one-way analysis of variance, P ≤ 0.05).
The difference in the sweetness of C. moschata was attributed to the content and composition ratio of sucrose (Wang et al., 2020). It indicated that C. moschata cultivars were sweeter than C. maxima due to the much higher sucrose contents and ratio after postharvest storage. The results confirmed that C. moschata cultivars tasted sweeter after storage. Studies reported the conversion of sucrose into glucose and fructose during the storage of apples caused a decrease in the sucrose content and an increase in glucose and fructose contents (Guan, Peace, Rudell, Verma, & Evans, 2015). Interestingly, the sucrose in C. maxima was converted into glucose and fructose at 30–60 days of postharvest, whereas this conversion did not occur in C. moschata during storage (Fig. 2).
3.4. Pumpkin postharvest starch contents and granule morphology
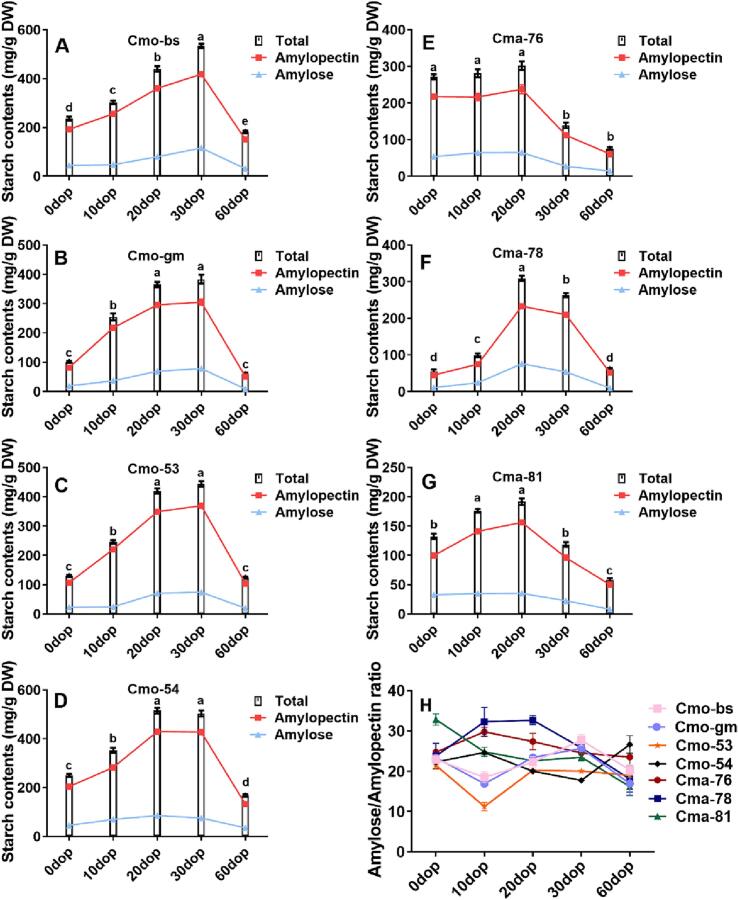
The starch contents of pumpkin cultivars showed an increasing and then decreasing trend from 0 to 60 dop in the seven pumpkin cultivars (Fig. 3). The cultivars of C. moschata and C. maxima showed maximum starch contents at 30 dop and 20 dop, respectively (Fig. 3). The texture of fruits was directly determined by their starch content, with a higher starch content resulting in a finer texture (Feng et al., 2022; Przetaczek-Rożnowska & Fortuna, 2017). This suggested that the best time for eating should not exceed 30 dop for C. moschata and 20 dop for C. maxima. In Cmo-53, the total starch content increased from 129.73 (0 dop) to 443.81 mg/g DW (30 dop), indicating a 2.4-fold increase (Fig. 3C). In Cma-78, the total starch content increased from 55.99 mg/g DW (0 dop) to 309.18 mg/g DW (20 dop), indicating an increase of 4.6-fold (Fig. 3F). At 20 dop and 30 dop, the total starch content was significantly higher in C. moschata than in C. maxima. At 30 dop, the starch content in Cmo-bs reached 534.13 mg/g DW, whereas it was 118.74 mg/g DW in Cmo-81. Starch in potatoes can be converted into soluble sugars (Hou et al., 2017), suggesting that the increase in the sugar content in postharvest pumpkins might be due to the conversion of starch (Fig. 2, Fig. 3).
Fig. 3.
Starch levels in various cultivars of C. moschata and C. maxima at different periods of harvest. (A) Cmo-bs, (B) Cmo-gm, (C) Cmo-53, (D) Cmo-54, (E) Cma-76, (F) Cma-78, and (G) Cma-81. (H) Amylose/Amylopectin ratio in C. moschata and C. maxima. Results were expressed as mean ± standard deviation. Means with different letters were significantly different from each other (one-way analysis of variance, P ≤ 0.05).
The trend of amylose/amylopectin ratio was different in these pumpkin cultivars. The structure of the branched-chain starch affects its digestive properties (Xie, Li, Li, & Yue, 2024). Amylose accounted for 12.9%–18.2% and 17.2% of the total starch content in C. maxima and C. pepo fruits, respectively (Singh, Singh, Kaur, Singh Sodhi, & Singh Gill, 2003; Stevenson, Yoo, Hurst, & Jane, 2005). The results also demonstrated that amylopectin was the main type of starch in pumpkin cultivars (Fig. 3). The amylose/amylopectin ratio was consistent with the glutinous flavor in pumpkin (Abbas, Huang, Yang, et al., 2020). The ratio of amylose/amylopectin decreased during 0–10 dop and 30–60 dop but increased during 10–30 dop in the large-fruited types of C. moschata. In contrast, the ratio increased during the early stage of storage and decreased during the later stage in green-skinned C. maxima, reaching its maximum at 10 dop. The amylose/amylopectin ratio in the red-skinned C. maxima consistently decreased from 32.86% at 0 dop to 16.21% at 60 dop, which was a reduction by half (Fig. 3H).
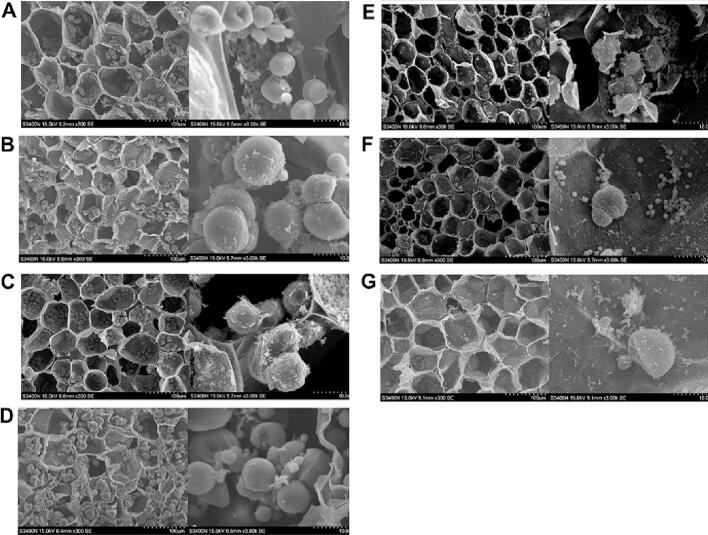
The size and shape of starch granules influence the physicochemical properties of the fruit. This is because small granules undergo extensive digestion, resulting in high viscosity with the presence of large granules (Abbas, Huang, Yang, et al., 2020). The size, shape, and smoothness of the starch granules affect the texture and mouthfeel of the pumpkin (Abbas, Huang, Yang, et al., 2020; Stevenson et al., 2005). Scanning electron microscopy was performed at 30 dop to explore the starch morphology of the seven cultivars. The starch grains were spherical, oval, polyhedral, irregular, and of other shapes with a smooth surface. Some of the starch granules also showed the depressions and cracks on their surfaces. The size of large granules ranged from 5 μm to 15 μm, and these granules were surrounded by small-sized (2-μm) granules. The results of this study showed that the number of starch granules was higher in C. moschata cultivars than in C. maxima cultivars. The large-fruited types of C. moschata (Cmo-bs and Cmo-gm) had larger starch granules compared with the red- and green-skinned C. maxima. In addition, the surface of the large-fruited types of C. moschata starch granules was smoother than that of the C. maxima starch granules (Fig. 4A-B, and 4E–G). The morphology of starch granules of small-fruited type of C. moschata Cmo-53 was similar to that of C. maxima (Fig. 4A–C), whereas the morphology of C. moschata Cmo-54 was similar to that of large-fruited types of C. moschata (Fig. 4D, E–G). Small-fruited types of C. moschata, Cmo-54, and C. maxima had fine texture and higher viscosity compared with other pumpkin cultivars, which might be related to their smaller starch granules and rougher surface.
Fig. 4.
Scanning electron microscopic images of C. moschata and C. maxima cultivars at 30 dop. (A) Cmo-bs, (B) Cmo-gm, (C) Cmo-53, (D) Cmo-54, (E) Cma-76, (F) Cma-78, and (G) Cma-81.
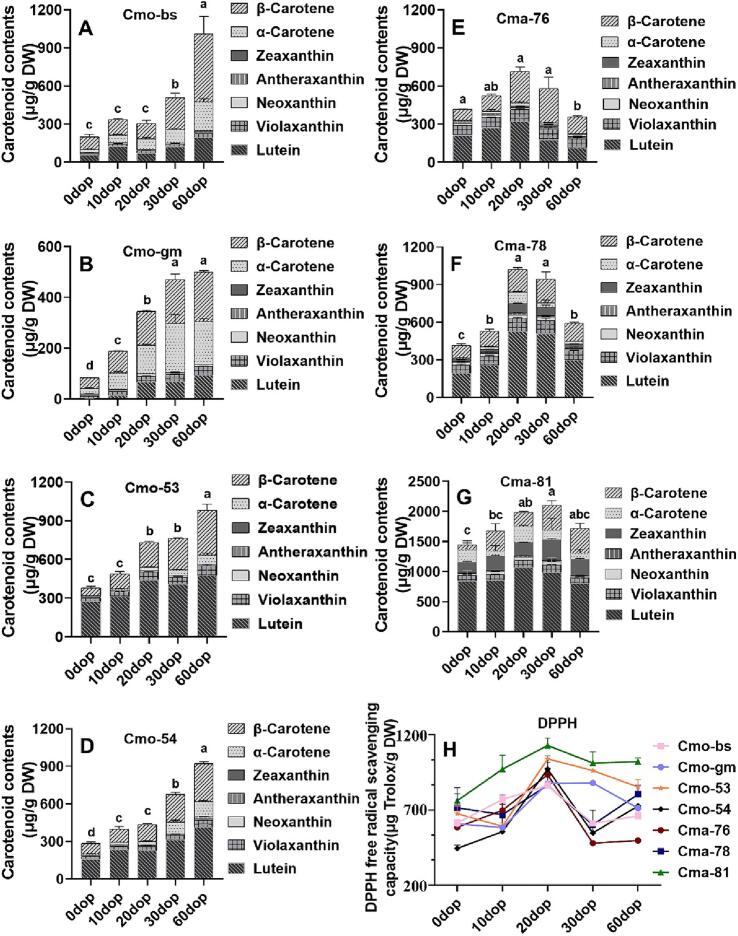
3.5. Comparison of carotenoid contents in different pumpkin cultivars after harvest
Carotenoids and their derivatives are closely related to the development of fruit appearance quality (color), nutritional quality (health functions), and flavor quality (aroma volatiles) (Meng et al., 2022). During fruit development, the total carotenoid contents of C. moschata and C. maxima showed an increasing trend (Huang et al., 2019). The carotenoid contents of kiwifruit increased with increasing postharvest time (0–35 days) under storage conditions at 20 °C (Nawirska-Olszańska et al., 2014). The results revealed that the carotenoid accumulation patterns of C. moschata and C. maxima were different after harvesting. The contents of total carotenoids in C. moschata showed an increasing trend with increasing postharvest time, whereas an initially increasing trend followed by a decreasing trend was detected in C. maxima (Fig. 5A–G). The total carotenoid contents ranged from 78.58 to 1011.72 μg/g DW in C. moschata samples and from 358.54 to 2105.09 μg/g DW in C. maxima. Nonspecific enzymes and nonenzymatic oxidation play an important role in the degradation of carotenoids during fruit storage (Li et al., 2023; Wyatt et al., 2016). The different trends in postharvest carotenoid contents in C. moschata and C. maxima might be associated with these factors. The carotenoid contents of large-fruited type of C. moschata was 5–6.4 times higher at 60 dop than at 0 dop, whereas the carotenoid content of small-fruited types of C. moschata was 2.6–3.7 times higher at 60 dop than at 0 dop (Fig. 5A–D). At 0–30 dop, the total carotenoid content of the large-fruited types of C. moschata (Cmo-bs and Cmo-gm) was lower than that of the small-fruited types of C. moschata (Cmo-53 and Cmo-54). The carotenoid contents of green-skinned C. maxima, Cmo-78, at 20 dop (959.55 μg/g DW) was approximately 2.4 times higher than the carotenoid contents at 0 dop (398.84 μg/g DW) (Fig. 5F).
Fig. 5.
Carotenoid contents and DPPH radical scavenging activities of C. moschata and C. maxima cultivars on different days of postharvest. Carotenoid contents of (A) Cmo-bs, (B) Cmo-gm, (C) Cmo-53, (D) Cmo-54, (E) Cma-76, (F) Cma-78, and (G) Cma-81 postharvest fruit. (H) DPPH radical scavenging activity of C. moschata and C. maxima cultivars at different times after harvesting. Values were expressed as mean ± standard deviation. Means with different letters were significantly different from each other (one-way analysis of variance, P ≤ 0.05).
Significant differences were found in the composition of carotenoids among the seven cultivars after harvest. The dominant carotenoid in large-fruited types of C. moschata was β-carotene, and the trend of β-carotene changes after harvest was consistent with that of total carotenoid changes. Similarly, an increase in carotenoids in postharvest small-fruited types of C. moschata and C. maxima was mainly due to lutein accumulation. The contents of α-carotene and zeaxanthin in large-fruited types of C. moschata and the red-skinned C. maxima were significantly higher than those in other cultivars. Zeaxanthin was detected in C. maxima cultivars but not in C. moschata cultivars at 30–60 dop (Fig. 5A–G). The flesh color of Cma-81 had a deeper orange color than other cultivars in different storage stages, which had approximately 4.5 times higher carotenoid levels (2105.09 μg/g DW) compared with Cmo-gm (464.25 μg/g DW) at 30 dop (Fig. 5G). In red-skinned C. maxima (Cma-81), the zeaxanthin contents ranged from 183.27 to 336.84 mg/g DW during the postharvest period (Fig. 5G). At 30 dop, the carotenoid contents of the Cma-81 cultivar were 41.38 mg/100 g FW, which was even higher than that of the Dutch orange breeding material known as “high carotene” with a carotenoid content of 40 mg/100 g FW (Baranski, Allender, & Klimek-Chodacka, 2012). Red-skinned C. maxima Cma-81 contained various carotenoids, such as α-carotene, β-carotene, lutein, zeaxanthin, antheraxanthin, neoxanthin, and violaxanthin, especially lutein and zeaxanthin. This demonstrated its potential as a comprehensive source of carotenoids that could effectively meet the body's needs and utilization efficiency, making it a promising prospect for carotenoid intake and extraction in the future.
3.6. Postharvest analysis of antioxidant activity in various pumpkin cultivars
In vitro antioxidant capacity of different cultivars during postharvest was analyzed. An increasing followed by a decreasing trend was observed during storage in all pumpkin cultivars. The DPPH activity ranges varied among different pumpkin cultivars, with the large-fruited C. moschata ranging from 583.46 to 879.92 μg Trolox/g DW, the small-fruited C. moschata ranging from 444.61 to 1039.41 μg Trolox/g DW, the green-skinned C. maxima ranging from 479.32 to 934.33 μg Trolox/g DW, and the red-skinned C. maxima ranging from 761.71 to 1128.53 μg Trolox/g DW. Cma-81 exhibited the highest DPPH activity compared with other cultivars throughout all postharvest stages, reaching 1128.53 μg Trolox/g DW at 20 dop. Meanwhile, a high DPPH activity was observed in C. moschata and C. maxima species at 20 dop (Fig. 5H). The flesh of C. moschata could be dried and ground into a powder to develop bread, cakes, and other baked goods as ingredients (Al-Ghamdi, Hong, Qu, & Sablani, 2020). This study confirmed that both C. moschata and C. maxima exhibited DPPH activity, indicating their suitability as accompaniments to rice dishes.
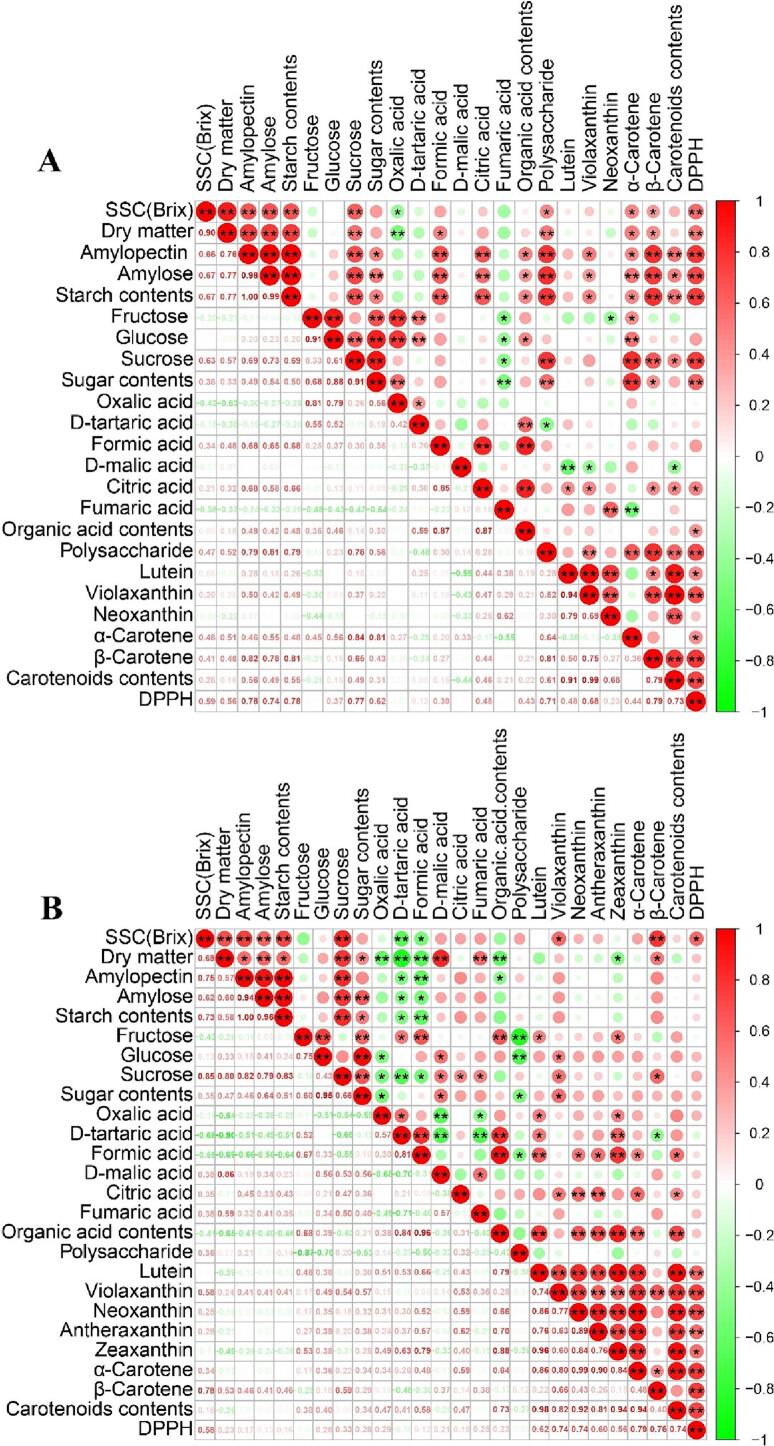
At 20 days of postharvest (dop), all pumpkin cultivars exhibited high DPPH radical scavenging activity. Consequently, a correlation analysis of pumpkin quality parameters at 20 dop was carried out to determine the relationship between the high DPPH activity and metabolic compounds, as well as to analyze the correlations among various quality parameters. Carotenoids and polysaccharides have been recognized as effective antioxidant compounds (Hussain et al., 2022). In C. moschata, DPPH activity was found to be associated with both polysaccharides and carotenoids. Meanwhile, in C. maxima, DPPH radical scavenging activity was positively correlated with carotenoids, as illustrated in Fig. 6.
Fig. 6.
Correlation analysis among fruit quality factors in the seven pumpkin cultivars during storage. Fruit quality factors in a pumpkin at harvest (0 days) and after storage (20 days) of C. moschata(A) and C. maxima(B), respectively. Data were analyzed using Pearson's correlation coefficients (⁎P ≤ 0.05, ⁎⁎P ≤ 0.01). Fig. 6A-B visualized the correlation, with larger circles, deeper colors, and more asterisks in the upper right corner indicating stronger correlations. The bottom left corner showed the correlation coefficient, with darker colors indicating stronger correlations.
3.7. Correlation analysis of postharvest quality factors in the seven pumpkin cultivars
The correlations among fruit quality factors, including DM, SSC, polysaccharides, organic acids, sugar, starch, carotenoids, and DPPH, were analyzed for the seven cultivars at 0, 10, 20, 30, and 60 dop. The results showed that C. moschata and C. maxima clustered together in each of the five postharvest testing periods (Fig. S2). At 20 dop, all pumpkin cultivars, except Cma-81, clustered together, possibly due to the significantly lower starch contents of Cma-81 compared with the other six pumpkin cultivars after 20 dop (Fig. S3). In tomatoes and watermelons, SSC was related to soluble sugars and organic acids (Tieman et al., 2017). However, in pumpkins, SSC was related to not only the sugar content but also starch and DM contents. In this study, a positive correlation was detected between starch and sucrose contents (Fig. 6). Regarding starch synthesis, sucrose is hydrolyzed into glucose—a necessary step for starch production (Nakkanong et al., 2012). This enzymatic process may underlie the observed positive association between the contents of starch and sucrose. Previously, Corrigan et al. (2001) explored the sensory qualities of C. maxima cultivars, noting substantial taste variations. They found that starch and DM contents were related to the sensory quality of pumpkin fruits. In this study, a correlation between the DM and starch content was found in the pumpkin cultivars of C. moschata and C. maxima (Fig. 6). In C. moschata, polysaccharides showed a positive correlation with starch, sucrose, carotenoids, and DPPH contents. However, in C. maxima, polysaccharides negatively correlated with glucose and fructose contents (Fig. 6).
4. Conclusions
The postharvest patterns of quality-related compounds varied between the two pumpkin species, C. moschata and C. maxima. Specifically, the total organic acid content in C. maxima remained higher than in most C. moschata cultivars during storage. Notably, the Cmo-bs cultivar of C. moschata exhibited increased polysaccharide levels at 30 days of postharvest (dop), while the Cma-81 cultivar of C. maxima was particularly rich in carotenoids at 20 dop. In C. moschata, the sucrose contents demonstrated a continuous increasing trend, leading to an overall increase in the total sugar content. In C. maxima, the glucose contents showed an increasing trend, resulting in an increasing trend of total sugar contents. C. moschata and C. maxima showed maximum starch contents at 30 dop and 20 dop, respectively. The carotenoid contents increased constantly in C. moschata cultivars, whereas they showed an initially increasing followed by a decreasing trend in all C. maxima cultivars. This study also found that some C. maxima began to rot after >60 dop. The external appearance and nutritional changes in the fruits indicate that C. moschata had better storage resistance than C. maxima.
Above all, pumpkin storage was necessary for taste improvement, with C. moschata showing the best taste and nutrition at 20–30 dop. However, C. maxima indicated that 10–20 dop was the best choice for commercial use.
CRediT authorship contribution statement
Yingchao Xu: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Data curation. Manman Wang: Writing – original draft, Investigation, Data curation. Hafiz Muhammad Khalid Abbas: Writing – review & editing. Shudan Xue: Validation, Methodology. Jitong Zhu: Methodology, Data curation. Qitao Meng: Methodology, Data curation. Qingmin Jin: Supervision, Methodology, Data curation. Manqin Fu: Validation, Investigation. Shuping Qu: Writing – review & editing, Supervision. Yujuan Zhong: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Food Nutrition and Health Collaborative Innovation Center of GDAAS (XT202226), the Special Found for Scientific Innovation Strategy-construction of Guangdong Academy of Agricultural Sciences (R2023PY-JG005). Guangdong Basic and Applied Basic Research Foundation (Grant No. 2023A1515110876 and 2024A1515012737), Guangzhou Basic and Applied Basic Research Foundation (Grant No. 2024A04J4363), the National Natural Science Foundation of China (Grant No.32172604), and Special Foundation for Introduction of Scientific Talents of Guangdong Academy of Agricultural Sciences (R2022YJ-YB3012).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101383.
Contributor Information
Shuping Qu, Email: spqu@neau.edu.cn.
Yujuan Zhong, Email: zhongyujuan@gdaas.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Abbas H.M.K., Huang H.X., Wang A.J., Wu T.Q., Xue S.D., Ahmad A.…Zhong Y.J. Metabolic and transcriptomic analysis of two Cucurbita moschata germplasms throughout fruit development. BMC Genomics. 2020;21:1–13. doi: 10.1186/542483-020-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas H.M.K., Huang H.X., Wu T.Q., Wang R., Du H., Lu S.…Zhong Y.J. High-density genetic mapping identified a major locus for environmental sex expression in pumpkin (Cucurbita moschata Duch.) Horticultural Plant Journal. 2022;8(5):593–601. doi: 10.1016/j.hpj.2022.05.006. [DOI] [Google Scholar]
- Abbas H.M.K., Huang H.X., Yang Y.F., Xie Y.H., Zou J.F., Xue S.D.…Zhong Y.J. Characterization of starch in Cucurbita moschata germplasms throughout fruit development. Journal of Agricultural and Food Chemistry. 2020;68(36):9690–9696. doi: 10.1021/acs.jafc.0c03181. [DOI] [PubMed] [Google Scholar]
- Akwaowo E.U., Ndon B.A., Etuk E.U. Minerals and antinutrients in fluted pumpkin (Telfairia occidentalis Hook f.) Food Chemistry. 2000;70:235–240. doi: 10.1016/S0308-8146(99)00207-1. [DOI] [Google Scholar]
- Al-Ghamdi S., Hong Y.K., Qu Z., Sablani S.S. State diagram, water sorption isotherms and color stability of pumpkin (Cucurbita pepo L.) Journal of Food Engineering. 2020;273 doi: 10.1016/j.jfoodeng.2019.109820. [DOI] [Google Scholar]
- Baranski R., Allender C., Klimek-Chodacka M. Towards better tasting and more nutritious carrots: Carotenoid and sugar content variation in carrot genetic resources. Food Research International. 2012;47:182–187. doi: 10.1016/j.foodres.2011.05.006. [DOI] [Google Scholar]
- Chavan P., Lata K., Kaur T., Jambrak A.R., Sharma S., Roy S.…Aayush K. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chemistry. 2023 doi: 10.1016/j.foodchem.2023.135916. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang G.L. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. International Jouranl Biological Macromolecules. 2018;118:770–774. doi: 10.1016/j.ijbiomac.2018.06.148. [DOI] [PubMed] [Google Scholar]
- Corrigan V.K., Hurst P.L., Potter J.F. Winter squash (Cucurbita maxima) texture: Sensory, chemical, and physical measures. New Zealand Journal of Crop and Horticultural Science. 2001;29:111–124. doi: 10.1080/01140671.2001.9514169. [DOI] [Google Scholar]
- Duan X.W., Cheng G.P., Yang E., Yi C., Ruenroengklin N., Lu W.J., Luo Y.B., Jiang Y.M. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chemistry. 2008;111:144–149. doi: 10.1016/j.foodchem.2008.03.049. [DOI] [Google Scholar]
- Feng S., Bi J., Yi J., Li X., Li J., Ma Y. Cell wall polysaccharides and mono−/disaccharides as chemical determinants for the texture and hygroscopicity of freeze-dried fruit and vegetable cubes. Food Chemistry. 2022;395 doi: 10.1016/j.foodchem.2022.133574. [DOI] [PubMed] [Google Scholar]
- Guan Y., Peace C., Rudell D., Verma S., Evans K. QTLs detected for individual sugars and soluble solids content in apple. Molecular Breeding. 2015;35:1–13. doi: 10.1007/s11032-015-0334-1. [DOI] [Google Scholar]
- Guo Q.B., Wang N.F., Liu H.H., Li Z.J., Lu L.F., Wang C.L. The bioactive compounds and biological functions of Asparagus officinalis L. Journal of Functional Foods. 2020;65 doi: 10.1016/j.jff.2019.103727. [DOI] [Google Scholar]
- Hou J., Zhang H.L., Liu J., Reid S., Liu T.F., Xu S.J.…Xie C. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. Journal of Experimental Botany. 2017;68:2317–2331. doi: 10.1093/jxb/erx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.X., Yu T., Li J.X., Qu S.P., Wang M.M., Wu T.Q., Zhong Y.J. Characterization of Cucurbita maxima fruit metabolomic profiling and transcriptome to reveal fruit quality and ripening gene expression patterns. Journal Of Plant Biology. 2019;62:203–216. doi: 10.1007/s12374-019-0015-4. [DOI] [Google Scholar]
- Huang L.L., Zhao J., Wei Y.L., Yu G.Y., Li F., Li Q.H. Structural characterization and mechanisms of macrophage immunomodulatory activity of a pectic polysaccharide from Cucurbita moschata Duch. Carbohydrate Polymers. 2021;269 doi: 10.1016/j.carbpol.2021.118288. [DOI] [PubMed] [Google Scholar]
- Hurkman W.J., Wood D.F. High temperature during grain fill alters the morphology of protein and starch deposits in the starchy endosperm cells of developing wheat (Triticum aestivum L.) grain. Journal of Agricultural and Food Chemistry. 2011;59:4938–4946. doi: 10.1021/jf102962t. [DOI] [PubMed] [Google Scholar]
- Hussain A., Kausar T., Sehar S., Sarwar A., Ashraf A.H., Jamil M.A.…Quddoos M.Y. A comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chemistry Advances. 2022;100067 doi: 10.1016/j.focha.2022.100067. [DOI] [Google Scholar]
- Jacobo-Valenzuela N., Maróstica-Junior M.R., Zazueta-Morales J.D.J., Gallegos-Infante J.A. Physicochemical, technological properties, and health-benefits of Cucurbita moschata Duchense vs. Cehualca. Food Research International. 2011;44:2587–2593. doi: 10.1016/j.foodres.2011.04.039. [DOI] [Google Scholar]
- Jiao X., Li F., Zhao J., Wei Y., Zhang L.L., Wang H.J.…Li Q.H. Structural diversity and physicochemical properties of polysaccharides isolated from pumpkin (Cucurbita moschata) by different methods. Food Research International. 2023;163 doi: 10.1016/j.foodres.2022.112157. [DOI] [PubMed] [Google Scholar]
- Karaağaç O., Balkaya A. Interspecific hybridization and hybrid seed yield of winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.) lines for rootstock breeding. Scientia Horticulturae. 2013;149:9–12. doi: 10.1016/j.scienta.2012.10.021. [DOI] [Google Scholar]
- Li C.L., Dougherty L., Coluccio A.E., Meng D., El-Sharkawy I., Borejsza-Wysocka…Cheng L.L. Apple ALMT9 requires a conserved C-terminal domain for malate transport underlying fruit acidity. Plant Physiology. 2020;182:992–1006. doi: 10.1104/pp.19.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.L., Sun H.N., Zhang M., Mu T.H., Khan N.M., Ahmad S., Validov S.Z. Fungal communities, nutritional, physiological and sensory characteristics of sweet potato under three Chinese representative storages. Postharvest Biology and Technology. 2023;201 doi: 10.1016/j.postharvbio.2023.112366. [DOI] [Google Scholar]
- Meng F., Li Y., Li S., Chen H., Shao Z., Jian Y., Wang Q. Carotenoid biofortification in tomato products along whole agro-food chain from field to fork. Trends in Food Science and Technology. 2022 doi: 10.1016/j.tifs.2022.04.023. [DOI] [Google Scholar]
- Nakkanong K., Yang J.H., Zhang M.F. Starch accumulation and starch related genes expression in novel inter-specific inbred squash line and their parents during fruit development. Scientia Horticulturae. 2012;136:1–8. doi: 10.1016/j.scienta.2011.12.020. [DOI] [Google Scholar]
- Nawirska-Olszańska A., Biesiada A., Sokół-Łętowska A., Kucharska A.Z. Characteristics of organic acids in the fruit of different pumpkin species. Food Chemistry. 2014;148:415–419. doi: 10.1016/j.foodchem.2013.10.080. [DOI] [PubMed] [Google Scholar]
- Provesi J.G., Dias C.O., Amante E.R. Changes in carotenoids during processing and storage of pumpkin puree. Food Chemistry. 2011;128(1):195–202. doi: 10.1016/j.foodchem.2011.03.027. doi:10.1016j.foodchem.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Przetaczek-Rożnowska I., Fortuna T. Effect of conditions of modification on thermal and rheological properties of phosphorylated pumpkin starch. International Journal of Biology Macromolecules. 2017;104:339–344. doi: 10.1016/j.ijbiomac.2017.06.048. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Miaruddin M., Khan M.H.H., Masud M.A.T., Begum M.M. Effect of storage periods on postharvest quality of pumpkin. Bangladesh Journal of Agricultural Research. 2013;38(2):247–255. doi: 10.3329/bjar.v38i2.15888. [DOI] [Google Scholar]
- Ren R., Li Z., Zhang L., Zhou H., Jiang X., Liu Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell, Tissue and Organ Culture. 2021;144(1):233–246. [Google Scholar]
- Rosul M., Deric N., Misan A., Pojic M., Simurina O., Halimi C.…Reboul E. Bioaccessibility and uptake by Caco-2 cells of carotenoids from cereal-based products enriched with butternut squash (Cucurbita moschata L.) Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132595. [DOI] [PubMed] [Google Scholar]
- Sanjur O.I., Piperno D.R., Andres T.C., Wessel-Beaver L. Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: Implications for crop plant evolution and areas of origin. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:535–540. doi: 10.1073/pnas.012577299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Singh J., Kaur L., Singh Sodhi N., Singh Gill B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chemistry. 2003;81:219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Stevenson D., Yoo S., Hurst P., Jane J. Structural and physicochemical characteristics of winter squash (Cucurbita maxima D.) fruit starches at harvest. Carbohydrate Polymers. 2005;59:153–163. doi: 10.1016/j.carbpol.2004.08.030. [DOI] [Google Scholar]
- Tieman D., Zhu G., Resende M.F., Jr., Lin T., Nguyen C., Bies D.…Zhang B. A chemical genetic roadmap to improved tomato flavor. Science. 2017;355:391–394. doi: 10.1126/science.aal1556. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang Y., Wang M., Han H., Luo Y., Ding W., Qu S. Soluble sugars accumulation and related gene expression during fruit development in Cucurbita maxima Duchesne. Scientia Horticulturae. 2020;272 doi: 10.1016/j.scienta.2020.109520. [DOI] [Google Scholar]
- Wang J.P., Li Y., Tian Y.Q., Xu X.M., Jin Z.Y. A novel triple-wavelength colorimetric method for measuring amylose and amylopectin contents. Starch-Starke. 2010;62:508–516. doi: 10.1002/star.200900242. [DOI] [Google Scholar]
- Wyatt L.E., Strickler S.R., Mueller L.A., Mazourek M. Comparative analysis of Cucurbita pepo metabolism throughout fruit development in acorn squash and oilseed pumpkin. Scientia Horticulturae. 2016;3 doi: 10.1038/hortres.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A.J., Li M.H., Li Z.W., Yue X.Q. A preparation of debranched waxy maize starch derivatives: Effect of drying temperatures on crystallization and digestibility. International Journal of Biological Macromolecules. 2024;264 doi: 10.1016/j.ijbiomac.2024.130684. [DOI] [PubMed] [Google Scholar]
- Yuan T., Ye F., Chen T., Li M., Zhao G. Structural characteristics and physicochemical properties of starches from winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch. Ex Poir.) Food Hydrocolloids. 2022;122 doi: 10.1016/j.carres.2006.04.013. [DOI] [Google Scholar]
- Zhao J., Zhang F.M., Liu X.Y., Ange K.S., Zhang A.Q., Li Q.H., Linhardt R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydrate Polymers. 2017;163:330–336. doi: 10.1016/j.carbpol.2017.01.067. [DOI] [PubMed] [Google Scholar]
- Zhong Y.J., Huang J.C., Liu J., Li Y., Jiang Y., Xu Z.F.…Chen F. Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis. Journal of Experimental Botany. 2011;62:3659–3669. doi: 10.1093/jxb/err070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.