Abstract
Background
Ischaemic intestines could be a driver of critical illness through an inflammatory response. We have previously published reports on a biomarker for intestinal injury, plasma Intestinal Fatty Acid Binding Protein (IFABP), and inflammatory biomarkers after out-of-hospital cardiac arrest (OHCA). In this post-hoc study we explored the potential indirect effects of intestinal injury mediated through the inflammatory response on organ dysfunction and mortality.
Methods
We measured IFABP and twenty-one inflammatory biomarkers in 50 patients at admission to intensive care unit after OHCA. First, we stratified patients on median IFABP and compared biomarkers between “low” and “high” IFABP. Second, by causal mediation analysis, we assessed effects of IFABP through the two most important inflammatory biomarkers, interleukin (IL)-6 and terminal complement complex (TCC), on day two circulatory variables, Sequential Organ Failure Assessment (SOFA)-score, and 30-day mortality.
Results
Cytokines and complement activation were higher in the high IFABP group. In mediation analysis, patients on the 75th percentile of IFABP, compared to the 25th percentile, had 53% (95% CI, 33–74; p < 0.001) higher risk of dying, where 13 (95% CI, 3–23; p = 0.01) percentage points were mediated through an indirect effect of IL-6. Similarly, the indirect effect of IFABP through IL-6 on SOFA-score was significant, but smaller than potential other effects. Effects through IL-6 on circulatory variables, and all effects through TCC, were not statistically significant and/or small.
Conclusion
Effects of intestinal injury mediated through inflammation on organ dysfunction and mortality were limited. Small, but significant, effects through IL-6 were noted.
Trial registration: ClinicalTrials.gov: NCT02648061.
Keywords: Cardiac arrest, Intestinal ischaemia, Intestinal fatty acid binding protein, Multiple organ dysfunction, Inflammation, Mediation
Introduction
After being initially successfully resuscitated from cardiac arrest, two-thirds of patients suffer from severe multiple organ dysfunction. Mortality increases with severity of extra-cerebral organ dysfunction and is around 90% in the most severe cases.1, 2, 3 While two-thirds die of neurological injury overall, cardiovascular dysfunction is the most common cause of death the first three days.4 The mechanisms leading from whole-body ischaemia to multiple organ dysfunction and death have not been determined, but the immune system is suggested to play a central role.5 Moreover, intestinal ischaemia might be a “driver” of the subsequent inflammatory response and multiple organ dysfunction.6, 7 Indeed, when and how intestinal injury leads to multiple organ dysfunction are highlighted as research questions by the European Society of Intensive Care Medicine.8
Both plasma Intestinal Fatty Acid Binding Protein (IFABP), a biomarker for small bowel injury, and inflammatory biomarkers, including cytokines and complement activation products, are elevated after cardiac arrest.5, 9, 10, 11 The origin of inflammatory biomarker release is not established, but some studies suggest that ischaemic intestines might initiate the inflammatory cascade.12, 13, 14, 15 However, there are few reports investigating the associations between intestinal injury and systemic inflammatory biomarkers in general.15, 16, 17, 18, 19 Previously, we have reported that both IFABP and some of the analysed inflammatory biomarkers, including interleukin (IL)-6 and the terminal complement complex (TCC), were associated with multiple organ dysfunction and mortality after out-of-hospital cardiac arrest (OHCA).20, 21
Causal mediation analysis is an emerging statistical method which contributes to new insights into pathophysiologic processes.22 The method combines conventional regression models and enables a decomposition of the total effect of an exposure on outcome into an indirect effect through a mediator and a direct effect which is not attributed to the mediator. To draw firm causal conclusions, rigorous assumptions are required.23, 24
The aim of this study was to explore, by causal mediation analysis, the potential indirect effects of intestinal injury mediated through the inflammatory response on organ dysfunction and mortality after out-of-hospital cardiac arrest (OHCA).
Methods
Study design and setting
This is a post hoc analysis of a prospective cohort included at St. Olav’s University Hospital, Norway. The inclusion period was January 2016 to November 2017. In addition to the inflammatory biomarkers (including IL-6 and TCC) and intestinal injury (IFABP), reports on circulatory characteristics in this cohort have been published previously20, 21, 25, 26. The AGReMA Statement for mediation analysis reporting published in JAMA, guided this study report.27
Participants
Inclusion criteria were successfully resuscitated patients admitted to the Intensive Care Unit (ICU) after OHCA. Exclusion criteria were age < 18 years, pregnancy, sepsis or anaphylaxis as assumed causes of arrest, transferal from other hospitals, decision upon arrival to limit life-sustaining therapy, or the following before admission to the ICU: application of extracorporeal membranous oxygenation (ECMO), or a ventricular assist device (VAD), or cardiothoracic surgery. Patients were followed for five days or until they were transferred to ward or died, ECMO or VAD was applied, cardio-thoracic surgery was performed, or withdrawing of life-prolonging therapies was decided. Day “zero” lasted from admission until next morning at 06:00, and thus had variable length.
Initial management
Targeted temperature management (TTM) at 36 °C for 24 hours was the standard treatment of comatose patients. Patients presenting with clinical signs of hypoperfusion and/or hypotension were given fluids, vasopressors and/or inotropic drugs. If indicated, percutaneous coronary intervention was done. Comprehensive information regarding the clinical care has been reported earlier.28
Data sources and measurement
We gathered data from the pre-hospital report in accordance with the Utstein cardiac arrest template, and information on assessment and treatment from the hospital record.29 We scored Sequential Organ Failure Assessment (SOFA) daily.30 Lactate was obtained from the first arterial blood gas sample after admission.
In all comatose patients without contraindications, we inserted a pulmonary artery catheter (Swan-Ganz CCOmbo, Edwards Lifesciences, Irvine, CA) for continuous haemodynamic measurements. We obtained all circulatory variables and medications from the electronic critical care information system (Picis CareSuite, Optum Inc. Peabody, MA). The circulatory variables were mean arterial pressure (MAP), cardiac output (CO), systemic vascular resistance (SVR), dose of noradrenaline (norepinephrine), and amount of fluid infusion. MAP, CO, SVR and dose of noradrenaline presented are the mean values over the period beginning 30 minutes before each blood sampling and ending 30 minutes after. Amount of fluid given is the mean for the previous 24 hours. We obtained 30-day mortality from the medical records and the Norwegian death registry.
Biomarkers
Blood sampling and plasma preparation
Blood samples were drawn at inclusion and every morning, gently mixed, placed vertical in ambient temperature for 30 minutes and then centrifuged at 2200g for 10 minutes. Within 1 hour from sampling, EDTA-plasma was frozen to −80 °C.
Intestinal Fatty Acid Binding Protein (IFABP), endothelium and platelet markers
Plasma levels of IFABP, syndecan-1, vascular endothelial (VE)-cadherin and von Willebrand factor (vWF) were measured in duplicate by enzyme immunoassays (EIA) using commercially available antibodies (Cat# DY3078, R&D Systems, Minneapolis, MN) in a 384-format using a combination of a CyBi-SELMA pipetting robot (Analytik Jena, Germany) and an automatic dispenser/washer (BioTek, Winooski, VN). Absorption was registered at 450 nm with wavelength correction set to 540 nm applying an ELISA plate reader (BioTek).
Complement activation and cytokines
Initial C3 activation product (C3bc) and TCC were measured by ELISA, applying the Complement International Standard #2.31 Plasma levels of the following cytokines were analysed with Bio-Plex ProTM Human Cytokine 27-plex Assay (Bio-Rad, Hercules, CA): IL-1 receptor antagonist (IL-1ra), IL-6, IL-8, regulated on activation normal T-cell expressed and secreted (RANTES).
Statistical methods
Descriptive statistics are presented as mean (standard deviation [SD]), median (interquartile range [IQR]), or proportion (%). Of twenty-one biomarkers in the previous report, biomarkers consistently associated with outcome were included in the present (TCC, C3bc, IL-1ra, IL-8, IL-6, RANTES, Syndecan-1, VE-cadherin and vWF), and only the two variables associated with mortality in the multivariable analysis (IL-6 and TCC) were included in the mediation analyses.20 We used admission values of all biomarkers. IL-6 had one outlier which was changed to “missing” and omitted in the analyses.
To assess the associations between inflammatory biomarkers and intestinal injury, we divided the cohort in “low” and “high” by the median IFABP. Biomarkers were compared between the two groups using the Student t-test or the Wilcoxon rank-sum test. We displayed survival and the values of biomarkers first five days graphically.
Mediation analyses
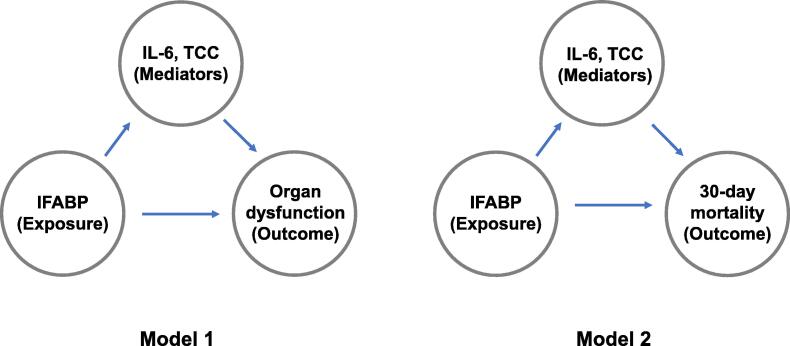
To assess the potential effects of IFABP through inflammatory biomarkers on organ dysfunction and death, we applied causal mediation analysis.22, 24 This approach is based on the potential outcomes framework and is implemented in Stata (StataCorp.2023. Stata 18 Causal mediation analysis Manual. Stata Press, College Station, TX). The potential total effect of an exposure on outcome is the sum of the natural indirect effect through a mediator and the natural direct effect, in the text simply named the “indirect” and “direct” effect. To make valid causal conclusions, these assumptions also must be fulfilled: There must be no unmeasured confounding in the exposure-outcome, the exposure-mediator, or the mediator-outcome relationships. In addition, there must be no confounders in the mediator-outcome relationship caused by the exposure. The mediation analyses, with assumed causes and effects, are presented in Fig. 1.
Fig. 1.
The two mediation models. IFABP is the exposure and inflammatory biomarkers are mediators in both models. Organ dysfunction is the outcome in Model 1 and 30-day mortality is the outcome in Model 2. Potential confounding factors such as whole-body ischaemia could affect all variables. IFABP: Intestinal Fatty Acid Binding Protein. IL: Interleukin; TCC: terminal complement complex.
We applied two mediation models using organ dysfunction (Model 1) and 30-day mortality (Model 2) as the outcomes. The indirect effect was our main interest. In both models IFABP was set as the exposure, and IL-6 and TCC were log2 transformed and entered one by one as mediators. Organ dysfunction at start of day two was evaluated by six outcome variables separately (SOFA-score, SVR, MAP, CO, dose of noradrenaline and amount of fluid given). Linear regression was applied for both the mediator and outcome functions in Model 1. In Model 2, we applied a probit-function for the dichotomous outcome. The effects (total, direct and indirect) were expressed as the regression coefficients (Model 1) and risk difference (RD) (Model 2) between the 75th percentile of IFABP, compared to the 25th percentile, with 95% confidence intervals (CI).
In Model 2, with IL-6 as the mediator, we included covariates that were plausible confounders (i.e. could affect both the exposure–mediator association and the mediator–outcome association).24 These covariates were initial non-shockable rhythm, time to return of spontaneous circulation (ROSC) and initial lactate at hospital admission. We added the covariates one at a time because of the low sample size. The Stata commands are displayed in Supplementary Table 1.
Sensitivity analysis
We performed several sensitivity analyses. First, we exchanged admission levels for day one levels of IL-6. Second, we applied two logistic regression models with IFABP, IL-6 and TCC as independent variables. In the first regression model, 30-day mortality was evaluated with confounding factors added one at a time, and in the second by excluding patients who were considered to die of irreversible brain injury.21
This was a descriptive study, and no sample size was calculated.28 All tests were two-sided and statistical significance was defined as p < 0.05. We did not adjust for multiple testing. Data were extracted with the software Matlab (Mathworks Inc., Natick, MA), and the statistical analyses were performed with Stata 18.0 (StataCorp LCC, Collage Station, TX).
Ethics
The Regional Committee for Medical and Health Research Ethics, Central Norway Health Region (REK Midt, No. 2015/1807) approved the study. Participants or their proxies provided written consent. The study is registered in ClinicalTrials.gov (Identifier: NCT02648061).
Results
Participants
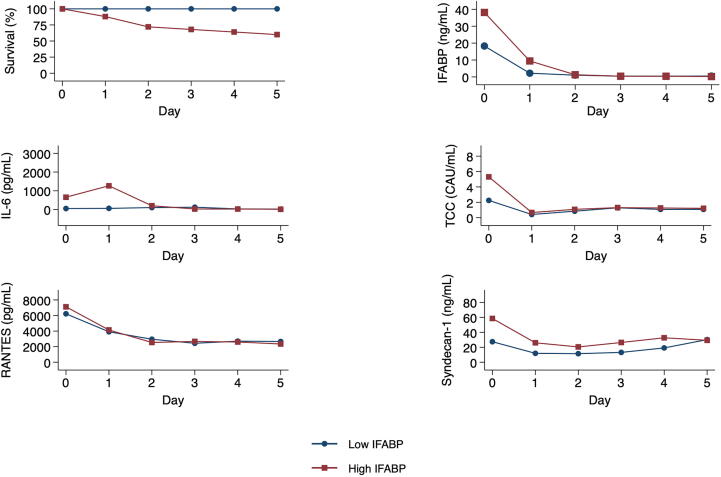
Sixty-five consecutive patients were assessed for eligibility; seven were excluded due to immediate withdrawal of life-support, two had septic etiology, two were not in need of ICU admission, three received VAD or ECMO and one underwent immediate surgery. Fifty patients were included in the study. Patient characteristics are shown in Table 1. Detailed demographic data have been published previously.21, 26 At start of day two (mean 36 hours after admission), seven patients had died, all in the “high” IFABP group (Fig. 2), and six patients had been transferred to ward, all in the “low” IFABP group.
Table 1.
Characteristics of successfully resuscitated OHCA patients.
| Low IFABP (n = 25) | High IFABP (n = 25) | |
|---|---|---|
| Demographic variables | ||
| Age | 67 [54–73] | 65 [52–76] |
| Sex, male | 22 (88%) | 18 (72%) |
| Prehospital Utstein variables | ||
| Witnessed cardiac arrest | 22 (88%) | 20 (80%) |
| Bystander CPR | 23 (100%) | 21 (84%) |
| Shockable initial rhythm | 24 (96%) | 15 (60%) |
| Adrenaline, total dose in mg | 0 [0–0] | 2 [1–3] |
| Time to ROSC (min) | 18 [10–28] | 28 [20.5–35.5] |
| Presumed cardiac etiology | 24 (96%) | 18 (72%) |
| At admission | ||
| Initial arterial lactate (mmol/L) | 3.8 [2.1–6.6] | 9.3 [5.2–12] |
| Certain pulmonary aspiration | 2 (8%) | 7 (28%) |
| Mechanically ventilation | 18 (72%) | 24 (96%) |
| Circulatory shock in ER* | 4 (16%) | 13 (52%) |
| Dose of noradrenaline (microg/kg/min) | 0.02 [0–0.1] | 0.1 [0.04–0.13] |
| During ICU stay | ||
| Treatment with antibiotics | 15 (60%) | 15 (60%) |
| Tratment with inotropic drugs† | 1 (4%) | 9 (36%) |
| Ventilator, hours | 49 [1–173] | 65 [21–137] |
| Cause of death, by day 30 | ||
| Circulatory | 0 (0%) | 2 (8%) |
| Multiple organ failure | 0 (0%) | 3 (12%) |
| Irreversible brain injury | 1 (4%) | 9 (36%) |
| Other/unknown | 1 (4%) | 0 (0%) |
The cohort is divided by the median IFABP (31.4 ng/mL). Variables are expressed as median (interquartile range [Q1–Q3]) or numbers (%). OHCA: Out-of-hospital cardiac arrest; IFABP: Intestinal Fatty Acid Binding Protein; CPR: Cardiopulmonary resuscitation; ROSC: Return of spontaneous circulation; ER: Emergency room.
Shock defined as systolic blood pressure < 90 mmHg or in need of fluids and/or vasopressors to maintain systolic blood pressure > 90 mmHg. Have been published previously.
Inotropic drugs: Adrenaline, dobutamine, dopamine.
Fig. 2.
Survival and trajectories of selected biomarkers. Biomarkers are expressed as means by “low” or “high” IFABP, at the start of the first five days. Only patients still treated in ICU are included (n = 50, 45, 38, 31, 27 and 25 for day 0–5, respectively). Day one started mean 11 hours after admission. OHCA: Out-of-hospital cardiac arrest; IFABP: Intestinal Fatty Acid Binding Protein; IL: interleukin; RANTES: regulated on activation normal T-cell expressed and secreted; TCC: terminal complement complex.
Association between IFABP and inflammatory biomarkers at admission
Median of IFABP was 31.4 (IQR, 21–38) ng/mL at admission, rapidly declining thereafter (Fig. 2).21 The high IFABP group (i.e. >31.4 ng/mL) had higher absolute levels of cytokines (IL-1ra, IL-6, IL-8) and complement activation (TCC, C3bc) (Table 2). Differences in markers of platelet degranulation and endothelial injury were not statistically significant. The trajectories of IL-6, TCC, syndecan-1 and RANTES during the first five days are shown in Fig. 2, while C3bc, IL-1ra, IL-8, VE-cadherin and vWF are shown in Supplementary Fig. 1.
Table 2.
Biomarkers on admission of successfully resuscitated OHCA patients.
| Low IFABP (n = 25) | High IFABP (n = 25) | p value | |
|---|---|---|---|
| Cytokines | |||
| IL-6 (pg/mL) | 26.9 [9.6–74.0] | 100.7 [23.8–447.3] | 0.004 |
| IL-8 (pg/mL) | 12.1 [7.5–17.3] | 58.3 [16.2–93.3] | <0.001 |
| IL-1ra (pg/mL) | 1213.5 [377–3961] | 3533 [1500–9862] | 0.02 |
| Complement activation | |||
| TCC (CAU/mL) | 0.7 [0.4–2.2] | 2.7 [1.0–4.7] | 0.004 |
| C3bc (CAU/mL) | 10.0 [6.4–16.0] | 21.0 [13.0–57.0] | 0.002 |
| Platelet degranulation | |||
| RANTES (pg/mL) | 5926 [5026–6698] | 7040 [5487–8289] | 0.10 |
| Endothelial injury | |||
| Syndecan-1 (ng/mL) | 21.6 [9.9–34.7] | 30.8 [15.1–79.7] | 0.05 |
| VE-Cadherin (ng/mL) | 4055 (999) | 3450 (1334) | 0.08 |
| vWF (U/mL) | 0.79 [0.49–1.747] | 1.14 [0.66–1.88] | 0.26 |
The cohort is divided by the median IFABP (31.4 ng/mL). Variables are expressed as mean (standard deviation) and compared by Student's t-Test, or median (interquartile range [Q1–Q3]) and compared by Wilcoxon rank-sum test. OHCA: Out-of-hospital cardiac arrest; IFABP: Intestinal Fatty Acid Binding Protein; IL: interleukin; RANTES: regulated on activation normal T-cell expressed and secreted; VE: vascular endothelial; U: Unit; vWF: von Willebrand factor; TCC: terminal complement complex; C3bc: activated complement C3.
Mediation analysis
Effects of IFABP on organ dysfunction at start of day two – Model 1
For SOFA-score as a dependent variable, the regression coefficient for the indirect effect through IL-6 was 0.9 (95% CI, 0.2–1.5; p = 0.009) SOFA-points. I.e. patients with IFABP 38 ng/mL had 0.9 points higher SOFA score than patients with 21 ng/mL. The total effect was 3.1 (95% CI, 1.6–4.6; p < 0.001), and the direct effect was 2.3 (95% CI, 0.7 to 3.9; p = 0.006). When TCC was the mediator, the indirect effect through TCC was not statistically significant (0.45 [95% CI, −0.46 to 1.36]; p = 0.33).
The estimated indirect effects of IFABP through IL-6 and TCC on circulatory variables were not statistically significant and/or small (Supplementary Table 2).
Effects of IFABP on 30-day mortality – Model 2
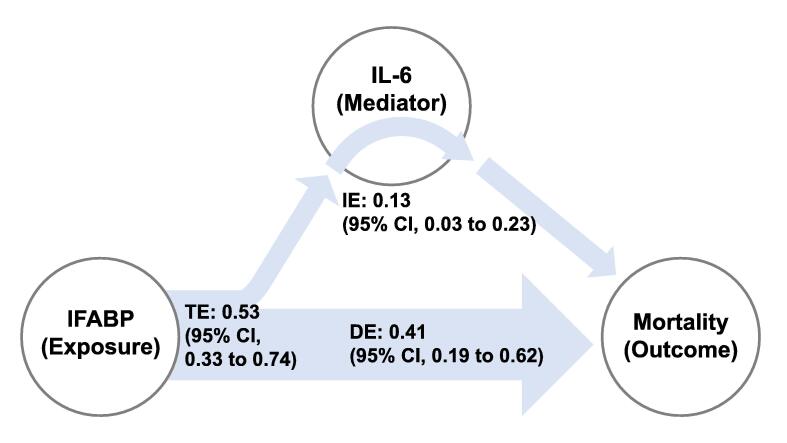
Patients on the 75th percentile of IFABP, compared to the 25th percentile, had an estimated indirect effect through IL-6 of 0.13 (RD; 95% CI, 0.03–0.23; p = 0.01) RD for dying within 30 days (Fig. 3). The total effect was 0.53 (RD; 95% CI, 0.33–0.74; p < 0.001) and the direct effect was 0.41 (RD; 95% CI, 0.19–0.62; p < 0.001). The effects were reduced when we added the covariates “time to ROSC” and “non-shockable rhythm” to Model 2 (Supplementary Table 3). When lactate was added as a covariate, estimates could not be computed due to non-convergence of the calculations.
Fig. 3.
Effects of IFABP and IL-6 on 30-day mortality in causal mediation analysis (Model 2). The total effect (TE) of IFABP on mortality is the sum of the indirect effect (IE) of IFABP through IL-6 and the direct effect (DE). The reported effects are risk differences between the 75th percentile of IFABP (38 ng/mL), compared to 25th percentile (21 ng/mL). IFABP: Intestinal Fatty Acid Binding Protein; IL: Interleukin; CI: Confidence interval.
When TCC was the mediator, the indirect effect was not statistically significant (0.12 [95% CI, −0.04 to 0.28]; p = 0.13). The total effect was 0.54 (95% CI, 0.32–0.76; p < 0.001) and the direct effect was 0.42 (95% CI, 0.17–0.66; p = 0.001).
Sensitivity analyses
When IL-6 at day one was exchanged for IL-6 at admission, the indirect effect through IL-6 on mortality was lower and not significant. In multivariable logistic regression, using death within 30 days as the dependent variable, IFABP was associated with outcome in both models, but IL-6 and TCC in none (Supplementary Tables 4–6).
Discussion
In this post-hoc study, IFABP was associated with cytokines and complement activation at admission, but not with other inflammatory biomarkers. Only the two biomarkers most consistently associated with mortality, IL-6 and TCC, were included in the causal mediation analyses.20, 24 Compared with the direct effects of intestinal injury on SOFA-score and mortality, the indirect effects mediated through IL-6 were significant, but substantially smaller than the direct effects. The indirect effects through IL-6 on circulatory variables, as well as all indirect effects through TCC on circulatory variables, SOFA-score and mortality, were not statistically significant and/or small.
We applied causal mediation analysis to explore if detrimental effects of intestinal injury could be mediated through inflammation. Thus, the direct effect of the exposure on outcome should not be interpreted as a causal effect, rather potential effects not attributed to the mediator. Further, we cannot firmly conclude on causal indirect effects, since the assumption of no unmeasured confounders is not likely to hold.24 Conversely, after analysing twenty-one inflammatory biomarkers and selecting the two biomarkers most consistently associated with outcome, few indirect effects were significant, and these were substantially smaller than the direct effects.20 When we added possible confounders, the indirect effects were reduced. Therefore, an important inflammatory response caused by intestinal injury seems unlikely in this cohort.
Intestinal ischemia may be a clinical concern related to low-flow and high-dose epinephrine given during resuscitation.9, 32 Intestinal ischemia is an even more pronounced concern when applying novel therapies in cardiac arrest like resuscitative endovascular balloon occlusion of the aorta (REBOA) or extracorporeal cardiopulmonary resuscitation (E-CPR).33, 34, 35 The presented findings and our previous paper explore the relationship between intestinal ischemia and organ failure and mortality.21 This relationship is not fully understood and may indicate that more underlying information is needed before we start new clinical trials. This is supported by the fact that clinical trials investigating the use of the IL-6 inhibitor tocilizumab and prehospital methylprednisolone had no effect on survival compared to placebo.36, 37
Causal mediation analysis combines regression models, and the limited mediated effects could be because of weak associations between IFABP and inflammatory biomarkers, between inflammatory biomarkers and outcome, or both.24 To answer this was not part of the study, but we found associations between IFABP, IL-6 and TCC. In other studies, associations between IFABP and inflammatory biomarkers have been found in malaria, but not in sepsis and cardiac surgery.15, 17, 18, 19, 38 Further, IFABP has been convincingly associated with mortality after cardiac arrest, while IL-6 are among the few inflammatory biomarkers which are associated with poor outcome after adjustment for confounding factors.9, 39, 40, 41, 42, 43, 44 Importantly, IFABP and inflammatory biomarkers could merely increase in parallel with the degree of whole-body ischaemia.20, 21
There are a few caveats with this study. First, small indirect effects through separate biomarkers could add up to a large effect in total, although IL-6 and TCC are central in the inflammatory response.5, 20 Second, perhaps only the most severely injured intestines cause a sustained inflammatory response.45 However, after excluding patients who died of irreversible brain injury, leaving patients with extra-cerebral organ dysfunction as assumed cause of death, inflammatory biomarkers were still not associated with mortality, while IFABP was. Third, we analysed both IFABP and inflammatory biomarkers at admission since previous studies have shown that IFABP increased before IL-6 during cardiac surgery, and both declined early.15, 17, 18 Both biomarkers have also declined early after cardiac arrest.10, 11, 42, 43, 44, 46, 47, 48, 49 In the sensitivity analysis, the effect through IL-6 at day one was less pronounced than through IL-6 at admission, suggesting against a delayed inflammatory response.
The concept of “gut origin sepsis” denotes intestinal injury leading to a “sepsis-like” syndrome in the critically ill patient.6 The term “sepsis-like syndrome” has also been applied on patients after cardiac arrest who share some features with septic patients, such as vasodilation, intravascular volume depletion, and endothelial injury.5, 49 We found limited effects of IFABP through inflammatory biomarkers on SOFA-score and variables reflecting vasodilation (SVR, dose of noradrenaline and MAP) and volume depletion (fluid infusion), while markers of endothelial injury were not consistently associated with outcome and excluded from the analysis.20 Thus, our findings indicate that intestinal injury did not lead to a “sepsis like syndrome” in this cohort, and contrast with the concept of “gut origin sepsis”.
In summary, a limited mediated effect of intestinal injury through IL-6 on organ dysfunction and death is possible. Our findings suggest, however, that intestinal injury is not a “driver” of critical illness acting through an inflammatory response after cardiac arrest.7, 8
Limitations
The main limitations of this study are the post hoc design, single center study, and the small sample size. Specific features of this cohort, such as a high proportion of bystander CPR, could limit the generalizability. Further, the validity of IFABP has not been established in the cardiac arrest population, and although lL-6 is extensively studied, different analytical methods can make comparisons challenging. Lastly, the immune system is complex, there may be an interplay between different organ failures, and other factors not studied in this analysis may be important.
Conclusion
To our knowledge, this is the first report on the relationship between intestinal injury and inflammation after cardiac arrest. By causal mediation analysis, effects of intestinal injury mediated through the inflammatory response on organ dysfunction and mortality were limited. Small, but significant, effects through IL-6 were noted.
Funding
Author BHF has received PhD-funding by the Norwegian Air Ambulance Foundation.
CRediT authorship contribution statement
Bjørn Hoftun Farbu: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Stian Lydersen: Writing – review & editing, Methodology, Formal analysis, Conceptualization. Randi Marie Mohus: Writing – review & editing, Methodology, Formal analysis. Thor Ueland: Writing – review & editing, Investigation, Formal analysis, Data curation. Tom Eirik Mollnes: Writing – review & editing, Investigation, Formal analysis, Data curation. Pål Klepstad: Writing – review & editing, Supervision, Methodology, Conceptualization. Halvor Langeland: Writing – review & editing, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘Bjoern Hoftun Farbu reports financial support and administrative support were provided by Norwegian Air Ambulance Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper’.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100639.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- 1.Roberts B.W., Kilgannon J.H., Chansky M.E., et al. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Read Online: Crit Care Med|Soc Crit Care Med. 2013;41:1492–1501. doi: 10.1097/CCM.0b013e31828a39e9. [DOI] [PubMed] [Google Scholar]
- 2.Nobile L., Taccone F.S., Szakmany T., et al. The impact of extracerebral organ failure on outcome of patients after cardiac arrest: An observational study from the ICON database. Crit Care. 2016;20 doi: 10.1186/s13054-016-1528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pekkarinen P.T., Bäcklund M., Efendijev I., et al. Association of extracerebral organ failure with 1-year survival and healthcare-associated costs after cardiac arrest: An observational database study. Crit Care. 2019;23 doi: 10.1186/s13054-019-2359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemiale V., Dumas F., Mongardon N., et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 5.Nolan J.P., Sandroni C., Bottiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Assimakopoulos S.F., Triantos C., Thomopoulos K., et al. Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infection. 2018;46:751–760. doi: 10.1007/s15010-018-1178-5. [DOI] [PubMed] [Google Scholar]
- 7.Meng M., Klingensmith N.J., Coopersmith C.M. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23:143–148. doi: 10.1097/MCC.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reintam Blaser A., Preiser J.C., Fruhwald S., et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24:224. doi: 10.1186/s13054-020-02889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krychtiuk K.A., Richter B., Lenz M., et al. Epinephrine treatment but not time to ROSC is associated with intestinal injury in patients with cardiac arrest. Resuscitation. 2020;155:32–38. doi: 10.1016/j.resuscitation.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Grimaldi D., Guivarch E., Neveux N., et al. Markers of intestinal injury are associated with endotoxemia in successfully resuscitated patients. Resuscitation. 2013;84:60–65. doi: 10.1016/j.resuscitation.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Piton G., Belin N., Barrot L., et al. Enterocyte damage: a piece in the puzzle of post-cardiac arrest syndrome. Shock. 2015;44:438–444. doi: 10.1097/SHK.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 12.Yi J., Slaughter A., Kotter C.V., et al. A “clean case” of systemic injury: mesenteric lymph after hemorrhagic shock elicits a sterile inflammatory response. Shock. 2015;44:336–340. doi: 10.1097/SHK.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Q., Ren H., Ren J., et al. Released mitochondrial DNA following intestinal ischemia reperfusion induces the inflammatory response and gut barrier dysfunction. Sci Rep. 2018;8:7350. doi: 10.1038/s41598-018-25387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grootjans J., Lenaerts K., Derikx J.P.M., et al. Human intestinal ischemia-reperfusion–induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanssen S.J., Derikx J.P., Vermeulen I.C., et al. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117–125. doi: 10.1097/SLA.0b013e3181784cc5. [DOI] [PubMed] [Google Scholar]
- 16.de Haan J.J., Lubbers T., Derikx J.P., et al. Rapid development of intestinal cell damage following severe trauma: a prospective observational cohort study. Crit Care. 2009;13:R86. doi: 10.1186/cc7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habes Q.L.M., Linssen V., Nooijen S., et al. Markers of intestinal damage and their relation to cytokine levels in cardiac surgery patients. Shock. 2017;47:709–714. doi: 10.1097/SHK.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 18.Habes Q.L.M., Kant N., Beunders R., et al. Relationships between systemic inflammation, intestinal damage and postoperative organ dysfunction in adults undergoing low-risk cardiac surgery. Heart Lung Circ. 2023;32:395–404. doi: 10.1016/j.hlc.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Ngai M., Hawkes M.T., Erice C., et al. Intestinal injury in Ugandan children hospitalized with malaria. J Infect Dis. 2022;226:2010–2020. doi: 10.1093/infdis/jiac340. [DOI] [PubMed] [Google Scholar]
- 20.Langeland H., Kristian Damas J., Eirik Mollnes T., et al. The inflammatory response is related to circulatory failure after out-of-hospital cardiac arrest: a prospective cohort study. Resuscitation. 2021 doi: 10.1016/j.resuscitation.2021.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Hoftun Farbu B., Langeland H., Ueland T., et al. Intestinal injury in cardiac arrest is associated with multiple organ dysfunction: A prospective cohort study. Resuscitation. 2023;185 doi: 10.1016/j.resuscitation.2023.109748. [DOI] [PubMed] [Google Scholar]
- 22.Lee H., Herbert R.D., McAuley J.H. Mediation analysis. JAMA. 2019;321:697–698. doi: 10.1001/jama.2018.21973. [DOI] [PubMed] [Google Scholar]
- 23.Hayes A.F., Rockwood N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57. doi: 10.1016/j.brat.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 24.VanderWeele T.J. Mediation analysis: A practitioner's guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 25.Langeland H., Bergum D., Loberg M., et al. Characteristics of circulatory failure after out-of-hospital cardiac arrest: a prospective cohort study. Open Heart. 2022;9 doi: 10.1136/openhrt-2021-001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langeland H., Bergum D., Nordseth T., et al. Circulatory trajectories after out-of-hospital cardiac arrest: a prospective cohort study. BMC Anesthesiol. 2021;21:219. doi: 10.1186/s12871-021-01434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H., Cashin A.G., Lamb S.E., et al. A guideline for reporting mediation analyses of randomized trials and observational studies: the AGReMA statement. JAMA. 2021;326:1045–1056. doi: 10.1001/jama.2021.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langeland H., Bergum D., Løberg M., et al. Transitions between circulatory states after out-of-hospital cardiac arrest: protocol for an observational, prospective cohort study. JMIR Res Protoc. 2018;7:e17. doi: 10.2196/resprot.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins Gavin D., Jacobs Ian G., Nadkarni Vinay M., et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the utstein resuscitation registry templates for out-of-hospital cardiac arrest. Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 30.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 31.Bergseth G., Ludviksen J.K., Kirschfink M., Giclas P.C., Nilsson B., Mollnes T.E. An international serum standard for application in assays to detect human complement activation products. Mol Immunol. 2013;56:232–239. doi: 10.1016/j.molimm.2013.05.221. [DOI] [PubMed] [Google Scholar]
- 32.Bjorck M., Koelemay M., Acosta S., et al. Editor's choice – management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the European society of vascular surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53:460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Brede J.R., Skulberg A.K., Rehn M., et al. REBOARREST, resuscitative endovascular balloon occlusion of the aorta in non-traumatic out-of-hospital cardiac arrest: a study protocol for a randomised, parallel group, clinical multicentre trial. Trials. 2021;22:511. doi: 10.1186/s13063-021-05477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H.E., Chu S.-E., Jo Y.H., et al. Effect of resuscitative endovascular balloon occlusion of the aorta in nontraumatic out-of-hospital cardiac arrest: a multinational, multicenter, randomized, controlled trial. Trials. 2024;25:118. doi: 10.1186/s13063-024-07928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renaudier M., de Roux Q., Bougouin W., et al. Acute mesenteric ischaemia in refractory shock on veno-arterial extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2020;10:62–70. doi: 10.1177/2048872620915655. [DOI] [PubMed] [Google Scholar]
- 36.Meyer M.A.S., Wiberg S., Grand J., et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (The IMICA Trial) a double-blinded, placebo-controlled, single-center, randomized, clinical trial. Circulation. 2021;143:1841–1851. doi: 10.1161/CIRCULATIONAHA.120.053318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obling L.E.R., Beske R.P., Meyer M.A.S., et al. Prehospital high-dose methylprednisolone in resuscitated out-of-hospital cardiac arrest patients (STEROHCA): a randomized clinical trial. Intensive Care Med. 2023 doi: 10.1007/s00134-023-07247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habes Q.L.M., van Ede L., Gerretsen J., Kox M., Pickkers P. Norepinephrine contributes to enterocyte damage in septic shock patients: a prospective cohort study. Shock. 2018;49:137–143. doi: 10.1097/SHK.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 39.Seppä A.M.J., Skrifvars M.B., Pekkarinen P.T. Inflammatory response after out-of-hospital cardiac arrest—Impact on outcome and organ failure development. Acta Anaesthesiol Scand. 2023;67:1273–1287. doi: 10.1111/aas.14291. [DOI] [PubMed] [Google Scholar]
- 40.Bro-Jeppesen J., Johansson P.I., Hassager C., et al. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation. 2016;107:71–79. doi: 10.1016/j.resuscitation.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Bro-Jeppesen J., Kjaergaard J., Wanscher M., et al. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: a substudy of the target temperature management trial. Crit Care Med. 2015;43:1223–1232. doi: 10.1097/CCM.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 42.Peberdy M.A., Andersen L.W., Abbate A., et al. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-A prospective multicenter observational study. Resuscitation. 2016;103:117–124. doi: 10.1016/j.resuscitation.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Chaban V., Nakstad E.R., Staer-Jensen H., et al. Complement activation is associated with poor outcome after out-of-hospital cardiac arrest. Resuscitation. 2021;166:129–136. doi: 10.1016/j.resuscitation.2021.05.038. [DOI] [PubMed] [Google Scholar]
- 44.Vaahersalo J., Skrifvars M.B., Pulkki K., et al. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of-hospital ventricular fibrillation. Resuscitation. 2014;85:1573–1579. doi: 10.1016/j.resuscitation.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 45.Schellekens D.H.S.M., Grootjans J., Dello S.A.W.G., et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253–260. doi: 10.1097/MCG.0b013e3182a87e3e. [DOI] [PubMed] [Google Scholar]
- 46.Bisschops L.L.A., Hoedemaekers C.W.E., Mollnes T.E., van der Hoeven J.G. Rewarming after hypothermia after cardiac arrest shifts the inflammatory balance*. Crit Care Med. 2012;40:1136–1142. doi: 10.1097/CCM.0b013e3182377050. [DOI] [PubMed] [Google Scholar]
- 47.Fries M., Stoppe C., Brucken D., Rossaint R., Kuhlen R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care. 2009;24:453–457. doi: 10.1016/j.jcrc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Bro-Jeppesen J., Kjaergaard J., Wanscher M., et al. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33 °C or 36 °C. Resuscitation. 2014;85:1480–1487. doi: 10.1016/j.resuscitation.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Adrie C., Adib-Conquy M., Laurent I., et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.