Abstract
Radiation-induced lung injury (RILI) is a common and fatal complication of chest radiotherapy. The underlying mechanisms include radiation-induced oxidative stress caused by damage to the deoxyribonucleic acid (DNA) and production of reactive oxygen species (ROS), resulting in apoptosis of lung and endothelial cells and recruitment of inflammatory cells and myofibroblasts expressing NADPH oxidase to the site of injury, which in turn contribute to oxidative stress and cytokine production. Nuclear factor erythroid 2-related factor 2 (Nrf-2) is a vital transcription factor that regulates oxidative stress and inhibits inflammation. Studies have shown that Nrf-2 protects against radiation-induced lung inflammation and fibrosis. This review discusses the protective role of Nrf-2 in RILI and its possible mechanisms.
Keywords: RILI, Nrf-2, Inflammatory response, Fibrosis, Ferroptosis
1. Introduction
Radiotherapy is one of the main treatments for malignant tumors, especially lung cancer. It can be potentially helpful in different types and stages of lung cancer, both in controlling cancer progression and palliative care [1,2]. With advances in treatment techniques and improvements in radiotherapy (RT), the adverse effects of RT have gradually decreased, and treatment outcomes have improved. However, radiation-induced lung injury (RILI) is inevitable in sensitive, normal lung tissue [3]. RILI can be divided into early radiation pneumonitis (RP) and late radiation pulmonary fibrosis (RPF). Early RILI is usually short-term and occurs approximately six months after the end of radiation. The pathological manifests as alveolar fluid exudation, alveolar wall congestion, edema, inflammatory cell exudation, megakaryocytic interstitial infiltration, and alveolar membrane damage, and the imaging manifests as ground-glass opacity. Advanced RPF usually appears 6–12 months after radiation exposure and is characterized by an irreversible alveolar wall or interstitial fibrosis [4]. Current treatment techniques are minimal because of the widespread involvement of the lungs in pulmonary fibrosis (PF), and the treatment mainly consists of glucocorticoids in combination with antibiotics, cough and sputum suppression, and other symptomatic treatments; there is no precise, effective treatment, which seriously affects the survival and quality of life of patients [3,5]. Therefore, reducing and preventing RILI represents a critical and unmet medical need that will provide significant clinical benefits to numerous patients.
Nrf-2, a critical regulator of antioxidant, drug, carbohydrate, lipid, heme, and iron metabolism, is a transcription factor susceptible to oxidative stress. It binds to the nucleus's antioxidant response element (ARE) and promotes the transcription of various antioxidant genes. The antioxidant pathway of Nrf-2 is essential in multiple lung diseases, including acute lung injury/acute respiratory distress syndrome, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, asthma, and allergy. It is widely considered a new therapeutic target for inflammatory lung diseases [6]. It has been shown that Nrf-2 is involved in radiation-induced oxidative stress and has protective effects against radiation-induced acute lung injury and inflammation [[7], [8], [9]]. The regulation of Nrf-2 could serve as a novel and more efficacious approach to treating radiation-induced lung damage (RILI). This article discusses the possible mechanism of RILI and the protective role of Nrf-2 in lung damage. It also explores the potential protective mechanism of activating Nrf-2 against RILI and provides an update on the progress of antioxidant therapy research in this field.
2. The mechanisms of radiation-induced lung injury
It is generally accepted that there are two main mechanisms of ionizing radiation damage: direct damage to the deoxyribonucleic acid (DNA) and indirect damage through the production of reactive oxygen species (ROS) [10,11]and release of corresponding cytokines and molecules through intracellular signal transduction to promote inflammation and the immune response [12].
In a very short period after radiation exposure, water molecules ionize to produce ROS, such as nitrogen species (NGS), hydroxyl radicals, and superoxide, which interact with proteins, nuclei, organelles, and the extracellular matrix, leading to DNA damage [[13], [14], [15]]. Damage to the alveolar epithelial cells and vascular endothelial cells following radiation exposure can lead to impaired barrier function. Most of these damaged cells can repair themselves, and the rest may undergo apoptosis or mutations [16]. These damaged cells are sensed by inflammatory cells, causing the proliferation of leukocytes and lymphocytes along with the release of inflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukin family members, and transforming growth factor (TGF-β1) [17]. The persistence of the inflammatory state eventually leads to early reversible toxicity (RP), which can progress to irreversible late toxicity (RPF).
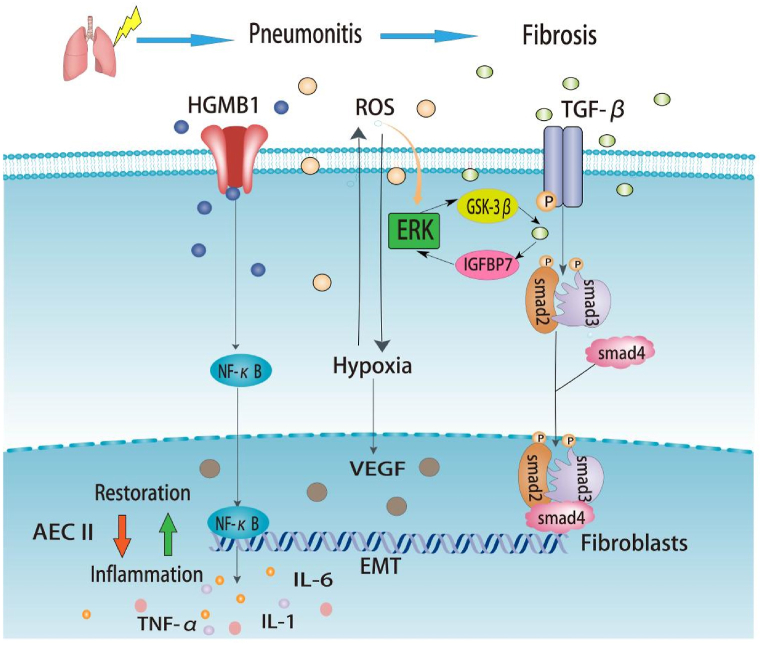
After apoptosis, cells release damage-associated molecular patterns (DAMPs), activating the innate immune system (neutrophils, macrophages, white blood cells, and lymphocytes); immune effector cells are recruited and infiltrate the damaged lung tissues. The inflammatory factors produced by these cells lead to the activation and proliferation of fibroblasts [18,19]. Simultaneously, the utilization of oxygen by the immune cells leads to tissue hypoxia. Hypoxia increases ROS production, regulates TGF-β, and promotes collagen formation, thereby reducing alveolar elasticity [20]. In addition, ROS can cause cell loss, alveolar wall edema, increased vascular permeability, and protein exudation, further reducing lung elasticity, destroying vascular integrity, and increasing the apoptosis of alveolar type I epithelial cells, thereby promoting alveolar type II epithelial cell proliferation and aggravating lung inflammation [10,21]. Under long-term cytokine action, fibroblast recruitment and myofibroblast proliferation lead to the remodeling of the extracellular matrix (ECM) and epithelial-mesenchymal transition (EMT), resulting in fibrogenesis and scar formation, eventually replacing the normal lung tissue with the development of advanced PF [22,23]. The specific signaling pathways are shown in Fig. 1.
Fig. 1.
Signaling pathways in radiation-induced lung injury. Radiation induces lung injury in these ways, showed in the figure. Under ionizing radiation, ROS are rapidly produced, and ROS can activate TGF-β. Activated TGF-β can bind to TGF-βRII, phosphorylating Smad2 and Smad3, which can form a complex with Smad4. Additionally, Radiation-induced ROS stimulates downstream signaling via the ERK/GSK-3β/snail axis. Increased GSK-3β then activates TGF-β and leads to an increase in β-catenin levels, which maintains stemness of type II AEC and promotes its differentiation into fibroblasts. IGFBP7 is enhanced by TGF-β and is involved in the EMT of AECs through the ERK signaling pathway. The complex can regulate gene expression to promote fibrosis. Activated HMGB1 leads to NF-kB into the nucleus and interacts with DNA, promoting TNF-α, IL-6, and IL-1 expression, which can cause lung inflammation.
Abbreviations: ROS: Reactive oxygen species; HMGB1: High-mobility group box 1; TNF: Tumor necrosis factor; IL: Interleukin; TGF: Transforming growth factor; AEC: Alveolar epithelial cells; VEGF: Vascular endothelial growth factor; ERK: Extracellular regulated protein kinases; IGFBP7: Insulin-like growth factor binding protein 7; GSK-3β: Glycogen synthase kinase-3 beta; EMT: Epithelial-mesenchymal transformation; ET-1: Endothelin-1.
With the emergence of stereotactic radiotherapy (SBRT) or stereotactic ablative radiotherapy (SABR), the indications for thoracic RT are expanding; however, RILI remains the most common complication after RT in patients with thoracic tumors. RILI is classified into early- and delayed-radiation PF. Although RP occurs in the early stages of RT and may be cured, there is a limited treatment currently for widespread RP, and late PF is considered irreversible. There is still no effective treatment for the prevention and treatment of RILI and radiation-induced PF [24,25]. However, although researchers have a new understanding of the mechanism, clinical evaluation, and treatment of RILI, more is needed to solve the need for effective clinical treatment of RILI. Hence, while radiotherapy may effectively treat cancer patients, the occurrence of severe RILI can significantly impact their quality of life and perhaps lead to fatal outcomes. Suppose somebody can explore more targeted therapy can be explored based on the known mechanism of RILI. In that case, minimizing radiation damage to normal lung tissue may enable a broader application of chest radiotherapy.
3. Introduction to Nrf-2 and its role in disease models
Nrf-2 is an intracellular transcription factor that is degraded in the cytoplasm under normal conditions by interacting with Kelch ECH-binding protein 1 (Keap1) inhibitors and then acts as an activator of ubiquitination factor [26]. The Nrf-2/Keap1 axis plays a significant role in the cellular regulation of redox homeostasis, mitochondrial physiology, autophagy, protein homeostasis, the immune system, and metabolism [27].
Studies in mouse models have shown that Nrf-2-mediated gene expression is an essential regulator of cellular response to radiation. Recent research indicates that cellular Nrf-2 absence enhances the sensitivity to radiation, resulting in reduced radioresistance. This effect is achieved by activating Nrf-2 overexpression, which helps mitigate radiation-induced damage. In conclusion, several preclinical experiments have verified that increased Nrf-2 expression can minimize radiation damage to normal tissues. However, the current study has not yet answered whether Nrf-2 expression affects the radiation sensitivity of normal human tissues, as it does in mice. Nevertheless, we expect additional relevant clinical trials to be carried out in the next few years, which may provide new treatment options for RILI. We briefly summarized some preclinical studies on the relationship between Nrf-2 expression and tissue radiation injury response, as shown in Table 1.
Table 1.
The role of Nrf-2 in the response to normal tissue radiation injury.
| Intervention | Effect | Mechanism | Outcome | Reference | |
|---|---|---|---|---|---|
| Hematopoietic system | Theaflavin | Nrf-2 activation | Alleviated radiation-induced DNA damage | Ameliorate radiation-induced hematopoietic system injury | [28] |
| Vam3 | Nrf-2 activation | Decreased the cellular ROS level | Ameliorate radiation-induced hematopoietic system injury | [29] | |
| TMC | Nrf-2 activation | Notch pathway activation | Ameliorate radiation-induced hematopoietic system injury | [30] | |
| Tongue | CDDO-Im | Nrf-2 activation | Alleviated radiation-induced DNA damage | Ameliorate radiation-induced oral mucositis | [31] |
| Gastrointestinal tract | 3,3′-Diindolylmethane | Nrf-2 activation | Decreased the cellular ROS level | Ameliorate radiation-induced intestinal injury | [32] |
| CDDO | Nrf-2 activation | Alleviated radiation-induced DNA damage | Ameliorate radiation-induced intestinal injury | [33] | |
| Quercetin | Nrf-2 activation | Decreased the cellular ROS level | Ameliorate radiation-induced intestinal injury | [34] | |
| Skin | Curcumin | Nrf-2 activation | Down-regulation of both inflammatory and fibrogenic cytokines | Ameliorate radiation-induced cutaneous cytotoxicity | [35] |
| Lung | Thalidomide | Nrf-2 activation | Inhibition of TGF-β1/Smad3 pathway | Ameliorate radiation-induced lung fibrosis | [36] |
| EASM | Nrf-2 activation | Inhibition of TGF-β1/Smad3 pathway | Ameliorate radiation-induced lung fibrosis | [37] |
Abbreviations:Nrf-2:nuclear factor erythroid 2-related factor 2; ROS:Reactive oxygen species; TBI:total body irradiation; TMC:2-trifluoromethyl-2′-methoxychalone; TGF: Transforming growth factor; HSC:hematopoietic stem cell; CDDO:1-(2-cyano-3,12-dioxooleana-1,9-dien-28-oyl); EASM:Salvia miltiorrhiza.
Numerous studies have demonstrated that Nrf-2 protects against oxidative lung disorders such as COPD, asthma, IPF, ARDS, respiratory syncytial virus disease, etc. Based on the above studies, Nrf-2 also has a specific protective effect against RILI, which undoubtedly provides new prevention and treatment ideas for RILI.
COPD is a progressive respiratory disease characterized by permanent alveolar wall destruction with loss of lung elasticity and ultimately irreversible airflow limitation, which is associated with a high mortality rate [38]. Smoking and oxidative stress are considered to be significant risk factors for COPD [39,40]. Impaired Nrf-2 has been shown to contribute potentially to the development of COPD [41]; this could be due to the Nrf-2 pathway's role in increasing antioxidant defense and decreasing lung inflammation and alveolar apoptosis. These mechanisms help protect alveolar cells from the harmful effects of tobacco smoke [[42], [43], [44]]. At the same time, recent studies have also shown that activation of the Nrf-2 pathway can balance redox reactions in COPD and restore macrophage function, thus playing a protective role [45].
Asthma is a genetic disease characterized by chronic inflammation and extensive and variable reversible airway obstruction [46]. It has been reported that in mouse models, disruption of the Nrf-2 pathway results in enhanced severity of the asthmatic response, possibly due to reduced basal Nrf-2 expression, leading to reduced antioxidant activity in the lungs, as well as significant attenuation of the transcription of multiple antioxidant genes [47,48]. Several recent studies have demonstrated the protective effects of sulforaphane against asthma. Sulforaphane-activated Nrf-2 signaling plays improves the bronchial protective response to methacholine (MCh) [48,49].
IPF is a chronic progressive lung disease associated with fibroplasia and excessive ECM deposition, which can eventually lead to irreversible interstitial lung fibrosis and respiratory failure. Early studies using mouse models revealed that patients with Nrf-2 deficiency are more susceptible to IPF-like PF and bleomycin-induced effects, resulting in more pronounced lung inflammation and fibrosis [50]. Nrf-2 signaling was also found to enhance antioxidant activity and inhibit bleomycin-induced inflammation in experimental PF [51,52].
ARDS is a severe clinical condition characterized by dyspnea, refractory hypoxemia, and noncardiogenic pulmonary edema. Nrf-2-deficient mice are more likely to develop ARDS with enhanced lung permeability, epithelial damage, and inflammation in response to stimulation than wild-type mice [53]. Recently, several studies have reported the protective effects of Nrf-2 activators against ARDS [[54], [55], [56]].
The respiratory syncytial virus (RSV) is currently considered the leading cause of acute respiratory infections in infants and children. RSV infection can significantly reduce the Nrf-2 levels and antioxidant enzymes in the airways and lungs of mice and nasopharyngeal secretions in children, which may be due to RSV-induced Nrf-2 degradation [57,58]. Previous experiments have demonstrated that Nrf-2-deficient mice develop more severe bronchopulmonary inflammation, epithelial damage, and reduced viral clearance in the presence of RSV infection [59].
4. The potential role and mechanisms of Nrf-2 in radiation induced lung injury
The Nrf-2 signaling pathway is key in regulating cell and tissue homeostasis and protecting cells from oxidative and pro-electrical stresses [60,61]. Electrophilic reagents or ROS can lead to conformational changes in Keap1, resulting in the dissociation of Nrf-2 from Keap1 and its subsequent translocation to the nucleus to activate the antioxidant genes [62,63]. Nrf-2 deficiency impairs ΔNp63 stem/progenitor cell mobilization after irradiation and promotes EMT of alveolar type 2 cells to myofibroblasts [36,64,65]. In addition, recent studies have shown that Nrf-2 plays a crucial role in oxidative homeostasis and inhibition of ferroptosis [[66], [67], [68]], strongly suggesting that Nrf-2 is closely associated with the onset and development of RILI.
4.1. Nrf-2 is related to radiation-induced inflammatory response
Several studies have shown that the Keap1-Nrf-2 pathway plays an essential role in the cytoprotective response to oxidative and pro-electrical stress, and the critical signaling factor in this pathway is the transcription factor Nrf-2 [[69], [70], [71]]. As previously described, Nrf-2 remains inactive by forming a complex with Keap1 in the mammalian cytoplasm. Keap1 is a cysteine-rich protein. When exposed to ionizing radiation, the reactive cysteine residues in Keap1 are covalently modified, damaging the structural integrity of the Keap1-Cul3 E3 ligase complex [72,73]. Subsequently, Nrf-2 dissociates from Keap1, translocates to the nucleus, heterodimerizes with the small Maf protein, and activates the target gene through the antioxidant/electrophilic response element (ARE/EpRE). It participates in glutathione synthesis, eliminates ROS, and inhibits oxidative stress to promote cell protection [[74], [75], [76]]. Thus, Nrf-2 can mitigate radiation-induced acute lung injury.
The lethal damage caused by RT is primarily the result of direct DNA damage by ionizing radiation [11]. It has also been suggested that Nrf-2 can repair radiation-induced DNA damage through the basal excision repair pathway and repair of broken DNA duplexes [77,78]. The basal excision repair pathway has been described in a study by Singh et al. Nrf-2 binds to the OGG1 promoter, and Nrf-2 deficiency inhibits OGG1 expression [79]. It has been demonstrated that the human OGG1 promoter has an ARE of 29 bp from the transcription start site. That alternate splicing of OGG1 leads to the expression of mitochondrial and nuclear proteins [80,81]. Nrf-2 repairs broken DNA duplexes primarily by regulating 53BP1 [33]; this is in line with previous studies showing that 53BP1 is involved in the repair of DNA double-strand breaks and that 53BP1 deficiency increases radiosensitivity [[82], [83], [84], [85]].
Furthermore, it has been shown that Nrf-2 deficiency decreases the levels of radiation-induced serum anti-inflammatory cytokines, IL-10, and antioxidant proteins, exacerbating the radiation-induced imbalance of serum inflammatory cytokines, thus, the inflammatory response [86,87]. All the above findings suggest that Nrf-2 has a considerable protective effect against RILI and inflammation.
4.2. Nrf-2 is involved in the regulation of pulmonary fibrosis
In the late stage of RILI, PF is a consequence of delayed radiation effects and is often considered an irreversible hazard [4,88]. Recent clinical studies have shown that Nrf-2 is associated with radiation-induced PF, and Nrf-2 activators have demonstrated anti-fibrotic effects [89]; this is mainly because Nrf-2 deficiency inhibits the mobilization of ΔNp63 stem/progenitor cells while amplifying the tendency of alveolar type 2 cells to convert into myofibroblasts under radiation induction [64]. In the injured lung, Δp63+/Krt5+ stem cells are significantly mobilized and proliferate. Diphtheria toxin targets the stem cells to damage the regeneration of injured lungs and pulmonary oxygenation while promoting fibrosis. Studies have shown that targeting Nrf-2 can promote epithelial cell repair and activate the BRCA1/Nrf-2/miR-140 signaling pathway to reduce the self-renewal of lung fibroblasts while increasing their migration and contraction, thereby reducing the risk of pulmonary fibrosis [9,90,91]. Xi et al. claimed that under hypoxic conditions in the lung, Notch signaling and Krt5POS basal-like cell expansion are driven by hypoxia-inducible factor (HIF1α) to re-encode Δp63+/Krt5+ cells to form basal-like metaplasia [92]. Nrf-2 activation improves the antioxidant capacity of fibroblasts and myofibroblast dedifferentiation in IPF [93].
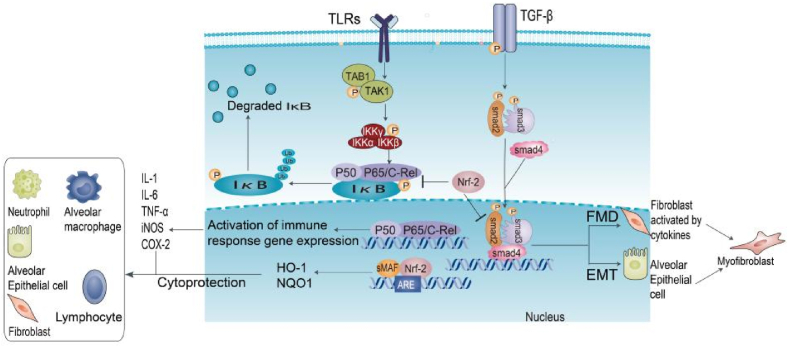
The anti-fibrotic function of Nrf-2 is also reflected in the inhibition of EMT and TGF-β1/Smad signaling [[94], [95], [96]]. Nrf-2 deficiency promotes TGF-β/Smad signaling, a key factor in promoting EMT. At the molecular level, it has been shown that Nrf-2 can form a nuclear complex with nuclear pSmad3 at the CAGA site of the proximal promoter of the TGF-β target gene, thereby inhibiting gene expression [97,98]. TGF-β/Smad signaling induces ATF3. The ATF2/Nrf-2 complex binds to the ARE site in the Nrf-2 target gene promoter and inhibits the recruitment of CBP to the ARE and expression of Nrf-37 target genes [99,100]. It is generally believed that EMT is another major source of myofibroblasts and that Nrf-2 alleviates PF by blocking EMT progression [101]. Moreover, Nrf-2 attenuates TGF-β1-induced EMT by down-regulating high mobility group box 1 (HMGB1) [102]. Nrf-1, Nrf-148, and Nrf-155 also help to alleviate EMT progression [96,103,104]. The current Nrf-2-related signaling pathways and lung inflammation and fibrosis-related signaling pathways can be seen in Fig. 2.
Fig. 2.
Nrf-2 and signaling pathways. In classical TLRs/NF-κB signal transduction, TLR activates the TAK1-TAB1 kinase complex, leading to the synergistic release of NF-κB dimers and IκB phosphorylation by the IKK complex. However, the activation of Nrf-2 can inhibit the phosphorylation of IκB in the typical NF-κB pathway, thereby reducing the nuclear accumulation of NF-κB dimers and inhibiting the expression of downstream immune response genes, such as IL-1, IL-6, TNF-α, iNOS, and COX-2. Activation of the TGF-β1/Smad pathway leads to a disturbance in the steady-state microenvironment, essential for promoting FMD and EMT processes. Activation of Nrf-2 specifically blocks these two processes and protects against pulmonary fibrosis.
Abbreviations: TLR: Toll-like receptor; IκB: inhibitory kappa B; NQO1: NADH quinone oxidoreductase 1; HO-1: Heme oxygenase-1; IL: Interleukin; TNF: Tumor necrosis factor; iNOS: inducible nitric oxide synthase; COX-2: Cyclooxygenase-2; Ub: Ubiquitination; TGF: Transforming growth factor; EMT: Epithelial-mesenchymal transformation; FMD: fibroblast-myofibroblast differentiation.
Mont et al. demonstrated that iso-L-prostaglandin (IsoLG) adducts of proteins are formed in radiation-induced and oxidant-mediated lung injury and that oxidative stress caused by the loss of Nrf-2 or NADPH oxidase activity can promote IsoLG adduct formation [105]. Chronic oxidative stress leads to the accumulation of IsoLG adducts, which can lead to protein toxicity or apoptotic events [64]. While this theory presents an alternative hypothesis for regulating pulmonary fibrosis by Nrf-2, it is essential to note that both studies are small-sample preclinical studies, which inherently possess certain limitations regarding objectivity. This argument would be more convincing if more large-scale clinical studies based on this theory could be carried out.
4.3. Nrf-2 regulates radiation induced cell ferroptosis
Ferroptosis is an oxidative stress-dependent cell death process characterized by iron accumulation and lipid peroxidation [106]. Research has shown that ferroptosis is important in radiation-induced cell death [107]. ROS overload is the basis of ferroptosis, which kills cells by amplifying oxidative stress or inhibiting the antioxidant system [108]. Studies have shown that after treatment of acute RILI with ferroptosis inhibitors, the level of ROS in the lungs and serum inflammatory cytokines (TNF-α, IL-6, IL-10, and TGF-β1) decreased significantly, leading to reduced radiation damage [109,110]. Ferroptosis can be induced by inhibiting GSH synthesis and disrupting the redox balance, thereby increasing the radiosensitivity of tumor cells [111]. A report by Ling et al. suggested that ferroptosis plays a vital role in regulating EMT in PF [112]. A recent report also demonstrated that miR-let-7, an exosome derived from menstrual blood stem cells, can inhibit ferroptosis and improve pulmonary fibrosis through the Sp3/HDAC2/Nrf2 signaling pathway [113].
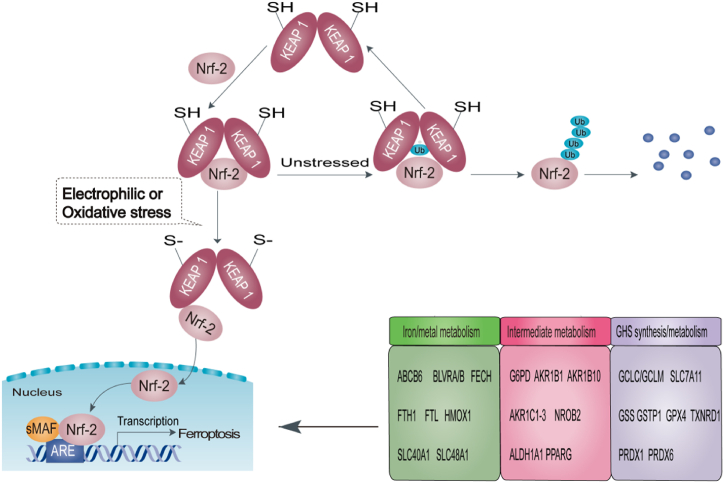
According to recent research, Nrf-2 is also closely involved in ferroptosis [114,115]. Firstly, Nrf-2 is essential for iron metabolism [116]. Nrf-2 positively regulates the transcription of heme oxygenase 1 (HMOX1), increases the storage of iron, and reduces intracellular free iron by rapidly upregulating the transcription of ferritin light chain (FLH) and ferritin heavy chain (FTH) [117]. Nrf-2 can control FTL/FTH, and the iron transporter (SLC40A1) is responsible for iron transport out of cells [[118], [119], [120]]. Secondly, Nrf-2 can participate in the catabolism/detoxification of reactive intermediates, such as AKR1B1, inhibiting the de novo synthesis of glutathione [121] and ALDH1A1, enhancing DNA repair [122]. In addition, Nrf-2 directly promotes the expression of GPX4, regulates the GCH1/BH4 pathway to mediate the cell redox reaction, and inhibits ferroptosis [123,124]. Studies have revealed that Nrf-2 can also mediate glutathione synthesis by promoting the expression of SLC7A11, glutamate cysteine ligase (GCLC/GLCM), and glutathione synthetase (GSS), which play crucial roles in preventing ferroptosis [[125], [126], [127]]. Therefore, Nrf-2 can activate downstream genes to regulate ferroptosis, and its target genes can be divided into three categories, as shown in Fig. 3.
Fig. 3.
Nrf-2 is involved in ferroptosis. Under stress-free conditions, Keap1 homodimers promote Nrf-2 ubiquitination. Under oxidative or electrophilic stress, the reactive cysteine residues in Keap1 are covalently modified, allowing Nrf-2 to dissociate from Keap1 and translocate to the nucleus, where it regulates ferroptosis through activation of the target genes by the antioxidant ARE.
Abbreviations: Nrf-2: nuclear factor erythroid 2-related factor 2; Keap1: Kelch ECH binding protein 1; sMAF: small Maf; ARE: antioxidant response element; Ub: Ubiquitination.
In addition, HIF-1α acts as a regulator of ferroptosis. The up-regulation of HIF-1α can buffer radiation-induced ROS and reduce ferroptosis, thereby enhancing the radioresistance of cells [128]. Nrf-2 is also involved in HIF-1α-mediated ferroptosis inhibition. Nrf-2 silencing blocks the accumulation of HIF-1α in hypoxic cancer cells, weakens its regulatory effect on cell metabolism, and leads to an imbalance of ROS homeostasis [129]. These results suggest that Nrf-2 can inhibit ferroptosis by regulating proteins associated with iron metabolism and ROS-scavenging pathways, thereby reducing radiation-induced oxidative stress and enhancing radiosensitivity.
5. Therapeutic potential of targeting of Nrf-2 in RILI
In conclusion, Nrf-2 is an essential predictor of RILI and may play an important role in preventing RILI. Nrf-2 and its target genes play a vital role in the development of RILI, and studies have suggested that inhibition of Nrf-2 activity may be a viable strategy for improving the radiation response and reducing radiation resistance in cancer [130]. Therefore, antioxidant therapy that activates Nrf-2 may be an effective intervention for preventing and treating RILI. Table 2 summarizes the common activators and inhibitors of Nrf-2 and their mechanisms of action.
Table 2.
Activators and inhibitors of Nrf-2.
| Compound | Mechanism of action | Reference | |
|---|---|---|---|
| Nrf-2 activators | Resveratrol | Modification of Keap1-Cys-151 | [94,131] |
| Sulforaphane | Modification of Keap1-Cys-151 | [98] | |
| Oltipraz | Modification of Keap1-Cys-151 | [132] | |
| Dimethyl fumarate | Modification of Keap1-Cys-151 | [133] | |
| CDDO; CDDO-Im | Modification of Keap1-Cys-151 | [134,135] | |
| Curcumin | Modification of Keap1-Cys-151 | [136] | |
| Diallyl trisulfide | Modification of Keap1-Cys-288 | [137] | |
| Apigenin | Epigenetic modifications of Nrf-2 | [138] | |
| Epigallocatechin-3-gallate | Oxidizing the cysteine thiols of Keap1 | [139] | |
| Nrf-2 inhibitors | Retinoic Acid | Prevention of nuclear translocation of Nrf-2 | [140,141] |
| Trigonelline | Prevention of nuclear translocation of Nrf-2 | [142] | |
| Chrysin | Prevention of nuclear translocation of Nrf-2 | [143] | |
| Luteolin | Nrf-2 mRNA degradation, Reduction of Nrf2 binding to AREs | [144] | |
| Brusatol | Stimulation of Nrf-2 poly-ubiquitination | [145] |
Abbreviations: Nrf-2: nuclear factor erythroid 2-related factor 2; Keap1: Kelch ECH binding protein 1; ARE: antioxidant response element; Cys: cysteine.
Antioxidants known to help prevent and treat RILI include thiol compounds, plant antioxidants, antioxidant enzymes, etc [146].The commonly used sulfhydryl compounds mainly include GSH and its precursor N-acetylcysteine (NAC). The primary mechanism is to use the active sulfhydryl group of its side chain to combine with free radicals and neutralize free radicals to protect the sulfhydryl group on its protein from oxidation. As mentioned above, Nrf-2 can regulate the expression of the downstream genes to mediate the synthesis of GSH from glutathione to regulate cellular ferroptosis; GSH can also be reduced to H2O by binding to H2O2 by the enzyme glutathione peroxidase to minimize oxidation. NAC, a precursor of GSH, can also act as a direct ROS scavenger and regulate the redox state of the cells [147]. Another study demonstrated that NAC may affect mucin expression and act as a mucolytic agent during oxidative stress and inflammation [148]. The application of NAC reportedly reduced sputum production in patients with RP, thereby reducing the use of expectorants [149].
At present, plant antioxidants have gradually gained attention in the prevention and treatment of RILI due to their advantages of comprehensive source, low side effects, and high patient acceptance, and have potential effects on radiosensitization of cancer cells and radiation protection of non-cancer cells [150]. Polyphenols in the diet, such as curcumin, resveratrol, and myrtol, can regulate the activation of Nrf-2 and biosynthesis of glutathione to remove ROS, thereby regulating inflammatory factors in macrophages and lung epithelial cells to play a therapeutic role [7,151,152]. Hesperidin and naringenin, antioxidants found in citrus peels and seeds, have been shown to significantly reduce the inflammatory response of the lung tissue after RT in rats and are potential therapeutic drugs for RILI [153,154].
The antioxidant enzyme system maintains the redox balance and protects against ROS, including heme oxygenase (HO-1), catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD). Increased levels of ROS activate Nrf-2 signaling, induce the expression of antioxidant enzymes, and protect cells from oxidative stress. At present, there are many studies on SOD in the field of RILI prevention and treatment, especially on SOD small-molecule analogs (AEOL 10150). Several studies have revealed that SOD can reduce lung injury caused by radiation and significantly improve survival rates of lung cancer patients [155,156]. Antonic et al. found that subcutaneous injection of 15 mg/kg bovine SOD 15 h after radiotherapy significantly improved the increased respiratory rate, pathological changes in the lung tissue, oxidative stress, macrophage activation, and TGF-β expression in rats [157]. Although this study verified that superoxide dismutase (SOD) can reduce reactive oxygen species (ROS) levels, the production of ROS may vary with the volume, dose, segmentation, course of treatment, and type of concurrent radiotherapy. If this study had included a comparison of the results at different administration times after radiotherapy, it would be of greater relevance.
Based on these results, we can infer that Nrf-2 promotes the expression of SOD in RILI, which is clinically significant since it can reduce the damage and inflammatory response of the normal lung tissue to ROS. These results indicate that Nrf-2 enhances the expression of SOD in RILI; this has clinical significance as it could reduce the adverse effects and inflammatory reactions caused by ROS in healthy lung tissue. Although Nrf-2 has significant therapeutic potential, somebody should adjust the timing and course of treatment with antioxidants to achieve absolute radiation protection. Therefore, the issues of individualization and standardization need to be further explored.
6. Perspective and conclusion
Injury to normal lung tissue is almost inevitable when cancer patients receive chest radiotherapy. The clinical needs for effective prevention or treatment of RILI have not been met. Therefore, it is necessary to consider previously unrecognized mechanisms of RILI to determine new effective therapies. The expression of Nrf-2 affects the recovery of radiation injury in a tissue-dependent manner. It has a specific protective effect on the skin, hematopoietic system, oral cavity, gastrointestinal tract, and lung. Especially in the lungs, Nrf-2 attenuates inflammatory response, oxidative stress, fibrosis, and cell death, ultimately having a protective effect against the development of lung diseases. It is considered an important target for clinical prevention of RILI.
At present, increasing evidence shows that targeting Nrf-2 may be very effective in the treatment or prevention of RILI. However, further standardized and large-scale clinical research evidence is needed to clarify the role of Nrf-2 in RILI after radiotherapy. In addition, although it has been determined that Nrf-2 is involved in the up-regulation pathway of inhibiting ROS production and down-regulating the expression of numerous genes, there is still a lack of understanding of the many downstream pathways mediated by Nrf-2; this hinders the transformation of preclinical evidence into clinical practice, providing suggestions for our further research ideas. Suppose somebody can control the expression or activity of Nrf-2 to increase radiotherapy's damage to tumor tissues and reduce the radiation damage to normal lung tissues. In that case, it will be of great clinical significance for treating and prognosis patients with thoracic tumors.
Funding
This work was supported by Hubei Province Natural Science Foundation of China (2022BCE038 to Jun Cai).
Ethics declarations
All participants provided informed consent to participate in the study.Review and/or approval by an ethics committee was not needed for this study because it does not directly involve humans or animals. It is a comprehensive analysis and summary of the existing literature.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
CRediT authorship contribution statement
Yuan-Yuan Chen: Visualization, Writing – original draft. Meng Wang: Investigation, Writing – original draft. Chen-Yang Zuo: Investigation. Meng-Xia Mao: Visualization. Xiao-Chun Peng: Supervision, Conceptualization, Writing – review & editing. Jun Cai: Project administration, Writing – review & editing, Funding acquisition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Xiao-Chun Peng, Email: pxcwd789@sina.com.
Jun Cai, Email: caijun0540@163.com.
References
- 1.Allaeys T., Berzenji L., Lauwers P., Yogeswaran S.K., Hendriks J.M.H., Billiet C., et al. Multimodality treatment including surgery related to the type of N2 involvement in locally advanced non-small cell lung cancer. Cancers. 2022;14(7) doi: 10.3390/cancers14071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayadi M., Zahra N., Thariat J., Bouilhol G., Boissard P., Van Houtte P., et al. [Intensity-modulated radiation therapy in non-small cell lung cancers] Cancer Radiother. 2014;18(5–6):406–413. doi: 10.1016/j.canrad.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Jin H., Yoo Y., Kim Y., Kim Y., Cho J., Lee Y.S. Radiation-induced lung fibrosis: preclinical animal models and therapeutic strategies. Cancers. 2020;12(6) doi: 10.3390/cancers12061561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolek V., Vašáková M., Šterclová M., Cwiertka K., Vrána D., Kudláček A., et al. [Radiotherapy of lung tumours in idiopathic pulmonary fibrosis] Klin. Onkol. 2017;30(4):303–306. doi: 10.14735/amko2017303. [DOI] [PubMed] [Google Scholar]
- 5.Kong F.M., Hayman J.A., Griffith K.A., Kalemkerian G.P., Arenberg D., Lyons S., et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(4):1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 6.Lee J., Jang J., Park S.M., Yang S.R. An update on the role of Nrf2 in respiratory disease: molecular mechanisms and therapeutic approaches. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao K., Lei X., Liu H., Zhao H., Guo J., Chen Y., et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial-mesenchymal transition. J. Cell Mol. Med. 2017;21(12):3264–3276. doi: 10.1111/jcmm.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J., Wang Y., Wang Z., Chen W., Luo M., Zhang C., et al. Near-infrared Nrf2 activator IR-61 dye alleviates radiation-induced lung injury. Free Radic. Res. 2022;56(5–6):411–426. doi: 10.1080/10715762.2022.2132942. [DOI] [PubMed] [Google Scholar]
- 9.Duru N., Gernapudi R., Zhang Y., Yao Y., Lo P.K., Wolfson B., et al. NRF2/miR-140 signaling confers radioprotection to human lung fibroblasts. Cancer Lett. 2015;369(1):184–191. doi: 10.1016/j.canlet.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., et al. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuranno L., Ient J., De Ruysscher D., Vooijs M.A. Radiation-induced lung injury (RILI) Front. Oncol. 2019;9:877. doi: 10.3389/fonc.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z., Zhou J., Verma V., Liu X., Wu M., Yu J., et al. Crossed pathways for radiation-induced and immunotherapy-related lung injury. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.774807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzam E.I., Jay-Gerin J.P., Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1–2):48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tak J.K., Park J.W. The use of ebselen for radioprotection in cultured cells and mice. Free Radic. Biol. Med. 2009;46(8):1177–1185. doi: 10.1016/j.freeradbiomed.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Najafi M., Motevaseli E., Shirazi A., Geraily G., Rezaeyan A., Norouzi F., et al. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int. J. Radiat. Biol. 2018;94(4):335–356. doi: 10.1080/09553002.2018.1440092. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y., Fu J., Kowalchuk R.O., Wright C.M., Zhang R., Li X., et al. Exploration of radiation-induced lung injury, from mechanism to treatment: a narrative review. Transl. Lung Cancer Res. 2022;11(2):307–322. doi: 10.21037/tlcr-22-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao H., Dong Z., Gong X., Dong J., Zhang Y., Wei W., et al. Effects of various radiation doses on induced T-helper cell differentiation and related cytokine secretion. J. Radiat. Res. 2018;59(4):395–403. doi: 10.1093/jrr/rry011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barthelemy-Brichant N., Bosquée L., Cataldo D., Corhay J.L., Gustin M., Seidel L., et al. Increased IL-6 and TGF-beta1 concentrations in bronchoalveolar lavage fluid associated with thoracic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):758–767. doi: 10.1016/s0360-3016(03)01614-6. [DOI] [PubMed] [Google Scholar]
- 19.Darby I.A., Hewitson T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007;257:143–179. doi: 10.1016/s0074-7696(07)57004-x. [DOI] [PubMed] [Google Scholar]
- 20.Fleckenstein K., Zgonjanin L., Chen L., Rabbani Z., Jackson I.L., Thrasher B., et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2007;68(1):196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S., Zhu J., Zhou P.K., Gu Y. Alveolar type 2 epithelial cell senescence and radiation-induced pulmonary fibrosis. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.999600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P., Yan Z., Zhou P.K., Gu Y. The promising therapeutic approaches for radiation-induced pulmonary fibrosis: targeting radiation-induced mesenchymal transition of alveolar type II epithelial cells. Int. J. Mol. Sci. 2022;23(23) doi: 10.3390/ijms232315014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Y., Lin Z., Lin X., Lu J., Wang N., Huang S., et al. IGFBP7 contributes to epithelial-mesenchymal transition of HPAEpiC cells in response to radiation. J. Cell. Biochem. 2019;120(8):12500–12507. doi: 10.1002/jcb.28516. [DOI] [PubMed] [Google Scholar]
- 24.Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallard A., Rancoule C., Le Floch H., Guy J.B., Espenel S., Le Péchoux C., et al. [Medical prevention and treatment of radiation-induced pulmonary complications] Cancer Radiother. 2017;21(5):411–423. doi: 10.1016/j.canrad.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi K., Yamamoto M. The KEAP1-NRF2 system in cancer. Front. Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M.Y., Lee D.Y., Chun K.S., Kim E.H. The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22094376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., Zhang J., Xue X., Zhao Y., Lu L., Cui M., et al. Theaflavin ameliorates ionizing radiation-induced hematopoietic injury via the NRF2 pathway. Free Radic. Biol. Med. 2017;113:59–70. doi: 10.1016/j.freeradbiomed.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Xue X., Han X., Yao C., Lu L., Li D., et al. Vam3 ameliorates total body irradiation-induced hematopoietic system injury partly by regulating the expression of Nrf2-targeted genes. Free Radic. Biol. Med. 2016;101:455–464. doi: 10.1016/j.freeradbiomed.2016.10.501. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.H., Thimmulappa R.K., Kumar V., Cui W., Kumar S., Kombairaju P., et al. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J. Clin. Invest. 2014;124(2):730–741. doi: 10.1172/jci70812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakamori S., Taguchi K., Nakayama Y., Ohkoshi A., Sporn M.B., Ogawa T., et al. Nrf2 protects against radiation-induced oral mucositis via antioxidation and keratin layer thickening. Free Radic. Biol. Med. 2022;188:206–220. doi: 10.1016/j.freeradbiomed.2022.06.239. [DOI] [PubMed] [Google Scholar]
- 32.Lu L., Jiang M., Zhu C., He J., Fan S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3'-Diindolylmethane (DIM) Free Radic. Biol. Med. 2019;130:244–255. doi: 10.1016/j.freeradbiomed.2018.10.410. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.B., Pandita R.K., Eskiocak U., Ly P., Kaisani A., Kumar R., et al. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. U. S. A. 2012;109(43):E2949–E2955. doi: 10.1073/pnas.1207718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X., Li Y., Yue L., Zhou X., Li J., Zhang Y., et al. Quercetin mitigates radiation-induced intestinal injury and promotes intestinal regeneration via nrf2-mediated antioxidant Pathway1. Radiat. Res. 2023;199(3):252–262. doi: 10.16667/rade-22-00090.1. [DOI] [PubMed] [Google Scholar]
- 35.Okunieff P., Xu J., Hu D., Liu W., Zhang L., Morrow G., et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(3):890–898. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Bian C., Qin W.J., Zhang C.Y., Zou G.L., Zhu Y.Z., Chen J., et al. Thalidomide (THD) alleviates radiation induced lung fibrosis (RILF) via down-regulation of TGF-β/Smad3 signaling pathway in an Nrf2-dependent manner. Free Radic. Biol. Med. 2018;129:446–453. doi: 10.1016/j.freeradbiomed.2018.10.423. [DOI] [PubMed] [Google Scholar]
- 37.Peng L.Y., An L., Sun N.Y., Ma Y., Zhang X.W., Liu W.H., et al. Salvia miltiorrhiza restrains reactive oxygen species-associated pulmonary fibrosis via targeting Nrf2-Nox4 redox balance. Am. J. Chin. Med. 2019;47(5):1113–1131. doi: 10.1142/s0192415x19500575. [DOI] [PubMed] [Google Scholar]
- 38.Pandey R., Singh M., Singhal U., Gupta K.B., Aggarwal S.K. Oxidative/Nitrosative stress and the pathobiology of chronic obstructive pulmonary disease. J. Clin. Diagn. Res. 2013;7(3):580–588. doi: 10.7860/jcdr/2013/4360.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantin A.M. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc. Am. Thorac. Soc. 2010;7(6):368–375. doi: 10.1513/pats.201001-014AW. [DOI] [PubMed] [Google Scholar]
- 40.Miglino N., Roth M., Tamm M., Borger P. Asthma and COPD - the C/EBP connection. Open Respir. Med. J. 2012;6:1–13. doi: 10.2174/1874306401206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki M., Betsuyaku T., Ito Y., Nagai K., Nasuhara Y., Kaga K., et al. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2008;39(6):673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 42.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W., et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114(9):1248–1259. doi: 10.1172/jci21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boutten A., Goven D., Artaud-Macari E., Boczkowski J., Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol. Med. 2011;17(7):363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L., Jian T., Wan Y., Huang R., Fang H., Wang Y., et al. Luteolin alleviates oxidative stress in chronic obstructive pulmonary disease induced by cigarette smoke via modulation of the TRPV1 and CYP2A13/NRF2 signaling pathways. Int. J. Mol. Sci. 2023;25(1) doi: 10.3390/ijms25010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan E.M., Sadiku P., Coelho P., Watts E.R., Zhang A., Howden A.J.M., et al. NRF2 activation reprograms defects in oxidative metabolism to restore macrophage function in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2023;207(8):998–1011. doi: 10.1164/rccm.202203-0482OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szefler S.J., Dakhama A. New insights into asthma pathogenesis and treatment. Curr. Opin. Immunol. 2011;23(6):801–807. doi: 10.1016/j.coi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Rangasamy T., Guo J., Mitzner W.A., Roman J., Singh A., Fryer A.D., et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005;202(1):47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J.H., Kim J.W., Lee C.M., Kim Y.D., Chung S.W., Jung I.D., et al. Sulforaphane inhibits the Th2 immune response in ovalbumin-induced asthma. BMB Rep. 2012;45(5):311–316. doi: 10.5483/bmbrep.2012.45.5.311. [DOI] [PubMed] [Google Scholar]
- 49.Brown R.H., Reynolds C., Brooker A., Talalay P., Fahey J.W. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir. Res. 2015;16(1):106. doi: 10.1186/s12931-015-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho H.Y., Reddy S.P., Yamamoto M., Kleeberger S.R. The transcription factor NRF2 protects against pulmonary fibrosis. Faseb j. 2004;18(11):1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 51.Sriram N., Kalayarasan S., Sudhandiran G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharmacol. Ther. 2009;22(3):221–236. doi: 10.1016/j.pupt.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Nie Y., Li J., Zhai X., Wang Z., Wang J., Wu Y., et al. Elamipretide(SS-31) attenuates idiopathic pulmonary fibrosis by inhibiting the nrf2-dependent NLRP3 inflammasome in macrophages. Antioxidants. 2023;12(12) doi: 10.3390/antiox12122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho H.Y., Jedlicka A.E., Reddy S.P., Kensler T.W., Yamamoto M., Zhang L.Y., et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002;26(2):175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 54.Sun Z., Niu Z., Wu S., Shan S. Protective mechanism of sulforaphane in Nrf2 and anti-lung injury in ARDS rabbits. Exp. Ther. Med. 2018;15(6):4911–4915. doi: 10.3892/etm.2018.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei J., Chen G., Shi X., Zhou H., Liu M., Chen Y., et al. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018;500(3):790–796. doi: 10.1016/j.bbrc.2018.04.161. [DOI] [PubMed] [Google Scholar]
- 56.Qiu Y.L., Cheng X.N., Bai F., Fang L.Y., Hu H.Z., Sun D.Q. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed. Pharmacother. 2018;106:192–199. doi: 10.1016/j.biopha.2018.05.070. [DOI] [PubMed] [Google Scholar]
- 57.Ivanciuc T., Sbrana E., Casola A., Garofalo R.P. Protective role of nuclear factor erythroid 2-related factor 2 against respiratory syncytial virus and human metapneumovirus infections. Front. Immunol. 2018;9:854. doi: 10.3389/fimmu.2018.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komaravelli N., Tian B., Ivanciuc T., Mautemps N., Brasier A.R., Garofalo R.P., et al. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic. Biol. Med. 2015;88(Pt B):391–403. doi: 10.1016/j.freeradbiomed.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho H.Y., Imani F., Miller-DeGraff L., Walters D., Melendi G.A., Yamamoto M., et al. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009;179(2):138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vomund S., Schäfer A., Parnham M.J., Brüne B., von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017;18(12) doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 62.Sajadimajd S., Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr. Cancer Drug Targets. 2018;18(6):538–557. doi: 10.2174/1568009617666171002144228. [DOI] [PubMed] [Google Scholar]
- 63.Chen B., Lu Y., Chen Y., Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015;225(3):R83–R99. doi: 10.1530/joe-14-0662. [DOI] [PubMed] [Google Scholar]
- 64.Traver G., Mont S., Gius D., Lawson W.E., Ding G.X., Sekhar K.R., et al. Loss of Nrf2 promotes alveolar type 2 cell loss in irradiated, fibrotic lung. Free Radic. Biol. Med. 2017;112:578–586. doi: 10.1016/j.freeradbiomed.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cameron B.D., Sekhar K.R., Ofori M., Freeman M.L. The role of Nrf2 in the response to normal tissue radiation injury. Radiat. Res. 2018;190(2):99–106. doi: 10.1667/rr15059.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong H., Xia Y., Jin S., Xue C., Wang Y., Hu R., et al. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. 2021;12(11):1027. doi: 10.1038/s41419-021-04307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong H., Qiang Z., Chai D., Peng J., Xia Y., Hu R., et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY) 2020;12(13):12943–12959. doi: 10.18632/aging.103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiang Z., Dong H., Xia Y., Chai D., Hu R., Jiang H. Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by regulating SLC7A11. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5146982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinkova-Kostova A.T., Kostov R.V., Canning P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017;617:84–93. doi: 10.1016/j.abb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sihvola V., Levonen A.L. Keap1 as the redox sensor of the antioxidant response. Arch. Biochem. Biophys. 2017;617:94–100. doi: 10.1016/j.abb.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 71.Kansanen E., Jyrkkänen H.K., Levonen A.L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012;52(6):973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 72.Shibata T., Ohta T., Tong K.I., Kokubu A., Odogawa R., Tsuta K., et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl. Acad. Sci. U. S. A. 2008;105(36):13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uruno A., Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25(2):153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Duan C., Wang H., Jiao D., Geng Y., Wu Q., Yan H., et al. Curcumin restrains oxidative stress of after intracerebral hemorrhage in rat by activating the Nrf2/HO-1 pathway. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.889226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Gene Cell. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 76.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U. S. A. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekhar K.R., Freeman M.L. Nrf2 promotes survival following exposure to ionizing radiation. Free Radic. Biol. Med. 2015;88(Pt B):268–274. doi: 10.1016/j.freeradbiomed.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., Xu C., Liu Q. Roles of NRF2 in DNA damage repair. Cell. Oncol. 2023;46(6):1577–1593. doi: 10.1007/s13402-023-00834-5. [DOI] [PubMed] [Google Scholar]
- 79.Singh B., Chatterjee A., Ronghe A.M., Bhat N.K., Bhat H.K. Antioxidant-mediated up-regulation of OGG1 via NRF2 induction is associated with inhibition of oxidative DNA damage in estrogen-induced breast cancer. BMC Cancer. 2013;13:253. doi: 10.1186/1471-2407-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhénaut A., Boiteux S., Radicella J.P. Characterization of the hOGG1 promoter and its expression during the cell cycle. Mutat. Res. 2000;461(2):109–118. doi: 10.1016/s0921-8777(00)00042-2. [DOI] [PubMed] [Google Scholar]
- 81.Boiteux S., Radicella J.P. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch. Biochem. Biophys. 2000;377(1):1–8. doi: 10.1006/abbi.2000.1773. [DOI] [PubMed] [Google Scholar]
- 82.Stewart G.S., Stankovic T., Byrd P.J., Wechsler T., Miller E.S., Huissoon A., et al. RIDDLE immunodeficiency syndrome is linked to defects in 53BP1-mediated DNA damage signaling. Proc. Natl. Acad. Sci. U. S. A. 2007;104(43):16910–16915. doi: 10.1073/pnas.0708408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakamura K., Sakai W., Kawamoto T., Bree R.T., Lowndes N.F., Takeda S., et al. Genetic dissection of vertebrate 53BP1: a major role in non-homologous end joining of DNA double strand breaks. DNA Repair. 2006;5(6):741–749. doi: 10.1016/j.dnarep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Iwabuchi K., Hashimoto M., Matsui T., Kurihara T., Shimizu H., Adachi N., et al. 53BP1 contributes to survival of cells irradiated with X-ray during G1 without Ku70 or Artemis. Gene Cell. 2006;11(8):935–948. doi: 10.1111/j.1365-2443.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 85.Shibata A., Jeggo P.A. Roles for 53BP1 in the repair of radiation-induced DNA double strand breaks. DNA Repair. 2020;93 doi: 10.1016/j.dnarep.2020.102915. [DOI] [PubMed] [Google Scholar]
- 86.Tian X., Wang F., Luo Y., Ma S., Zhang N., Sun Y., et al. Protective role of nuclear factor-erythroid 2-related factor 2 against radiation-induced lung injury and inflammation. Front. Oncol. 2018;8:542. doi: 10.3389/fonc.2018.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anuranjani Bala M. Concerted action of Nrf2-ARE pathway, MRN complex, HMGB1 and inflammatory cytokines - implication in modification of radiation damage. Redox Biol. 2014;2:832–846. doi: 10.1016/j.redox.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giridhar P., Mallick S., Rath G.K., Julka P.K. Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev. 2015;16(7):2613–2617. doi: 10.7314/apjcp.2015.16.7.2613. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Wei J., Deng H., Zheng L., Yang H., Lv X. The role of Nrf2 in pulmonary fibrosis: molecular mechanisms and treatment approaches. Antioxidants. 2022;11(9) doi: 10.3390/antiox11091685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hiebert P., Werner S. Targeting NRF2 to promote epithelial repair. Biochem. Soc. Trans. 2023;51(1):101–111. doi: 10.1042/bst20220228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sisson T.H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;181(3):254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xi Y., Kim T., Brumwell A.N., Driver I.H., Wei Y., Tan V., et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 2017;19(8):904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Artaud-Macari E., Goven D., Brayer S., Hamimi A., Besnard V., Marchal-Somme J., et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18(1):66–79. doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 94.Zeng H., Wang Y., Gu Y., Wang J., Zhang H., Gao H., et al. Polydatin attenuates reactive oxygen species-induced airway remodeling by promoting Nrf2-mediated antioxidant signaling in asthma mouse model. Life Sci. 2019;218:25–30. doi: 10.1016/j.lfs.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Wang T., Dai F., Li G.H., Chen X.M., Li Y.R., Wang S.Q., et al. Trans-4,4'-dihydroxystilbene ameliorates cigarette smoke-induced progression of chronic obstructive pulmonary disease via inhibiting oxidative stress and inflammatory response. Free Radic. Biol. Med. 2020;152:525–539. doi: 10.1016/j.freeradbiomed.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z., Qu J., Zheng C., Zhang P., Zhou W., Cui W., et al. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 2018;9(2):83. doi: 10.1038/s41419-017-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Travis E.L., Rachakonda G., Zhou X., Korhonen K., Sekhar K.R., Biswas S., et al. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic. Biol. Med. 2011;51(6):1175–1183. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oh C.J., Kim J.Y., Min A.K., Park K.G., Harris R.A., Kim H.J., et al. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic. Biol. Med. 2012;52(3):671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michaeloudes C., Chang P.J., Petrou M., Chung K.F. Transforming growth factor-β and nuclear factor E2–related factor 2 regulate antioxidant responses in airway smooth muscle cells: role in asthma. Am. J. Respir. Crit. Care Med. 2011;184(8):894–903. doi: 10.1164/rccm.201011-1780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou W., Mo X., Cui W., Zhang Z., Li D., Li L., et al. Nrf2 inhibits epithelial-mesenchymal transition by suppressing snail expression during pulmonary fibrosis. Sci. Rep. 2016;6 doi: 10.1038/srep38646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qu J., Zhang Z., Zhang P., Zheng C., Zhou W., Cui W., et al. Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF. J. Cell. Physiol. 2019;234(6):8862–8872. doi: 10.1002/jcp.27548. [DOI] [PubMed] [Google Scholar]
- 103.Feng F., Cheng P., Xu S., Li N., Wang H., Zhang Y., et al. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via Nrf2-mediated inhibition of EMT and TGF-β1/Smad signaling. Chem. Biol. Interact. 2020;319 doi: 10.1016/j.cbi.2020.109024. [DOI] [PubMed] [Google Scholar]
- 104.Ding Z., Wu X., Wang Y., Ji S., Zhang W., Kang J., et al. Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3β/Nrf2 pathway. Biomed. Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110827. [DOI] [PubMed] [Google Scholar]
- 105.Mont S., Davies S.S., Roberts Second L.J., Mernaugh R.L., McDonald W.H., Segal B.H., et al. Accumulation of isolevuglandin-modified protein in normal and fibrotic lung. Sci. Rep. 2016;6 doi: 10.1038/srep24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lang X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.cd-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu J., Xiong Y., Zhang Y., Wen J., Cai N., Cheng K., et al. The molecular mechanisms of regulating oxidative stress-induced ferroptosis and therapeutic strategy in tumors. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8810785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X., Zhuang X., Qiao T. Role of ferroptosis in the process of acute radiation-induced lung injury in mice. Biochem. Biophys. Res. Commun. 2019;519(2):240–245. doi: 10.1016/j.bbrc.2019.08.165. [DOI] [PubMed] [Google Scholar]
- 110.Li X., Duan L., Yuan S., Zhuang X., Qiao T., He J. Ferroptosis inhibitor alleviates Radiation-induced lung fibrosis (RILF) via down-regulation of TGF-β1. J. Inflamm. 2019;16:11. doi: 10.1186/s12950-019-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Z., Lu M., Chen C., Tong X., Li Y., Yang K., et al. Holo-lactoferrin: the link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics. 2021;11(7):3167–3182. doi: 10.7150/thno.52028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ling H., Xiao H., Luo T., Lin H., Deng J. Role of ferroptosis in regulating the epithelial-mesenchymal transition in pulmonary fibrosis. Biomedicines. 2023;11(1) doi: 10.3390/biomedicines11010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun L., He X., Kong J., Yu H., Wang Y. Menstrual blood-derived stem cells exosomal miR-let-7 to ameliorate pulmonary fibrosis through inhibiting ferroptosis by Sp3/HDAC2/Nrf2 signaling pathway. Int Immunopharmacol. 2024;126 doi: 10.1016/j.intimp.2023.111316. [DOI] [PubMed] [Google Scholar]
- 114.Shakya A., McKee N.W., Dodson M., Chapman E., Zhang D.D. Anti-ferroptotic effects of Nrf2: beyond the antioxidant response. Mol Cells. 2023;46(3):165–175. doi: 10.14348/molcells.2023.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23 doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duarte T.L., Talbot N.P., Drakesmith H. NRF2 and hypoxia-inducible factors: key players in the redox control of systemic iron homeostasis. Antioxid Redox Signal. 2021;35(6):433–452. doi: 10.1089/ars.2020.8148. [DOI] [PubMed] [Google Scholar]
- 117.Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274(37):26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 118.Agyeman A.S., Chaerkady R., Shaw P.G., Davidson N.E., Visvanathan K., Pandey A., et al. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012;132(1):175–187. doi: 10.1007/s10549-011-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harada N., Kanayama M., Maruyama A., Yoshida A., Tazumi K., Hosoya T., et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch. Biochem. Biophys. 2011;508(1):101–109. doi: 10.1016/j.abb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 120.Marro S., Chiabrando D., Messana E., Stolte J., Turco E., Tolosano E., et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95(8):1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang K.R., Zhang Y.F., Lei H.M., Tang Y.B., Ma C.S., Lv Q.M., et al. Targeting AKR1B1 inhibits glutathione de novo synthesis to overcome acquired resistance to EGFR-targeted therapy in lung cancer. Sci. Transl. Med. 2021;13(614) doi: 10.1126/scitranslmed.abg6428. [DOI] [PubMed] [Google Scholar]
- 122.Liu L., Cai S., Han C., Banerjee A., Wu D., Cui T., et al. ALDH1A1 contributes to PARP inhibitor resistance via enhancing DNA repair in BRCA2(-/-) ovarian cancer cells. Mol Cancer Ther. 2020;19(1):199–210. doi: 10.1158/1535-7163.mct-19-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xue J., Yu C., Sheng W., Zhu W., Luo J., Zhang Q., et al. The nrf2/GCH1/BH4 Axis ameliorates radiation-induced skin injury by modulating the ROS cascade. J. Invest. Dermatol. 2017;137(10):2059–2068. doi: 10.1016/j.jid.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 124.Zhu K., Zhu X., Liu S., Yu J., Wu S., Hei M. Glycyrrhizin attenuates hypoxic-ischemic brain damage by inhibiting ferroptosis and neuroinflammation in neonatal rats via the HMGB1/GPX4 pathway. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8438528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan J.Y., Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta. 2000;1517(1):19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 126.Kwak M.K., Itoh K., Yamamoto M., Kensler T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell Biol. 2002;22(9):2883–2892. doi: 10.1128/mcb.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li M., Chiu J.F., Kelsen A., Lu S.C., Fukagawa N.K. Identification and characterization of an Nrf2-mediated ARE upstream of the rat glutamate cysteine ligase catalytic subunit gene (GCLC) J. Cell. Biochem. 2009;107(5):944–954. doi: 10.1002/jcb.22197. [DOI] [PubMed] [Google Scholar]
- 128.Semenza G.L. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004;5(5):405–406. doi: 10.1016/s1535-6108(04)00118-7. [DOI] [PubMed] [Google Scholar]
- 129.Kim T.H., Hur E.G., Kang S.J., Kim J.A., Thapa D., Lee Y.M., et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Cancer Res. 2011;71(6):2260–2275. doi: 10.1158/0008-5472.can-10-3007. [DOI] [PubMed] [Google Scholar]
- 130.Zhou S., Ye W., Shao Q., Zhang M., Liang J. Nrf2 is a potential therapeutic target in radioresistance in human cancer. Crit. Rev. Oncol. Hematol. 2013;88(3):706–715. doi: 10.1016/j.critrevonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 131.Alavi M., Farkhondeh T., Aschner M., Samarghandian S. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int. 2021;21(1):579. doi: 10.1186/s12935-021-02280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu Z., Shao W., Chiang Y., Foltz W., Zhang Z., Ling W., et al. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia. 2011;54(4):922–934. doi: 10.1007/s00125-010-2001-8. [DOI] [PubMed] [Google Scholar]
- 133.Scannevin R.H., Chollate S., Jung M.Y., Shackett M., Patel H., Bista P., et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341(1):274–284. doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]
- 134.Cao M., Onyango E.O., Williams C.R., Royce D.B., Gribble G.W., Sporn M.B., et al. Novel synthetic pyridyl analogues of CDDO-Imidazolide are useful new tools in cancer prevention. Pharmacol. Res. 2015;100:135–147. doi: 10.1016/j.phrs.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 135.Liby K., Hock T., Yore M.M., Suh N., Place A.E., Risingsong R., et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65(11):4789–4798. doi: 10.1158/0008-5472.can-04-4539. [DOI] [PubMed] [Google Scholar]
- 136.Khor T.O., Huang Y., Wu T.Y., Shu L., Lee J., Kong A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82(9):1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 137.Tsai C.Y., Wang C.C., Lai T.Y., Tsu H.N., Wang C.H., Liang H.Y., et al. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013;168(2):1286–1297. doi: 10.1016/j.ijcard.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 138.Paredes-Gonzalez X., Fuentes F., Su Z.Y., Kong A.N. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. AAPS J. 2014;16(4):727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Na H.K., Surh Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008;46(4):1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 140.Magesh S., Chen Y., Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012;32(4):687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu J., Wang H., Chen F., Fu J., Xu Y., Hou Y., et al. An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy. Free Radic. Biol. Med. 2016;99:544–556. doi: 10.1016/j.freeradbiomed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 142.Qin W., Guan D., Ma R., Yang R., Xing G., Shi H., et al. Effects of trigonelline inhibition of the Nrf2 transcription factor in vitro on Echinococcus granulosus. Acta Biochim. Biophys. Sin. 2017;49(8):696–705. doi: 10.1093/abbs/gmx067. [DOI] [PubMed] [Google Scholar]
- 143.Gao A.M., Ke Z.P., Shi F., Sun G.C., Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013;206(1):100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 144.Chian S., Thapa R., Chi Z., Wang X.J., Tang X. Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochem. Biophys. Res. Commun. 2014;447(4):602–608. doi: 10.1016/j.bbrc.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 145.Ren D., Villeneuve N.F., Jiang T., Wu T., Lau A., Toppin H.A., et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. U. S. A. 2011;108(4):1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Y., Song Q., Yao Y., Dong Y., Gao Y., Wu B. [Progression of anti-oxygen therapy in radiation-induced lung injury] Zhongguo Fei Ai Za Zhi. 2019;22(9):579–582. doi: 10.3779/j.issn.1009-3419.2019.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sadowska A.M., Manuel Y.K.B., De Backer W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm. Pharmacol. Ther. 2007;20(1):9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 148.Sadowska A.M. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2012;6(3):127–135. doi: 10.1177/1753465812437563. [DOI] [PubMed] [Google Scholar]
- 149.Han D.W., Ji W., Lee J.C., Song S.Y., Choi C.M. Efficacy of nebulized acetylcysteine for relieving symptoms and reducing usage of expectorants in patients with radiation pneumonitis. Thorac Cancer. 2019;10(2):243–248. doi: 10.1111/1759-7714.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang J.Y., Chuang Y.T., Shiau J.P., Yen C.Y., Chang F.R., Tsai Y.H., et al. Connection between radiation-regulating functions of natural products and miRNAs targeting radiomodulation and exosome biogenesis. Int. J. Mol. Sci. 2023;24(15) doi: 10.3390/ijms241512449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72(11):1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 152.Zhao D.Y., Qu H.J., Guo J.M., Zhao H.N., Yang Y.Y., Zhang P., et al. Protective effects of myrtol standardized against radiation-induced lung injury. Cell. Physiol. Biochem. 2016;38(2):619–634. doi: 10.1159/000438655. [DOI] [PubMed] [Google Scholar]
- 153.Haddadi G.H., Rezaeyan A., Mosleh-Shirazi M.A., Hosseinzadeh M., Fardid R., Najafi M., et al. Hesperidin as radioprotector against radiation-induced lung damage in rat: a histopathological study. J. Med. Phys. 2017;42(1):25–32. doi: 10.4103/jmp.JMP_119_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang C., Zeng W., Yao Y., Xu B., Wei X., Wang L., et al. Naringenin ameliorates radiation-induced lung injury by lowering IL-1β level. J Pharmacol Exp Ther. 2018;366(2):341–348. doi: 10.1124/jpet.118.248807. [DOI] [PubMed] [Google Scholar]
- 155.MacVittie T.J., Gibbs A., Farese A.M., Barrow K., Bennett A., Taylor-Howell C., et al. AEOL 10150 mitigates radiation-induced lung injury in the nonhuman primate: morbidity and mortality are administration schedule-dependent. Radiat. Res. 2017;187(3):298–318. doi: 10.1667/rr4413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rabbani Z.N., Batinic-Haberle I., Anscher M.S., Huang J., Day B.J., Alexander E., et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int. J. Radiat. Oncol. Biol. Phys. 2007;67(2):573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Antonic V., Rabbani Z.N., Jackson I.L., Vujaskovic Z. Subcutaneous administration of bovine superoxide dismutase protects lungs from radiation-induced lung injury. Free Radic. Res. 2015;49(10):1259–1268. doi: 10.3109/10715762.2015.1066501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.