Key Points
Question
Is administration of antenatal corticosteroids to individuals at risk of late preterm delivery, originally shown to improve short-term neonatal respiratory outcomes but with an increased rate of hypoglycemia, associated with adverse childhood neurodevelopmental outcomes at age 6 years or older?
Findings
There were no statistically significant differences in the primary outcome, a General Conceptual Abilities score of less than 85, between the betamethasone (17.1%) and placebo (18.5%) groups. No differences in any secondary outcomes were observed.
Meaning
In this follow-up study of a randomized clinical trial, antenatal corticosteroids in persons at risk of late preterm delivery were not associated with adverse effects on childhood neurodevelopmental outcomes at age 6 years or older.
Abstract
Importance
The Antenatal Late Preterm Steroids (ALPS) trial changed clinical practice in the United States by finding that antenatal betamethasone at 34 to 36 weeks decreased short-term neonatal respiratory morbidity. However, the trial also found increased risk of neonatal hypoglycemia after betamethasone. This follow-up study focused on long-term neurodevelopmental outcomes after late preterm steroids.
Objective
To evaluate whether administration of late preterm (34-36 completed weeks) corticosteroids affected childhood neurodevelopmental outcomes.
Design, Setting, and Participants
Prospective follow-up study of children aged 6 years or older whose birthing parent had enrolled in the multicenter randomized clinical trial, conducted at 13 centers that participated in the Maternal-Fetal Medicine Units (MFMU) Network cycle from 2011-2016. Follow-up was from 2017-2022.
Exposure
Twelve milligrams of intramuscular betamethasone administered twice 24 hours apart.
Main Outcome and Measures
The primary outcome of this follow-up study was a General Conceptual Ability score less than 85 (−1 SD) on the Differential Ability Scales, 2nd Edition (DAS-II). Secondary outcomes included the Gross Motor Function Classification System level and Social Responsiveness Scale and Child Behavior Checklist scores. Multivariable analyses adjusted for prespecified variables known to be associated with the primary outcome. Sensitivity analyses used inverse probability weighting and also modeled the outcome for those lost to follow-up.
Results
Of 2831 children, 1026 enrolled and 949 (479 betamethasone, 470 placebo) completed the DAS-II at a median age of 7 years (IQR, 6.6-7.6 years). Maternal, neonatal, and childhood characteristics were similar between groups except that neonatal hypoglycemia was more common in the betamethasone group. There were no differences in the primary outcome, a general conceptual ability score less than 85, which occurred in 82 (17.1%) of the betamethasone vs 87 (18.5%) of the placebo group (adjusted relative risk, 0.94; 95% CI, 0.73-1.22). No differences in secondary outcomes were observed. Sensitivity analyses using inverse probability weighting or assigning outcomes to children lost to follow-up also found no differences between groups.
Conclusion and Relevance
In this follow-up study of a randomized clinical trial, administration of antenatal corticosteroids to persons at risk of late preterm delivery, originally shown to improve short-term neonatal respiratory outcomes but with an increased rate of hypoglycemia, was not associated with adverse childhood neurodevelopmental outcomes at age 6 years or older.
This prospective follow-up study of a randomized clinical trial evaluates whether antenatal corticosteroids administered to birthing parents at risk of late preterm delivery were associated with adverse neurodevelopmental effects on their offspring.
Introduction
The Antenatal Late Preterm Steroids randomized trial noted decreased short-term neonatal respiratory morbidity after antenatal exposure to betamethasone compared with placebo.1 The trial also found an increased risk of neonatal hypoglycemia after exposure to betamethasone. Prolonged and persistent hypoglycemia has been associated with adverse childhood neurodevelopment,2 but there is no consensus on a safe range of neonatal blood glucose concentrations.3
Preclinical models as early as the 1990s have suggested adverse effects of corticosteroids on fetal brain development,4,5 and follow-up studies on multiple courses of antenatal corticosteroids have suggested potential adverse childhood neurodevelopment, particularly with more than 4 courses.6,7 However, these findings have not been corroborated in humans exposed to a single course of antenatal corticosteroids8 or to late preterm steroid exposure,9 although this distinction has not always been clear in reviews on potential steroid risks.10 The findings of the ALPS trial reignited an interest in childhood neurodevelopment following antenatal steroid exposure, with subsequent observational studies suggesting adverse cognitive outcomes after some period of antenatal glucocorticoid exposure.11,12 Because of a benefit to steroid administration, which subsequently has been incorporated into clinical practice in many centers,13,14 this study was designed to evaluate whether administration of late preterm (34-36 weeks) corticosteroids affected childhood neurodevelopmental outcomes at age 6 years or older. Based on prior human data,8,9,15 it was hypothesized that children exposed to antenatal betamethasone would have a lower frequency of cognitive function 1 SD below the mean at age 6 years or older compared with children exposed to placebo.
Methods
The investigators have adhered to the US Department of Health and Human Services policies for protection of human subjects as prescribed in 45 CFR 46.
This was a prospective, follow-up study of the children of the participants in the trial,1 a multicenter, double-blind, placebo-controlled trial conducted at 17 Maternal-Fetal Medicine Units (MFMU) Network centers of the National Institute of Child Health and Human Development from 2010 to 2015, over 2 different MFMU funding cycles (see the study protocol in Supplement 1). Participants were eligible if they were at high risk of preterm delivery from 34 weeks, 0 days to 36 weeks, 6 days, based on specific previously reported criteria.1 Consenting participants were randomly assigned to receive 12 mg of intramuscular betamethasone for fetal lung maturity. A second, final dose was given if the participant had not delivered at 24 hours. During the parent trial, all participants were given the option to consent for future research.
Children of former participants were eligible if they were 6 years or older and if the original participant (1) enrolled at 1 of the 13 centers that participated in the MFMU Network in the 5-year cycle from 2011 to 2016 and (2) had agreed to future contact. Research staff received center-specific lists of eligible participants and used a multimodal approach (phone, email, social media, cards) to contact them. A website was created to provide information about the follow-up study to parent trial participants, and the web address was included in cards mailed to potential participants. Children were ineligible if they were unable to participate in a study visit or if their family declined future contact or participation. Medical records were reviewed for all excluded children whether the exclusion was due to death or custody issues or for the presence of any neurocognitive conditions. The parent or guardian provided signed consent. The child provided either verbal or signed assent, as required by the local centers’ institutional review board. Baseline demographics were derived both from the parent trial and the follow-up study. Birthing parent race and ethnicity were assigned by self-report, and the child’s race and ethnicity were based on parent or guardian report.
Enrolled children participated in cognitive testing performed by a licensed psychologist certified in the study procedures. The evaluation included the Differential Ability Scales, 2nd Edition (DAS-II),16 which reports an overall General Conceptual Ability (GCA) score and cluster scores including verbal, nonverbal reasoning, and spatial ability. The DAS-II measures cognitive function and the GCA correlates well (ρ = 0.89) with the full scale IQ measured by the Wechsler Preschool and Primary Scale of Intelligence.17 To be certified, psychologists recorded themselves administering the DAS-II to a child between the ages of 5 years, 11 months and 7 years, 11 months. The video as well as a self-assessment evaluating their administration was sent for central review and approval by the study criterion standard examiner prior to initiating study examinations on participants. Study visits were performed at the clinical center, the participant’s home, or other arranged location. Additionally, the Gross Motor Function Classification System was assessed by certified staff.18
The parent or guardian completed 3 questionnaires related to child health and behavior including the Social Responsiveness Scale, 2nd Edition,19 which assesses social abilities and characteristic traits of autism spectrum disorder, the Child Behavior Checklist for ages 6 through 18 years,20 which assesses behavioral and emotional problems in children, and additional questions about the child’s health. Guardians, research staff, and investigators remained blinded to treatment assignment.
Study Outcomes
The DAS-II GCA and cluster age–standardized scores have a mean (SD) of 100 (15). Higher scores reflect greater abilities. The primary outcome of this follow-up study, the proportion of GCA scores of less than 85, represents 1 SD below the mean. Secondary outcomes included the cluster scores of the DAS-II (verbal ability, nonverbal reasoning ability, and spatial ability); the Gross Motor Function Classification System level greater than level 1, indicating a functional impairment equal to or greater than the inability to walk without limitations; the Social Responsiveness Scale t score of more than 65, representing moderate to severe impairment in reciprocal social behavior indicative of autistic traits; and Child Behavior Checklist scores. Child Behavior Checklist t scores for the summary measures and the number and percent within the score range (normal, borderline, clinical) were reported, where higher scores indicate greater problems. Values on syndrome scales (anxious or depressed, attention problems, and aggressive behavior) between the 93rd and 97th percentile are classified as borderline, and higher than the 97th percentile as clinical, indicating a potential need for referral. Values for internalizing behavior, externalizing behavior, and total problems between the 84th and 90th percentile are classified as borderline, and higher than the 90th as clinical.
Statistical Analysis
Baseline characteristics were compared between participants and their birthing parents who were and were not enrolled in the follow-up study and by original treatment assignment among those enrolled. Log-binomial regression was used to estimate relative risks and 95% CIs for categorical outcomes. Quantile regression was used to estimate median differences and 95% CIs for nonnormally distributed continuous outcomes and general linear models were used to estimate mean differences and 95% CIs for normally distributed outcomes. Analyses were adjusted for prespecified baseline characteristics known to be associated with the primary outcome (the birthing parent’s age and educational level, gestational age at delivery, and child sex and age at study visit) or to account for potential variation in clinical care and study population (study center). A post hoc subgroup analysis of the primary outcome among children born preterm (<37 weeks’ gestational age) and term was conducted. Children who consented for the study and either declined the DAS-II or had invalid scores due to examiner error were considered lost to follow-up.
Several sensitivity analyses were performed. The first included participants lost to follow-up with different assumptions regarding the primary outcome (either all had the primary outcome or all did not have the primary outcome). The other sensitivity analysis included patients who were excluded or refused to participate due to a neuromotor, neurological, cognitive, or behavioral condition and assumed that all patients with one of these conditions had the primary outcome.
An additional sensitivity analysis was conducted post hoc at the request of the editors. Logistic regression was used to calculate the probability of enrollment in the follow-up study for characteristics that significantly differed by inclusion in the study. Only characteristics that were not missing in the full cohort were used to calculate the weights. The probability of enrollment was then used to calculate inverse probability weights, and the analyses were rerun using the weighted cohort. Bootstrapping was used to construct 95% CIs for relative risks (RRs) and mean differences. All analyses were conducted using SAS statistical software version 9.4 (SAS Institute Inc).
Sample Size Considerations
The parent trial included 2831 birthing parents and their infants, of which 186 (6.6%) declined future contact, 209 (7.4%) were from centers no longer participating in the MFMU Network, 9 (0.32%) had infant or neonatal deaths, 4 (0.14%) were lost to follow-up in the parent trial, and 1 (0.04%) had a maternal death (Figure). This left 2422 potentially eligible participants. The sample size was estimated based on prior experience of enrolling 80% of eligible children when the follow-up was not preplanned.21 Using these data, we planned to enroll 2000 participants, providing 83% power to detect a 25% reduction (ie, from 20% in the placebo group to 15% in the betamethasone group) in the proportion of children with a GCA score of less than 85 in the betamethasone group.
Figure. Antenatal Late Preterm Steroids (ALPS) Follow-Up Flow Diagram.
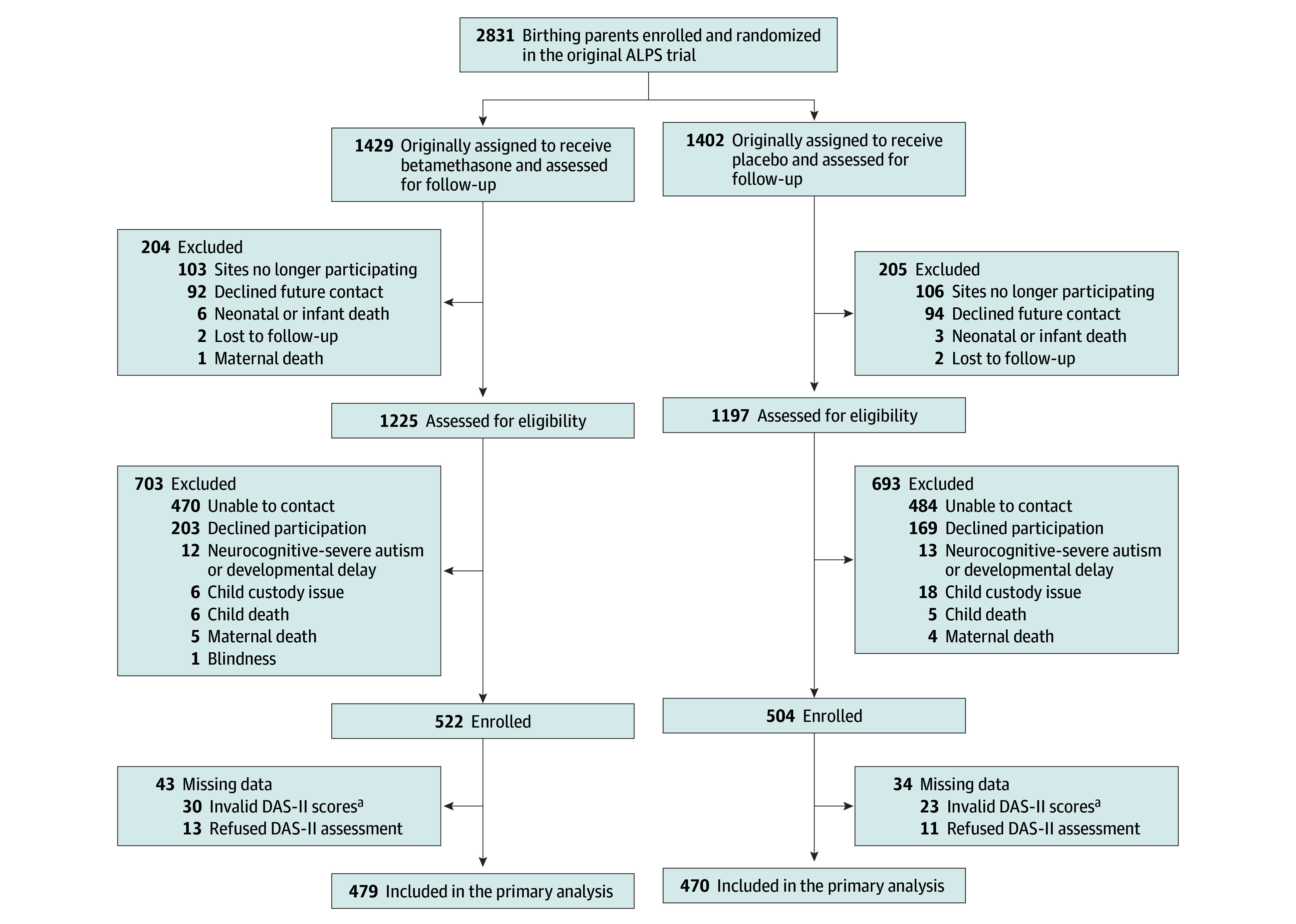
aThe Differential Ability Scales, 2nd Edition (DAS-II) General Conceptual Ability score has a mean (SD) of 100 (15); a score of at least 85 is average, and less than 85 is below average, with higher scores indicating greater abilities. Invalid DAS-II scores include 20 examinations that were performed by a noncertified examiner and 33 with errors in administration involving incorrect start and stop points.
Results
Of 2422 potentially eligible participants from the parent trial, 1396 were excluded, mostly because of an inability to contact or because they declined participation (Figure). The birthing parents of children who participated in the follow-up study were older; less likely to smoke during pregnancy; and more likely to be employed, have private insurance, and a college degree. Otherwise, they were similar in most characteristics (eTable 1 in Supplement 2). There were 25 participants excluded for severe autism or developmental delay, 12 (0.84%) in the betamethasone group and 13 (0.93%) in the placebo group. Deaths were also similar between groups (eTable 2 in Supplement 2). Of the 1026 enrolled between December 2017 and August 2022, there were 522 in the betamethasone group and 504 in the placebo group (Figure).
Of those included in this follow-up study, maternal and neonatal characteristics for those in the betamethasone group were similar to those in the placebo group, aside from maternal race, neonatal hypoglycemia, and major congenital anomaly (Table 1). Notably, neonatal hypoglycemia was more common in the betamethasone group, similar to the parent trial. Children enrolled in the follow-up study were similar by treatment assignment aside from race, with the placebo group having a higher proportion of Black children (Table 1). The median child age at the study visit was 7 years in each group: 7 (IQR, 6.5-7.7) years in the betamethasone group and 7 (IQR, 6.5-7.6) years in the placebo group (Table 1).
Table 1. Maternal and Child Characteristics by Treatment Assignment for Included Follow-Up Participants.
| Characteristic | No. (%) | |
|---|---|---|
| Betamethasone (n = 522) | Placebo (n = 504) | |
| Maternal | ||
| Race and ethnicitya | ||
| American Indian or Alaska Native | 2 (0.4) | 0 |
| Asian | 21 (4.0) | 11 (2.2) |
| Hispanic | 147 (28.2) | 162 (32.1) |
| Native Hawaiian or Pacific Islander | 3 (0.6) | 0 |
| Non-Hispanic Black | 124 (23.8) | 134 (26.6) |
| Non-Hispanic White | 222 (42.5) | 195 (38.7) |
| ≥ 1 Self-reported race | 0 | 2 (0.4) |
| Unknown or not reported | 3 (0.6) | 0 |
| Age, mean (SD), y | 29 (6.3) | 29 (6.1) |
| ≥College degree (maternal), No./total (%) | 192/517 (37.1) | 187/503 (37.2) |
| Employed at randomization, No./total (%) | 217/521 (41.7) | 228/503 (45.3) |
| No. of prior pregnancies ≥20 wk | ||
| 0 | 209 (40.0) | 208 (41.3) |
| 1 | 131 (25.1) | 146 (29.0) |
| ≥2 | 182 (34.9) | 150 (29.8) |
| Smoked during pregnancy | 55 (10.5) | 53 (10.5) |
| Preeclampsia or gestational hypertension | 168 (32.2) | 157 (31.2) |
| Gestational diabetes | 54 (10.3) | 59 (11.7) |
| Indication for trial entry | ||
| Preterm labor with intact membranes | 143 (27.4) | 146 (29.0) |
| Ruptured membranes | 119 (22.8) | 108 (21.4) |
| Expected delivery | ||
| Gestational hypertension or preeclampsia | 150 (28.7) | 126 (25.0) |
| Fetal growth restriction | 13 (2.5) | 17 (3.4) |
| Oligohydramnios | 15 (2.9) | 10 (2.0) |
| Other indication | 82 (15.7) | 97 (19.2) |
| Gestational age at delivery, median (IQR), wk | 36 (35.4-36.7) | 36 (35.3-36.7) |
| Delivery ≥ 37 wk | 86 (16.5) | 83 (16.5) |
| Neonatal | ||
| Small for gestational age (<10th percentile) | 90 (17.2) | 79 (15.7) |
| Hypoglycemia, No./total (%) | 144/519 (27.7) | 90/501 (18.0) |
| Major congenital anomalyb | 1 (0.2) | 7 (1.4) |
| Enrolled child | (n = 521) | (n = 504) |
| Race and ethnicitya | ||
| American Indian or Alaska Native | 0 | 0 |
| Asian | 14 (2.7) | 8 (1.6) |
| Hispanic | 174 (33.3) | 174 (34.5) |
| Native Hawaiian or Pacific Islander | 2 (0.4) | 0 |
| Non-Hispanic Black | 112 (21.5) | 127 (25.2) |
| Non-Hispanic White | 197 (37.7) | 185 (36.7) |
| ≥ 1 Self-reported race | 22 (4.2) | 10 (2.0) |
| Unknown or not reported | 0 | 0 |
| Age, median (IQR), y | 7 (6.5-7.7) | 7 (6.5-7.6) |
| Sex | ||
| Female | 233 (44.6) | 237 (47.0) |
| Male | 289 (55.4) | 267 (53.0) |
| Private insurance, No./total (%)c | 229/521 (44.0) | 220/504 (43.7) |
Maternal race and ethnicity were based on self-report; the child’s race and ethnicity on parent or guardian report.
Although the presence of a major congenital anomaly was an exclusion criterion in the parent trial, these disorders were not discovered until birth.
Those without private insurance have government-assisted insurance or are self-pay or uninsured.
Of the 1026 enrolled, 949 had valid scores on the DAS-II. Of the 77 who did not, 24 declined to complete the assessment after enrollment, 20 examinations were performed by a noncertified examiner, and 33 had invalid scores due to errors in administration involving incorrect start and stop points. The use of different start and/or stop points results in inconsistencies in scoring, so these examinations were considered invalid. The proportion with the primary outcome, a GCA score less than 85, was similar between groups: 82 of 479 (17.1%) in the betamethasone group and 87 of 470 (18.5%) in the placebo group (adjusted RR, 0.94 [95% CI, 0.73-1.22]). The mean (SD) GCA score was also similar between groups: 96.6 (13.8) for the betamethasone group and 96.6 (13.7) for the placebo group (adjusted mean difference, 0.00 [95% CI, −1.47 to 1.47]). There were no differences in any of the secondary outcomes (Table 2).
Table 2. Primary and Secondary Outcomes.
| Outcome | No. (%) | Relative risk (95% CI)a | ||
|---|---|---|---|---|
| Betamethasone (n = 479) | Placebo (n = 470) | Unadjusted | Adjustedb | |
| Primary outcome | ||||
| DAS-II General Conceptual Ability <85c | 82 (17.1) | 87 (18.5) | 0.92 (0.70 to 1.22) | 0.94 (0.73 to 1.22) |
| Secondary outcomes | Mean difference (95% CI) | |||
| DAS-II General Conceptual Ability | 96.6 (13.8) | 96.6 (13.7) | 0.00 (−1.76 to 1.76) | 0.00 (−1.47 to 1.47) |
| Verbal ability | 97.7 (20.0) | 97.0 (19.7) | 0.69 (−1.85 to 3.22) | 0.59 (−1.61 to 2.79) |
| Nonverbal reasoning | 95.3 (4.9) | 96.3 (15.4) | −1.00 (−2.94 to 0.93) | −1.08 (−2.85 to 0.69) |
| Spatial ability | 98.2 (14.3) | 97.7 (14.4) | 0.46 (−1.37 to 2.29) | 0.64 (−1.00 to 2.28) |
| (n =522) | (n = 504) | Relative risk (95% CI) | ||
| Social Responsiveness Scale t score >65, No./total (%)d | 73/518 (14.1) | 77/504 (15.3) | 0.92 (0.69 to 1.24) | 0.91 (0.68 to 1.22) |
| >Level I Gross Motor Function Classification Systeme | 1 (0.2) | 1 (0.2) | ||
| Child Behavior Checklist t score f | Mean difference (95% CI) | |||
| Total problems | 48.5 (11.0) | 49.1 (11.2) | −0.58 (−1.94 to 0.78) | −0.81 (−2.15 to 0.52) |
| Normal | 433 (83.4) | 413 (81.9) | ||
| Borderline | 36 (6.9) | 44 (8.7) | ||
| Clinical | 50 (9.6) | 47 (9.3) | ||
| Median difference (95% CI) | ||||
| Internalizing behavior, median (IQR) | 48 (41 to 57) | 48 (43 to 57) | 0.00 (−1.45 to 1.45) | −0.61 (−1.97 to 0.75) |
| Normal | 422 (81.3) | 411 (81.5) | ||
| Borderline | 36 (6.9) | 39 (7.7) | ||
| Clinical | 61 (11.8) | 54 (10.7) | ||
| Externalizing behavior, median (IQR) | 48 (41 to 54) | 49 (41 to 57) | −1.00 (−2.88 to 0.88) | −0.60 (−2.00 to 0.80) |
| Normal | 438 (84.4) | 409 (81.2) | ||
| Borderline | 35 (6.7) | 39 (7.7) | ||
| Clinical | 46 (8.9) | 56 (11.1) | ||
| Dysregulation profile, median (IQR) | 156 (151 to 167) | 158 (151 to 171) | −1.00 (−3.34 to 1.34) | −0.68 (−2.05 to 0.68) |
| Anxious/depressed, median (IQR) | 51 (50 to 57) | 51 (50 to 57) | 0.00 (−0.22 to 0.22) | −0.27 (−0.52 to −0.03) |
| Normal | 473 (91.1) | 469 (93.1) | ||
| Borderline | 33 (6.4) | 21 (4.2) | ||
| Clinical | 13 (2.5) | 14 (2.8) | ||
| Attention problems, median (IQR) | 52 (50 to 57) | 52 (50 to 57) | 0.00 (−0.22 to 0.22) | 0.02 (−0.46 to 0.49) |
| Normal | 458 (88.2) | 450 (89.3) | ||
| Borderline | 42 (8.1) | 34 (6.7) | ||
| Clinical | 19 (3.7) | 20 (4.0) | ||
| Aggressive behavior, median (IQR) | 51 (50 to 56) | 51 (50 to 57) | 0.00 (0.00 to 0.00) | 0.00 (−0.27 to 0.27) |
| Normal | 479 (92.3) | 450 (89.3) | ||
| Borderline | 20 (3.9) | 29 (5.8) | ||
| Clinical | 20 (3.9) | 25 (5.0) | ||
Data are relative risk (95% CI) for categorical outcomes and mean difference (95% CI) or median difference (95% CI) for continuous outcomes.
Adjusted for maternal age, gestational age at delivery, child age, sex, maternal education, and center.
Differential Ability Scales 2nd Edition (DAS-II) General Conceptual Ability score has a mean (SD) of 100 (15); scores of 85 or higher are average and less than 85, below average, with higher scores indicating greater abilities.
Score range: less than 60, normal; 60 to 65, mild; 66 to 75, moderate; and 76 or higher, severe, with higher scores indicating greater problems.
There are 5 levels with greater functional impairment at increasing levels: walks without limitations, walks with limitations, walks with handheld mobility device, self-mobility with limitations; may use powered mobility, and transported in a manual wheelchair.
Score range: less than 60, normal; 60 to 63, borderline (84th-90th percentile); and 64 or higher, clinical (>90th percentile), with higher scores indicating greater problems. Values on syndrome scales (anxious or depressed, attention problems, and aggressive behavior) between the 93rd and 97th percentile are classified as borderline. Scores higher than the 97th percentile are clinical, indicating a potential need for referral. Values for internalizing behavior and externalizing behavior between the 84th and 90th percentile are classified as borderline. Scores higher than the 90th percentile are clinical. Child Behavior Checklist score is missing for 3 children.
To adjust for differences in the parent trial participants and the current study’s participants in maternal age, private insurance, and smoking status during pregnancy, the post hoc sensitivity analysis using inverse probability weighting did not alter the estimates, and none of the primary or secondary outcomes changed significance. The primary outcome of this follow-up study, the proportion of GCA scores less than 85, remained similar between groups: 238 of 1315 (18.1%) in the betamethasone group and 266 of 1305 (20.3%) in the placebo group (adjusted RR, 0.92 [95% CI, 0.71-1.25]). Secondary outcomes also remained similar between groups (Table 3). A comparison of characteristics in the original, unweighted analysis, and weighted analysis cohorts is located in eTable 3 in Supplement 2. Results demonstrate that the weighted cohort approximates the makeup of the original cohort via maternal age, insurance status, and smoking status during pregnancy.
Table 3. Post Hoc Sensitivity Analysis of Primary and Secondary Outcomes Weighted to Reflect Original Antenatal Late Preterm Steroids Trial Populationa.
| Outcome | No. (%) | Inverse probability weighting, relative risk (95% CI)b | ||
|---|---|---|---|---|
| Betamethasone | Placebo | Unadjusted | Adjustedc | |
| Primary outcome | (n = 1315) | (n = 1305) | ||
| DAS-II General Conceptual Ability <85d | 238 (18.1) | 266 (20.3) | 0.89 (0.67 to 1.16) | 0.92 (0.71 to 1.25) |
| Secondary outcomes | (n = 1430) | (n = 1402) | Relative risk (95% CI) | |
| Social Responsiveness Scale t score >65, No./total (%)e | 210/1416 (14.8) | 232/1402 (16.5) | 0.90 (0.65 to 1.19) | 0.89 (0.66 to 1.20) |
| >Level I Gross Motor Function Classification Systemf | 3 (0.2) | 3 (0.2) | ||
| Child Behavior Checklistg | Mean difference | |||
| Total problems t score | 48.8 (18.2) | 49.5 (18.8) | −0.70 (−2.16 to 0.73) | −0.91 (−2.23 to 0.51) |
Comparison of original cohorts and cohorts remaining at 6 years or older, including corrections with weighting, are shown in eTable 3 in Supplement 1.
Inverse probability weighting accounts for maternal age, insurance status, and smoking status during pregnancy. Data are relative risk (95% CI) for categorical outcomes and mean difference (95% CI) for continuous outcomes.
Adjusted for gestational age at delivery, child age, sex, and center.
The Differential Ability Scales 2nd Edition (DAS-II) General Conceptual Ability score has a mean (SD) of 100 (15); scores 85 or higher are average and less than 85, below average, with higher scores indicating greater abilities.
Score range: less than 60, normal; 60 to 65, mild; 66 to 75, moderate; and 76 or higher, severe, with higher scores indicating greater problems.
There are 5 levels with greater functional impairment at increasing levels: walks without limitations, walks with limitations, walks with handheld mobility device, self-mobility with limitations; may use powered mobility, and transported in a manual wheelchair.
Score range: less than 60, normal; 60 to 63, borderline (84th-90th percentile); and 64 or higher clinical (>90th percentile), with higher scores indicating greater problems.
Results were similar when including patients who were lost to follow-up (Table 4). There were no differences in a sensitivity analysis that assumed that all lost to follow-up had the primary outcome: 125 of 522 (23.9%) in the betamethasone group and 121 of 504 (24.0%]) in the placebo group (adjusted RR, 0.99 [95% CI, 0.80-1.21]) or assuming all lost to follow-up did not have the primary outcome: 82 of 522 (15.7%) for the betamethasone group and 87 of 504 (17.0%) for the placebo group (adjusted RR, 0.94 [95% CI, 0.73-1.22]). There were also similar results when including those who did not participate due to a neuromotor, neurological, cognitive, or behavioral condition, as having the primary outcome: 96 of 493 (19.5%) for the betamethasone group and 103 of 486 (21.0%) for the placebo group (adjusted RR, 0.94 [95% CI, 0.73-1.22]). Evaluating the outcomes of those who delivered at term vs preterm also revealed no difference in primary or secondary outcomes (eTable 4 in Supplement 2).
Table 4. Results of Prespecified Sensitivity Analyses.
| Outcome | No. (%) | Relative risk (95% CI) | ||
|---|---|---|---|---|
| Betamethasone (n = 522) | Placebo (n = 504) | Unadjusted | Adjusteda | |
| Lost to follow-up sensitivity analysis | ||||
| DAS-II General Conceptual Ability <85 all lost to follow-up assumed to have the primary outcome | ||||
| Yes | 125 (23.9) | 121 (24.0) | 1.00 (0.80-1.24) | 0.99 (0.80-1.21) |
| No | 82 (15.7) | 87 (17.0) | 0.91 (0.69-1.20) | 0.94 (0.73-1.22) |
| Neurocognitive sensitivity analysis b | (n = 493) | (n = 486) | ||
| DAS-II General Conceptual Ability <85 | 96 (19.5) | 103 (21.0) | 0.92 (0.72-1.18) | 0.94 (0.73-1.22) |
Abbreviation: DAS-II, Differential Ability Scales, 2nd Edition.
Adjusted by prespecified baseline characteristics including maternal age, gestational age at delivery, child age, sex, maternal education, and center.
All who did not participate due to a neuromotor, neurological, cognitive, or behavioral condition were assumed to have the primary outcome.
Discussion
In this prospective follow-up of the ALPS randomized clinical trial, no significant differences were found in neurodevelopment as assessed by the DAS-II generalized conceptual ability score among children who were exposed antenatally to betamethasone or placebo in the late preterm period. There were also no differences in the other secondary outcomes assessing autistic traits, gross motor function, and problem behaviors. These findings were noted despite a higher rate of hypoglycemia in the betamethasone group in the included cohort and are in line with other clinical data on children formerly exposed to a single course of antenatal betamethasone for fetal lung maturity.8,9
These findings are consistent with those of MacArthur and colleagues15 who completed a 6-year follow-up study on children whose birthing parents had been enrolled in the original randomized trial of antenatal betamethasone by Liggins and Howie.22 In their parent trial, individuals at risk of preterm delivery from 24 weeks, 0 days of gestation to 36 weeks, 6 days of gestation were randomized to betamethasone vs placebo. The majority of follow-up participants were born in the late preterm period, and there were no differences in the 4 measures of cognitive testing performed at age 6 years.15 The same cohort was followed up to age 30 years. No differences in cognitive testing were noted.9
The current study’s findings contrast Räikkönen et al11 who performed a retrospective register-linkage study using a Finnish data set to associate childhood mental and behavioral disorders with antenatal corticosteroid exposure. Importantly, they did not have information on the gestational age of antenatal corticosteroid exposure but reported that the practice at the time was to administer antenatal steroids only up to 34 weeks, 6 days’ gestation.23 There were also no data on the indication for administration, which is important because the indication may be an independent risk factor for an adverse outcome. Finally, as others have also mentioned, death was a competing outcome but was not accounted for, limiting the robustness of the associations observed.24 Given the limitations of studies by Räikkön and colleagues11 and others,25,26 there has been a call for “high-evidence level randomized data” from the ALPS trial follow-up study to help answer this question.10
The current study has important strengths. Investigators, research staff, certified psychologists, and participants remained blinded to original treatment assignment. This study was able to evaluate a myriad of neurocognitive domains. Most importantly, this is a follow-up of a randomized clinical trial, so there is no bias by indication for steroids or by gestational age of administration, which is invariably true for observational studies.
Several studies have shown that being born late preterm leads to adverse neurodevelopment compared with birth at term.27,28,29 Although effective strategies to decrease the prevalence of preterm birth worldwide will have the largest impact on neonatal and childhood outcomes, this study provides evidence that steroids do not contribute to adverse neurodevelopment in this group.
Limitations
These findings have a few notable limitations. First, the group enrolled included just more than half of the intended sample size of 2000 participants, likely due to both the effects of the COVID-19 pandemic and difficulty finding original trial participants. After enrolling participants, some ultimately declined DAS-II testing. Nevertheless, sensitivity analyses, in which multiple assumptions were tested with regard to the primary outcome for this study, resulted in conclusions similar to the primary analysis. In addition, the final sample size still provided more than 80% power to show a 36% relative decrease (range, 18.5%-11.8%) in the primary outcome and more than 80% power to show a 42% relative increase (range, 18.5%-26.3%) in the primary outcome. Furthermore, the achieved sample size makes this the largest neurocognitive follow-up study of the use of late preterm steroids and the largest follow-up study of a single course of antenatal corticosteroids.30 Second, this study had relatively small numbers of neonates born at term, limiting the ability to detect small differences between the betamethasone and placebo groups for infants born at term. Third, instruments for evaluating neurodevelopment, such as the standardized testing used in this study, may not capture subjective differences in school performance or other more subtle outcomes.
Conclusions
In this follow-up study of a randomized clinical trial, administration of antenatal corticosteroids to persons at risk of late preterm delivery, originally shown to improve short-term neonatal respiratory outcomes but with an increased rate of hypoglycemia, was not associated with adverse effects on childhood neurodevelopmental outcomes at age 6 years or older using a variety of metrics. These data provide reassurance with regard to the administration of antenatal corticosteroids in the late preterm period.
Trial Protocol
Trial Sites and Study Personnel
eTable 1. Maternal/Neonatal Characteristics for Enrolled vs. Non-Enrolled Follow-Up Participants
eTable 2. Participants Excluded for Death or Developmental Delay
eTable 3. Comparison of Original Cohorts and Cohorts Remaining at ≥ 6 years
eTable 4. Subgroup Analysis among Preterm and Term Deliveries
Data Sharing Statement
References
- 1.Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. ; NICHD Maternal–Fetal Medicine Units Network . Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320. doi: 10.1056/NEJMoa1516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65-74. doi: 10.1542/peds.2007-2822 [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Davis A, Shekhawat PS. Hypoglycemia in the preterm neonate: etiopathogenesis, diagnosis, management and long-term outcomes. Transl Pediatr. 2017;6(4):335-348. doi: 10.21037/tp.2017.10.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uno H, Lohmiller L, Thieme C, et al. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques, I: hippocampus. Brain Res Dev Brain Res. 1990;53(2):157-167. doi: 10.1016/0165-3806(90)90002-G [DOI] [PubMed] [Google Scholar]
- 5.Slotkin TA, Zhang J, McCook EC, Seidler FJ. Glucocorticoid administration alters nuclear transcription factors in fetal rat brain: implications for the use of antenatal steroids. Brain Res Dev Brain Res. 1998;111(1):11-24. doi: 10.1016/S0165-3806(98)00115-1 [DOI] [PubMed] [Google Scholar]
- 6.Wapner RJ, Sorokin Y, Mele L, et al. ; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357(12):1190-1198. doi: 10.1056/NEJMoa071453 [DOI] [PubMed] [Google Scholar]
- 7.Asztalos EV, Murphy KE, Willan AR, et al. ; MACS-5 Collaborative Group . Multiple Courses of Antenatal Corticosteroids for Preterm Birth Study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102-1110. doi: 10.1001/jamapediatrics.2013.2764 [DOI] [PubMed] [Google Scholar]
- 8.Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental outcome after a single course of antenatal steroids in children born preterm: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(6):1385-1396. doi: 10.1097/AOG.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 9.Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665. doi: 10.1136/bmj.38576.494363.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidaeff AC, Belfort MA, Kemp MW, et al. Updating the balance between benefits and harms of antenatal corticosteroids. Am J Obstet Gynecol. 2023;228(2):129-132. doi: 10.1016/j.ajog.2022.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. 2020;323(19):1924-1933. doi: 10.1001/jama.2020.3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao S, Du J, Chi X, et al. ; China National Birth Cohort (CNBC Study Group) . Associations between antenatal corticosteroid exposure and neurodevelopment in infants. Am J Obstet Gynecol. 2022;227(5):759.e1-759.e15. doi: 10.1016/j.ajog.2022.05.060 [DOI] [PubMed] [Google Scholar]
- 13.Committee on Obstetric Practice . Committee Opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130(2):e102-e109. doi: 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 14.Reddy UM, Deshmukh U, Dude A, Harper L, Osmundson SS; Society for Maternal-Fetal Medicine (SMFM) . Society for Maternal-Fetal Medicine Consult Series #58: use of antenatal corticosteroids for individuals at risk for late preterm delivery: replaces SMFM statement #4, implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery, August 2016. Am J Obstet Gynecol. 2021;225(5):B36-B42. doi: 10.1016/j.ajog.2021.07.023 [DOI] [PubMed] [Google Scholar]
- 15.MacArthur BA, Howie RN, Dezoete JA, Elkins J. School progress and cognitive development of 6-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics. 1982;70(1):99-105. doi: 10.1542/peds.70.1.99 [DOI] [PubMed] [Google Scholar]
- 16.Elliott CD, Salerno JD, Dumont R, Willis JO. The Differential Ability Scales. In: Flanagan DP, McDonough EM, eds. Contemporary Intellectual Assessment: Theories, Tests, and Issues. 2nd ed. TGuilford Press; 2018:360-382. [Google Scholar]
- 17.Welscher D. The Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. Psychological Corp; 2002. [Google Scholar]
- 18.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 19.Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the social responsiveness scale-2. Autism. 2014;18(1):31-44. doi: 10.1177/1362361313500382 [DOI] [PubMed] [Google Scholar]
- 20.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 21.Northen AT, Norman GS, Anderson K, et al. Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo. Obstet Gynecol. 2007;110(4):865-872. doi: 10.1097/01.AOG.0000281348.51499.bc [DOI] [PubMed] [Google Scholar]
- 22.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515-525. doi: 10.1542/peds.50.4.515 [DOI] [PubMed] [Google Scholar]
- 23.Gyamfi-Bannerman C, Saade GR, Rouse DJ. Maternal antenatal corticosteroid treatment and childhood mental and behavioral disorders. JAMA. 2020;324(15):1569-1570. doi: 10.1001/jama.2020.15446 [DOI] [PubMed] [Google Scholar]
- 24.Dehaene I. Updating the balance between benefits and harms of antenatal corticosteroids requires appropriate causal inference. Am J Obstet Gynecol. 2023;229(1):80-81. doi: 10.1016/j.ajog.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 25.Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067-1069. doi: 10.1111/1471-0528.13853 [DOI] [PubMed] [Google Scholar]
- 26.Ninan K, Liyanage SK, Murphy KE, Asztalos EV, McDonald SD. Evaluation of long-term outcomes associated with preterm exposure to antenatal corticosteroids: a systematic review and meta-analysis. JAMA Pediatr. 2022;176(6):e220483. doi: 10.1001/jamapediatrics.2022.0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams BL, Dunlop AL, Kramer M, Dever BV, Hogue C, Jain L. Perinatal origins of first-grade academic failure: role of prematurity and maternal factors. Pediatrics. 2013;131(4):693-700. doi: 10.1542/peds.2012-1408 [DOI] [PubMed] [Google Scholar]
- 28.Lipkind HS, Slopen ME, Pfeiffer MR, McVeigh KH. School-age outcomes of late preterm infants in New York City. Am J Obstet Gynecol. 2012;206(3):222.e1-6. doi: 10.1016/j.ajog.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 29.Mirzakhani H, Kelly RS, Yadama AP, et al. Stability of developmental status and risk of impairment at 24 and 36 months in late preterm infants. Infant Behav Dev. 2020;60:101462. doi: 10.1016/j.infbeh.2020.101462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454. doi: 10.1002/14651858.CD004454.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Trial Sites and Study Personnel
eTable 1. Maternal/Neonatal Characteristics for Enrolled vs. Non-Enrolled Follow-Up Participants
eTable 2. Participants Excluded for Death or Developmental Delay
eTable 3. Comparison of Original Cohorts and Cohorts Remaining at ≥ 6 years
eTable 4. Subgroup Analysis among Preterm and Term Deliveries
Data Sharing Statement


