Keywords: 60-kDa glycoprotein, glutamate dehydrogenase, internal transcribed spacer region of rRNA, intestinal protists, opportunistic pathogens, PCR, small ribosomal subunit rRNA, subtyping, triosephosphate isomerase, β-giardin
Abstract
Cryptosporidium spp., Giardia intestinalis and microsporidia are unicellular opportunistic pathogens that can cause gastrointestinal infections in both animals and humans. Since companion animals may serve as a source of infection, the aim of the present screening study was to analyse the prevalence of these intestinal protists in fecal samples collected from dogs living in 10 animal shelters in central Europe (101 dogs from Poland and 86 from the Czech Republic), combined with molecular subtyping of the detected organisms in order to assess their genetic diversity. Genus-specific polymerase chain reactions were performed to detect DNA of the tested species and to conduct molecular subtyping in collected samples, followed by statistical evaluation of the data obtained (using χ2 or Fisher's tests). The observed prevalence was 15.5, 10.2, 1 and 1% for G. intestinalis, Enterocytozoon bieneusi, Cryptosporidium spp. and Encephalitozoon cuniculi, respectively. Molecular evaluation has revealed the predominance of dog-specific genotypes (Cryptosporidium canis XXe1 subtype; G. intestinalis assemblages C and D; E. cuniculi genotype II; E. bieneusi genotypes D and PtEbIX), suggesting that shelter dogs do not pose a high risk of human transmission. Interestingly, the percentage distribution of the detected pathogens differed between both countries and individual shelters, suggesting that the risk of infection may be associated with conditions typical of a given location.
Introduction
Dogs play an important role in human life as companion animals; however, they may be carriers of various pathogens, constituting a potential reservoir of zoonotic infections for their owners. Many of these infectious agents reside in the intestinal tract; therefore, their dispersive forms are excreted with animal stool and may easily be spread to other hosts by fecal–oral transmission through direct contact or indirectly via water or food contamination. This includes unicellular enteric organisms such as Giardia intestinalis (syn. duodenalis or lamblia), Cryptosporidium spp. or microsporidia from genera Encephalitozoon and Enterocytozoon, which can be responsible for gastrointestinal symptoms like abdominal pain, diarrhoea or flatulence (Xiao, 2010; Liao et al., 2020). Since these organisms belong to the group of opportunistic pathogens, infection is of particular importance for individuals with impaired immunity (e.g. HIV-infected patients, cancer-treated patients or transplant recipients), in whom it may lead to the development of hazardous, even life-threatening symptoms.
Giardia intestinalis is the most common intestinal pathogenic protozoan in humans and animals (Kváč et al., 2017), consisting of 8 distinct assemblages (A–H) differing in host specificities. Assemblages A and B display a broad host range and are most commonly reported in humans, while the remaining 6 seem to be host-specific for non-human species, with assemblages C and D predominantly found in dogs (Bouzid et al., 2015; Ryan and Zahedi, 2019). Among nearly 51 valid Cryptosporidium species (Tůmová et al., 2023), Cryptosporidium hominis and Cryptosporidium parvum represent the major causes of human cryptosporidiosis, whereas Cryptosporidium meleagridis, Cryptosporidium mortiferum, Cryptosporidium felis and Cryptosporidium canis are rare causative agents of zoonotic infections, with the latter being the most prevalent in dogs (Xu et al., 2016; Li et al., 2021; Alderisio et al., 2023). Out of over 1200 microsporidian species described so far, Enterocytozoon bieneusi and Encephalitozoon genus, including Encephalitozoon intestinalis, Encephalitozoon cuniculi and Encephalitozoon hellem, represent the species causing human microsporidiosis (Didier et al., 2000), especially in persons with impaired immunity (Kicia et al., 2014, 2016). These species may also be detected in a broad range of other hosts (livestock, wildlife and domesticated animals) (Dengjel et al., 2001; Mathis et al., 2005). The phylogenetic analysis of E. bieneusi allows for distinction of various genotypes differing in a host specificity. Genotypes D, EbpC and type IV are characterized by the widest host range and are also most frequently reported in humans (Li et al., 2019). In turn, genotype PtEbIX seems to be restricted to canine host population (Li et al., 2019), although dogs can serve as a reservoir of many other zoonotic genotypes as well, including those most often reported in humans (Li et al., 2020). Regarding the genus Encephalitozoon, the ability of these 3 species to inhabit a wide variety of organisms has been shown, with E. cuniculi having the widest host range, mainly among mammals and birds. Four E. cuniculi strains have been identified (I–IV); ‘canine’ strain III has been shown to cause high mortality in dogs, while the recently discovered ‘human’ strain IV has so far been documented in humans, cats and dogs. Although there appears to be some host preference in each strain, this specificity is not exact; humans have been found to be infected with all known strains (though rarely with strain III). In turn, E. hellem is the most common species among birds, while E. intestinalis is the most prevalent Encephalitozoon species in humans (Hinney et al., 2016).
The dispersive forms of the discussed pathogens are very resistant, and many species of wild and domesticated animals, as well as humans, may serve as their hosts which facilitates their spread and maintenance in the environment. Previous research on the occurrence of these species in central Europe, including Poland and the Czech Republic, shows different data depending on the population studied and the detection methods used. According to a review by Plutzer et al., the reported incidence of Cryptosporidium spp. and G. intestinalis per 100 000 inhabitants is 0.006 and 5.43 for Poland and 0.01 and 0.51 for the Czech Republic, respectively (Plutzer et al., 2018). In Poland, estimates of the prevalence of these 2 species in humans are available based on the results of research limited to specific population groups and regions (Plutzer et al., 2018). However, studies of Czech residents regarding the seroprevalence of Cryptosporidium spp. show the frequency of antibodies at the level of approximately 67–72% (Kozisek et al., 2008). In turn, the prevalence of Cryptosporidium spp. in dogs in Poland ranges from approximately 3.5 to 12.5% (Bajer and Bednarska, 2007; Piekara-Stępińska et al., 2021a), and G. intestinalis from 6 to 36%, with mainly canid-specific genotypes detected, which suggests that they do not represent an important source of Giardia infection for humans (Bajer and Bednarska, 2007; Piekarska et al., 2016; Piekara-Stępińska et al., 2021b). A similar prevalence refers to dogs from the Czech Republic, especially those from animal shelters (Zemanová et al., 2005; Dubná et al., 2007). Microsporidia, however, occur in dogs at a low level, only a few per cent, which may nevertheless pose a risk for immunodeficient individuals, as zoonotic genotypes are often detected (Piekarska et al., 2017). Importantly, frequent exposure to microsporidia has been confirmed among immunocompetent people in the Czech Republic (Sak et al., 2011), while in studies conducted in Poland, up to 26% of tested immunocompromised individuals were found to be infected with at least 1 microsporidian species (Kicia et al., 2016, 2019).
It has been previously shown that specific genotypes and assemblages of these enteric pathogens may be detected in both humans and animals (Xiao et al., 2007; Soliman et al., 2011; Karim et al., 2014a, 2014b; Hinney et al., 2016), suggesting the possible zoonotic route of transmission. One of their sources may be shelter dogs, which often live in poor sanitary conditions and crowded spaces that favour the spread of such microorganisms (Raza et al., 2018). Currently, there are 226 registered animal shelters in Poland (General Veterinary Inspectorate, 2023) and 248 in the Czech Republic (State Veterinary Administration, 2024). Therefore, the aim of the present study was to investigate the occurrence of Cryptosporidium spp., G. intestinalis, Encephalitozoon spp. and E. bieneusi in dogs living in animal shelters in central Europe (Poland and the Czech Republic) and to assess the host specificity and zoonotic potential of these organisms at the genotype level.
Materials and methods
Samples
Individual fresh fecal samples were collected from dogs in animal shelters in Poland and the Czech Republic. Samples were collected directly from the floor by study research staff immediately after defecation, with care taken to avoid sampling fecal material that came into contact with the ground (concrete surface, without contact with the soil). They were collected in the morning, before daily cleaning routinely performed in each shelter. Each sample was individually placed in a sterile tube with animal ID, refrigerated at 4°C without preservatives and transported to laboratory. None of the collected stool had an apparent diarrhoeal symptom at the time of sampling. Where possible, information about the animal, such as sex and age, was also recorded during material collection (see Supplementary Table 1). Control of intestinal protozoa in dogs and cats in both Polish and Czech shelters is carried out according to current ESCCAP guidelines (ESCCAP, 2018) – in all facilities, pyrantel and fenbendazole were routinely used once every 3 months as part of antiparasitic prophylaxis.
Molecular analysis
Stool samples were stored up to 2 months in 4°C without preservatives until DNA extraction. Initial homogenization of 200 mg of each stool sample was performed by bead disruption for 60 s at 5.5 m s−1 with 0.5 mm glass beads using a Precellys 24 Instrument (Bertin Technologies, Montigny le Bretonneux, France), followed by genomic DNA (gDNA) extraction using a GeneMATRIX Stool DNA Purification Kit (EurX, Gdańsk, Poland). Molecular detection was based on the nested polymerase chain reaction (PCR) protocols for the amplification of the chosen genes of E. bieneusi (ITS), Encephalitozoon spp. (ITS), Cryptosporidium spp. (18S rRNA) and G. intestinalis (TPI) (Didier et al., 1995; Katzwinkel-Wladarsch et al., 1996; Xiao et al., 1999; Buckholt et al., 2002; Sulaiman et al., 2003). Additional PCRs amplifying selected loci were performed for subtyping in order to assess intra-species genetic diversity in the case of samples positive for Cryptosporidium spp. (partial 60-kDa glycoprotein gene – gp60) and G. intestinalis (β-giardin – BG and glutamate dehydrogenase – GDH) (see Supplementary Table 2) (Cacciò et al., 2002, 2008; Lalle et al., 2005; Jiang et al., 2021). Each PCR contained 0.25–2.0 μL of DNA, 200 μm each of deoxynucleoside triphosphate (dNTP), 1× PCR buffer (DreamTaq™ Green Buffer, ThermoFisher Scientific, Waltham, MA, USA), 3.0 mm MgCl2, 0.125 U of Taq polymerase (ThermoFisher Scientific), 10 μg of bovine serum albumin (BSA) and 200 nm of each primer in a total of 20–25 μL reaction. The reactions were performed in a C1000 Bio-Rad thermocycler, with an initial hot start (94°C for 5 min) and a final extension (72°C for 10 min), according to the conditions described in Supplementary Table 2. An aliquot of primary PCR was used as a template for the secondary PCR. Its conditions were identical to the primary PCR, except that BSA was not added to the secondary reaction. Negative (molecular grade water) and positive controls (DNA extracted from E. bieneusi genotype CZ3, E. cuniculi genotype III spores, Cryptosporidium serpentis oocysts or G. intestinalis assemblage F cysts) were included in each PCR amplification. Secondary PCR products were electrophoresed on a 1% agarose gel containing 0.2 mg mL−1 Midori Green DNA stain in TAE buffer at 75 V for approximately 1 h. Bands of the predicted sizes were visualized using a UV light source, cut from the gel, extracted using a Zymoclean Gel DNA Recovery Kit (Zymo Research, Irvine, CA, USA) and sequenced bi-directionally by a company offering this service commercially (Genomed S.A., Warsaw, Poland). The nucleotide sequences obtained were processed using Chromas Pro 2.4.1 software (Technelysium, Pty, Ltd., South Brisbane, Australia). Subsequently, BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was performed to verify the identity of the sequences. The edited and aligned sequences were further processed using BioEdit v.7.0.5 (Hall, 1999). To align the obtained sequences with reference sequences from GenBank, the online server MAFFT version 7 was used (http://mafft.cbrc.jp/alignment/software). The best model for DNA/protein phylogeny for each alignment was selected based on the Bayesian information criterion in MEGA 7 (Kumar et al., 2016). Tamura's 3-parameter model + G + I was used for the alignments. The maximum likelihood (ML) approach was carried out in MEGA7 software. Bootstrap support was calculated based on 1000 replications to evaluate the robustness of tree branching. Finally, the resulting trees were visualized using Corel Draw X7 software (https://www.corel.draw.com). Representative nucleotide sequences of all loci used as markers for subtyping of isolates obtained in the current study were deposited in GenBank with the accession numbers OR791083, OR791084, OR791770, OR791659, OR791771–OR791785, OR807726 and OR807727.
Statistical analysis
Statistical analysis was performed using χ2 or Fisher's tests to compare the frequency of occurrence of the tested pathogens between Polish and Czech shelters (Statistica software, TIBCO Software Inc., USA). A P < 0.05 was considered significant.
Results
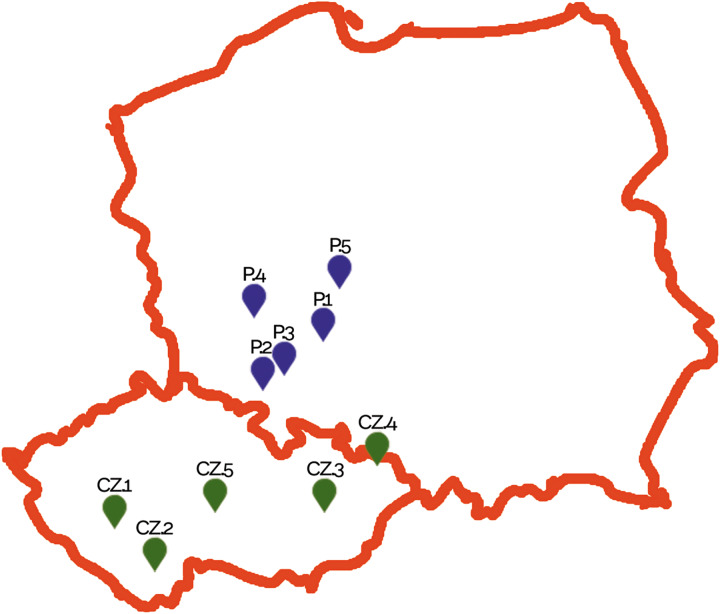
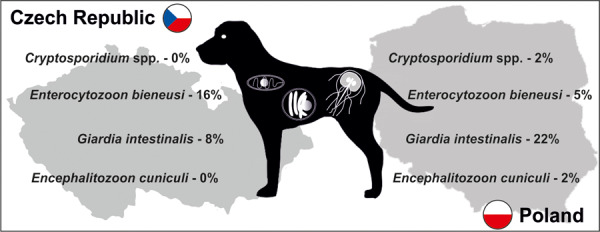
A total of 187 apparently healthy dogs from 10 shelters (Fig. 1), 5 in Poland (101 dogs) and 5 in the Czech Republic (86 dogs), were studied (Table 1). Specific DNA of targeted parasites was detected in 50 of 187 animals (26.7%), with higher occurrence observed in Polish than in Czech animals (32.6 vs 24.4%, χ2 = 1.0254; P = 0.3112; Table 1). Most of the detected infections were monoinfections; only 2 dogs (ID 2966_CZ.1 and 3031_CZ.5) had a coinfection, E. bieneusi and G. intestinalis. Giardia intestinalis (29 dogs, 15.5%) followed by E. bieneusi (19 dogs, 10.2%) were the most frequently detected parasites, whereas each Encephalitozoon spp. and Cryptosporidium spp. were found in 2 dogs (Table 1). Overall, there was a significant trend towards more frequent occurrence of G. intestinalis in Polish (21.8%) vs Czech animals (8.1%, χ2 = 6.5979; P = 0.0102), while E. bieneusi was seen more often in dogs from Czech shelters (16.3%) than in Polish ones (4.9%, χ2 = 6.5305; P = 0.0106). Since detailed demographic data were obtained only from a small number of individuals, they were not subjected to statistical analysis.
Figure 1.
Schematic arrangement of shelters in Poland (P.1–P.5) and the Czech Republic (CZ.1–CZ.5) from which samples were collected.
Table 1.
Occurrence of Cryptosporidium spp., Giardia intestinalis, Encephalitozoon cuniculi and Enterocytozoon bieneusi in individual dogs kept in animal shelters in Poland and the Czech Republic
| Country | Location | No. of tested animals | Dogs ID | No. of dogs positive for screened parasites | |||
|---|---|---|---|---|---|---|---|
| Cryptosporidium spp. | G. intestinalis | E. cuniculi | E. bieneusi | ||||
| Poland | P.1 | 39 | 2806 | 1 | – | – | – |
| 2803, 2807 | – | 2 | – | – | |||
| 2839 | – | – | 1 | – | |||
| 2808, 2834 | 2 | ||||||
| P.2 | 32 | 2728, 2732, 2733, 2742, 2744, 2747, 2748, 2751, 2753, 2757 | – | 10 | – | – | |
| 2735 | 1 | – | – | – | |||
| 2727 | – | – | – | 1 | |||
| P.3 | 13 | 2768, 2771, 2772, 2773, 2775, 2778, 2779, 2780 | – | 8 | – | – | |
| P.4 | 11 | 2783 | – | – | 1 | – | |
| 2787 | – | 1 | – | – | |||
| 2789 | – | – | – | 1 | |||
| P.5 | 6 | 2696 | – | 1 | – | – | |
| 2699 | – | – | – | 1 | |||
| Czech Republic | CZ.1 | 20 | 2966 | – | 1 | – | 1 |
| 2965, 2973, 2977 | – | – | – | 3 | |||
| CZ.2 | 7 | 2985, 2986, 2987, 2989 | – | – | – | 4 | |
| CZ.3 | 6 | – | – | – | – | – | |
| CZ.4 | 30 | 3010, 3027 | – | 2 | – | – | |
| CZ.5 | 23 | 3031 | – | 1 | – | 1 | |
| 3030, 3036, 3041 | – | 3 | – | – | |||
| 3032, 3044, 3047, 3048, 3049 | – | – | – | 5 | |||
| Total | 187 | 2 (1.1%) | 29 (15.5%) | 2 (1.1%) | 19 (10.2%) | ||
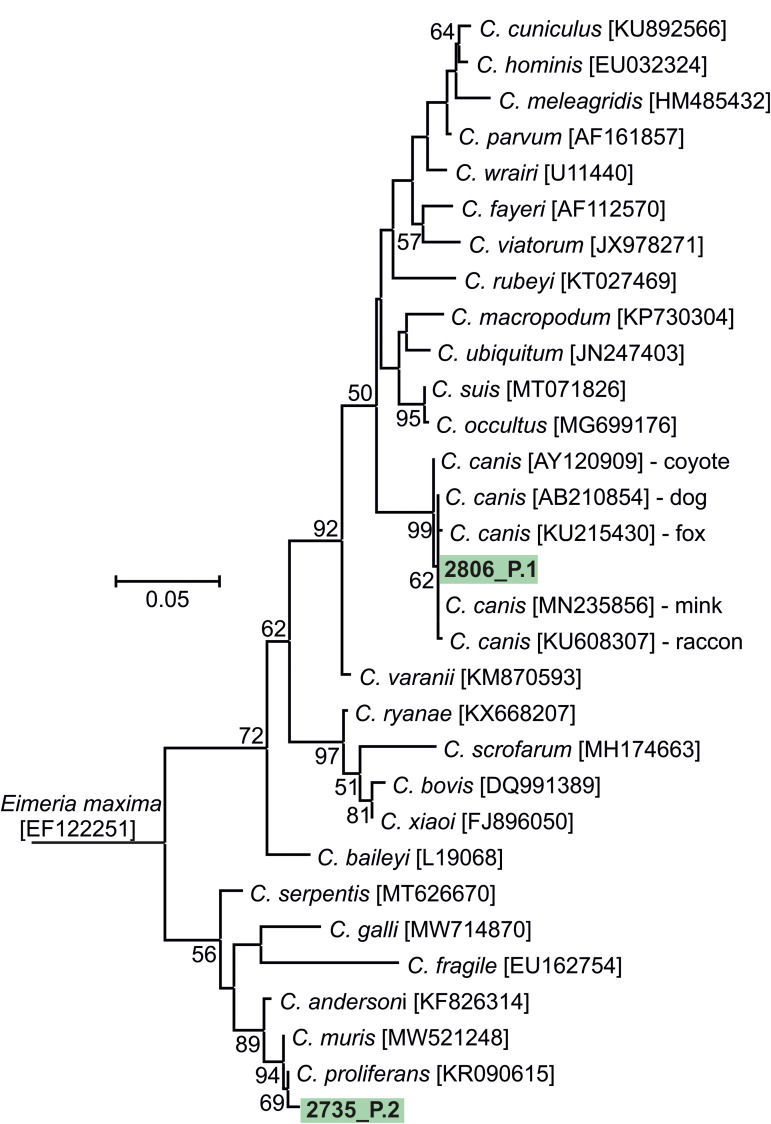
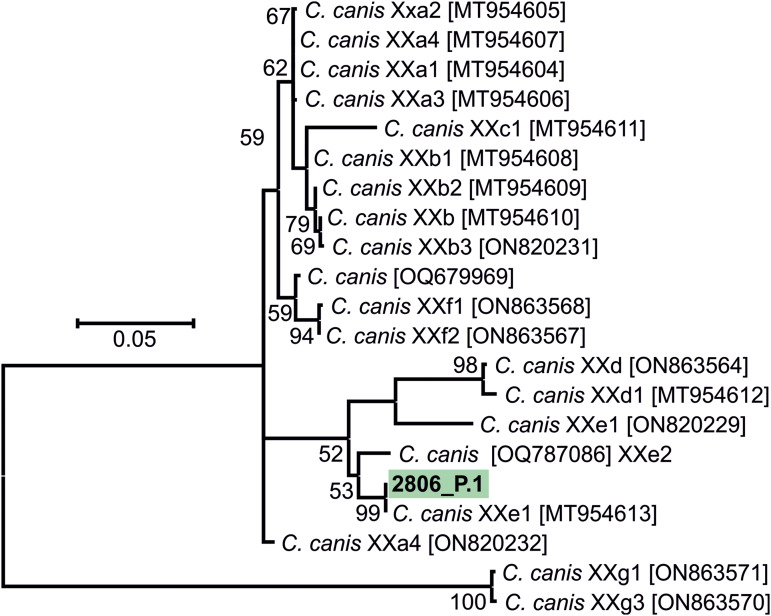
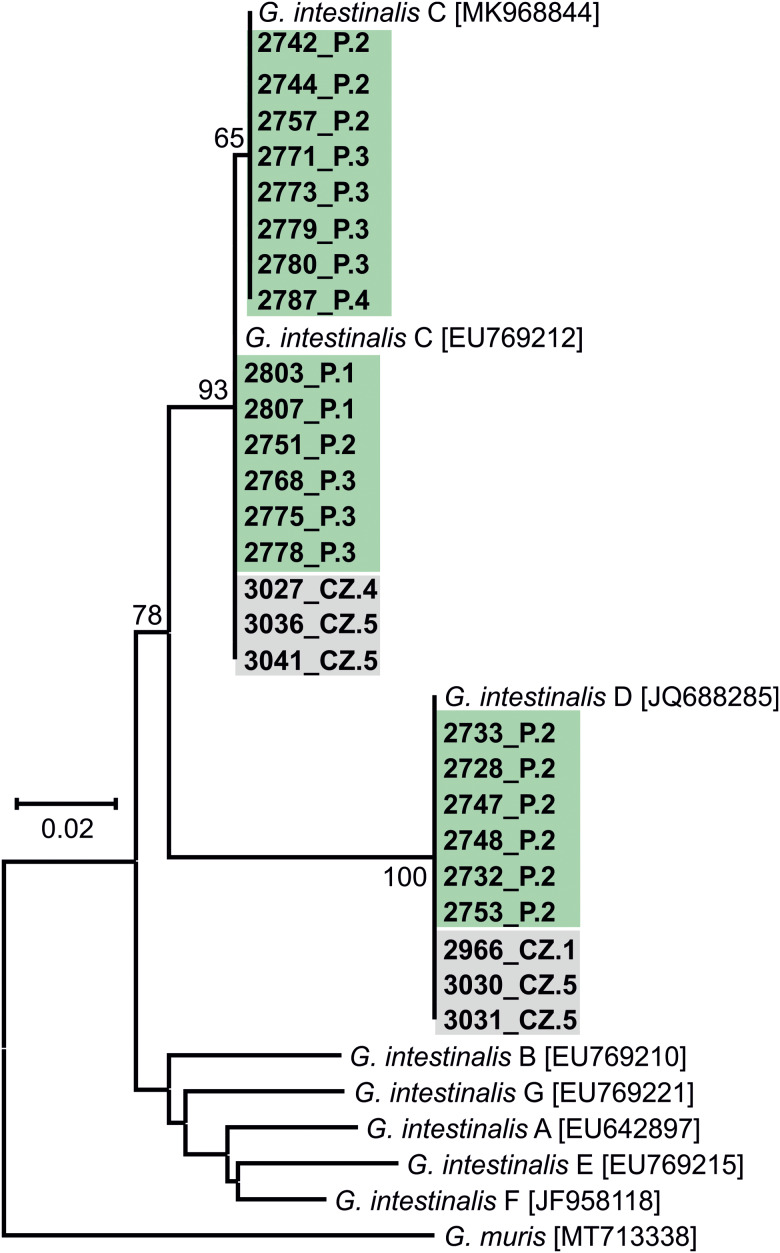
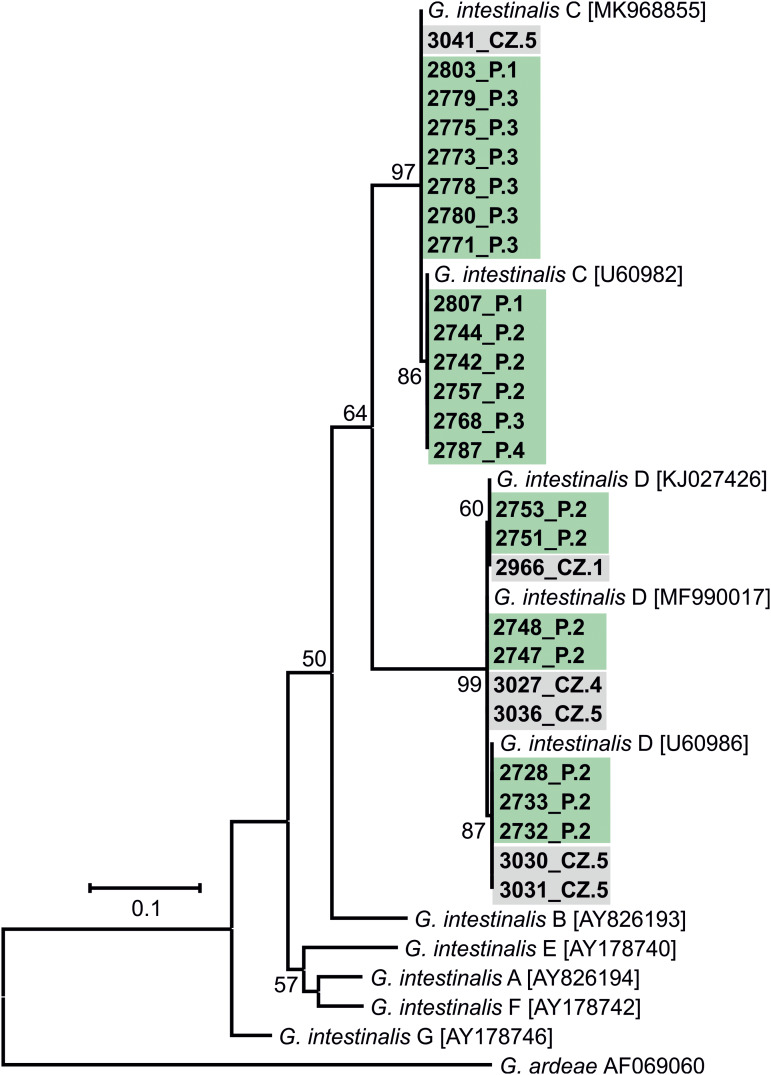
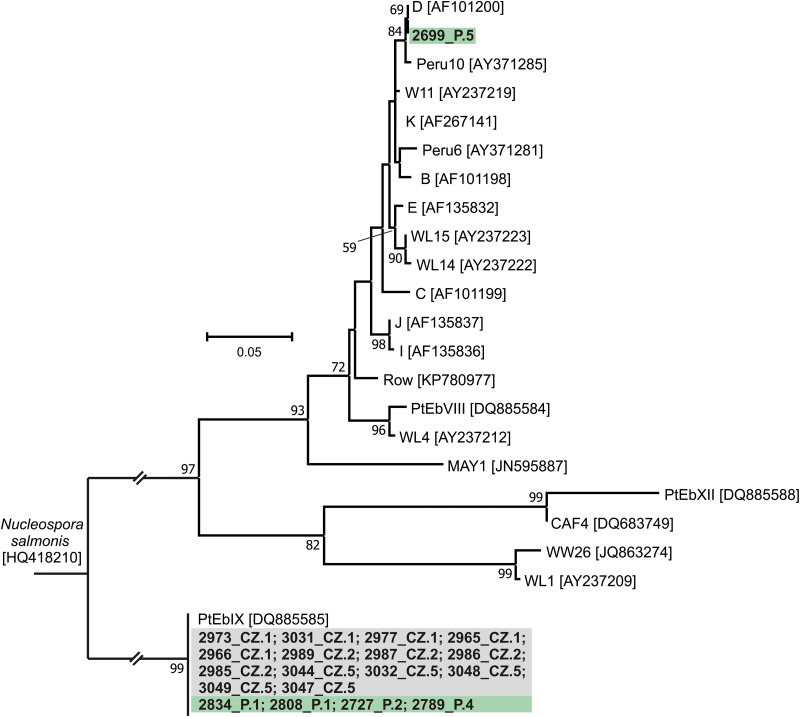
The results of genotyping of all pathogens performed in this study are presented in Table 2. Phylogeny analysis of partial sequences of 18S rRNA of Cryptosporidium showed the presence of C. canis identical to C. canis dog genotype (GenBank Acc. No. AB120909) in isolate 2806_P.1 and in a Cryptosporidium sp. isolate 2735_P.2, phylogenetically clustered near the gastric Cryptosporidium spp. (Fig. 2). Cryptosporidium sp. isolate 2735_P.2 differed from Cryptosporidium proliferans in 3 single-nucleotide polymorphisms (SNPs) with 99.6% sequence identity. Based on C. canis gp60 locus subtyping, isolate 2806_P.1 was assigned to the XXe family (Fig. 3). Genotype II, determined at ITS sequences, was detected in both dogs positive for E. cuniculi (Table 2; phylogeny is not shown). Phylogenetic analysis of Giardia showed different results in 12 isolates, depending on the marker used (Table 2). Subtyping based on TPI gene revealed the presence of only assemblage C in all samples examined (Fig. 4), while for BG (Fig. 5) and GDH (Fig. 6) loci the presence of both C and D assemblages has been shown. Subtyping of BG and GDH failed in 2 and 3 isolates, respectively. With the exception of genotype D, which was detected in dog 2699_P.5, all other E. bieneusi sequences were identical to the PtEbIX genotype (Fig. 7).
Table 2.
Results of genotyping of Cryptosporidium spp., G. intestinalis, E. cuniculi and E. bieneusi in all tested samples
| G. intestinalis assemblage | Cryptosporidium spp. species/subtype | E. cuniculi genotype ITS | E. bieneusi genotype ITS | |||||
|---|---|---|---|---|---|---|---|---|
| Country | Dog ID_ Location | TPI | BG | GDH | 18S rRNA | gp60 | ||
| Poland | 2803_P.1 | C [OR791776] | C [OR791779] | C | ||||
| 2806_P.1 | Cryptosporidium canis [OR791083] | XXe1 [OR791770] | ||||||
| 2807_P.1 | C [OR791774] | C | C [OR791782] | |||||
| 2808_P.1 | PtEbIX | |||||||
| 2834_P.1 | PtEbIX [OR807727] | |||||||
| 2839_P.1 | II [OR791659] | |||||||
| 2728_P.2 | C | D | D [OR791785] | |||||
| 2732_P.2 | C | D | D | |||||
| 2733_P.2 | C | D [OR791780] | D | |||||
| 2735_P.2 | Cryptosporidium proliferansa [OR791084] | |||||||
| 2742_P.2 | C | C [OR791778] | C | |||||
| 2744_P.2 | C | C | C | |||||
| 2747_P.2 | C | D | D | |||||
| 2748_P.2 | C | D | D [OR791784] | |||||
| 2751_P.2 | C [OR791775] | C | D | |||||
| 2753_P.2 | C | D | D [OR791783] | |||||
| 2757_P.2 | C | C | C | |||||
| 2768_P.3 | C | C | C | |||||
| 2771_P.3 | C | C | C | |||||
| 2772_P.3 | C | N/Ab | N/Ab | |||||
| 2773_P.3 | C | C | C | |||||
| 2775_P.3 | C | C | C | |||||
| 2778_P.3 | C [OR791772] | C | C | |||||
| 2779_P.3 | C | C | C | |||||
| 2780_P.3 | C | C | C | |||||
| 2783_P.4 | II | |||||||
| 2787_P.4 | C | C | C | |||||
| 2789_P.4 | PtEbIX | |||||||
| 2696_P.5 | C | N/Ab | N/Ab | |||||
| 2699_P.5 | D [OR807726] | |||||||
| Czech Republic | 2965_CZ.1 | PtEbIX | ||||||
| 2966_CZ.1 | C [OR791771] | D | D | PtEbIX | ||||
| 2973_CZ.1 | PtEbIX | |||||||
| 2977_CZ.1 | PtEbIX | |||||||
| 2985_CZ.2 | PtEbIX | |||||||
| 2986_CZ.2 | PtEbIX | |||||||
| 2987_CZ.2 | PtEbIX | |||||||
| 2989_CZ.2 | PtEbIX | |||||||
| 3010_CZ.4 | C | D | N/Ab | |||||
| 3027_CZ.4 | C [OR791777] | C | D | |||||
| 3030_CZ.5 | C | D | D | |||||
| 3031_CZ.5 | C | D | D | PtEbIX | ||||
| 3032_CZ.5 | PtEbIX | |||||||
| 3036_CZ.5 | C [OR791773] | C | D | |||||
| 3041_CZ.5 | C | C | C [OR791781] | |||||
| 3044_CZ.5 | PtEbIX | |||||||
| 3047_CZ.5 | PtEbIX | |||||||
| 3048_CZ.5 | PtEbIX | |||||||
| 3049_CZ.5 | PtEbIX | |||||||
Accession numbers in square brackets indicate the isolates deposited in GenBank as representative nucleotide sequences derived from the present study.
TPI, triosephosphate isomerase; BG, β-giardin; GDH, glutamate dehydrogenase; 18S rRNA, small ribosomal subunit rRNA; gp60, 60-kDa glycoprotein; ITS, internal transcribed spacer region of rRNA.
Cryptosporidium sp. isolate 2735_P.2, phylogenetically clustered near the gastric Cryptosporidium spp., differed from C. proliferans in 3 SNPs with 99.6% sequence identity of the 18S rRNA region.
N/A, assemblage not available (subtyping failed).
Figure 2.
Phylogenetic relationships between Cryptosporidium spp. detected in dogs in this study (highlighted in green) and other Cryptosporidium available in GenBank using an ML analysis of partial sequences of 18S rRNA (sequence alignment length: 820 bp). Percentage supports (>50%) from 1000 pseudoreplicates are indicated next to the supported node. The branch length scale bar indicates the number of substitutions per site. Sequences from this study are identified by an isolate number (e.g. 2806) followed by region and location (P.1, Poland location 1).
Figure 3.
Phylogenetic relationship between Cryptosporidium canis detected in 1 dog in this study (highlighted in green) and other C. canis available in GenBank using an ML analysis of a region of gp60 gene (sequence alignment length: 540 bp). Percentage supports (>50%) from 1000 pseudoreplicates are indicated next to the supported node. The branch length scale bar indicates the number of substitutions per site. Sequences from this study are identified by an isolate number (2806) followed by region and location (P.1, Poland location 1).
Figure 4.
Phylogenetic relationships between Giardia intestinalis assemblages detected in dogs in this study (highlighted in green – Poland or in grey – Czech Republic) and other G. intestinalis assemblages available in GenBank using an ML analysis of a region of TPI gene (sequence alignment length: 467 bp). Percentage supports (>50%) from 1000 pseudoreplicates are indicated next to the supported node. The branch length scale bar indicates the number of substitutions per site. Sequences from this study are identified by an isolate number (e.g. 2966) followed by region and location (P.1, Poland location 1, CZ.1, Czech Republic location 1).
Figure 5.
Phylogenetic relationships between G. intestinalis assemblages detected in dogs in this study (highlighted in green – Poland or in grey – Czech Republic) and other G. intestinalis assemblages available in GenBank using an ML analysis of a region of BG gene (sequence alignment length: 820 bp). Percentage supports (>50%) from 1000 pseudoreplicates are indicated next to the supported node. The branch length scale bar indicates the number of substitutions per site. Sequences from this study are identified by an isolate number (e.g. 2966) followed by region and location (P.1, Poland location 1, CZ.1, Czech Republic location 1).
Figure 6.
Phylogenetic relationships between G. intestinalis assemblages detected in dogs in this study (highlighted in green – Poland or in grey – Czech Republic) and other G. intestinalis assemblages available in GenBank using an ML analysis of a region of GDH gene (sequence alignment length: 439 bp). Percentage supports (>50%) from 1000 pseudoreplicates are indicated next to the supported node. The branch length scale bar indicates the number of substitutions per site. Sequences from this study are identified by an isolate number (e.g. 2966) followed by region and location (P.1, Poland location 1, CZ.1, Czech Republic location 1).
Figure 7.
Phylogenetic relationships between Enterocytozoon bieneusi genotypes detected in dogs in this study (highlighted in green – Poland or in grey – Czech Republic) and other E. bieneusi genotypes available in GenBank using an ML analysis of ITS region of rRNA gene (sequence alignment length: 309 bp). Percentage supports (>50%) from 1000 pseudoreplicates are indicated next to the supported node. The branch length scale bar indicates the number of substitutions per site. Sequences from this study are identified by an isolate number (e.g. 2973) followed by region and location (P.1, Poland location 1, CZ.1, Czech Republic location 1).
Discussion
In the present study, the screening of fecal samples collected from shelter dogs in central Europe was performed in order to analyse the prevalence of zoonotic unicellular pathogens (Cryptosporidium spp., G. intestinalis, E. bieneusi and Encephalitozoon spp.) at the molecular level. Over a quarter of the tested dogs were carriers of at least one of the studied pathogens, among which the most often observed was G. intestinalis – one of the most common intestinal parasites infecting humans and animals (Kváč et al., 2017). Nevertheless, its prevalence in other European canine populations, including shelter dogs, has shown to be even higher [27–36.5% in various regions of Spain (Gil et al., 2017; Adell-Aledón et al., 2018; Remesar et al., 2022), 33.8% in Portugal (Pereira et al., 2021), over 45% in Serbia (Sommer et al., 2017), while in central Italy this value ranged from about 7% (Scaramozzino et al., 2018) to 41% (Agresti et al., 2022)]. Generally, such a high prevalence is most likely due to the simple and direct life cycle of Giardia, with easily spread dispersive forms excreted in feces, which facilitates transmission in highly dense populations, such as those found in animal shelters. Enterocytozoon bieneusi was also found to be a common pathogen in dogs in the present study, with an overall prevalence of 10.2%, which agrees with previous reports considering European dogs, with infection rates ranging between 4.9 and 11.7% (Mathis et al., 2005; Santín and Fayer, 2011; Piekarska et al., 2017). In turn, considerably low prevalences were observed for Encephalitozoon ssp. and Cryptosporidium spp. (~1% for both), comparably to previous reports regarding canine populations: 0–2.4% for Encephalitozoon spp. (Piekarska et al., 2017; Delrobaei et al., 2019) and 0.6–4.9% for Cryptosporidium spp. (Giangaspero et al., 2006; Simonato et al., 2017; Yu et al., 2018; Piekara-Stępińska et al., 2021a), although in 1 German study the Cryptosporidium prevalence was as high as 10% (Murnik et al., 2022). Notwithstanding, the true prevalence of these pathogens among healthy hosts may, in fact, be higher as their forms are excreted periodically and irregularly, which may be overlooked with a single sampling (Sak et al., 2010). It would therefore be recommended to collect samples several times from the same animals, which may prove difficult due to the conditions specific to the shelters, such as the rotation of animals or irregular hours of cleaning the excrement. Nevertheless, the fact that the demonstrated prevalence of pathogens such as G. intestinalis was high even with only 1 sampling underscores the importance of their likely distribution in the population and the wide reservoir of the pathogen. Differences in the frequencies of the studied species between Polish and Czech dogs, as well as the higher prevalence of specific pathogens in individual facilities, may be related to some specific conditions typical of a particular location.
Sequence analyses of detected pathogens showed that most infections involved dog-specific genotypes or species, the transmission of which may be favoured by intensive contact among large numbers of dogs living together. In the case of 12 isolates, assignment to the appropriate G. intestinalis assemblage was difficult because subtyping results varied depending on the locus used. Similar discrepancies have been reported in previous studies (Read et al., 2004; Traub et al., 2004), emphasizing the importance of using multilocus genotyping in the molecular analysis of Giardia diversity. Nevertheless, all G. intestinalis-positive samples harboured assemblage C or D, which have a strong host specificity for dogs and other canines (Feng and Xiao, 2011). These assemblages were also found to be highly prevalent in different dog populations in Europe (Simonato et al., 2015; Adell-Aledón et al., 2018; Pereira et al., 2021) and although sporadically they have been reported in humans as well (Broglia et al., 2013; Liu et al., 2014; Villamizar et al., 2019), their zoonotic relevance seems to be low and limited to individuals at risk, for instance, children or immunocompromised persons. Likewise, cases of C. canis colonization in humans were described in individuals at increased risk (children, HIV-infected adults) and immunocompetent people as well (Learmonth et al., 2004; Gatei et al., 2007; Feng et al., 2012; Liao et al., 2020). However, due to the relatively transient nature of these infections in humans, dogs do not seem to represent an important source of cryptosporidiosis for people (Villamizar et al., 2019; Liao et al., 2020). To date, 9 families of C. canis subtypes (XXa–XXi) have been identified based on gp60 locus subtyping, occurring not only in canids, but also in minks, foxes and humans (Jiang et al., 2021; Murnik et al., 2022; Wang et al., 2022). According to the study of Jiang et al., the zoonotic potential may concern XXa family, detected in both dog and human samples (Jiang et al., 2021). In our study, analysis of the C. canis gp60 locus in the 2806_P.1 isolate revealed that it belongs to the XXe family (Fig. 3), which was also the most prevalent among dogs in the report from Germany (Murnik et al., 2022). In turn, the newly detected Cryptosporidium sp. isolate 2735_P.2 also does not pose a significant risk to humans and probably not to dogs as well. An incidental infection/contamination was likely caused by rodent feces. Cryptosporidium sp. isolate 2735_P.2 is closely related to Cryptosporidium muris and C. proliferans, whose hosts are rodents. However, the phylogenetic position is not related to host specificity, and therefore another host cannot be excluded (Kváč et al., 2018). To summarize the subtyping results, as in previous studies (de Lucio et al., 2017; Rehbein et al., 2019), zoonotic transmission of giardiasis or cryptosporidiosis between dogs and humans is most likely a rare event.
All E. bieneusi isolates detected in the studied dogs, except for 1 clustering to genotype D, were identical to E. bieneusi genotype PtEbIX. This genotype appears to be specific to dogs; to date, it has been detected almost exclusively in dogs and sporadically in wolves, cats and swans (Santín et al., 2008; Abe et al., 2009; Santín and Fayer, 2011; Mori et al., 2013; Karim et al., 2014a; Piekarska et al., 2017; Kváč et al., 2021). In turn, both E. bieneusi genotype D and E. cuniculi genotype II detected in the present study have been reported in a broad range of hosts so far, including humans (Li et al., 2012; Kváč et al., 2017; Piekarska et al., 2017; Delrobaei et al., 2019). Observation of microsporidian genotypes with a human-infection capacity in companion animals suggests that pets may be of importance as one of the potential sources of infection. However, the presented results do not indicate that dogs in shelters in Poland and the Czech Republic represent a significant source of zoonotic species and genotypes of the studied parasites for humans.
Our study had some limitations. Firstly, due to its screening nature, a detailed analysis in the context of the demographic data of the tested dogs or the drugs used was not possible to conduct. Moreover, the study groups differed in size – in Poland it was possible to collect material from a larger number of animals than in the Czech Republic, which may have an impact on the differences in prevalence.
This study included clinically healthy animals without signs of intestinal infection, yet dispersive forms of potentially pathogenic and infectious organisms were observed. It should also be borne in mind that asymptomatic hosts could shed cysts, oocysts or spores occasionally and irregularly, and thus their screening with multiple sampling could increase the real observed prevalence (Sak et al., 2010). Nevertheless, since infections of the studied pathogens in dogs can often be asymptomatic, they may not be detected by routine veterinary examinations, and their occurrence in companion animals may be underestimated. On the contrary, the majority of species and genotypes observed in canine samples herein are not commonly associated with human infections, and aforesaid transmission routes seem to be rare. The exceptions are genotypes D (E. bieneusi) and II (E. cuniculi) observed in the present study, whose zoonotic potential should be emphasized due to their occurrence in a wide range of different hosts (Hinney et al., 2016; Li et al., 2019, 2020). Despite the low likelihood of transmission of the studied pathogens and due to the fact that they mainly affect immunosuppressed individuals, in whom the consequences of opportunistic infections may be life-threatening, the awareness among new dog owners is recommended, especially those with various levels of immunosuppression, on the relevance of diagnosing and treating zoonotic diseases.
Supporting information
Szydłowicz et al. supplementary material
Szydłowicz et al. supplementary material
Acknowledgements
The authors acknowledge the staff of dog shelters for their assistance in sample collection and the technician staff of the Laboratory of Veterinary and Medical Protistology, Biology Centre CAS, for sample processing during this study.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118202400009X
Data availability statement
The data that support the findings of this study are available from the corresponding author, MS, upon reasonable request.
Author's contribution
M. S., M. Ki. and Ż. Z. conceived and designed the study. M. S., M. Ki., Ż. Z., M. Ka., M. Kv., B. S. and N. H. conducted data gathering. M. S., M. Ki., Ż. Z., M. Ka., B. Ł. and A. L. prepared the samples and conducted molecular analysis. M. S. conducted statistical analyses. M. Kv. prepared phylogenetic analyses. M. S., M. Ki. and M. Kv. wrote the article.
Financial support
This work was supported with a subvention by the Polish Ministry of Health (M. S., grant number SUBK.A060.23.027 from the IT Simple system of Wroclaw Medical University) and the Grant Agency of the Czech Republic (M. Kv., GACR 21-23773S and B. S. 23-06571S).
Competing interests
None.
Ethical standards
The collection of samples carried out in a non-invasive way, without interfering with the organism of the animals included in the study.
References
- Abe N, Kimata I and Iseki M (2009) Molecular evidence of Enterocytozoon bieneusi in Japan. Journal of Veterinary Medical Science 71, 217–219. [DOI] [PubMed] [Google Scholar]
- Adell-Aledón M, Köster PC, de Lucio A, Puente P, Hernández-de-Mingo M, Sánchez-Thevenet P, Dea-Ayuela MA and Carmena D (2018) Occurrence and molecular epidemiology of Giardia duodenalis infection in dog populations in eastern Spain. BMC Veterinary Research 14, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A, Berrilli F, Maestrini M, Procesi IG, Loretti E, Vonci N and Perrucci S (2022) Prevalence, risk factors and genotypes of Giardia duodenalis in sheltered dogs in Tuscany (Central Italy). Pathogens (Basel, Switzerland) 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderisio KA, Mergen K, Moessner H and Madison-Antenucci S (2023) Identification and evaluation of Cryptosporidium species from New York City cases of cryptosporidiosis (2015 to 2018): a watershed perspective. Microbiology Spectrum 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer A and Bednarska M (2007) Cryptosporidium spp. and Giardia spp. infections in sled dogs. Medycyna Weterynaryjna 63, 681–687. [Google Scholar]
- Bouzid M, Halai K, Jeffreys D and Hunter PR (2015) The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples. Veterinary Parasitology 207, 181–202. [DOI] [PubMed] [Google Scholar]
- Broglia A, Weitzel T, Harms G, Cacció SM and Nöckler K (2013) Molecular typing of Giardia duodenalis isolates from German travellers. Parasitology Research 112, 3449–3456. [DOI] [PubMed] [Google Scholar]
- Buckholt MA, Lee JH and Tzipori S (2002) Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Applied and Environmental Microbiology 68, 2595–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò SM, De Giacomo M and Pozio E (2002) Sequence analysis of the β-giardin gene and development of a polymerase chain reaction–restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. International Journal for Parasitology 32, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Cacciò SM, Beck R, Lalle M, Marinculic A and Pozio E (2008) Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. International Journal for Parasitology 38, 1523–1531. [DOI] [PubMed] [Google Scholar]
- Delrobaei M, Jamshidi S, Shayan P, Ebrahimzade E, Ashrafi Tamai I, Rezaeian M and Mirjalali H (2019) Molecular detection and genotyping of intestinal microsporidia from stray dogs in Iran. Iranian Journal of Parasitology 14, 159–166. [PMC free article] [PubMed] [Google Scholar]
- de Lucio A, Bailo B, Aguilera M, Cardona GA, Fernández-Crespo JC and Carmena D (2017) No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Álava, northern Spain. Acta Tropica 170, 48–56. [DOI] [PubMed] [Google Scholar]
- Dengjel B, Zahler M, Hermanns W, Heinritzi K, Spillmann T, Thomschke A, Löscher T, Gothe R and Rinder H (2001) Zoonotic potential of Enterocytozoon bieneusi. Journal of Clinical Microbiology 39, 4495–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES, Vossbrinck CR, Baker MD, Rogers LB, Bertucci DC and Shadduck JA (1995) Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 111, 411–421. [DOI] [PubMed] [Google Scholar]
- Didier ES, Didier PJ, Snowden KF and Shadduck JA (2000) Microsporidiosis in mammals. Microbes and Infection 2, 709–720. [DOI] [PubMed] [Google Scholar]
- Dubná S, Langrová I, Nápravník J, Jankovská I, Vadlejch J, Pekár S and Fechtner J (2007) The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Veterinary Parasitology 145, 120–128. [DOI] [PubMed] [Google Scholar]
- European Scientific Counsel Companion Animal Parasites (ESCCAP) (2018) Control of intestinal protozoa in dogs and cats. Available at https://www.esccap.org/guidelines/gl6/ (accessed 13 January 2024).
- Feng Y and Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clinical Microbiology Reviews 24, 110–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Wang L, Duan L, Gomez-Puerta LA, Zhang L, Zhao X, Hu J, Zhang N and Xiao L (2012) Extended outbreak of cryptosporidiosis in a pediatric hospital, China. Emerging Infectious Diseases 18, 312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei W, Das P, Dutta P, Sen A, Cama V, Lal AA and Xiao L (2007) Multilocus sequence typing and genetic structure of Cryptosporidium hominis from children in Kolkata, India. Infection, Genetics and Evolution 7, 197–205. [DOI] [PubMed] [Google Scholar]
- General Veterinary Inspectorate (2023) Annual report on visits to animal shelters for 2022. Available at https://www.wetgiw.gov.pl/nadzor-weterynaryjny/schroniska-dla-bezdomnych-zwierzat (accessed 13 January 2024).
- Giangaspero A, Iorio R, Paoletti B, Traversa D and Capelli G (2006) Molecular evidence for Cryptosporidium infection in dogs in central Italy. Parasitology Research 99, 297–299. [DOI] [PubMed] [Google Scholar]
- Gil H, Cano L, de Lucio A, Bailo B, de Mingo MH, Cardona GA, Fernández-Basterra JA, Aramburu-Aguirre J, López-Molina N and Carmena D (2017) Detection and molecular diversity of Giardia duodenalis and Cryptosporidium spp. in sheltered dogs and cats in northern Spain. Infection, Genetics and Evolution 50, 62–69. [DOI] [PubMed] [Google Scholar]
- Hall, T A (1999) BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 95–98.
- Hinney B, Sak B, Joachim A and Kváč M (2016) More than a rabbit's tale – Encephalitozoon spp. in wild mammals and birds. International Journal for Parasitology: Parasites and Wildlife 5, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Roellig DM, Guo Y, Li N, Feng Y and Xiao L (2021) Development of a subtyping tool for zoonotic pathogen Cryptosporidium canis. Journal of Clinical Microbiology 59, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Dong H, Yu F, Jian F, Zhang L, Wang R, Zhang S, Rume FI, Ning C and Xiao L (2014a) Genetic diversity in Enterocytozoon bieneusi isolates from dogs and cats in China: host specificity and public health implications. Journal of Clinical Microbiology 52, 3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Wang R, Dong H, Zhang L, Li J, Zhang S, Rume FI, Qi M, Jian F, Sun M, Yang G, Zou F, Ning C and Xiao L (2014b) Genetic polymorphism and zoonotic potential of Enterocytozoon bieneusi from nonhuman primates in China. Applied and Environmental Microbiology 80, 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzwinkel-Wladarsch S, Lieb M, Helse W, Löscher T and Rinder H (1996) Direct amplification and species determination of microsporidian DNA from stool specimens. Tropical Medicine & International Health 1, 373–378. [DOI] [PubMed] [Google Scholar]
- Kicia M, Wesolowska M, Jakuszko K, Kopacz Z, Sak B, Květonova D, Krajewska M and Kváč M (2014) Concurrent infection of the urinary tract with Encephalitozoon cuniculi and Enterocytozoon bieneusi in a renal transplant recipient. Journal of Clinical Microbiology 52, 1780–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicia M, Wesolowska M, Kopacz Z, Jakuszko K, Sak B, Květonová D, Krajewska M and Kváč M (2016) Prevalence and molecular characteristics of urinary and intestinal microsporidia infections in renal transplant recipients. Clinical Microbiology and Infection 22, 462.e5–462.e9. [DOI] [PubMed] [Google Scholar]
- Kicia M, Szydłowicz M, Cebulski K, Jakuszko K, Piesiak P, Kowal A, Sak B, Krajewska M, Hendrich AB, Kváč M and Kopacz Ż (2019) Symptomatic respiratory Encephalitozoon cuniculi infection in renal transplant recipients. International Journal of Infectious Diseases 79, 21–25. [DOI] [PubMed] [Google Scholar]
- Kozisek F, Craun GF, Cerovska L, Pumann P, Frost F and Muller T (2008) Serological responses to Cryptosporidium-specific antigens in Czech populations with different water sources. Epidemiology and Infection 136, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G and Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kváč M, Hofmannová L, Ortega Y, Holubová N, Horčičková M, Kicia M, Hlásková L, Květoňová D, Sak B and McEvoy J (2017) Stray cats are more frequently infected with zoonotic protists than pet cats. Folia Parasitologica 64, 034. doi: 10.14411/fp.2017.034 [DOI] [PubMed] [Google Scholar]
- Kváč M, Vlnatá G, Ježková J, Horčičková M, Konečný R, Hlásková L, McEvoy J and Sak B (2018) Cryptosporidium occultus sp. n. (Apicomplexa: Cryptosporidiidae) in rats. European Journal of Protistology 63, 96–104. [DOI] [PubMed] [Google Scholar]
- Kváč M, Myšková E, Holubová N, Kellnerová K, Kicia M, Rajský D, McEvoy J, Feng Y, Hanzal V and Sak B (2021) Occurrence and genetic diversity of Cryptosporidium spp. in wild foxes, wolves, jackals, and bears in central Europe. Folia Parasitologica 68, 1–8. [DOI] [PubMed] [Google Scholar]
- Lalle M, Jimenez-Cardosa E, Cacciò SM and Pozio E (2005) Genotyping of Giardia duodenalis from humans and dogs from Mexico using a β-giardin nested polymerase chain reaction assay. Journal of Parasitology 91, 203–205. [DOI] [PubMed] [Google Scholar]
- Learmonth JJ, Ionas G, Ebbett KA and Kwan ES (2004) Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Applied and Environmental Microbiology 70, 3973–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xiao L, Wang L, Zhao S, Zhao X, Duan L, Guo M, Liu L and Feng Y (2012) Molecular surveillance of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Neglected Tropical Diseases 6, e1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Y and Santin M (2019) Host specificity of Enterocytozoon bieneusi and public health implications. Trends in Parasitology 35, 436–451. [DOI] [PubMed] [Google Scholar]
- Li W, Feng Y and Xiao L (2020) Diagnosis and molecular typing of Enterocytozoon bieneusi: the significant role of domestic animals in transmission of human microsporidiosis. Research in Veterinary Science 133, 251–261. [DOI] [PubMed] [Google Scholar]
- Li J, Ryan U, Guo Y, Feng Y and Xiao L (2021) Advances in molecular epidemiology of cryptosporidiosis in dogs and cats. International Journal for Parasitology 51, 787–795. [DOI] [PubMed] [Google Scholar]
- Liao S, Lin X, Sun Y, Qi N, Lv M, Wu C, Li J, Hu J, Yu L, Cai H, Xiao W, Sun M and Li G (2020) Occurrence and genotypes of Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. in household, shelter, breeding, and pet market dogs in Guangzhou, southern China. Scientific Reports 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Shen Y, Yin J, Yuan Z, Jiang Y, Xu Y, Pan W, Hu Y and Cao J (2014) Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infectious Diseases 14, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Weber R and Deplazes P (2005) Zoonotic potential of the microsporidia. Clinical Microbiology Reviews 18, 423–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Mahittikorn A, Thammasonthijarern N, Chaisiri K, Rojekittikhun W and Sukthana Y (2013) Presence of zoonotic Enterocytozoon bieneusi in cats in a temple in central Thailand. Veterinary Parasitology 197, 696–701. [DOI] [PubMed] [Google Scholar]
- Murnik L-C, Daugschies A and Delling C (2022) Cryptosporidium infection in young dogs from Germany. Parasitology Research 121, 2985–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A, Teixeira J, Sousa S, Parreira R, Campino L, Meireles J and Maia C (2021) Giardia duodenalis infection in dogs from the metropolitan area of Lisbon, Portugal: prevalence, genotyping and associated risk factors. Journal of Parasitic Diseases 45, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekara-Stępińska A, Piekarska J and Gorczykowski M (2021a) Cryptosporidium spp. in dogs and cats in Poland. Annals of Agricultural and Environmental Medicine 28, 345–347. [DOI] [PubMed] [Google Scholar]
- Piekara-Stępińska A, Piekarska J, Gorczykowski M and Bania J (2021b) Genotypes of Giardia duodenalis in household dogs and cats from Poland. Acta Parasitologica 66, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarska J, Bajzert J, Gorczykowski M, Kantyka M and Podkowik M (2016) Molecular identification of Giardia duodenalis isolates from domestic dogs and cats in Wroclaw, Poland. Annals of Agricultural and Environmental Medicine 23, 410–415. [DOI] [PubMed] [Google Scholar]
- Piekarska J, Kicia M, Wesołowska M, Kopacz Ż, Gorczykowski M, Szczepankiewicz B, Kváč M and Sak B (2017) Zoonotic microsporidia in dogs and cats in Poland. Veterinary Parasitology 246, 108–111. [DOI] [PubMed] [Google Scholar]
- Plutzer J, Lassen B, Jokelainen P, Djurković-Djaković O, Kucsera I, Dorbek-Kolin E, Šoba B, Sréter T, Imre K, Omeragić J, Nikolić A, Bobić B, Živičnjak T, Lučinger S, Stefanović LL, Kučinar J, Sroka J, Deksne G, Keidāne D, Kváč M, Hůzová Z and Karanis P (2018) Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Eurosurveillance 23, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Rand J, Qamar AG, Jabbar A and Kopp S (2018) Gastrointestinal parasites in shelter dogs: occurrence, pathology, treatment and risk to shelter workers. Animals 8, 108. doi: 10.3390/ani8070108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read CM, Monis PT and Andrew Thompson RC (2004) Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infection, Genetics and Evolution 4, 125–130. [DOI] [PubMed] [Google Scholar]
- Rehbein S, Klotz C, Ignatius R, Müller E, Aebischer A and Kohn B (2019) Giardia duodenalis in small animals and their owners in Germany: a pilot study. Zoonoses and Public Health 66, 117–124. [DOI] [PubMed] [Google Scholar]
- Remesar S, García-Dios D, Calabuig N, Prieto A, Díaz-Cao JM, López-Lorenzo G, López C, Fernández G, Morrondo P, Panadero R and Díaz P (2022) Cardiorespiratory nematodes and co-infections with gastrointestinal parasites in new arrivals at dog and cat shelters in north-western Spain. Transboundary and Emerging Diseases 69, e3141–e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U and Zahedi A (2019) Molecular epidemiology of giardiasis from a veterinary perspective. Advances in Parasitology 106, 209–254. [DOI] [PubMed] [Google Scholar]
- Sak B, Kašičková D, Kváč M, Květoňová D and Ditrich O (2010) Microsporidia in exotic birds: intermittent spore excretion of Encephalitozoon spp. in naturally infected budgerigars (Melopsittacus undulatus). Veterinary Parasitology 168, 196–200. [DOI] [PubMed] [Google Scholar]
- Sak B, Brady D, Pelikánová M, Květoňová D, Rost M, Kostka M, Tolarová V, Hůzová Z and Kváč M (2011) Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. Journal of Clinical Microbiology 49, 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santín M and Fayer R (2011) Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Research in Veterinary Science 90, 363–371. [DOI] [PubMed] [Google Scholar]
- Santín M, Vecino JAC and Fayer R (2008) Enterocytozoon bieneusi genotypes in dogs in Bogota, Colombia. The American Journal of Tropical Medicine and Hygiene 79, 215–217. [PubMed] [Google Scholar]
- Scaramozzino P, Carvelli A, Iacoponi F and De Liberato C (2018) Endoparasites in household and shelter dogs from central Italy. International Journal of Veterinary Science and Medicine 6, 45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato G, Frangipane di Regalbono A, Cassini R, Traversa D, Beraldo P, Tessarin C and Pietrobelli M (2015) Copromicroscopic and molecular investigations on intestinal parasites in kenneled dogs. Parasitology Research 114, 1963–1970. [DOI] [PubMed] [Google Scholar]
- Simonato G, Frangipane di Regalbono A, Cassini R, Traversa D, Tessarin C, Di Cesare A and Pietrobelli M (2017) Molecular detection of Giardia duodenalis and Cryptosporidium spp. in canine faecal samples contaminating public areas in northern Italy. Parasitology Research 116, 3411–3418. [DOI] [PubMed] [Google Scholar]
- Soliman RH, Fuentes I and Rubio JM (2011) Identification of a novel assemblage B subgenotype and a zoonotic assemblage C in human isolates of Giardia intestinalis in Egypt. Parasitology International 60, 507–511. [DOI] [PubMed] [Google Scholar]
- Sommer MF, Zdravković N, Vasić A, Grimm F and Silaghi C (2017) Gastrointestinal parasites in shelter dogs from Belgrade, Serbia. Veterinary Parasitology: Regional Studies and Reports 7, 54–57. [DOI] [PubMed] [Google Scholar]
- State Veterinary Administration (2024) Registered animal shelters. Available at https://www.svscr.cz/registrovane-subjekty-svs/registrovane-utulky-pro-zvirata/ (accessed 13 January 2024).
- Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA and Xiao L (2003) Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerging Infectious Diseases 9, 1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ, Monis PT, Robertson I, Irwin P, Mencke N and Thompson RCA (2004) Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology 128, 253–262. [DOI] [PubMed] [Google Scholar]
- Tůmová L, Ježková J, Prediger J, Holubová N, Sak B, Konečný R, Květoňová D, Hlásková L, Rost M, McEvoy J, Xiao L, Santín M and Kváč M (2023) Cryptosporidium mortiferum n. sp. (Apicomplexa: Cryptosporidiidae), the species causing lethal cryptosporidiosis in Eurasian red squirrels (Sciurus vulgaris). Parasites & Vectors 16, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamizar X, Higuera A, Herrera G, Vasquez-A LR, Buitron L, Muñoz LM, Gonzalez-C FE, Lopez MC, Giraldo JC and Ramírez JD (2019) Molecular and descriptive epidemiology of intestinal protozoan parasites of children and their pets in Cauca, Colombia: a cross-sectional study. BMC Infectious Diseases 19, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wei Y, Cao S, Wu W, Zhao W, Guo Y, Xiao L, Feng Y and Li N (2022) Divergent Cryptosporidium species and host-adapted Cryptosporidium canis subtypes in farmed minks, raccoon dogs and foxes in Shandong, China. Frontiers in Cellular and Infection Microbiology 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L (2010) Molecular epidemiology of cryptosporidiosis: an update. Experimental Parasitology 124, 80–89. [DOI] [PubMed] [Google Scholar]
- Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, Shulaw W, Thompson RC, Fayer R and Lal AA (1999) Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Applied and Environmental Microbiology 65, 3386–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Cama VA, Cabrera L, Ortega Y, Pearson J and Gilman RH (2007) Possible transmission of Cryptosporidium canis among children and a dog in a household. Journal of Clinical Microbiology 45, 2014–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jin Y, Wu W, Li P, Wang L, Li N, Feng Y and Xiao L (2016) Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasites & Vectors 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Ruan Y, Zhou M, Chen S, Zhang Y, Wang L, Zhu G and Yu Y (2018) Prevalence of intestinal parasites in companion dogs with diarrhea in Beijing, China, and genetic characteristics of Giardia and Cryptosporidium species. Parasitology Research 117, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanová I, Husník R and Svobodová V (2005) Giardia intestinalis u psů – výskyt, zoonotický potenciál a využití endoskopické diagnostiky. [Giardia intestinalis in dogs – occurrence, zoonotic potential and use of endoscopic diagnostic]. Veterinarstvi 55, 319–325. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Szydłowicz et al. supplementary material
Szydłowicz et al. supplementary material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MS, upon reasonable request.