Abstract
Despite major advances, our understanding of the neurobiology of life course socioeconomic conditions is still scarce. This study aimed to provide insight into the pathways linking socioeconomic exposures—household income, last known occupational position, and life course socioeconomic trajectories—with brain microstructure and cognitive performance in middle to late adulthood. We assessed socioeconomic conditions alongside quantitative relaxometry and diffusion-weighted magnetic resonance imaging indicators of brain tissue microstructure and cognitive performance in a sample of community-dwelling men and women (N = 751, aged 50–91 years). We adjusted the applied regression analyses and structural equation models for the linear and nonlinear effects of age, sex, education, cardiovascular risk factors, and the presence of depression, anxiety, and substance use disorders. Individuals from lower-income households showed signs of advanced brain white matter (WM) aging with greater mean diffusivity (MD), lower neurite density, lower myelination, and lower iron content. The association between household income and MD was mediated by neurite density (B = 0.084, p = 0.003) and myelination (B = 0.019, p = 0.009); MD partially mediated the association between household income and cognitive performance (B = 0.017, p < 0.05). Household income moderated the relation between WM microstructure and cognitive performance, such that greater MD, lower myelination, or lower neurite density was only associated with poorer cognitive performance among individuals from lower-income households. Individuals from higher-income households showed preserved cognitive performance even with greater MD, lower myelination, or lower neurite density. These findings provide novel mechanistic insights into the associations between socioeconomic conditions, brain anatomy, and cognitive performance in middle to late adulthood.
Keywords: brain white matter microstructure, cognition, social inequalities
Significance Statement
Pathways linking socioeconomic conditions, brain anatomy, and cognitive performance have rarely been investigated. Using multicontrast imaging, we found that individuals from lower-income households had markers of advanced brain white matter (WM) aging with a lower neurite density, lower myelination, and lower iron content, alongside a greater mean diffusivity (MD). Greater MD (reflecting myelin and neurite density) contributed to the association between household income and cognitive performance. Household income also buffered the observed WM effects, such that a greater MD, lower index of myelin content, or lower neurite density were only associated with poorer cognitive performance among individuals from lower-income households. These findings provide a detailed neurobiological understanding of socioeconomic differences in the brain anatomy and associated cognitive performance.
Introduction
Exposure to chronic socioeconomic disadvantage is associated with dysregulation in multiple physiological systems, which contributes to age-related diseases, cognitive decline, and mortality (Cunliffe, 2016; Belsky et al., 2017; Kim et al., 2018; Berger et al., 2019; Schrempft et al., 2022). On the contrary, living in an enriched environment, characterized by, for example, higher levels of educational attainment and engagement in complex occupations, is thought to support neurodevelopment and compensate for age-related decline (Stern, 2009; Ihle et al., 2018).
Existing studies examining associations between socioeconomic conditions and brain health have primarily focused on macrostructural measures, such as brain volume, cortical thickness, and surface area rather than the underlying tissue microstructure. These studies have generally found that socioeconomic disadvantage in both childhood and adulthood predicts reduced total gray and white matter (WM) volume, reduced cortical surface area and cortical thickness, and reduced volume of regions implicated in cognitive and emotional processing (see Farah, 2017, and Yaple and Yu, 2020, for a review).
Changes in the underlying tissue microstructure may precede macrostructural and functional changes in brain health and may be better predictors of age and cognitive decline (Bartzokis, 2004; Metzler-Baddeley et al., 2019; Hayek et al., 2020). Indeed, with increasing age, there is significant demyelination in human WM (Draganski et al., 2011; Callaghan et al., 2014; Slater et al., 2019), as well as a decreased axonal density (Cox et al., 2016; Slater et al., 2019) and increased fiber dispersion (Slater et al., 2019). These tissue property changes lead to inefficient signal transmission between cortical regions and are believed to underlie aging-related cognitive decline, such as reduced processing speed, executive dysfunction, poorer memory, and decline in fluid intelligence (Bennett and Madden, 2014; Ritchie et al., 2015).
Although WM microstructure is known to be integral to cognitive function, little is known about the role it plays in the relationship between socioeconomic disadvantage and cognitive performance. Previous studies using diffusion tensor imaging (DTI) found that socioeconomic disadvantage in childhood (Gullick et al., 2016; Ursache and Noble, 2016; Dufford and Kim, 2017; Johnson et al., 2021) and adulthood (Gianaros et al., 2013; Johnson et al., 2013; Noble et al., 2013; Shaked et al., 2019) is associated with a lower fractional anisotropy (indicative of fiber orientation) in multiple brain regions. However, the robustness and interpretation of fractional anisotropy have recently been questioned given the inability of the underlying tensor model to deal with the abundant fiber crossings in the human brain's WM (Jeurissen et al., 2013; Figley et al., 2022). Differences in DTI-derived indices are open to several biological interpretations, including changes in axonal dispersion, myelination, and/or axonal densities (Beaulieu, 2002; Jones et al., 2013).
The advent of new, advanced magnetic resonance imaging (MRI) techniques can provide further insight into the neurobiology of processes relevant to aging. Quantitative magnetic resonance imaging (qMRI) relaxometry allows for the estimation of parameters indicative of the brain tissue myelin and iron content across cortical areas, subcortical areas, and within the WM (Draganski et al., 2011; Weiskopf et al., 2021). Additionally, it reduces the probability of spurious morphometric findings that may result when using the conventional T1-weighted MRI (Lorio et al., 2016). Neurite orientation dispersion and density imaging (NODDI) uses data collected at different diffusion weightings (shells) and a biophysical model where diffusion is modeled as isotropic in free water, hindered in the extracellular space, and restricted within neurites. The latter provides the microstructural metric of the intracellular volume fraction (ICVF), indicative of the packing density of neurites (Zhang et al., 2012). It is complementary to the widespread tensor model using single diffusion weighting (shell), which provides indices of mean diffusivity (MD) and fractional anisotropy (Basser and Pierpaoli, 2011). Here, the interpretation is that the MD increases in areas with structural (cell) loss, whereas differences in fractional anisotropy (indicative of fiber directionality) are open to a wider range of interpretations, particularly in WM areas with crossing fibers (Jones et al., 2013). Recent research using qMRI found that disadvantaged socioeconomic conditions are associated with lower levels of myelin (Loued-Khenissi et al., 2022) and reduced myelin growth across adolescence and young adulthood (Ziegler et al., 2020), thereby potentially contributing to socioeconomic differences in cognitive function (Schrempft et al., 2023).
The present study therefore aims to combine information from multicontrast MRI to provide novel insights into associations between socioeconomic conditions, brain WM anatomy, and cognitive performance in middle- and older-aged adults. Specifically, the study aims to examine whether (1) socioeconomic conditions are associated with more specific measures of brain microstructure including myelin, axonal density, and tract complexity, in addition to the magnitude of water diffusion; (2) differences in myelin, axonal density, and tract complexity explain (mediate) socioeconomic differences in the magnitude of water diffusion; (3) differences in WM microstructure explain (mediate) associations between socioeconomic conditions and cognitive performance; and (4) socioeconomic conditions shape (moderate) associations between WM microstructure and cognitive performance.
Materials and Methods
Data source
The data stemmed from the BrainLaus study (Trofimova et al., 2021, 2023; Loued-Khenissi et al., 2022), which is part of the CoLaus|PsyCoLaus longitudinal cohort (Firmann et al., 2008; Preisig et al., 2009) recruited from 35- to 75-year-old residents of the city of Lausanne, Switzerland (N = 6,734). Physical and psychiatric baseline assessments conducted between 2003 and 2006 were followed by first (2009–2013), second (2014–2018), and third (2018–2021) follow-up evaluations. All participants of the psychiatric evaluation were invited to take part in BrainLaus at the second and third follow-ups. At the third follow-up, BrainLaus participants completed a cognitive test battery at the time of the brain scan. In total, 1,324 individuals participated in BrainLaus at the second follow-up (mean age, 60.5 years; SD, 9.4; 52% women), and 823 individuals participated in BrainLaus at the third follow-up and provided cognitive data (mean age, 63.1 years; SD, 8.9; 50% women). When comparing those who participated in BrainLaus at the second follow-up (N = 1,324) with the 823 participants at the third follow-up, there were no significant differences in the distributions of age (5-year age groups), sex, educational level, last known occupational position, or gross household income.
The data from 71 participants were excluded after MRI data quality assessment (as described below), and 1 participant was missing a study ID, which resulted in 751 participants included in the analyses. There were no differences in age, sex, educational level, last known occupational position, or gross household income when comparing the analysis sample (N = 751) with those who did not pass the MRI data quality assessment (N = 71).
Given the absence of substantive differences in tract characteristics between right- and left-handed subjects (Cox et al., 2016), handedness was not assessed. The CoLaus|PsyCoLaus and BrainLaus studies received approval from the local ethics committee, and participants provided written informed consent prior to inclusion in the study.
Socioeconomic conditions
Measures of socioeconomic conditions in adulthood included educational level, last known occupation, and household gross income. Education level was categorized as primary (none or compulsory school), secondary (secondary school or apprenticeship), and tertiary (university). Occupation was measured using the European Socioeconomic Classification framework (Rose and Harrison, 2007) and categorized as lower (lower clerical, services, and sales workers, skilled, semiskilled, and unskilled workers), middle (small employers and self-employed, farmers, lower supervisors, and technicians), and higher (higher professionals and managers, higher clerical, services, and sales workers). The questionnaire item assessing household income had seven different response options with varying increments of income between them: <30,000 CHF, 30,000–49,999 CHF, 50,000–69,999 CHF, 70,000–89,999 CHF, 90,000–109,999 CHF, 110,000–199,999 CHF, and >200,000 CHF. As such, household income could not be implemented as a continuous variable and was instead categorized into tertiles, reflecting lower (≤69,999 CHF), middle (70,000–109,999 CHF), and higher (≥110,000 CHF) gross household incomes. A similar approach has been used in previous research examining links between socioeconomic indicators and brain markers (Dennis et al., 2022), and allowed us to simplify our statistical models without losing nuance. Individuals in the higher tertile had a gross household annual income close to or above the average amount for all households in the canton of Vaud in 2020 (117,000 CHF), at the time of data collection (Statistique Vaud, 2023), while those in the lower tertile were well below the average.
Life course socioeconomic trajectories were calculated using father's occupational position during childhood and participant's last known occupational position, as in previous international epidemiological research (Ben-Shlomo and Kuh, 2002; Melchior et al., 2006; Pearce et al., 2009; Stringhini et al., 2013, 2015; Rocha et al., 2020). Trajectories were defined as stable-low (disadvantaged in childhood and adulthood), downward (advantaged in childhood and average or disadvantaged in adulthood; or average in childhood and disadvantaged in adulthood), stable-mid (average in childhood and adulthood), upward (disadvantaged in childhood and average or advantaged in adulthood; or average in childhood and advantaged in adulthood), and stable-high (advantaged in childhood and adulthood). For analyses using father's occupational position and participant's last known occupational position, participants or fathers who had never worked (due to health or other reasons) were excluded. For each socioeconomic variable, values ranged from 0 (higher) to 1 (lower).
MRI data acquisition and processing
All brain MRI data were acquired on a 3T whole-body MRI system (Magnetom Prisma, Siemens Medical Systems), using a 64-channel radio-frequency (RF) receiver head coil and body coil for transmission.
Multiparametric mapping
The qMRI protocol comprised three multiecho 3D fast low-angle shot (FLASH) acquisitions with magnetization transfer-weighted (MTw; TR, 24.5 ms, α = 6°), proton density-weighted (PDw; TR, 24.5 ms, α = 6°), and T1-weighted (TR, 24.5 ms, α = 21°) contrasts at 1 mm isotropic resolution (Draganski et al., 2011). The B1 mapping data was acquired using a 3D EPI spin-echo/stimulated echo technique (Lutti et al., 2010, 2012) to correct for the effects of RF transmit field inhomogeneities on the qMRI maps (4 mm3 resolution; TE, 39.06 ms; TR, 500 ms). We acquired the B0-field mapping data using a 2D double-echo FLASH sequence to correct for distortions in the EPI scans of slice thickness, 2 mm; TR, 1,020 ms; TE1/TE2, 10/12.46 ms; and α = 90°.
The qMRI maps were calculated from the raw data using the voxel-based quantification toolbox (Draganski et al., 2011; Tabelow et al., 2019). The magnetization transfer (MT) maps were calculated as described in Helms et al. (2008a,b). The maps of the transverse relaxation rate (R2* = 1/T2*) were estimated from the regression of the log signal of the raw FLASH images with the corresponding echo times (Weiskopf et al., 2014).
Diffusion-weighted imaging (DWI)
The diffusion-weighted MRI data were acquired using a 2D EPI sequence with the following parameters: TR/TE, 7,420/69 ms; generalized autocalibrating partially parallel acquisition acceleration factor, 2; FOV, 192 × 212 mm2; matrix size, 96 × 106; 70 axial slices; 2 mm ISO voxel dimension; 118 isotropically distributed diffusion sensitization directions (15 at b = 650 s/mm2, 30 at b = 1,000 s/mm2, and 60 at b = 2,000 s/mm2); and 13 b = 0 images interleaved throughout the acquisition (Slater et al., 2019). The DWI data were preprocessed with MRtrix3 for denoising (Veraart et al., 2016) and Gibbs-ringing artifact removal (Kellner et al., 2016). To correct for eddy current distortions and subject motion, we used the FSL 5.0 EDDY tool (Andersson and Sotiropoulos, 2016), including correction of the gradient directions for subject movement (Leemans and Jones, 2009). We used the B0 maps acquired as part of the qMRI session to correct for EPI susceptibility distortions (Hutton et al., 2002). The bias field was estimated from the mean b = 0 images and corrected for in all the DWI data. The DWI data were then rigid body aligned to the MT images with the aid of the mean b = 0 image.
We estimated the MD maps using the b = 0 s/mm2, b = 650 s/mm2, and b = 1,000 s/mm2 data and MRtrix3 (Veraart et al., 2013). The NODDI model (Zhang et al., 2012) and the AMICO toolbox implementation (Daducci et al., 2015) used the multishell data (all acquired b values) to provide maps for ICVF (a measure of neurite density), isotropic volume fraction (ISOVF; a measure of extracellular water diffusion), and orientation dispersion index (OD; the degree of fanning or angular variation in neurite orientation).
For delineating WM tracts, we used the TractSeg convolutional neural network–based approach (Wasserthal et al., 2018). We estimated the average MD, MT, R2*, ICVF, ISOVF, and OD values across 20 bilateral and callosal tracts of interest: anterior, posterior, and superior thalamic radiations; arcuate, inferior longitudinal, inferior fronto-occipital, superior, and uncinate fasciculi; cingulum; and genu and splenium of the corpus callosum. Tracts were selected based on their hypothesized associations with cognitive performance. The number of voxels was used as a proxy for the tract volume.
All structural data were processed in the framework of Statistical Parametric Mapping 12 (SPM12; www.fil.ion.ucl.ac.uk/spm; Wellcome Trust Centre for Neuroimaging) using customized MATLAB tools (Mathworks). We performed automated tissue classification using the multichannel option of the SPM12 “unified segmentation” with MT and PD maps and enhanced tissue priors (Lorio et al., 2016) that yielded gray matter (GM), WM, and cerebrospinal fluid (CSF) maps. Total intracranial volumes were calculated as the sum of GM, WM, and CSF volumes.
Structural imaging data quality assessment
For the present study, we report the quality of the qMRI data using the validated motion degradation index (MDI; Castella et al., 2018). Consistently with Lutti et al. (2022), MDI values from the MT-weighted images are higher than those from the PD- and T1-weighted images by ∼1 s−1* due to the lower number of echo images and lower signal-to-noise of the raw data. The distribution of the MDI values across the cohort is shown in Extended Data Figure 1-1A. Datasets with MDI values below 6 s−1 for the PD- and T1-weighted images, and below 7 s−1 for the MT-weighted images are empirically considered of sufficient quality. For completion, we also provide values of the rigid body transformation parameters for coregistration of the MT- and T1-weighted images to the reference PD-weighted images, which are metrics of head motion between image volumes (Extended Data Fig. 1-1B; Tabelow et al., 2019).
A. Motion Degradation Index (MDI in s-1) estimated for each contrast: MT-weighted (MTw), PD-weighted (PDw) and T1-weighted (T1w) B. Rigid body transformation parameters (x,y, z translation; pitch roll yaw in mm) estimated for co-registration between MT-weighted and PD-weighted (MT2PD), and between T1-weighted and PD-weighted (T12PD). Download Figure 1, TIF file (212.2KB, tif) .
Cognitive performance
Processing speed was the time in seconds needed to correctly connect numbers 1–25 in ascending order [trail making test (TMT) part A; Reitan, 1958]. Cognitive flexibility was the time in seconds needed to correctly connect numbers 1–13 in ascending order and letters A to L in alphabetic order while alternating between numbers and letters (i.e., 1-A-2-…12-L-13; TMT B; Reitan, 1958). To assess abstract reasoning ability, we used performance on the abbreviated form of Raven’s Standard Progressive Matrices (nine matrices) to estimate performance on the full form of the test (60 matrices; Bilker et al., 2012). The scores indicate the estimated number of correct items out of 60.
Covariates
An aggregate measure of cardiovascular risk was calculated by counting instances of hypertension (blood pressure, ≥140/90 mmHg or antihypertensive drug treatment), diabetes (fasting plasma glucose, ≥7.0 mmol/L, or antidiabetic drug treatment), or dyslipidemia (high-density lipoprotein, <1.0 mmol/L; triglycerides, ≥2.2 mmol/L; low-density lipoprotein, ≥4.1 mmol/L; or hypolipidemic treatment), having ever smoked, having a BMI > 25, and having a high waist-to-hip ratio (WHR) (>0.85 for females and >0.90 for males; Cox et al., 2019). A description of the clinical data collection methods can be found elsewhere (Firmann et al., 2008). Depressive disorder (diagnosed current major depressive disorder or dysthymia), anxiety disorder (diagnosed current generalized anxiety disorder, panic disorder, agoraphobia, social phobia, specific phobias, posttraumatic stress disorder, or obsessive-compulsive disorder), and alcohol or illicit substance use disorder were assessed using the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994). Ethnicity was not included in the analyses as 94% of the sample were Caucasian and 6% were from several other ethnic subgroups.
Experimental design and statistical analyses
As previous research has reported a high degree of covariance among WM microstructural properties across the brain (Penke et al., 2010, 2012; Cox et al., 2016, 2019; Lee et al., 2017; Telford et al., 2017), measures of global WM tissue properties were derived using Principal Component Analysis (PCA). For each of the WM microstructure measures (MD, MT, R2*, ICVF, ISOVF, and OD), the 20 tracts were entered into a PCA, and the first unrotated component was extracted and used in further analyses. Within all WM measures, tracts correlated positively; for instance, those with higher MT in one tract tended to have higher MT in all other tracts. Means of between-tract correlations were 0.83 ± 0.07 for MD, 0.84 ± 0.06 for MT, 0.80 ± 0.09 for R2*, 0.88 ± 0.06 for ICVF, 0.71 ± 0.11 for ISOVF, and 0.48 ± 0.18 for OD. For MD, MT, R2*, ICVF, and ISOVF, initial scree plots of the tract data provided evidence for a strong single component capturing common variance across the tracts. This was less clear for OD, which had a comparatively weaker first component and a stronger second and third component than the other measures. The first component explained 84% of the total variance in MD, 85% in MT, 81% in R2*, 89% in ICVF, 73% in ISOVF, and 52% in OD. All variables within each PCA correlated highly with the first principal component (≥0.9 for MD, MT, ICVF; ≥0.8 for R2* and ISOVF; ≥0.5 for OD).
Associations between socioeconomic conditions and the global WM microstructure measures, and between socioeconomic conditions and cognitive performance, were first examined with regression models that included age, age2, and sex as covariates [plus total intracranial volume (TIV) to correct for head size when examining associations with WM microstructure]. As different socioeconomic factors represent distinct constructs that may have different roles in development and aging (Braveman et al., 2005; Duncan and Magnuson, 2012; Noble et al., 2015; Basto-Abreu et al., 2018), each socioeconomic indicator (last known occupational position, household income, and social mobility) was examined in separate regression models.
Structural equation modeling with maximum likelihood estimation was then used to examine (1) the degree to which the effect of socioeconomic conditions on the generally greater magnitude of water diffusion (MD) across tracts was attributable to neurite density (ICVF), the amount of tract complexity/fanning (OD), and/or myelin (MT) in a multiple mediator model, and (2) whether WM microstructure (MD) mediated associations between socioeconomic conditions and cognitive performance (represented by a latent variable, comprising TMT A, TMT B, and Raven's matrices performance). A mediating effect was indicated by the presence of a significant indirect effect (the product of the direct paths; Zhao et al., 2010). The Monte Carlo method (5,000 samples) was used to estimate standardized indirect effects with 95% CI (Mehmetoglu, 2018).
To examine whether socioeconomic conditions moderate associations between the WM microstructure and cognitive performance, interaction terms were created by multiplying the socioeconomic indicators by the WM component scores (MD, MT, R2*, ICVF, ISOVF, OD). A statistically significant interaction term in the prediction of cognitive performance indicates the presence of a moderating effect. Interaction effects were probed using simple slope analysis.
In each model, the socioeconomic indicator (household income, occupation, or social mobility) was included alongside age, age2, sex, education, and TIV (independent variables). A second series of models additionally included cardiovascular risk factors, anxiety disorder, depressive disorder, and substance use disorder as covariates.
Supplementary analyses included (1) the adjustment for the number of people depending on the household income, using the modified OECD equivalence scale (income bracket midpoints were weighted by the number of dependents, for example, the household income of a couple with two children is divided by 2.1 (1 + 0.5 + 0.3 + 0.3); (2) running the models by age group (<65 vs ≥65 years), as well as adding an interaction term (age group multipled by the socioeconomic conditions) in predicting WM microstructure characteristics, as research indicates that social inequalities in biological risk are reduced at older ages (Crimmins et al., 2009); (3) testing the other WM measures that were significantly associated with socioeconomic conditions as mediators (in separate models); (4) examining whether household income moderates the relationship between the WM microstructure and cognitive performance on each of the individual cognitive tasks. Analyses were performed using Stata version 16 (StataCorp).
Results
Descriptives
Participants were on average 63 years old, 49% were women, and 26% were educated to tertiary level. Of the total sample, 61% (N = 455) were younger than 65 years, and 39% (N = 296) were aged 65 years and above. The summary statistics for the total sample and by age group are presented in Table 1. Household income and last known occupational position were positively and moderately correlated (r = 0.40, p < 0.05), indicating that the constructs are not redundant measures of socioeconomic position.
Table 1.
Descriptive statistics for the total sample and by age group
| Total (N = 751) | <65 years, (N = 455) | ≥65 years, (N = 296) | p value <65 vs ≥65 years | N | |
|---|---|---|---|---|---|
| M (SD) or % (n) | |||||
| Age at MRI scan | 63.5 (8.9) | 57.5 (4.1) | 72.7 (5.6) | <0.001 | 751 |
| Sex | 0.003 | 751 | |||
| Women | 49.4 (371) | 45.1 (205) | 56.1 (166) | ||
| Men | 50.6 (380) | 54.9 (250) | 43.9 (130) | ||
| Educational level | 0.052 | 743 | |||
| Tertiary | 26.1 (194) | 29.2 (131) | 21.4 (63) | ||
| Secondary | 62.0 (461) | 59.8 (268) | 65.4 (193) | ||
| Primary | 11.8 (88) | 10.9 (49) | 13.2 (39) | ||
| Last known occupation | 0.026 | 688 | |||
| Higher | 15.8 (109) | 17.9 (75) | 12.6 (34) | ||
| Middle | 37.2 (256) | 39.0 (163) | 34.4 (93) | ||
| Lower | 46.9 (323) | 43.1 (180) | 53.0 (143) | ||
| Household gross income (CHF p.a.) | <0.001 | 579 | |||
| Higher (110,000+) | 33.2 (192) | 44.2 (157) | 15.6 (35) | ||
| Middle (70,000–109,999) | 34.0 (197) | 33.8 (120) | 34.4 (77) | ||
| Lower (≤69,999) | 32.8 (190) | 22.0 (78) | 50.0 (112) | ||
| Social mobility | <0.001 | 667 | |||
| Stable-high | 7.0 (47) | 6.6 (27) | 7.7 (20) | ||
| Upward | 19.2 (128) | 24.6 (100) | 10.8 (28) | ||
| Stable-mid | 19.2 (128) | 17.9 (73) | 21.2 (55) | ||
| Downward | 38.8 (259) | 36.4 (148) | 42.7 (111) | ||
| Stable-low | 15.7 (105) | 14.5 (59) | 17.7 (46) | ||
| Smoking status | 0.001 | 700 | |||
| Never | 45.1 (316) | 46.3 (198) | 43.4 (118) | ||
| Former | 38.1 (267) | 33.6 (144) | 45.2 (123) | ||
| Current | 16.7 (117) | 20.1 (86) | 11.4 (31) | ||
| BMI | 26.2 (4.6) | 26.1 (4.6) | 26.3 (4.6) | 0.71 | 735 |
| WHR | 0.88 (0.1) | 0.87 (0.1) | 0.89 (0.1) | 0.016 | 733 |
| Hypertensiona | <0.001 | 737 | |||
| Yes | 43.3 (319) | 33.1 (148) | 59.0 (171) | ||
| Dyslipidemiab | 0.092 | 732 | |||
| Yes | 22.7 (166) | 24.8 (110) | 19.4 (56) | ||
| Diabetesc | <0.001 | 733 | |||
| Yes | 7.4 (54) | 4.5 (20) | 11.8 (34) | ||
| Anxiety disorderd | 0.58 | 751 | |||
| Yes | 7.5 (56) | 7.0 (32) | 8.1 (24) | ||
| Depressive disordere | 0.045 | 751 | |||
| Yes | 6.7 (50) | 8.1 (37) | 4.4 (13) | ||
| Substance use disorder | 0.35 | 751 | |||
| Yes | 2.4 (18) | 2.0 (9) | 3.0 (9) | ||
| TMT A score, seconds | 41.1 (17.1) | 36.2 (12.5) | 48.6 (20.4) | <0.001 | 731 |
| TMT B score, seconds | 74.8 (43.2) | 66.2 (37.0) | 88.3 (48.5) | <0.001 | 726 |
| Raven's matrices estimated score | 39.3 (12.2) | 42.2 (11.5) | 34.9 (12.0) | <0.001 | 751 |
χ2 tests were used to compare categorical variables across cohorts; t tests were used to compare continuous variables; CHF, Swiss Francs; WHR, waist-to-hip ratio; TMT, trail making test.
Blood pressure ≥140/90 mmHg or receiving treatment for the condition.
High-density lipoprotein <1.0 mmol/L; triglycerides, ≥2.2 mmol/L; and/or low-density lipoprotein, ≥4.1 mmol/L.
Fasting plasma glucose ≥7.0 mmol/L or receiving treatment for the condition.
Diagnosed current generalized anxiety disorder, panic disorder, agoraphobia, social phobia, specific phobias, posttraumatic stress disorder, or obsessive-compulsive disorder.
Diagnosed current major depressive disorder or dysthymia.
The results below are based on single component measures of the WM microstructure, as described in Materials and Methods, Experimental design and statistical analyses.
Socioeconomic differences in WM microstructure
Table 2 shows the associations between the socioeconomic indicators and WM measures for the total sample. Of the different socioeconomic measures, only household income was associated with WM microstructure. Participants who had lower household income had a greater mean water diffusivity (MD), lower myelination (MT), lower iron content (R2*), and lower neurite density (ICVF), than those of participants who had higher household income. The extracellular water content (ISOVF) and tract complexity/fanning (OD) were not associated with household income. The results were the same when adjusting for the number of people depending on the household income (β range for MD, MT, R2*, and ICVF, 0.089–0.102; p < 0.05). See Extended Data Figure 1-2 for a graphical display of the associations between household income and the WM measures.
Table 2.
Associations between socioeconomic conditions and the measures of WM microstructure
| MD | MT | R2* | ICVF | ISOVF | OD | |
|---|---|---|---|---|---|---|
| Last known occupation | 0.041, (−0.035, 0.116) | −0.020, (−0.095, 0.054) | −0.027, (−0.112, 0.057) | −0.031, (−0.120, 0.057) | 0.017, (−0.058, 0.093) | −0.032, (−0.119, 0.055) |
| p value | 0.293 | 0.590 | 0.530 | 0.487 | 0.651 | 0.467 |
| N = 661 | ||||||
| Household income | 0.111 (0.036, 0.187) | −0.088 (−0.163, −0.012) | −0.099, (−0.184, −0.014) | −0.118, (−0.207, −0.029) | 0.040, (−0.038, 0.118) | −0.000, (−0.089, 0.088) |
| p value | 0.004 | 0.023 | 0.023 | 0.009 | 0.314 | 0.992 |
| N = 556 | ||||||
| Social mobility | 0.071, (−0.002, 0.145) | −0.032, (−0.105, 0.041) | −0.024, (−0.107, 0.058) | −0.048, (−0.134, 0.039) | 0.050, (−0.024, 0.124) | −0.012, (−0.098, 0.073) |
| p value | 0.058 | 0.389 | 0.564 | 0.279 | 0.183 | 0.782 |
| N = 641 |
The results are standardized regression coefficients and 95% CI in parentheses for the lowest versus highest socioeconomic group; models adjusted for age, age square, sex, and total intracranial volume; MD, mean diffusivity; MT, magnetization transfer; R2*, transverse relaxation rate; ICVF, intracellular volume fraction; ISOVF, isotropic volume fraction; OD, orientation dispersion index.
Graded associations between gross household income and the white matter measures (mean diffusivity, magnetization transfer saturation, intra-cellular volume fraction). Linear regression models adjusted for age, age square, sex, and total intracranial volume. Bar plots indicate coefficients and 95% confidence intervals for each level of household income (lower ≤69'999 CHF, middle 70'000–109'999 CHF, higher ≥110'000 CHF). Download Figure 2, TIF file (46.3KB, tif) .
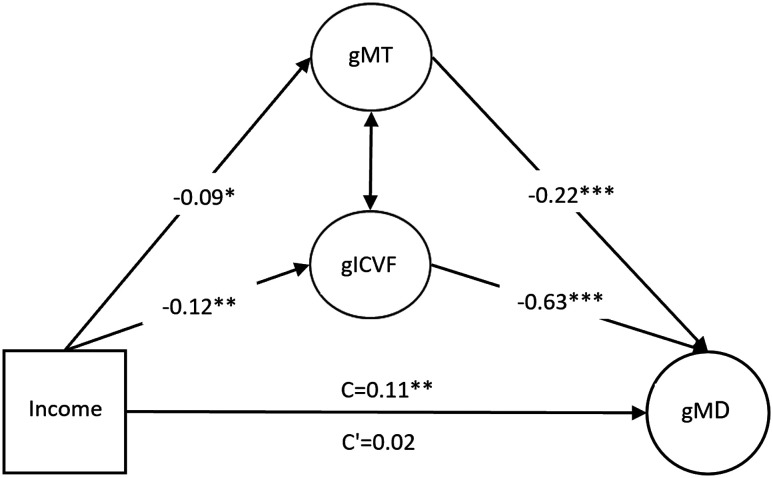
A mediation model, which included neurite density and myelin as mediators of the association between household income and MD had an acceptable fit (χ2(10) = 43.85; p < 0.001; comparative fit index (CFI), 0.97; Tucker-Lewis index (TLI), 0.92; root mean square error of approximation (RMSEA), 0.08), and there was a significant indirect effect, indicating that the association between household income and MD was mediated by neurite density (β = 0.084; 95% CI, 0.029–0.141; p = 0.003) and myelin (β = 0.019; 95% CI, 0.006–0.034; p = 0.009; Fig. 1).
Figure 1.
The association between household income and global MD (path C) is mediated (note the lower value of path C’) by a combination of global neurite density (ICVF) and global myelin (MT). The majority of the mediation takes place through gICVF rather than MT (respective indirect effects are β = −0.084 and β = 0.019). Standardized βs reported. *p < 0.01, **p < 0.01, ***p < 0.001.
Supplementary analyses showed that socioeconomic effects on WM were only observed in those younger than 65 years (Table 3); in this age group, social mobility also predicted MD. Participants who had experienced a stable-low occupational trajectory had a greater global water molecule diffusion than those who had experienced a stable-high trajectory (β = 0.132; 95% CI 0.018–0.245; p = 0.023). However, the interaction terms (age group multiplied by the socioeconomic conditions) did not reach statistical significance in predicting WM microstructure characteristics.
Table 3.
Associations between socioeconomic conditions and the measures of WM microstructure by age group
| MD | MT | R2* | ICVF | ISOVF | OD | |
|---|---|---|---|---|---|---|
| <65 years | ||||||
| Last known occupation | 0.113 (−0.002, 0.228) | −0.048 (−0.162, 0.066) | −0.061 (−0.178, 0.056) | −0.096 (−0.215, 0.024) | 0.041 (−0.072, 0.153) | −0.012 (−0.126, 0.101) |
| p value | 0.055 | 0.408 | 0.308 | 0.117 | 0.479 | 0.827 |
| N = 406 | ||||||
| Household income | 0.144 (0.036, 0.252) | −0.128 (−0.236, −0.020) | −0.143 (−0.254, −0.032) | −0.132 (−0.245, −0.018) | 0.051 (−0.053, 0.156) | −0.027 (−0.135, 0.082) |
| p value | 0.009 | 0.020 | 0.012 | 0.023 | 0.336 | 0.630 |
| N = 343 | ||||||
| Social mobility | 0.132 (0.018, 0.245) | −0.059 (−0.172, 0.054) | −0.028 (−0.144, 0.088) | −0.094 (−0.213, 0.024) | 0.089 (−0.021, 0.200) | 0.020 (−0.091, 0.132) |
| p value | 0.023 | 0.308 | 0.634 | 0.119 | 0.113 | 0.720 |
| N = 395 | ||||||
| ≥65 years | ||||||
| Last known occupation | −0.031 (−0.165, 0.103) | −0.011 (−0.146, 0.124) | −0.007 (−0.150, 0.137) | 0.065 (−0.084, 0.214) | 0.004 (−0.127, 0.136) | −0.074 (−0.210, 0.063) |
| p value | 0.652 | 0.869 | 0.925 | 0.391 | 0.947 | 0.288 |
| N = 255 | ||||||
| Household income | 0.097 (−0.037, 0.231) | −0.044 (−0.177, 0.090) | −0.049 (−0.191, 0.094) | −0.095 (−0.242, 0.052) | 0.043 (−0.093, 0.180) | 0.061 (−0.080, 0.202) |
| p value | 0.155 | 0.518 | 0.500 | 0.205 | 0.534 | 0.396 |
| N = 213 | ||||||
| Social mobility | 0.027 (−0.103, 0.156) | −0.017 (−0.148, 0.113) | −0.039 (−0.177, 0.100) | 0.014 (−0.131, 0.159) | 0.024 (−0.104, 0.152) | −0.063 (−0.197, 0.071) |
| p value | 0.687 | 0.794 | 0.584 | 0.849 | 0.714 | 0.355 |
| N = 246 | ||||||
The results are standardized β coefficients and 95% CI in parentheses for the lowest versus highest socioeconomic group; models adjusted for age, age square, sex, and total intracranial volume; MD, mean diffusivity; MT, magnetization transfer; R2*, transverse relaxation rate; ICVF, intracellular volume fraction; ISOVF, isotropic volume fraction; OD, orientation dispersion index.
Socioeconomic differences in cognitive performance mediated by WM microstructure
Table 4 shows associations between the socioeconomic and cognitive measures. Participants who experienced socioeconomic disadvantage performed worse on tests of processing speed, cognitive flexibility, and general cognitive ability than those who experienced more advantaged socioeconomic conditions; these effects were apparent in each age group. For household income, results were the same when adjusting for the number of people depending on the income (β range, 0.163–0.236; p < 0.001). See Extended Data Figure 1-3 for a graphical display of the associations between household income and cognitive performance.
Table 4.
Associations between life course socioeconomic conditions and cognitive performance
| TMT A | TMT B | Raven's matrices | |
|---|---|---|---|
| Last known occupation | 0.212 (0.141, 0.283) | 0.288 (0.217, 0.360) | −0.243 (−0.315, −0.171) |
| p value | <0.001 | <0.001 | <0.001 |
| N | 668 | 665 | 688 |
| Household income | 0.177 (0.097, 0.258) | 0.250 (0.168, 0.331) | −0.223 (−0.305, −0.140) |
| p value | <0.001 | <0.001 | <0.001 |
| N | 560 | 556 | 579 |
| Social mobility | 0.191 (0.119, 0.264) | 0.263 (0.190, 0.336) | −0.234 (−0.308, −0.161) |
| p value | <0.001 | <0.001 | <0.001 |
| N | 647 | 644 | 667 |
| <65 years | |||
| Last known occupation | 0.242 (0.145, 0.340) | 0.346 (0.252, 0.441) | −0.245 (−0.341, −0.150) |
| p value | <0.001 | <0.001 | <0.001 |
| N | 406 | 406 | 418 |
| Household income | 0.092 (−0.014, 0.199) | 0.201 (0.096, 0.306) | −0.218 (−0.322, −0.115) |
| p value | 0.090 | <0.001 | <0.001 |
| N | 344 | 344 | 355 |
| Social mobility | 0.223 (0.126, 0.324) | 0.340 (0.244, 0.436) | −0.250 (−0.347, −0.154) |
| p value | <0.001 | <0.001 | <0.001 |
| N | 395 | 395 | 407 |
| ≥65 years | |||
| Last known occupation | 0.200 (0.081, 0.319) | 0.217 (0.097, 0.337) | −0.261 (−0.381, −0.142) |
| p value | 0.001 | <0.001 | <0.001 |
| N | 262 | 259 | 270 |
| Household income | 0.294 (0.165, 0.423) | 0.319 (0.189, 0.449) | −0.223 (−0.359, −0.087) |
| p value | <0.001 | <0.001 | 0.001 |
| N | 216 | 212 | 224 |
| Social mobility | 0.172 (0.050, 0.293) | 0.154 (0.031, 0.277) | −0.234 (−0.357, −0.111) |
| p value | 0.006 | 0.014 | <0.001 |
| N | 252 | 249 | 260 |
TMT, trail making test; the results are standardized β coefficients and 95% CI in parentheses for the lowest versus highest socioeconomic group; models adjusted for age, age square, and sex.
Graded associations between household income and cognitive performance (standardized scores on the Trail Making Test part A, Trail Making Test part B, and abbreviated form of the Raven Standard Progressive Matrices). Linear regression models adjusted for age, age square, and sex. Bar plots indicate coefficients and 95% confidence intervals for each level of household income (lower ≤69'999 CHF, middle 70'000–109'999 CHF, higher ≥110'000 CHF). Download Figure 2, TIF file (45.7KB, tif) .
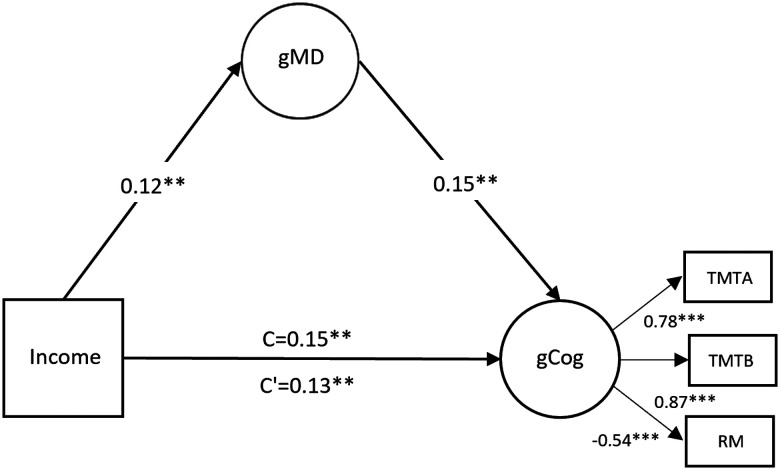
A mediation model, which included MD as a mediator of the association between household income and cognitive performance (represented as a latent factor) had an acceptable fit: χ2(10) = 43.66; p < 0.001; CFI, 0.97; TLI, 0.93; and RMSEA, 0.08. There was a significant indirect effect (β = 0.017; 95% CI, 0.003–0.035; p = 0.040), indicating that MD partially mediated the association between household income and cognitive performance (Fig. 2). This pathway became borderline significant when taking into account cardiovascular risk factors, anxiety disorder, depressive disorder, and substance use disorder (β = 0.012; 95% CI, 0.002–0.027; p = 0.064), and when examining the model by age group. Myelin or neurite density alone did not mediate the association between household income and cognitive performance.
Figure 2.
The association between household income and global cognitive performance (path C) is partially mediated (note lower value of path C’) by global MD. The indirect effect is β = 0.017. Standardized βs reported. **p < 0.01, ***p < 0.001. TMTA, trail making test part A; TMTB, trail making test part B; RM, abbreviated form of the Raven’s Standard Progressive Matrices.
Socioeconomic differences moderate associations between the WM microstructure and cognitive performance
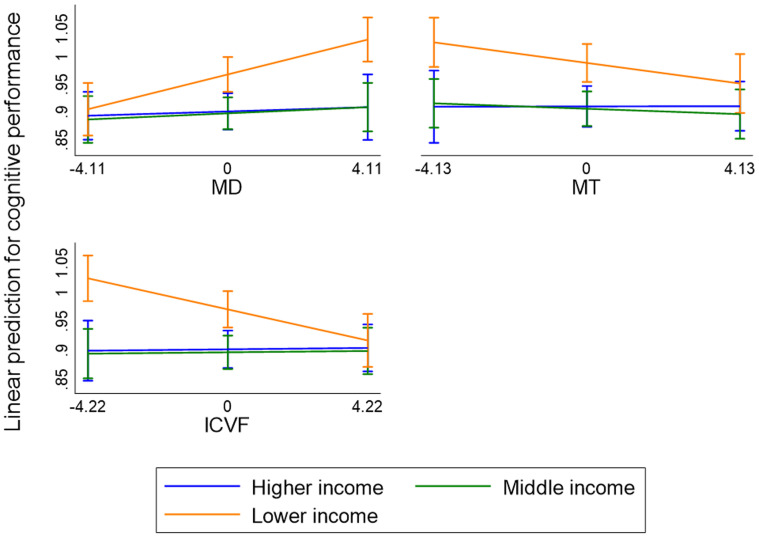
There was a significant income–WM interaction predicting cognitive performance for MD (β = 0.023; 95% CI, 0.009–0.037; p < 0.01), myelination (β = −0.015; 95% CI, −0.028 to −0.001; p < 0.05), and neurite density (β = −0.019; 95% CI, −0.032 to −0.007; p < 0.01), but not for the other components. Simple slope analysis showed that a greater MD (β = 0.015; 95% CI, 0.008–0.023; p < 0.001), lower myelination (β = −0.008; 95% CI, −0.016 to −0.000; p < 0.05), and lower neurite density (β = −0.013; 95% CI, −0.019 to −0.006; p < 0.001) were associated with poorer cognitive performance among those with a lower household income, but there were no significant associations between the WM measures and cognitive performance among those with a middle or higher household income. See Figure 3 for a graphical display of the interaction effects. These moderating effects remained when adding cardiovascular risk factors, anxiety disorder, depressive disorder, and substance abuse disorder to the models (apart from myelin). Supplementary analyses showed that moderating effects of household income on WM only reached statistical significance in those younger than 65 years. When examining the moderating effects for each cognitive test separately, the reported effects were apparent for TMT A and B (β range, −0.020 to 0.028; p < 0.05), but not Raven's alone. There were no statistically significant interaction effects when examining the other socioeconomic indicators (education, occupation, or social mobility).
Figure 3.
Predicted values of cognitive performance at each level of household income [higher (≥110,000 CHF per annum), middle (70,000–109,999 CHF per annum), lower (≤69,999 CHF per annum)] setting MD, MT, and ICVF at 1 SD below the mean, the mean, and 1 SD above the mean. Higher scores indicate poorer cognitive performance. Models adjusted for age, age square, sex, education, and total intracranial volume.
Discussion
Using information from multicontrast MRI, we provided an in-depth neurobiological understanding of associations between socioeconomic conditions, brain WM anatomy, and cognitive performance in middle- and older-aged adults. We found that individuals from lower-income households had markers of advanced brain aging, including lower levels of MRI parameters indicative of myelin and iron content, and reduced neurite density, paralleled by a greater MD of water. Differences in myelin and neurite density explained differences in the magnitude of water diffusion between lower- and higher-income households, and differences in the magnitude of water diffusion partially explained associations between household income and cognitive performance. Household income also shaped associations between the WM microstructure and cognitive performance, such that a greater MD, as well as lower myelin, and neurite density were associated with poorer cognitive performance among those from lower-income households. Individuals from higher-income households showed preserved cognitive performance in the face of greater MD, lower myelin, or neurite density.
Socioeconomic differences in WM microstructure
Our findings critically extend previous reports of socioeconomic differences in tract-specific and global measures of fractional anisotropy (Gianaros et al., 2013; Johnson et al., 2013, 2021; Noble et al., 2013; Gullick et al., 2016; Ursache and Noble, 2016; Dufford and Kim, 2017; Shaked et al., 2019), which are assumed to be due to differences in fiber density, diameter, or myelination but have limited neurobiological specificity and reliability (Jeurissen et al., 2013). We found socioeconomic differences in global MD, which is more robust to the issue of fiber crossing (Figley et al., 2022); and these differences were explained by global myelin and neurite density. We did not see socioeconomic differences in extracellular water content or neurite dispersion, suggesting socioeconomic effects in WM are not driven by these features. However, given the scarcity of research using NODDI to examine socioeconomic differences in WM microstructure, further research is needed to replicate this finding.
Socioeconomic differences in cognitive performance mediated by WM microstructure
Our study is one of the few to empirically assess potential neurobiological pathways linking socioeconomic conditions and cognitive performance and, to our knowledge, the first one to offer insights from the most recent levels of neurobiological specificity. Extending previous studies on children and young adults (Noble et al., 2013; Johnson et al., 2021), we found that the association between household income and cognitive performance was partially mediated by MD, but not by myelin or neurite density alone, suggesting that the combination of myelin and neurite density, reflected in MD, partially explains the association between income and cognitive performance. One interpretation of these results is that higher household income may contribute (directly or indirectly) to differences in WM development, which in turn supports cognitive performance. The mediation effect was not independent of cardiovascular risk factors and psychiatric disorders, as it became borderline significant when adding these factors to the model. This is unsurprising given that socioeconomic disadvantage is associated with an increased risk of psychiatric disorder and cardiovascular disease (Kivimäki et al., 2020), and the latter are also associated with poorer cognitive outcomes (Pal et al., 2018; Semkovska et al., 2019), as well as differences in brain macro- and microstructure (Cox et al., 2019; Grosu et al., 2022).
Socioeconomic differences moderate associations between the WM microstructure and cognitive performance
Our finding that income moderated associations between WM and cognitive performance dovetails with previous studies indicating that more advantaged socioeconomic conditions may buffer individuals from a range of potentially detrimental cognitive outcomes (Czernochowski et al., 2008; Mortamais et al., 2014; Ursache and Noble, 2016). Individuals from higher-income households may have increased access to means that enable them to use different neural resources or strategies in order to exhibit a high level of behavioral performance (Stern, 2009). From the present findings, we cannot identify the precise mechanisms by which household income might contribute to differences in WM and cognitive performance, as well as their association. However, there are a number of possible contributing factors that may accumulate over the life course (Evans and Kim, 2010), including differential access to appropriate healthcare (Schröder et al., 2016) and adequate nutrition (Prado and Dewey, 2014), exposure to psychosocial stressors (Chen and Miller, 2013) and environmental pollution (Evans and Kantrowitz, 2002; Binter et al., 2022), workplace conditions and cognitive demands (Then et al., 2014), and gene–environment interaction (Ryan et al., 2011). The experience of chronic stress can have deleterious effects on the brain. In animal models, early stress exposure has been associated with reduced neurogenesis and enhanced apoptosis (Lemaire et al., 2000; Heine et al., 2004; Mirescu et al., 2004), which may lead to a reduction in the density of neuronal axons. Stress exposure has also been shown to impair oligodendrogenesis in rodents (Teissier et al., 2020), which could explain the hypomyelination of WM tracts and cognitive impairment seen in animal models of stress (Bordner et al., 2011; Yang et al., 2017). On the contrary, environmental enrichment, such as training and stimulation, has been associated with increased neurogenesis (Nilsson et al., 1999) and promotes oligodendrogenesis and myelination (Gibson et al., 2014). Future studies should examine the potential operative pathways through which socioeconomic disadvantage impacts WM microstructure and cognitive performance.
Our supplementary analyses indicated that the moderating effects of household income were primarily evident for processing speed (TMT A) and cognitive flexibility (TMT B) performance, rather than abstract reasoning (Raven's matrices). However, previous research has reported moderating effects of socioeconomic conditions on other cognitive domains including memory (Czernochowski et al., 2008) and executive function (Ursache and Noble, 2016), as well as mild cognitive impairment (Mortamais et al., 2014), so we cannot conclude that the moderating effects are specific to any cognitive domain. Future research should examine whether the effects observed in this study generalize to other cognitive domains including episodic memory, verbal fluency, and inhibitory control.
Socioeconomic differences in WM microstructure by age group
Household income was the only socioeconomic measure that predicted WM microstructure in the total sample. Occupational position has been associated with brain structure and function among adults (Johnson et al., 2013; Chan et al., 2018) but is usually acquired in early- or mid-adulthood and therefore may not reflect socioeconomic resources as well as current household income. When stratifying by age group, associations between household income and WM were observed among those younger than 65 years, but not among those aged 65 years and above; and social mobility of occupational position additionally predicted WM in the younger age group. Similarly, another study found socioeconomic differences in cortical GM thickness in middle but not older adulthood (Chan et al., 2018). Social inequalities in the process of aging may be visible up to a certain age (Crimmins et al., 2009), since higher-risk individuals die at younger ages or become too unwell to participate in surveys, resulting in greater similarity among participants who reach old age. In the present study, the observed differences between age groups could also be due to differences in the sample size and statistical power, as a smaller proportion of the sample was aged 65 years and above, and the interaction terms (age group multiplied by the socioeconomic conditions) did not reach statistical significance in predicting WM microstructure characteristics. Wealth or indicators of deprivation are likely the most precise indicators of socioeconomic resources at older ages (Henretta and Campbell, 1978; Grundy and Holt, 2001) and should be included in future studies examining common and unique associations between different socioeconomic indicators and WM microstructure.
Strengths and limitations
Our study has several strengths, including the use of data from a large-scale monocentric cohort and data acquisition using the same MRI machine without any major hardware or software changes, alongside a unique multicontrast imaging approach. Methodological strengths include relaxometry-based MRI sensitive to brain tissue properties; the careful adjustment for transmit field inhomogeneities that ensures the quantitative character of the data; the use of individual diffusion-based tractography instead of atlas information to provide a reliable delineation and sampling across WM tracts; and the quantitative assessment of data quality (Castella et al., 2018).
We cannot rule out the possibility of cohort-specific effects, but it is noteworthy that the associations between socioeconomic factors and cognitive performance, and between socioeconomic factors and previously examined WM parameters, are consistent with findings from different cohorts (Gianaros et al., 2013; Johnson et al., 2013; Shaked et al., 2019; Schrempft et al., 2023). Our single-center study is complementary to multisite initiatives given that we provide a deeper brain anatomy phenotype due to fewer time constraints for MRI data acquisition. Multicenter studies can obtain a larger sample size, with greater power to detect true effects, but the harmonization of MRI data from different sites and scanners can be difficult and costly to coordinate (e.g., correction for different acquisition protocols may be needed). Although replication studies are needed, our study is an important first step in investigating neurobiological pathways linking social adversity and cognition.
The design of this study was cross-sectional, which limits the interpretation in terms of the direction of the observed effects. Longitudinal research is needed to test whether changes in socioeconomic conditions, including financial resources, in middle to late adulthood are associated with changes in the WM microstructure. Recent research in adults found no association between education and change in cortical or hippocampal volume (Nyberg et al., 2021). In other research, older adults without a college education showed a decline in resting-state brain system segregation over time compared with their college-educated peers (Chan et al., 2021). Across adolescence and young adulthood, neighborhood deprivation was associated with reduced myelin growth, but not with changes in macroscopic measures such as GM volume or cortical surface area (Ziegler et al., 2020). Alongside measures of brain function, measures of WM microstructure may be more sensitive markers of brain aging than macrostructural measures and should be included in future longitudinal studies.
The present study cannot identify the genetic and environmental contributions to the observed socioeconomic differences in the brain WM and cognitive performance, nor can it distinguish between social causation and social selection accounts of the findings. However, research indicates that both genetic and environmental factors contribute to brain and cognitive health (Belsky et al., 2018; Judd et al., 2020). For example, a recent study on adolescents found that brain and cognitive development was independently influenced by socioeconomic status and polygenic scores for educational attainment (Judd et al., 2020). Future research examining socioeconomic differences in the brain WM microstructure and cognitive performance should take into account genetic factors.
The data in this study were from the metropolitan city of Lausanne in Switzerland, home to 345,000 inhabitants, ∼40% of whom are foreign citizens with a variety of different nationalities. Switzerland has one of the highest average salaries among OECD countries, and the income gap is not as wide as other European countries and America (Swiss Inequality Database, 2023; World Inequality Database, 2023). However, the cost of living is also particularly high in Switzerland (Nakamura et al., 2020), with a large part of income being spent on taxes, housing, and compulsory health insurance (Federal Office for Statistics, 2020). The high cost of living in Switzerland puts lower-income families at a particular disadvantage. Households with a gross monthly salary of <5,000 CHF (60,000 CHF per year) can't set aside any savings (Federal Office for Statistics, 2020). Moreover, living costs tend to increase while wages remain stable, putting a strain on low-paid workers. Health inequalities are seen within high-income countries such as Switzerland, but the effects observed in this study may be stronger in other countries where income inequality is greater and lower-income families have limited access to adequate healthcare, education, and quality food.
Most participants in our study were Caucasian; therefore it was not possible to test for interaction effects between ethnicity and socioeconomic conditions. This is an important future research endeavor as stress related to ethnic minority status may accelerate aging, and we may see stronger effects among individuals with both ethnic minority status and lower household income. Our study focused on individual-level socioeconomic factors in relation to WM and cognitive performance. Research examining socioeconomic effects at the neighborhood level is also warranted.
Conclusion
An individual's household income relates to their WM brain anatomy and associated cognitive performance in middle to late adulthood. These findings provide novel insights into the neurobiological mechanisms underlying socioeconomic inequalities in brain anatomy and cognitive performance, and present an important baseline from which to further investigate pathways linking adversity, WM microstructure, and cognition.
References
- Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125:1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G (2004) Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 25:5–18. 10.1016/j.neurobiolaging.2003.03.001 [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (2011) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson 213:560–570. 10.1016/j.jmr.2011.09.022 [DOI] [PubMed] [Google Scholar]
- Basto-Abreu A, Barrientos-Gutiérrez T, Zepeda-Tello R, Camacho V, Gimeno Ruiz de Porras D, Hernández-Ávila M (2018) The relationship of socioeconomic status with body mass index depends on the socioeconomic measure used. Obesity 26:176–184. 10.1002/oby.22042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15:435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Belsky DW, et al. (2018) Genetic analysis of social-class mobility in five longitudinal studies. Proc Natl Acad Sci U S A 115:E7275–E7284. 10.1073/pnas.1801238115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Cohen HJ, Kraus WE, Ramrakha S, Poulton R, Moffitt TE (2017) Impact of early personal-history characteristics on the pace of aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16:644–651. 10.1111/acel.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D (2002) A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 31:285–293. 10.1093/ije/31.2.285 [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ (2014) Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience 276:187–205. 10.1016/j.neuroscience.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E, et al. (2019) Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat Commun 10:773. 10.1038/s41467-019-08732-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilker WB, Hansen JA, Brensinger CM, Richard J, Gur RE, Gur RC (2012) Development of abbreviated nine-item forms of the Raven’s standard progressive matrices test. Assessment 19:354–369. 10.1177/1073191112446655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binter A-C, Kusters MSW, van den Dries MA, Alonso L, Lubczyńska MJ, Hoek G, White T, Iñiguez C, Tiemeier H, Guxens M (2022) Air pollution, white matter microstructure, and brain volumes: periods of susceptibility from pregnancy to preadolescence. Environ Pollut 313:120109. 10.1016/j.envpol.2022.120109 [DOI] [PubMed] [Google Scholar]
- Bordner KA, et al. (2011) Functional genomic and proteomic analysis reveals disruption of myelin-related genes and translation in a mouse model of early life neglect. Front Psychiatry 2:18. 10.3389/fpsyt.2011.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S (2005) Socioeconomic status in health research: one size does not fit all. J Am Med Assoc 294:2879–2888. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- Callaghan MF, et al. (2014) Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol Aging 35:1862–1872. 10.1016/j.neurobiolaging.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella R, Arn L, Dupuis E, Callaghan MF, Draganski B, Lutti A (2018) Controlling motion artefact levels in MR images by suspending data acquisition during periods of head motion. Magn Reson Med 80:2415–2426. 10.1002/mrm.27214 [DOI] [PubMed] [Google Scholar]
- Chan MY, Han L, Carreno CA, Zhang Z, Rodriguez RM, LaRose M, Hassenstab J, Wig GS (2021) Long-term prognosis and educational determinants of brain network decline in older adult individuals. Nat Aging 1:1053–1067. 10.1038/s43587-021-00125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Na J, Agres PF, Savalia NK, Park DC, Wig GS (2018) Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc Natl Acad Sci U S A 115:E5144–E5153. 10.1073/pnas.1714021115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE (2013) Socioeconomic status and health: mediating and moderating factors. Annu Rev Clin Psychol 9:723–749. 10.1146/annurev-clinpsy-050212-185634 [DOI] [PubMed] [Google Scholar]
- Cox SR, et al. (2019) Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J 40:2290–2300. 10.1093/eurheartj/ehz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ (2016) Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun 7:13629. 10.1038/ncomms13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Seeman TE (2009) Poverty and biological risk: the earlier “aging” of the poor. J Gerontol A Biol Sci Med Sci 64A:286–292. 10.1093/gerona/gln010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe VT (2016) The epigenetic impacts of social stress: how does social adversity become biologically embedded? Epigenomics 8:1653–1669. 10.2217/epi-2016-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernochowski D, Fabiani M, Friedman D (2008) Use it or lose it? SES mitigates age-related decline in a recency/recognition task. Neurobiol Aging 29:945–958. 10.1016/j.neurobiolaging.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran J-P (2015) Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage 105:32–44. 10.1016/j.neuroimage.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Dennis E, Manza P, Volkow ND (2022) Socioeconomic status, BMI, and brain development in children. Transl Psychiatry 12:33. 10.1038/s41398-022-01779-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Ashburner J, Hutton C, Kherif F, Frackowiak RSJ, Helms G, Weiskopf N (2011) Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage 55:1423–1434. 10.1016/j.neuroimage.2011.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford AJ, Kim P (2017) Family income, cumulative risk exposure, and white matter structure in middle childhood. Front Hum Neurosci 11:547. 10.3389/fnhum.2017.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K (2012) Socioeconomic status and cognitive functioning: moving from correlation to causation. WIREs Cogn Sci 3:377–386. 10.1002/wcs.1176 [DOI] [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E (2002) Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health 23:303–331. 10.1146/annurev.publhealth.23.112001.112349 [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P (2010) Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci 1186:174–189. 10.1111/j.1749-6632.2009.05336.x [DOI] [PubMed] [Google Scholar]
- Farah MJ (2017) The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron 96:56–71. 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Federal Office for Statistics (2020) Household budget survey 2018. Available at: https://www.bfs.admin.ch/bfs/de/home/aktuell/neue-veroeffentlichungen.assetdetail.14963784.html.
- Figley CR, Uddin MN, Wong K, Kornelsen J, Puig J, Figley TD (2022) Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front Neurosci 15:799576. 10.3389/fnins.2021.799576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmann M, et al. (2008) The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord 8:6. 10.1186/1471-2261-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD (2013) Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex 23:2058–2071. 10.1093/cercor/bhs191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, et al. (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosu C, Trofimova O, Gholam-Rezaee M, Strippoli M-PF, Kherif F, Lutti A, Preisig M, Draganski B, Eap CB (2022) CYP2C19 expression modulates affective functioning and hippocampal subiculum volume—a large single-center community-dwelling cohort study. Transl Psychiatry 12:316. 10.1038/s41398-022-02091-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy E, Holt G (2001) The socioeconomic status of older adults: how should we measure it in studies of health inequalities? J Epidemiol Community Health 55:895–904. 10.1136/jech.55.12.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick MM, Demir-Lira ÖE, Booth JR (2016) Reading skill-fractional anisotropy relationships in visuospatial tracts diverge depending on socioeconomic status. Dev Sci 19:673–685. 10.1111/desc.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek D, Thams F, Flöel A, Antonenko D (2020) Dentate gyrus volume mediates the effect of fornix microstructure on memory formation in older adults. Front Aging Neurosci 12:79. 10.3389/fnagi.2020.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ (2004) Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci 19:131–144. 10.1046/j.1460-9568.2003.03100.x [DOI] [PubMed] [Google Scholar]
- Helms G, Dathe H, Dechent P (2008a) Quantitative FLASH MRI at 3T using a rational approximation of the Ernst equation. Magn Reson Med 59:667–672. 10.1002/mrm.21542 [DOI] [PubMed] [Google Scholar]
- Helms G, Dathe H, Kallenberg K, Dechent P (2008b) High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med 60:1396–1407. 10.1002/mrm.21732 [DOI] [PubMed] [Google Scholar]
- Henretta JC, Campbell RT (1978) Net worth as an aspect of status. Am J Sociol 83:1204–1223. 10.1086/226679 [DOI] [Google Scholar]
- Hutton C, Bork A, Josephs O, Deichmann R, Ashburner J, Turner R (2002) Image distortion correction in fMRI: a quantitative evaluation. NeuroImage 16:217–240. 10.1006/nimg.2001.1054 [DOI] [PubMed] [Google Scholar]
- Ihle A, Ghisletta P, Ballhausen N, Fagot D, Vallet F, Baeriswyl M, Sauter J, Oris M, Maurer J, Kliegel M (2018) The role of cognitive reserve accumulated in midlife for the relation between chronic diseases and cognitive decline in old age: a longitudinal follow-up across six years. Neuropsychologia 121:37–46. 10.1016/j.neuropsychologia.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier J-D, Jones DK, Sijbers J (2013) Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34:2747–2766. 10.1002/hbm.22099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Bathelt J, Akarca D, Crickmore G, Astle DE (2021) Far and wide: associations between childhood socio-economic status and brain connectomics. Dev Cogn Neurosci 48:100888. 10.1016/j.dcn.2020.100888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Gold BT (2013) Socioeconomic status is positively correlated with frontal white matter integrity in aging. Age 35:2045–2056. 10.1007/s11357-012-9493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73:239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Judd N, et al. (2020) Cognitive and brain development is independently influenced by socioeconomic status and polygenic scores for educational attainment. Proc Natl Acad Sci U S A 117:12411–12418. 10.1073/pnas.2001228117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner E, Dhital B, Kiselev VG, Reisert M (2016) Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76:1574–1581. 10.1002/mrm.26054 [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Chen E, Miller G, Seeman T (2018) How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan. In: Handbook of life course health development (Halfon N, Forrest CB, Lerner RM, Faustman EM. Cham (CH): Springer. [PubMed] [Google Scholar]
- Kivimäki M, et al. (2020) Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 5:e140–e149. 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Steiner RJ, Yu Y, Short SJ, Neale MC, Styner MA, Zhu H, Gilmore JH (2017) Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc Natl Acad Sci U S A 114:148–153. 10.1073/pnas.1604658114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349. 10.1002/mrm.21890 [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A 97:11032–11037. 10.1073/pnas.97.20.11032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorio S, et al. (2016) New tissue priors for improved automated classification of subcortical brain structures on MRI. NeuroImage 130:157–166. 10.1016/j.neuroimage.2016.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loued-Khenissi L, et al. (2022) Signatures of life course socioeconomic conditions in brain anatomy. Hum Brain Mapp 43:2582–2606. 10.1002/hbm.25807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutti A, Corbin N, Ashburner J, Ziegler G, Draganski B, Phillips C, Kherif F, Callaghan MF, Di Domenicantonio G (2022) Restoring statistical validity in group analyses of motion-corrupted MRI data. Hum Brain Mapp 43:1973–1983. 10.1002/hbm.25767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutti A, Hutton C, Finsterbusch J, Helms G, Weiskopf N (2010) Optimization and validation of methods for mapping of the radiofrequency transmit field at 3T. Magn Reson Med 64:229–238. 10.1002/mrm.22421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutti A, Stadler J, Josephs O, Windischberger C, Speck O, Bernarding J, Hutton C, Weiskopf N (2012) Robust and fast whole brain mapping of the RF transmit field B1 at 7T. PLoS One 7:e32379. 10.1371/journal.pone.0032379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmetoglu M (2018) Medsem: a Stata package for statistical mediation analysis. Int J Comput Econ 8:63–78. 10.1504/IJCEE.2018.088321 [DOI] [Google Scholar]
- Melchior M, Berkman LF, Kawachi I, Krieger N, Zins M, Bonenfant S, Goldberg M (2006) Lifelong socioeconomic trajectory and premature mortality (35-65 years) in France: findings from the GAZEL cohort study. J Epidemiol Community Health 60:937–944. 10.1136/jech.2005.042440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Mole JP, Sims R, Fasano F, Evans J, Jones DK, Aggleton JP, Baddeley RJ (2019) Fornix white matter glia damage causes hippocampal gray matter damage during age-dependent limbic decline. Sci Rep 9:1060. 10.1038/s41598-018-37658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E (2004) Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 7:841–846. 10.1038/nn1290 [DOI] [PubMed] [Google Scholar]
- Mortamais M, et al. (2014) Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry 22:1336–1345. 10.1016/j.jagp.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Harati R, Lall SV, Dikhanov YM, Hamadeh N, Oliver WV, Rissanen MO, Yamanaka M (2020) Comparing costs of living across world cities. World Bank Econ Rev 34:S79–S88. 10.1093/wber/lhz037 [DOI] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS (1999) Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 39:569–578. [DOI] [PubMed] [Google Scholar]
- Noble KG, et al. (2015) Family income, parental education and brain structure in children and adolescents. Nat Neurosci 18:773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM (2013) Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev Sci 16:653–664. 10.1111/desc.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (1994) Diagnostic interview for genetic studies: rationale, unique features, and training. Arch Gen Psychiatry 51:849–859. 10.1001/archpsyc.1994.03950110009002 [DOI] [PubMed] [Google Scholar]
- Nyberg L, et al. (2021) Educational attainment does not influence brain aging. Proc Natl Acad Sci U S A 118:e2101644118. 10.1073/pnas.2101644118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K, Mukadam N, Petersen I, Cooper C (2018) Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 53:1149–1160. 10.1007/s00127-018-1581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MS, Thomson WM, Walls AWG, Steele JG (2009) Lifecourse socio-economic mobility and oral health in middle age. J Dent Res 88:938–941. 10.1177/0022034509344524 [DOI] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Valdés Hernández MC, Murray C, Royle NA, Starr JM, Wardlaw JM, Deary IJ (2012) Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry 17:1026–1030. 10.1038/mp.2012.66 [DOI] [PubMed] [Google Scholar]
- Penke L, Muñoz Maniega S, Murray C, Gow AJ, Hernández MCV, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ (2010) A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci 30:7569–7574. 10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado EL, Dewey KG (2014) Nutrition and brain development in early life. Nutr Rev 72:267–284. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- Preisig M, et al. (2009) The PsyCoLaus study: methodology and characteristics of the sample of a population-based survey on psychiatric disorders and their association with genetic and cardiovascular risk factors. BMC Psychiatry 9:9. 10.1186/1471-244X-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1958) Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 8:271–276. 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- Ritchie SJ, et al. (2015) Coupled changes in brain white matter microstructure and fluid intelligence in later life. J Neurosci 35:8672–8682. 10.1523/JNEUROSCI.0862-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V, Stringhini S, Henriques A, Falcão H, Barros H, Fraga S (2020) Life-course socioeconomic status and lung function in adulthood: a study in the EPIPorto cohort. J Epidemiol Community Health 74:290–297. 10.1136/jech-2019-212871 [DOI] [PubMed] [Google Scholar]
- Rose D, Harrison E (2007) The European socio-economic classification: a new social class schema for comparative European research. Eur Soc 9:459–490. 10.1080/14616690701336518 [DOI] [Google Scholar]
- Ryan L, Walther K, Bendlin BB, Lue L-F, Walker DG, Glisky EL (2011) Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage 54:1565–1577. 10.1016/j.neuroimage.2010.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempft S, Belsky DW, Draganski B, Kliegel M, Vollenweider P, Marques-Vidal P, Preisig M, Stringhini S (2022) Associations between life course socioeconomic conditions and the pace of aging. J Gerontol A Biol Sci Med Sci 11:2257–2264. 10.1093/gerona/glab383 [DOI] [PubMed] [Google Scholar]
- Schrempft S, Trofimova O, Künzi M, Draganski B, Kliegel M, Stringhini S (2023) Life-course socioeconomic conditions and cognitive performance in older adults: a cross-cohort comparison. Aging Ment Health 27:745–754. 10.1080/13607863.2022.2084511 [DOI] [PubMed] [Google Scholar]
- Schröder SL, Richter M, Schröder J, Frantz S, Fink A (2016) Socioeconomic inequalities in access to treatment for coronary heart disease: a systematic review. Int J Cardiol 219:70–78. 10.1016/j.ijcard.2016.05.066 [DOI] [PubMed] [Google Scholar]
- Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, Knittle H, Ahern E, Gload T (2019) Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry 6:851–861. 10.1016/S2215-0366(19)30291-3 [DOI] [PubMed] [Google Scholar]
- Shaked D, Leibel DK, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, Erus G, Evans MK, Zonderman AB, Waldstein SR (2019) Disparities in diffuse cortical white matter integrity between socioeconomic groups. Front Hum Neurosci 13:198. 10.3389/fnhum.2019.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater DA, Melie-Garcia L, Preisig M, Kherif F, Lutti A, Draganski B (2019) Evolution of white matter tract microstructure across the life span. Hum Brain Mapp 40:2252–2268. 10.1002/hbm.24522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistique Vaud (2023) Annuaire statistique du canton de Vaud 2023, 46e édition. Available at: https://www.vd.ch/fileadmin/user_upload/organisation/dfin/statvd/Publications/Annuaire/A2023_eannuaire.pdf.
- Stern Y (2009) Cognitive reserve. Neuropsychologia 47:2015–2028. 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S, et al. (2015) Life-course socioeconomic status and DNA methylation of genes regulating inflammation. Int J Epidemiol 44:1320–1330. 10.1093/ije/dyv060 [DOI] [PubMed] [Google Scholar]
- Stringhini S, Batty GD, Bovet P, Shipley MJ, Marmot MG, Kumari M, Tabak AG, Kivimäki M (2013) Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the Whitehall II prospective cohort study. PLoS Med 10:e1001479. 10.1371/journal.pmed.1001479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiss Inequality Database (2023) Top 10% income share before taxes 1917 - 2019. Available at: https://www.iwp.swiss/sid/#graph-1.
- Tabelow K, et al. (2019) hMRI - a toolbox for quantitative MRI in neuroscience and clinical research. Neuroimage 194:191–210. 10.1016/j.neuroimage.2019.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A, Le Magueresse C, Olusakin J, Andrade da Costa BLS, De Stasi AM, Bacci A, Imamura Kawasawa Y, Vaidya VA, Gaspar P (2020) Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol Psychiatry 25:1159–1174. 10.1038/s41380-019-0493-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford EJ, Cox SR, Fletcher-Watson S, Anblagan D, Sparrow S, Pataky R, Quigley A, Semple SI, Bastin ME, Boardman JP (2017) A latent measure explains substantial variance in white matter microstructure across the newborn human brain. Brain Struct Funct 222:4023–4033. 10.1007/s00429-017-1455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then FS, Luck T, Luppa M, Thinschmidt M, Deckert S, Nieuwenhuijsen K, Seidler A, Riedel-Heller SG (2014) Systematic review of the effect of the psychosocial working environment on cognition and dementia. Occup Environ Med 71:358–365. 10.1136/oemed-2013-101760 [DOI] [PubMed] [Google Scholar]
- Trofimova O, et al. (2021) Brain tissue properties link cardio-vascular risk factors, mood and cognitive performance in the CoLaus|PsyCoLaus epidemiological cohort. Neurobiol Aging 102:50–63. 10.1016/j.neurobiolaging.2021.02.002 [DOI] [PubMed] [Google Scholar]
- Trofimova O, et al. (2023) Topography of associations between cardiovascular risk factors and myelin loss in the ageing human brain. Commun Biol 6:392. 10.1038/s42003-023-04741-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, Noble KG (2016) Socioeconomic status, white matter, and executive function in children. Brain Behav 6:e00531. 10.1002/brb3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E (2016) Denoising of diffusion MRI using random matrix theory. NeuroImage 142:394–406. 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B (2013) Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. NeuroImage 81:335–346. 10.1016/j.neuroimage.2013.05.028 [DOI] [PubMed] [Google Scholar]
- Wasserthal J, Neher P, Maier-Hein KH (2018) TractSeg - fast and accurate white matter tract segmentation. NeuroImage 183:239–253. 10.1016/j.neuroimage.2018.07.070 [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Callaghan MF, Josephs O, Lutti A, Mohammadi S (2014) Estimating the apparent transverse relaxation time (R2*) from images with different contrasts (ESTATICS) reduces motion artifacts. Front Neurosci 8:278. 10.3389/fnins.2014.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Edwards LJ, Helms G, Mohammadi S, Kirilina E (2021) Quantitative magnetic resonance imaging of brain anatomy and in vivo histology. Nat Rev Phys 3:570–588. 10.1038/s42254-021-00326-1 [DOI] [Google Scholar]
- World Inequality Database (2023) Top 10% national income share. Available at: https://wid.world/world/#sptinc_p90p100_z/US;FR;DE;CN;ZA;GB;WO/last/eu/k/p/yearly/s/false/22.6225/80/curve/false/country.
- Yang Y, Cheng Z, Tang H, Jiao H, Sun X, Cui Q, Luo F, Pan H, Ma C, Li B (2017) Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of wnt signaling. Cereb Cortex 27:2871–2884. 10.1093/cercor/bhw121 [DOI] [PubMed] [Google Scholar]
- Yaple ZA, Yu R (2020) Functional and structural brain correlates of socioeconomic status. Cereb Cortex 30:181–196. 10.1093/cercor/bhz080 [DOI] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016. 10.1016/j.neuroimage.2012.03.072 [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q (2010) Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Consum Res 37:197–206. 10.1086/651257 [DOI] [Google Scholar]
- Ziegler G, Moutoussis M, Hauser TU, Fearon P, Bullmore ET, Goodyer IM, Fonagy P, Jones PB, Lindenberger U, Dolan RJ (2020) Childhood socio-economic disadvantage predicts reduced myelin growth across adolescence and young adulthood. Hum Brain Mapp 41:3392–3402. 10.1002/hbm.25024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Motion Degradation Index (MDI in s-1) estimated for each contrast: MT-weighted (MTw), PD-weighted (PDw) and T1-weighted (T1w) B. Rigid body transformation parameters (x,y, z translation; pitch roll yaw in mm) estimated for co-registration between MT-weighted and PD-weighted (MT2PD), and between T1-weighted and PD-weighted (T12PD). Download Figure 1, TIF file (212.2KB, tif) .
Graded associations between gross household income and the white matter measures (mean diffusivity, magnetization transfer saturation, intra-cellular volume fraction). Linear regression models adjusted for age, age square, sex, and total intracranial volume. Bar plots indicate coefficients and 95% confidence intervals for each level of household income (lower ≤69'999 CHF, middle 70'000–109'999 CHF, higher ≥110'000 CHF). Download Figure 2, TIF file (46.3KB, tif) .
Graded associations between household income and cognitive performance (standardized scores on the Trail Making Test part A, Trail Making Test part B, and abbreviated form of the Raven Standard Progressive Matrices). Linear regression models adjusted for age, age square, and sex. Bar plots indicate coefficients and 95% confidence intervals for each level of household income (lower ≤69'999 CHF, middle 70'000–109'999 CHF, higher ≥110'000 CHF). Download Figure 2, TIF file (45.7KB, tif) .