Abstract
Monoclonal antibody (MAb) 190/4 blocks binding of hepatitis A virus (HAV) to the HAV cellular receptor 1 (havcr-1) and protects African green monkey kidney (AGMK) clone GL37 cells (GL37 cells) against HAV infection. BS-C-1 and CV-1 cells, two widely used AGMK cell lines, did not react with MAb 190/4 but expressed havcr-1, as judged by Western blot analysis. The cDNA coding for havcr-1 was amplified from BS-C-1 and CV-1 total cellular RNA by reverse transcription-PCR. Alignment of the amino acid sequences inferred from the cDNA nucleotide sequences showed that BS-C-1 and CV-1 havcr-1 differed from GL37 havcr-1 by having two substitutions in the Cys-rich region, N48H and K108Q, and 10 to 11 additional substitutions plus the insertion of 18 to 22 amino acids in the mucin-like region. Studies with chimeras of GL37 havcr-1 and BS-C-1 havcr-1 showed that the K108Q substitution was responsible for the lack of reaction of MAb 190/4 with BS-C-1 and CV-1 cells. Binding studies indicated that HAV bound to dog cell transfectants expressing the BS-C-1 havcr-1 as well as the GL37/BS-C-1 havcr-1 chimeras. These results indicate that antigenic variants of havcr-1 are expressed in AGMK cells and that binding of HAV to these havcr-1 variants tolerates changes in protective epitope 190/4.
Hepatitis A virus (HAV), the causative agent of acute hepatitis in humans, is the only member of the hepatovirus genus of the Picornaviridae, a family of small, nonenveloped, positive-strand RNA viruses that include human pathogens such as poliovirus and rhinovirus (for a review, see reference 2). We recently identified the glycoprotein encoded by the HAV cellular receptor 1 gene (HAVcr-1) as an African green monkey kidney (AGMK) cellular receptor for HAV (3). Nucleotide sequence analysis revealed that the HAVcr-1 cDNA codes for a novel mucin-like class I integral-membrane glycoprotein, which was termed havcr-1, whose extracellular domain contains four putative N-glycosylation sites and two distinctive regions: an N-terminal, Cys-rich region that displays homology to members of the immunoglobulin superfamily and a mucin-like, C-terminal region containing 27 repeats of the consensus sequence PTTTTL. Our knowledge about the interaction of HAV with havcr-1 is limited; however, Thompson et al. (10) have recently shown that the havcr-1 Cys-rich region and its first N-glycosylation site are required for HAV receptor function. Monoclonal antibody (MAb) 190/4, which was raised against the cell surfaces of AGMK clone GL37 cells (GL37 cells), protected these cells against HAV infection and was used as a probe to molecularly clone the HAVcr-1 cDNA. The lack of cross-reaction of MAb 190/4 with human HeLa cells (3) was puzzling; however, more intriguing was our preliminary result suggesting that this MAb did not react with the cell surfaces of other AGMK cell lines.
Protective epitope 190/4 is not conserved among AGMK cell lines.
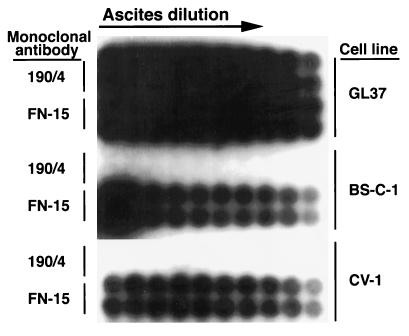
To confirm that the protective epitope 190/4 was not conserved among AGMK cell lines, we performed a highly sensitive cell surface radioimmunoassay (Fig. 1) of BS-C-1 and CV-1 cells obtained from the American Type Culture Collection (ATCC). Briefly, duplicate wells of unfixed cells grown in 96-well plates were treated with twofold dilutions of ascites fluid of MAb 190/4 or control anti-human fibronectin MAb FN-15 (Sigma Chemical Co.) for 1 h at room temperature, washed extensively, and treated with 0.1 μCi of 125I-labeled sheep anti-mouse antibody (Ab) (Amersham, Inc.) per well in 100 μl of phosphate-buffered saline–1% bovine serum albumin for 1 h at room temperature. After being washed extensively, the plates were autoradiographed at −70°C for 48 h with an intensifying screen. The control MAb FN-15 reacted with GL37, BS-C-1, and CV-1 cells, whereas MAb 190/4 reacted only with GL37 cells, which indicated that the 190/4 epitope was not present in the other two cell lines.
FIG. 1.
Cell surface radioimmunoassay of the expression of epitope 190/4 in AGMK cell lines. Duplicate wells of GL37, BS-C-1, and CV-1 cells grown in 96-well plates were treated with dilutions of MAb 190/4 or anti-human fibronectin MAb FN-15 ascites fluid, washed extensively, and treated with 125I-labeled sheep anti-mouse Ab. After being washed extensively, the plates were autoradiographed at −70°C for 48 h with an intensifying screen.
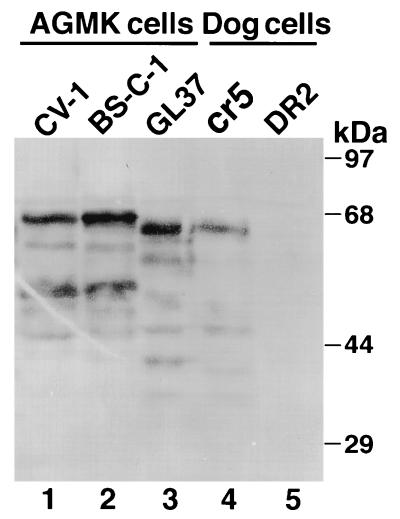
The lack of reaction with MAb 190/4 raised the possibility that the HAVcr-1 gene was not present in BS-C-1 and CV-1 cells. Southern blot analysis of genomic DNA probed with 32P-labeled, full-length HAVcr-1 cDNA showed similar patterns of HAVcr-1-specific bands in BS-C-1, CV-1, and GL37 cells (data not shown). Northern blot analysis of total cellular RNA probed with 32P-labeled, full-length HAVcr-1 cDNA (3) showed that GL37, BS-C-1, and CV-1 cells expressed a 2.1-kb HAVcr-1-specific message (data not shown). To further confirm that havcr-1 was expressed in the AGMK cell lines, a Western blot analysis (10) of cell extracts from BS-C-1, CV-1, and GL37 cells was done with the anti-GST2 Ab, which was raised against the mucin-like region of havcr-1 expressed in Escherichia coli (Fig. 2). Dog cells transfected with the GL37 HAV cr-1 cDNA, which were termed cr5 cells, or vector pDR2 (7, 9), which were termed DR2 cells, were included as controls (10). BS-C-1 and CV-1 cells expressed prominent 68-kDa havcr- 1-specific bands (lanes 1 and 2), whereas GL37 cells expressed a smaller major havcr-1 band with a molecular mass of 65 kDa (lane 3). The cr5 cells (lane 4) expressed a prominent 65-kDa band that comigrated with the major band expressed in GL37 cells. The DR2 cells (Fig. 2, lane 5) did not react with the anti-GST2 Ab, which indicated that the bands observed in the blot were havcr-1 specific. The remaining smaller and less conspicuous bands observed in the blot are probably different glycosylation forms or degradation products of havcr-1.
FIG. 2.
Western blot analysis of cytoplasmic extracts of AGMK cell lines. Cytoplasmic extracts of AGMK CV-1 (lane 1), BS-C-1 (lane 2), and GL37 (lane 3) cells and control dog cells transfected with GL37 HAVcr-1 cDNA (cr5 cells [lane 4]) and vector alone (DR2 cells [lane 5]) were prepared in RSB–1% Nonidet P-40. Cytoplasmic extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel), transferred to nylon membranes, and probed with rabbit anti-GST2 Ab. The positions and sizes of prestained molecular mass markers are shown on the right.
Molecular cloning of HAVcr-1 from BS-C-1 and CV-1 cells.
To further analyze the molecular basis for the lack of reaction of MAb 190/4 with BS-C-1 and CV-1 cells, we amplified the HAVcr-1 cDNAs from these two cell lines by reverse transcription (RT)-PCR. To do so, total RNA was extracted from mouse Ltk− cells (ATCC) and from GL37, BS-C-1, and CV-1 cells by using the RNASTAT-60 kit as suggested by the manufacturer (Tel-Test “B”, Inc.). First-strand cDNA was synthesized from 10 μg of total RNA with oligo(dT) and avian myeloblastosis virus reverse transcriptase as suggested by the manufacturer (Promega Corp.). The HAV cr-1 cDNAs were amplified by PCR with 10% of the RT reaction and a mixture of Taq and Pwo DNA polymerases in 30 cycles as recommended by the manufacturer (Expand High Fidelity PCR System; Boehringer Mannheim). Synthetic oligonucleotides (1 μg) HAVcr-15′end (5′-CGGATACGCGGATCCGCGCGTAGGTTTAGTTTTTGAAGTTCTTCTGTG-3′), which is positive sense and codes for a BamHI site adjacent to nucleotides (nt) 1 to 36 of the HAV cr-1 cDNA, and HAVcr-13′end (5′-AGAGCCTAGTCTAGA TTTTTAGGGTGAATTAAACTCACTTTATTTCCCCAT-3′), which is negative sense and codes for an XbaI site followed by five T residues complementary to the poly(A) tract and the complement of nt 2071 to 2035 of the HAVcr-1 cDNA, were used as PCR primers. The PCR was initiated by a hot start technique in a 50-μl reaction mixture without MgCl2 but containing wax beads which, upon melting, provided a final concentration of 1.5 mM MgCl2 (HotWax Mg+ beads; Invitrogen). HAVcr-1 cDNA PCR fragments of approximately 2.1 kb were amplified from BS-C-1, CV-1, and GL37 cells but not from Ltk− cells. The nucleotide sequences of the PCR fragments were determined as described previously (10) with positive- and negative-sense synthetic oligonucleotides spaced 300 to 400 bases apart, which revealed that BS-C-1 and CV-1 cells coded for HAVcr-1 cDNA variants of 2,127 and 2,139 bp, respectively, that shared approximately 95% identity with the 2,076-bp GL37 HAVcr-1 cDNA. Alignment of the nucleotide sequences of the AGMK HAVcr-1 cDNAs showed that the difference in the lengths of the cDNAs were mainly due to nucleotide insertions in the repeat area of the mucin-like region (data not shown).
Due to ambiguities in the 5′ end sequences, we amplified the 5′ ends of the AGMK HAVcr-1 cDNAs by RT-PCR by using the conditions mentioned above and PCR primers cr63-83+ (5′-GGTGGGAGACAGAGGAAACA-3′), a positive-sense synthetic oligonucleotide coding for nt 63 to 83 of the HAVcr-1 cDNA, and cr403-425− (5′-TAGCGTGTCTCCTTCCGATAGG-3′), a negative-sense synthetic oligonucleotide coding for the complement of nt 425 to 403 of the HAVcr-1 cDNA. This RT-PCR analysis resulted in the amplification of a single band of 352 nt in GL37 cells and two bands of 352 and 404 nt in BS-C-1 and CV-1 cells. Nucleotide sequence analysis revealed that the 404-nt band contained an insertion of 52 nt at position 182 of the 5′-UTR of the HAVcr-1 cDNA. These data indicated that BS-C-1 and CV-1 cells coded for two HAVcr-1 cDNA forms: a short HAVcr-1 cDNA form containing a 5′-UTR similar to that of the GL37 HAVcr-1 cDNA and a long HAVcr-1 cDNA form containing an insertion of 52 nt in the 5′-UTR.
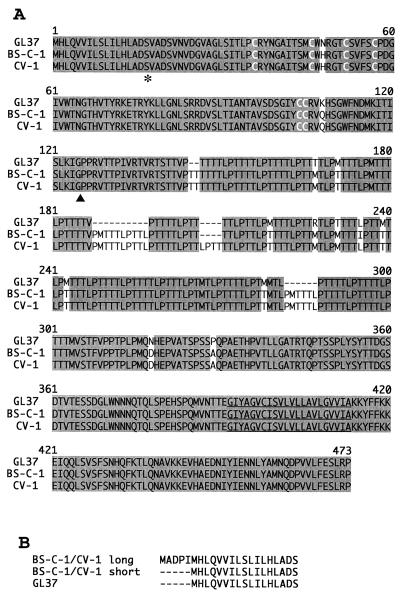
Alignment of the inferred amino acid sequences from the cDNA sequences (Fig. 3A) revealed that the short forms of the BS-C-1 HAVcr-1 and CV-1 HAVcr-1 cDNAs coded for receptors of 469 and 473 amino acids, respectively, which shared 99% identity. The BS-C-1 havcr-1 had 92% identity with the GL37 havcr-1 and contained 2 substitutions in the Cys-rich region (N48H and K108Q) and the insertion of 18 amino acids corresponding to three extra repeats plus 11 additional substitutions, i.e., T165M, M169T, M203T, R211M, T215M, L219I, M223T, M227T, M262T, N296D, and P307A, in the TSP-rich region. The CV-1 havcr-1 was similar to the BS-C-1 havcr-1; however, it contained a four-amino-acid insertion corresponding to an additional repeat and did not have the L219I substitution in the mucin-like region. No differences were found in the havcr-1 transmembrane and cytoplasmic domains of the three AGMK cell lines. The insertion of 52 nt in the long forms of the BS-C-1 HAVcr-1 and CV-1 HAVcr-1 cDNAs resulted in the putative insertion of five extra amino acids, MADPI, at the N terminus of the havcr-1, which increased the size of the putative signal sequence from 17 to 22 residues (Fig. 3B). Since the signal sequences are cleaved intracellularly, we expect that the mature havcr-1 encoded by the long and short forms of the HAVcr-1 mRNA are identical.
FIG. 3.
Alignment of havcr-1 from different AGMK cell lines. (A) Alignment of amino acid sequences predicted from the HAVcr-1 cDNAs of BS-C-1, CV-1, and GL37 cells was done with the Clustal W program. Gaps introduced in the sequences for the alignment are indicated by dashes; numbers of residues starting with the respective initiating methionine codons are indicated. Shading, identical amino acids; white letters, the six Cys residues of the Cys-rich region; asterisk, the end of the signal sequence; arrowhead, the beginning of the mucin-like region; underlining, the transmembrane region. (B) Alignment of the putative signal sequences predicted from the short and long forms of the BS-C-1 and CV-1 HAVcr-1 cDNAs compared to the GL37 havcr-1 signal sequence. The dashes indicate untranslated sequences. The amino acid sequences were inferred from the HAVcr-1 cDNA nucleotide sequences deposited in GenBank (see text).
The 2.1-kb HAVcr-1 cDNA PCR fragment amplified from BS-C-1 cells was gel purified, cut with XbaI, and cloned into pDR2 cut with BamHI, filled in with DNA polymerase I Klenow fragment (Pharmacia Biotech), and cut with XbaI. The nucleotide sequences of three clones were obtained; one clone, whose sequence was identical to that of the PCR fragments and contained a 52-nt insertion at the 5′-UTR, was termed pDR2BS-C-1 and was used as the source of BS-C-1 HAVcr-1 cDNA for further constructions.
The K108Q substitution in havcr-1 is responsible for the lack of reaction with MAb 190/4.
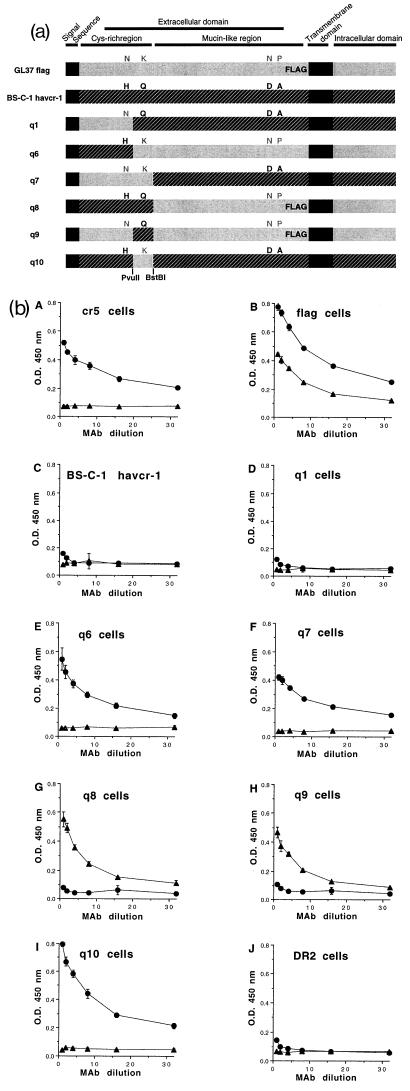
To determine which changes in the BS-C-1 havcr-1 and the CV-1 havcr-1 were responsible for the lack of reaction with protective MAb 190/4, we constructed chimeras between the GL37 havcr-1 and the BS-C-1 havcr-1 (Fig. 4a). Since the anti-GST2 Ab did not react with havcr-1 expressed at the cell surfaces of the AGMK cells and dog cell transfectants (10), we used a FLAG-tagged GL37 havcr- 1 (10), termed GL37 flag, to construct some of the chimeras and monitor their expression at the cell surface. The unique PvuII and BstBI cleavage sites at nt 482 and 607 of the GL37 HAVcr-1 cDNA were conserved in the BS-C-1 HAVcr-1 cDNA; therefore, we used them to swap cDNA fragments between the two cDNAs using standard methods as described previously (10). These cDNA constructs were cloned into the pDR2 vector, verified by automatic nucleotide sequence analysis, and transfected into dog cells. Hygromycin-resistant dog cell transfectants were selected as described previously (10). Western blot analysis with the anti-GST2 Ab showed that all chimeras were expressed in the dog cell transfectants and migrated with the expected molecular weights (data not shown). A cell surface enzyme-linked immunosorbent assay (ELISA) (10) of the dog cell transfectants with MAb 190/4 or anti-FLAG MAb M2 (Kodak Co.) (Fig. 4b) showed that cr5 cells reacted only with MAb 190/4, that cells expressing the GL37 flag construct reacted with MAb 190/4 and anti-FLAG MAb M2, and that DR2 cells did not react with either MAb. These data indicated that both MAbs reacted specifically against their corresponding epitopes at the cell surfaces of the dog cells. As expected, dog cells expressing the BS-C-1 havcr-1 did not react with either MAb. Dog cells expressing the q1 chimera, which differed from the BS-C-1 havcr-1 in only a H48N substitution, did not react with either MAb, indicating that this change did not result in the expression of epitope 190/4. Moreover, dog cells expressing the reciprocal construct, chimera q6, which differed from the GL37 havcr-1 in an N48H substitution, reacted with MAb 190/4, suggesting that amino acid 48 of the Cys-rich region probably does not form part of the 190/4 epitope. However, dog cells expressing chimera q7, which contained the whole Cys-rich region of the GL37 havcr-1 in the BS-C-1 havcr-1 background, resulted in expression of the 190/4 epitope. Dog cells expressing the reciprocal construct, chimera q8, in the background of the FLAG-tagged GL37 havcr-1 reacted with MAb M2, which indicated that this chimera was expressed at the cell surfaces of the dog cell transfectants. Because dog cells expressing the q8 chimera did not react with MAb 190/4, we concluded that this epitope was located in the Cys-rich region of the GL37 havcr-1. Since the N48H substitution failed to destroy the 190/4 epitope, the only other change present in the Cys-rich region which could account for the lack of reaction of MAb 190/4 with BS-C-1 havcr-1 was the K108Q substitution. To verify this, we constructed chimera q9 (containing a K108Q substitution in the FLAG-tagged GL37 havcr-1 background) and chimera q10 (containing a Q108K substitution in the BS-C-1 havcr-1 background). The cell surface ELISA showed that dog cells expressing chimera q9 contained the M2 but not the 190/4 epitope, which indicated that K108Q substitution was responsible for the lack of reaction with MAb 190/4. Dog cells expressing chimera q10 reacted with MAb 190/4, which clearly indicated that a Q108K substitution was sufficient to induce expression of the 190/4 epitope in BS-C-1 havcr-1. Considered together, these data showed that the K108Q substitution in BS-C-1 and CV-1 havcr-1 was responsible for the lack of reaction with MAb 190/4 and, therefore, suggested that amino acid residue 108 of the Cys-rich region of havcr-1 probably forms part of the 190/4 epitope.
FIG. 4.
Chimeras between BS-C-1 havcr-1 and GL37 havcr-1. (a) Schematic drawing of BS-C-1/GL37 havcr-1 chimeras. Chimeras between the BS-C-1 havcr-1 (black box with hatching) and the GL37 havcr-1 (grey box) containing an inserted FLAG peptide in the mucin-like region (GL37 flag) were constructed. The signal sequence and transmembrane domain are indicated by solid black boxes. The havcr-1 amino acid residues N48, K108, N296, and P307 of GL37 flag are in grey letters, and H48, Q108, D296, and A307 of BS-C-1 havcr-1 are in black. Additional substitutions and insertions in the repeat area of the mucin-like region were not marked (see text). Chimeras q1, q6, q7, q8, q9, and q10 contain different arrangements of the residues 48, 108, 296, and 307 of havcr-1. Chimera q6 was constructed without a FLAG tag. Restriction sites for PvuII and BstBI endonucleases used in the construction of the chimeras are indicated in boldface. (b) Expression of protective epitope 190/4 at the cell surfaces of dog cells transfectants. Expression of the 190/4 and M2 epitopes at the surfaces of dog cells expressing GL37 havcr-1 (cr5 cells), FLAG-tagged GL37 havcr-1 (flag cells), BS-C-1 havcr-1, and chimeras q1, q6, q7, q8, q9, and q10 was determined by ELISA with twofold dilutions of MAb 190/4 (circles) or anti-FLAG MAb M2 (triangles). Dog cells transfected with vector pDR2 alone (DR2 cells) were used as a negative control for the ELISA. Absorbance at 450 nm was plotted versus the MAb dilution starting at 0.4 μg/ml. Plotted values are means of triplicate wells ± standard errors of the means. The results correspond to one experiment which was repeated at least two times, with approximately 5 to 10% experimental error. O.D., optical density.
Binding of HAV is not disrupted by the K108Q substitution in BS-C-1 havcr-1.
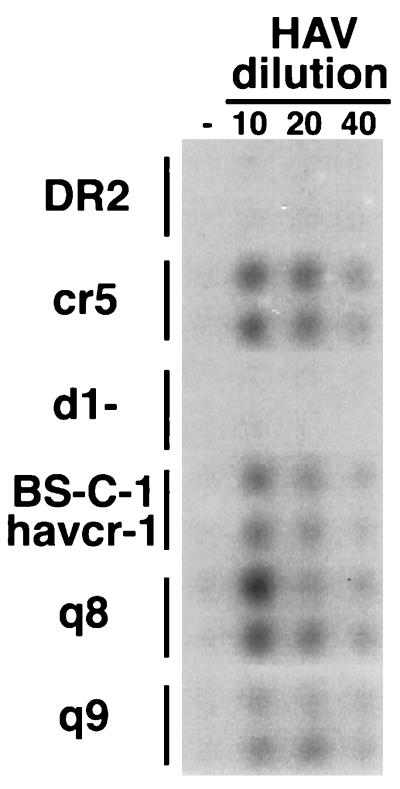
To determine whether the K108Q substitution that destroyed the 190/4 epitope also affected binding of HAV to BS-C-1 havcr-1, we performed a binding assay using the dog cell transfectants (10). To do so, duplicate wells of cells grown in 96-well plates were treated with different dilutions of tissue culture-adapted HAV strain HM175 for 1 h at 35°C and washed extensively, and bound HAV was detected by using 125I-labeled human anti-HAV Ab and autoradiography (Fig. 5). The control cr5 cells bound HAV in a concentration-dependent manner, whereas cells expressing GL37 havcr-1 containing a deletion of the Cys-rich region (d1-cells) (10) and DR2 cells did not bind virus. Dog cell transfectants expressing the BS-C-1 havcr-1, chimera q8, and chimera q9 also bound HAV in a concentration-dependent manner, which indicated that although the K108Q substitution destroyed the 190/4 epitope, it did not abrogate binding of HAV to BS-C-1 havcr-1.
FIG. 5.
Binding of HAV to dog cell transfectants. Dog cells transfected with vector pDR2 (DR2 cells) and dog cell transfectants expressing GL37 havcr-1 (cr5 cells), GL37 havcr-1 containing a deletion of the Cys-rich region (d1− cells), BS-C-1 havcr-1, chimera q8, and chimera q9 were grown in 96-well plates and infected with 1:10, 1:20, and 1:40 dilutions of purified HAV HM175 for 1 h at 35°C or mock infected (−). After extensive washing, monolayers were fixed and HAV bound to the cells was detected with 125I-labeled human anti-HAV Ab. Autoradiography of the 96-well plate showing duplicate wells for each treatment is presented.
Concluding remarks.
Our results showed that the BS-C-1 havcr-1 and CV-1 havcr-1 contained a K108Q substitution in the Cys-rich region that was responsible for the lack of reaction with MAb 190/4. Ashida and Hamada (1) have recently identified an havcr-1 variant in S.la/Ve-1 cells, a hybrid between marmoset liver and Vero cells, as an HAV receptor using the independently derived protective MAb 2H4, which suggested that havcr-1 is indeed a generic receptor for HAV in monkey cell lines. This S.la/Ve-1 havcr-1 has 95.7% identity with GL37 havcr-1, has some of the characteristic features of the BS-C-1/CV-1 havcr-1, and contains the K108Q substitution; therefore, it will probably not react with MAb 190/4. Unfortunately, our anti-GST2 Ab did not react with havcr-1 expressed at the cell surfaces of AGMK cells (10); therefore, it cannot be used to protect BS-C-1 and CV-1 cells against HAV infection and further prove that havcr-1 is a general HAV cellular receptor in AGMK and other primate cells. Since African green monkeys (AGM) (Cercopithecus aethiops) are a diverse group of animals that were classified into four geographically distinctive subspecies (5), it is possible that the above-mentioned havcr-1 variants correspond to different alleles of HAVcr-1 from one or more subspecies of AGM. This receptor variability in AGM does not seem to be unique to havcr-1, since a high degree of polymorphism has also been reported for CCR5, the major coreceptor for macrophage-tropic isolates of human immunodeficiency virus type 1 (4).
Anti-poliovirus receptor protective MAbs react with primate cells of different origins, such as HeLa, BS-C-1, and CV-1 cells (6, 8), which indicates that the protective epitope is conserved among primates. Our data indicated that the GL37 havcr-1 protective epitope 190/4 is conserved neither in human cells (3) nor in different AGMK cell lines. The natural antigenic variability of havcr-1 in AGM allowed us to map the 190/4 epitope to amino acid 108 of the Cys-rich region of havcr-1. The lack of reaction of MAb 190/4 with havcr-1 in Western blots suggested the possibility that the 190/4 epitope is discontinuous and that other residues located far from amino acid 108 also form part of this epitope. Further mutagenesis will be required to precisely map the 190/4 epitope and to determine which residues of havcr-1 are crucial for the HAV–havcr-1 interaction. Since MAb 190/4 blocks binding of HAV to havcr-1, it is possible that HAV interacts with the residues that form the 190/4 epitope. If this is the case, the K108Q change did not affect binding of HAV to havcr-1, which suggested that the HAV–havcr-1 interaction can withstand some degree of variability at this position. However, we cannot rule out the possibility that binding of MAb 190/4 to havcr-1 esterically blocks binding of HAV to a site different from epitope 190/4 or induces conformational changes in havcr-1 that inhibit binding of HAV.
Nucleotide sequence accession numbers.
The sequences obtained in this study have been assigned GenBank accession no. AF043446, AF043447, AF043448, and AF043449.
Acknowledgments
We thank Stephen Feinstone for encouragement and helpful advice and Sara Gagneten, Barry Falgout, and Hira Nakhasi for comments on the manuscript. We also thank Michael Klutch for automatic sequencing.
This research was supported in part by the appointment of D.F. to the Postgraduate Research Participation Program at the Center for Biologics Evaluation and Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
REFERENCES
- 1.Ashida M, Hamada C. Molecular cloning of the hepatitis A virus receptor from a simian cell line. J Gen Virol. 1997;78:1565–1569. doi: 10.1099/0022-1317-78-7-1565. [DOI] [PubMed] [Google Scholar]
- 2.Feinstone S M, Gust I. Hepatitis A virus. In: Richman D D, Whitley R J, Hayden F G, editors. Clinical virology. New York, N.Y: Churchill Livingstone; 1997. pp. 1049–1072. [Google Scholar]
- 3.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone S M. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhmann S E, Platt E J, Kozak S L, Kabat D. Polymorphisms in the CCR5 genes of African green monkeys and mice implicate specific amino acids in infections by simian and human immunodeficiency viruses. J Virol. 1997;71:8642–8656. doi: 10.1128/jvi.71.11.8642-8656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lernould J-M. Classification and geographical distribution of guenons: a review. In: Gautier-Hion A, Bourliere F, Gautier J-P, editors. A primate radiation: evolutionary biology of the African guenons. Cambridge, United Kingdom: Cambridge University Press; 1988. pp. 54–78. [Google Scholar]
- 6.Minor P D, Pipkin P A, Hockley D, Schild G C, Almond J W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1:203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy A J M, Kung A L, Swirski R A, Schimke R T. cDNA expression cloning in human cells using the pλDR2 episomal vector system. Methods (Orlando) 1992;4:111–131. [Google Scholar]
- 8.Nobis P, Zibirre R, Meyer G, Kühne J, Warnecke G, Koch G. Production of a monoclonal antibody against an epitope of HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985;66:2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- 9.Swirski R A, Van Den Berg D, Murphy A J M, Lambert C M, Friedberg E C, Schimke R T. Improvements in the Epstein-Barr-based shuttle vector system for direct cloning in human tissue culture cells. Methods (Orlando) 1992;4:133–142. [Google Scholar]
- 10.Thompson P, Lu J, Kaplan G G. The Cys-rich region of hepatitis A virus cellular receptor 1 is required for binding of hepatitis A virus and protective monoclonal antibody 190/4. J Virol. 1998;72:3751–3761. doi: 10.1128/jvi.72.5.3751-3761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]