Abstract
Background
Intrauterine fetal demise is a recognized complication of coronavirus disease 2019 in pregnant women and is associated with histopathological placental lesions. The pathological mechanism and virus-induced immune response in the placenta are not fully understood. A detailed description of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced inflammation in the placenta during fetal demise is crucial for improved clinical management.
Case presentation
We report the case of a 27-week gestation SARS-CoV-2-asymptomatic unvaccinated pregnant woman without comorbidities or other risk factors for negative pregnancy outcomes with a diagnosis of intrauterine fetal demise. Histopathological findings corresponded to patterns of subacute inflammation throughout the anatomic compartments of the placenta, showing severe chorioamnionitis, chronic villitis and deciduitis, accompanied by maternal and fetal vascular malperfusion. Our immunohistochemistry results revealed infiltration of CD68+ macrophages, CD56+ Natural Killer cells and scarce CD8+ T cytotoxic lymphocytes at the site of placental inflammation, with the SARS-CoV-2 nucleocapsid located in stromal cells of the chorion and chorionic villi, and in decidual cells.
Conclusion
This case describes novel histopathological lesions of inflammation with infiltration of plasma cells, neutrophils, macrophages, and natural killer cells associated with malperfusion in the placenta of a SARS-CoV-2-infected asymptomatic woman with intrauterine fetal demise. A better understanding of the inflammatory effects exerted by SARS-CoV-2 in the placenta will enable strategies for better clinical management of pregnant women unvaccinated for SARS-CoV-2 to avoid fatal fetal outcomes during future transmission waves.
Keywords: Intrauterine fetal demise, SARS-CoV-2, COVID-19 in pregnancy, Placental pathology, Placental inflammation, Maternal vascular malperfusion, Fetal vascular malperfusion
Background
Since the first cases of coronavirus disease 2019 (COVID-19) were reported in 2019, much has been learned about the effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy. Although SARS-CoV-2 is rarely transmitted transplacentally to the fetus, histopathological alterations have been described in a subset of placentas from SARS-CoV-2-infected mothers [1]. These pathologies in the placenta have been associated with negative pregnancy outcomes, including preterm birth and intrauterine fetal demise [1–4]. The term SARS-CoV-2 placentitis describes SARS-CoV-2-infected placentas in cases of fetal demise, with three specific pathological lesions, chronic histiocytic intervillositis, massive perivillous fibrin deposition and trophoblast necrosis, which were rare before the COVID-19 pandemic [5]. The different pathological mechanisms and viral-induced immune responses in the placenta associated with SARS-CoV-2-related fetal demise are not fully understood. We report a novel histopathological lesion of inflammation with a description of the immune cells associated with the presence of SARS-CoV-2 in the placenta of a pregnant woman who was diagnosed with intrauterine fetal demise.
Case presentation
A 20-year-old woman, gravida 3, para 2, presented with a pregnancy of 27 weeks to Hospital Santo Tomas with a 3-day history of absent fetal movements without other medical complaints. She had no significant past medical history or comorbidities for negative pregnancy outcomes. The maternal physical examination was unremarkable, and vital signs were within normal limits (Table 1). Obstetric sonography confirmed intrauterine fetal demise by revealing a 27-week fetus with an estimated body weight of 968 g and no fetal heart rate. There were no signs of placental abruption or preterm rupture of membranes. Maternal laboratory values were normal, and TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex) infections were excluded (Table 1).
Table 1.
Maternal and neonatal clinical characteristics of COVID-19-positive pregnant woman with fetal demise diagnosis. Hospital Santo Tomas, Panama 2020
| Maternal information | |
|---|---|
| Date of stillbirth | September 2020 |
| Maternal age (years) | 20 |
| Maternal history | Unremarkable |
| Gravidity | 3 |
| Parity | 2 |
| Gestational age (weeks) | 27 |
| Clinical severity | Asymptomatic |
| Type of delivery | Vaginal |
| Physical exam | |
| Temperature (°C) | 36.8 |
| Blood pressure (mmHg) | 108/64 |
| Heart rate (BPM) | 81 |
| Respiratory rate (CPM) | 16 |
| Oxygen saturation (%) | 97 |
| Laboratory findings | Normal range |
| Hemoglobin (g/dL) | 11.9 (10.9–14.3) |
| Hematocrit (%) | 35 (31.2–41.9) |
| Leukocytes (× 10^3/µL) | 10.6 (3.8–11.8) |
| Neutrophils (× 10^3/µL) | 3 (1.9–8.2) |
| Lymphocytes (× 10^3/µL) | 2.4 (1.1–3.1) |
| Platelets (× 10^3/µL) | 374 (179–408) |
| Fibrinogen (mg/dL) | 492 (248–506) |
| Virology and microbiology | |
| Nasopharingeal SARS CoV-2 RT PCR | Positive |
| Syphillis | Negative |
| HIV | Negative |
| Other TORCH | Negative a |
| Ultrasound findings | |
| Fetal growth | Normal |
| Fetal weight (g) | 968 |
| Amniotic fluid volume | Normal |
| Placental abruption | No sign |
| Preterm rupture of membranes | No sign |
| Neonatal information | Normal range |
| Sex | Male |
| Birthweight (g) | 992 |
| Birthweight percentile (%) | 40 (10–90) |
| Fronto-occipital circumference (cm) | 24 |
| Fronto-occipital circumference percentile (%) | 25 (10–90) |
| Crown-rump length (cm) | 24 |
| Virology | |
| SARS-CoV-2 RT‒PCR on fetus | Not done |
| SARS-CoV-2 RT‒PCR on placental tissue | Negative |
| Fetal autopsy | Not perfomed |
aPatient declared negative for TORCH agents based on clinical history and hospital laboratory results during the data collection. Abbreviations: BPM Beats per minute, COVID-19 Coronavirus disease 2019, CPM Cycles per minute, HIV Human immunodeficiency virus, RT‒PCR Reverse transcription polymerase chain reaction, SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2, TORCH Toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex
The mother was positive for SARS-CoV-2 through viral RNA nasopharyngeal swab analysis via reverse transcription polymerase chain reaction (RT–PCR) performed on all pregnant women before admission. She was admitted for labor induction via the use of Misoprostol. The patient delivered a male stillborn fetus of 992 g, corresponding to the 40th weight percentile, with a fronto-occipital circumference of 24 cm, equivalent to the 25th percentile for his gestational age, and a crown-rump length of 24 cm (Table 1). Macroscopic evaluation of the fetus did not show apparent morphological abnormalities. The patient was asymptomatic, and she was discharged without complications. The patient was not vaccinated against SARS-CoV-2 due to the unavailability of vaccines during the last quarter of 2020, when the major circulating variant in the country was the Panamanian endemic A.2.4. The National Bioethics Research Committee approved this study (EC-CNBI-2020–04-52), and written informed consent was obtained from the patient.
The placenta was collected, and histopathological analysis was performed through hematoxylin and eosin staining by using diagnostic criteria from the Amsterdam Placental Workshop Group Consensus Statement. Severe chorioamnionitis was present throughout the chorion, subchorionic fibrin and decidua of the membranes (Fig. 1A), as was the identification of mononuclear cells that morphologically resembled plasma cells and lymphocytes within the fetal membranes (Fig. 1B). Maternal vascular malperfusion, which consisted of scarce intervillous fibrin deposition and accelerated villi maturation with the formation of a vasculo-syncytial membrane in chorionic villi, was observed (Fig. 1C). The chorionic villi showed inflammation with infiltrating plasma cells (Fig. 1D). The decidua basalis was necrotic with severe inflammation composed of lymphocytes and neutrophils (Fig. 1E). Stem villi with vessel obliteration and thrombi were observed, consistent with fetal vascular malperfusion (Fig. 1F).
Fig. 1.
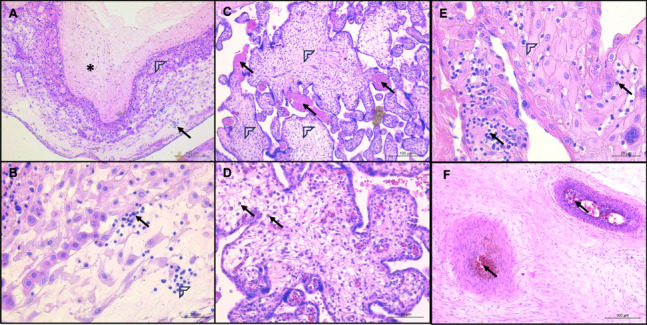
Histopathological findings in the placenta of a patient with fetal demise. A Severe chorioamnionitis shown in the chorion (asterisk), subchorionic fibrin (arrowhead) and decidua (arrow); H&E stain, 10 × magnification, scale bar: 100 µm; B Inflammatory infiltration in the fetal membranes, which consists of plasma cells (arrowhead) and lymphocytes (arrow); H&E stain, 40 × magnification; scale bar: 20 µm; C, Arrows show intervillous fibrin deposition and arrowheads, villitis; H&E stain, 10 × magnification, scale bar: 100 µm; D Villitis with infiltration of plasma cells (arrows), 20 × magnification; scale bar: 50 µm; E Infiltration of neutrophils (arrowhead) and lymphocytes (arrow) in the decidua basalis; H&E stain, 40 × magnification, scale bar: 20 µm; F Thrombi in stem villi (arrows); H&E stain, 10 × magnification, scale bar: 100 µm. Abbreviations: H&E: hematoxylin and eosin
The immunohistochemistry results demonstrated inflammation with infiltration of CD68+ macrophages at the chorionic plate (Fig. 2A), the chorionic villi (Fig. 2B) and the decidua (Fig. 2C) and of CD56+ natural killer (NK) cells (Fig. 2D) and scarce CD8+ cytotoxic T lymphocytes (Fig. 2E) in the decidua basalis. Placental tissue from the SARS-CoV-2-infected patient expressed the SARS-CoV-2 nucleocapsid (1:500, anti–SARS-CoV-2 nucleocapsid protein, Santa Cruz Biotechnologies, Santa Cruz, California) in stromal cells of the chorion (Fig. 2F), stromal cells of the chorionic villi (Fig. 2G), and decidual cells (Fig. 2H). SARS-CoV-2 viral RNA was not detected by RT‒PCR (Table 1) in the placental tissue.
Fig. 2.
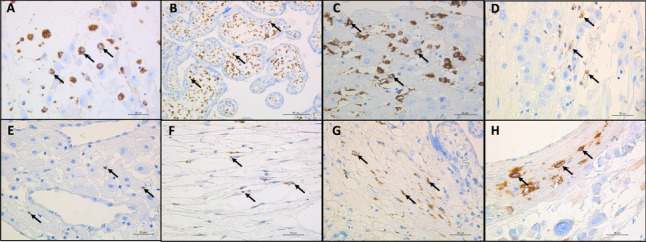
Immunohistochemistry of immune cell markers and the SARS-CoV-2 antigen in the placenta of a patient with fetal demise. A Macrophages detected at the interphase between the decidua of membranes and chorion (arrow); anti-CD68 immunohistochemical stain, 40 × magnification, scale bar: 20 µm; B Hyperplasia of macrophages in chorionic villi showed by arrows; anti-CD68 immunohistochemical stain, 20 × magnification, scale bar: 50 µm; C, Macrophages detected in the decidua represented by arrows; anti-CD68 immunohistochemical stain, 40 × magnification, scale bar: 20 µm; D, Natural killer cells in decidua basalis showed by arrows; anti-CD56 immunohistochemical stain, 40 × magnification, scale bar: 20 µm; E CD8 T cytotoxic lymphocytes detected in the decidua basalis represented by arrows; anti-CD8 immunohistochemical stain, 40 × magnification, scale bar: 20 µm; F SARS-COV2-infected stromal cells of the chorion represented by arrows; anti-SARS-CoV-2 nucleocapsid immunohistochemical stain, 40 × magnification, scale bar: 20 µm; G, SARS-CoV-2-infected stromal cells of chorionic villi represented by arrows; anti-SARS-CoV-2 nucleocapsid immunohistochemical stain, 40 × magnification, scale bar: 20 µm; H SARS-CoV-2-infected decidual cells represented by arrows; anti-SARS-CoV-2 nucleocapsid immunohistochemical stain, 40 × magnification, scale bar: 20 µm. Abbreviations: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
None of the compartments of the placenta stained positive for CD19+ B lymphocytes (Fig. 3A to C), CD4+ T lymphocytes (Fig. 3D to F), or p53+ apoptotic cells (Fig. 3G and I).
Fig. 3.
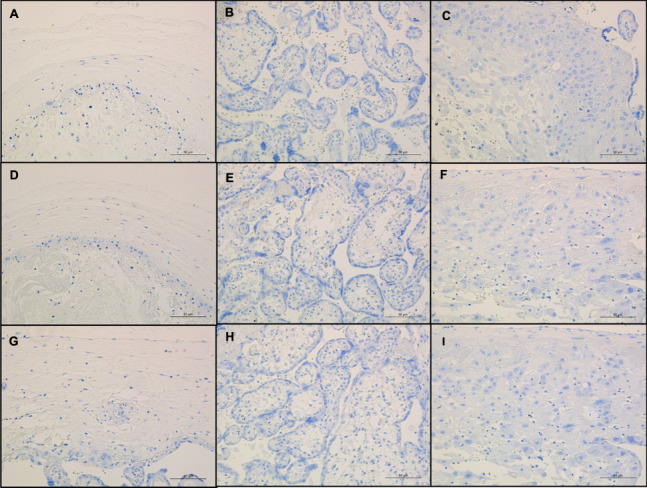
Immunohistochemical staining for CD19, CD4 and p53. A-C Chorion, chorionic villi and decidua basalis; anti-CD19 immunohistochemical stain, 20 × magnification; scale bar: 50 µm; D-F Chorion, chorionic villi and decidua basalis; anti-CD4 immunohistochemical stain, 20 × magnification; scale bar: 50 µm; G-I Chorion, chorionic villi and decidua basalis; anti-P53 immunohistochemical stain, 20 × magnification; scale bar: 50 µm
Discussion and conclusion
Intrauterine fetal demise is a rare complication of COVID-19 in pregnant women and is associated with histopathological lesions induced by SARS-CoV-2 infection in the placenta. The pathological mechanism and virus-induced immune response in the placenta are not fully understood. We report the case of a 27-week gestation, SARS-CoV-2-infected asymptomatic woman with fetal demise. Studies have reported that SARS-CoV-2-associated fetal demise is not linked to the severity of the disease in pregnant mothers [6–8] and occurs between 21 and 39 weeks of gestation [7, 9], consistent with our case. Reports suggest that unvaccinated women with different clinical manifestations may develop placental pathological features with rapid deleterious fetal outcomes occurring between 3 and 15 days after receiving a COVID-19 diagnosis [10–12]. In our patient, maternal SARS-CoV-2 detection was followed by the diagnosis of fetal demise, due to asymptomatic status. This case arises before the emergence of the alpha and delta variants in Panama, which are linked to increased reports of fetal demise in SARS-CoV-2-infected mothers [7, 9].
The placental pathology present in cases of fetal demise in COVID-19 patients generally consists of three lesions: chronic histiocytic intervillositis, massive perivillous fibrin deposition [13, 14], and trophoblast necrosis; this triad is termed SARS-CoV-2 placentitis [15, 16]. It is suggested that SARS-CoV-2 placentitis may affect > 75% of the placenta [6], negatively compromising its function in gas and nutrient exchange and leading to severe fetal hypoxic-ischemic injury and fetal demise through malperfusion and placenta insufficiency [5, 9, 16]; many of these findings were rare before the COVID-19 pandemic. Our histopathological findings corresponded to patterns of subacute inflammation throughout the anatomic compartments of the placenta, showing severe chorioamnionitis, chronic villitis and deciduitis, accompanied by maternal and fetal vascular malperfusion with scarce intervillous fibrin deposition. In this patient, necrosis of trophoblasts, massive perivillous fibrin deposition, and histiocytic intervillositis were not observed, suggesting that other histopathological features in the placenta may be involved in the mechanisms that led to fetal demise. It is not clear whether the scarce fibrin deposition we observed was enough to compromise gas and nutrient exchange from the intervillous space to the chorionic villi; however, as we observed both maternal and fetal vascular malperfusion, we suggest that the sum of the findings played a role in the outcome of pregnancy.
Inflammation of the placental compartments is likely also crucial [8]. We identified CD68+ macrophages in the chorionic plate, chorionic villi, and decidua basalis. Other reports have identified macrophages in the intervillous space of the chorionic villi in SARS-CoV-2-associated fetal demises, consistent with one criterion of the SARS-CoV-2 placentitis triad [6, 14, 17, 18]. We additionally reported the presence of other immune cells, such as neutrophils, plasma cells, CD56 + NK cells and a few CD8+ T lymphocytes, which are implicated in antiviral responses, at sites of placental inflammation. Most cases reported the localization of SARS-CoV-2 spike and nucleocapsid antigens in syncytiotrophoblasts [9, 19], cytotrophoblasts [19], within villous stromal cells including Hofbauer cells and villous capillary endothelial cells [20, 21], and on the maternal side of the placenta [18, 21]. We detected the SARS-CoV-2 nucleocapsid in both maternal decidual cells and fetal stromal cells of the chorion and chorionic villi, consistent with the sites of inflammation.
Two possible mechanisms could be involved in fetal demise, one linked to placental vascular malperfusion and the other to an abnormal inflammatory immune response that leads to a cascade of fatal consequences. The fact that SARS-CoV-2 infection of the placenta and fetal demise are rare events but are consistently described in unvaccinated pregnant women demonstrates the importance of preventive measures in unvaccinated populations and highlights the urgency of vaccination in pregnant women, as we confirm that healthy patients can present SARS-CoV-2 placental lesions with fatal consequences.
Acknowledgements
We thank Dimelza Araúz, Maria Chen-Germán and Melissa Gaitán from the Department of Research in Virology and Biotechnology at the Gorgas Memorial Institute for Health Studies for their technical support.
Abbreviations
- COVID-19
Coronavirus disease 2019
- HIV
Human immunodeficiency virus
- NK
Natural killer
- RT‒PCR
Reverse transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TORCH
Toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex
Authors’ contributions
MAN, MAS, and SLV conceptualized and wrote the manuscript, and analyzed and interpreted the data. MAN and RV performed pathological evaluation and final interpretation. JS, PVDG recruited patient, and collected and analyzed clinical data. DL performed the histopathological technical support. CF and EG performed placental collection and sampling. MAS and SLV obtained the research funding. All the authors read and approved the final manuscript.
Funding
This report was supported by the British Embassy in Panama City Research Grant (MAS), the L’Óreal UNESCO for Women in Science Panama National Prize 2020 (MAS), and the Sistema Nacional de Investigación de Panamá (SNI, SENACYT) (SNI242-2022 MAS, SNI21-2020 SLV).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This patient was enrolled in a study approved by the National Bioethics Research Committee (EC-CNBI-2020–04-52), and written informed consent was obtained from the patient.
Consent for publication
Written informed consent for publication was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maricarmen Abrego-Navarro and Rodrigo Villalobos equally contributed to the work as first authors.
Mairim A. Solis and Sandra López-Vergès are equally senior authors.
Contributor Information
Sandra López-Vergès, Email: slopez@gorgas.gob.pa.
Mairim A. Solis, Email: msolis@gorgas.gob.pa
References
- 1.Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Can Med Assoc J. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226:177–186. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, Manuck TA, Miodovnik M, Sowles A, Clark K, Gyamfi-Bannerman C, Mendez-Figueroa H, Sehdev HM, Rouse DJ, Tita ATN, Bailit J, Costantine MM, Simhan HN, Macones GA, Health ftEKSNIoC, Network HDM-FMU. Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease, COVID-19. Obstet Gynecol. 2019;2021(137):571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, Roggero P, Prefumo F, do Vale MS, Cardona-Perez JA, Maiz N, Cetin I, Savasi V, Deruelle P, Easter SR, Sichitiu J, Soto Conti CP, Ernawati E, Mhatre M, Teji JS, Liu B, Capelli C, Oberto M, Salazar L, Gravett MG, Cavoretto PI, Nachinab VB, Galadanci H, Oros D, Ayede AI, Sentilhes L, Bako B, Savorani M, Cena H, García-May PK, Etuk S, Casale R, Abd-Elsalam S, Ikenoue S, Aminu MB, Vecciarelli C, Duro EA, Usman MA, John-Akinola Y, Nieto R, Ferrazzi E, Bhutta ZA, Langer A, Kennedy SH, Papageorghiou AT. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz DA, Avvad-Portari E, Babál P, Baldewijns M, Blomberg M, Bouachba A, Camacho J, Collardeau-Frachon S, Colson A, Dehaene I, Ferreres JC, Fitzgerald B, Garrido-Pontnou M, Gergis H, Hargitai B, Helguera-Repetto AC, Holmström S, Irles CL, Leijonhfvud Å, Libbrecht S, Marton T, McEntagart N, Molina JT, Morotti R, Nadal A, Navarro A, Nelander M, Oviedo A, Otani ARO, Papadogiannakis N, Petersen AC, Roberts DJ, Saad AG, Sand A, Schoenmakers S, Sehn JK, Simpson PR, Thomas K, Valdespino-Vázquez MY, van der Meeren LE, Van Dorpe J, Verdijk RM, Watkins JC, Zaigham M. Placental Tissue Destruction and Insufficiency From COVID-19 Causes Stillbirth and Neonatal Death From Hypoxic-Ischemic Injury. Arch Pathol Lab Med. 2022;146:660–676. doi: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 6.Konstantinidou AE, Angelidou S, Havaki S, Paparizou K, Spanakis N, Chatzakis C, Sotiriadis A, Theodora M, Donoudis C, Daponte A, Skaltsounis P, Gorgoulis VG, Papaevangelou V, Kalantaridou S, Tsakris A. Stillbirth due to SARS-CoV-2 placentitis without evidence of intrauterine transmission to fetus: association with maternal risk factors. Ultrasound Obstet Gynecol. 2022;59:813–822. doi: 10.1002/uog.24906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nkobetchou M, Leruez-Ville M, Guilleminot T, Roux N, Petrilli G, Guimiot F, Saint-Frison MH, Deryabin I, Ville Y, Faure-Bardon V. SARS-CoV-2 infection as cause of in-utero fetal death: regional multicenter cohort study. Ultrasound Obstet Gynecol. 2023;62:867–874. doi: 10.1002/uog.27439. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D, Peyvandipour A, Bhatti G, Galaz J, Gershater M, Levenson D, Pusod E, Tao L, Kracht D, Florova V, Leng Y, Motomura K, Para R, Faucett M, Hsu CD, Zhang G, Tarca AL, Pique-Regi R, Gomez-Lopez N. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13:320. doi: 10.1038/s41467-021-27745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Yamaguchi K, Maegawa Y, Komine-Aizawa S, Kondo E, Ikeda T. Intrauterine fetal death during COVID-19 pregnancy: Typical fetal heart rate changes, coagulopathy, and placentitis. J Obstet Gynaecol Res. 2022;48:1978–1982. doi: 10.1111/jog.15302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merriel A, Fitzgerald B, O'Donoghue K. SARS-CoV-2-Placental effects and association with stillbirth. BJOG. 2024;131(4):385–400. doi: 10.1111/1471-0528.17698. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz DA. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses. 2022;14(3):458. Published 2022 Feb 23. 10.3390/v14030458. [DOI] [PMC free article] [PubMed]
- 12.Zaigham M, Gisselsson D, Sand A, Wikström AK, von Wowern E, Schwartz DA, Iorizzo L, Nelander M, Blomberg M, Papadogiannakis N, Holmström S, Leijonhfvud Å, Sengpiel V. Clinical-pathological features in placentas of pregnancies with SARS-CoV-2 infection and adverse outcome: case series with and without congenital transmission. BJOG. 2022;129:1361–1374. doi: 10.1111/1471-0528.17132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nonn O, Bonstingl L, Sallinger K, Neuper L, Fuchs J, Gauster M, Huppertz B, Brislinger D, El-Heliebi A, Fluhr H, Kampelmühler E, Klaritsch P. Maternal COVID-19 causing intrauterine foetal demise with microthrombotic placental insufficiency: a case report. BMC Pregnancy Childbirth. 2023;23:653. doi: 10.1186/s12884-023-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosič N, Luzar B, Pečlin P, Druškovič M, Rus KR, Županc TA, Sršen TP. Fetal death from SARS-CoV-2 mediated acute placental failure. J Reprod Immunol. 2023;158:103958. doi: 10.1016/j.jri.2023.103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz DA, Mulkey SB, Roberts DJ. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: clinical-pathologic correlations. Am J Obstet Gynecol. 2023;228:261–269. doi: 10.1016/j.ajog.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debelenko L. SARS-CoV-2 Infection in Late Pregnancy and Childbirth from the Perspective of Perinatal Pathology. J Dev Biol. 2023;11. [DOI] [PMC free article] [PubMed]
- 17.Daza M, Corchuelo S, Osorio J, Alberto Gómez L, Parra E, Alarcón Á, Mercado M. Fetal demise and SARS-CoV-2 infection during pregnancy: Histopathological and immunohistochemical findings of three cases referred to the Colombian National Institute of Health. Clin Infect Pract. 2023;17:100219. doi: 10.1016/j.clinpr.2023.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corn M, Pham T, Kemp W. Adverse Fetal Outcomes and Histopathology of Placentas Affected by COVID-19: A Report of Four Cases. Cureus. 2023;15:e44402. doi: 10.7759/cureus.44402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz DA, Bugatti M, Santoro A, Facchetti F. Molecular Pathology Demonstration of SARS-CoV-2 in Cytotrophoblast from Placental Tissue with Chronic Histiocytic Intervillositis, Trophoblast Necrosis and COVID-19. Journal of Developmental Biology. 2021;9:33. doi: 10.3390/jdb9030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz DA, Baldewijns M, Benachi A, Bugatti M, Bulfamante G, Cheng K, Collins RRJ, Debelenko L, De Luca D, Facchetti F, Fitzgerald B, Levitan D, Linn RL, Marcelis L, Morotti D, Morotti R, Patanè L, Prevot S, Pulinx B, Saad AG, Schoenmakers S, Strybol D, Thomas K, Tosi D, Toto V, van der Meeren LE, Verdijk RM, Vivanti AJ, Zaigham M. Hofbauer Cells and COVID-19 in Pregnancy: Molecular Pathology Analysis of Villous Macrophages, Endothelial Cells, and Placental Findings From 22 Placentas Infected by SARS-CoV-2 With and Without Fetal Transmission. Arch Pathol Lab Med. 2021;145:1328–1340. doi: 10.5858/arpa.2021-0296-SA. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez J, Vigil-De Gracia P, Guerrero E, Gaitán M, Fu C, Chen-Germán M, Villalobos R, Coronado L, Martínez AA, Araúz D, Saenz L, Chavarría O, Góndola J, Moreno A, González C, Vega S, Campana S, Ng Chinkee J, López-Vergès S, Solís MA. Severe acute respiratory syndrome coronavirus 2 detected in placentas of 2 coronavirus disease 2019-positive asymptomatic pregnant women-case report. AJOG Glob Rep. 2021;1:100001. doi: 10.1016/j.xagr.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


