Abstract
Background
Many older adults with cancer have ≥2 impairments on geriatric assessment, which affects present and future frailty status, treatment tolerability, and outcomes. Our objective was to identify and describe distinct geriatric assessment impairment classes using latent class analysis (LCA) in older patients with gastrointestinal malignancies and assess 1-year mortality.
Methods
We used the Cancer & Aging Resilience Evaluation (CARE) Study, a registry of older adults (≥60 years) at University of Alabama at Birmingham. The analytic cohort included patients with gastrointestinal malignancies who completed a self-administered geriatric assessment (CARE tool) before chemotherapy and had ≥1 geriatric assessment impairment. Thirteen geriatric assessment impairments were used as indicators in LCA. Resultant classes were described, mortality was estimated, and risk contrasts (differences and hazard ratios) were calculated with 95% confidence intervals. For comparison, estimates were provided for frailty categories (robust, prefrail, and frail) determined from 44 items in the CARE tool. Stratified analyses included high-risk (pancreatic, hepatobiliary, and esophageal) versus low-risk gastrointestinal cancers, and stage (IV vs I–III).
Results
Six geriatric assessment impairment classes were identified: Mild impairment (LC1); Social support impairment (LC2); Weight loss alone (LC3); Impaired, low anxiety/depression (LC4); Impaired with anxiety/depression (LC5); and Global impairment (LC6). One-year mortality was 14%, 22%, 29%, 34%, 50%, and 50% for LC1–LC6, respectively. For frailty categories, estimates ranged from 18% (robust) to 40% (frail). In stratified analyses, LC4–LC6 consistently had higher mortality estimates compared to LC1.
Conclusions
The 6 geriatric assessment impairment classes showed a wider spread of mortality estimates compared to frailty categories and could be used to identify vulnerable patients and to plan interventions.
Keywords: Epidemiology, Frailty, Geriatric assessment, Geriatric oncology, Latent class analysis
Cancer is broadly considered a disease of aging with half of new diagnoses occurring among adults age 65 or older (1). Although age is not a contraindication for chemotherapy treatment, older patients often have co-occurring impairments that warrant consideration (2,3). It is estimated that more than half of older adults with cancer have ≥2 health impairments (4,5). Thus, the presence of multiple impairments is common for older adults with cancer and contributes to heterogeneity between patients and variable treatment tolerability.
In acknowledging these challenges, the American Society of Clinical Oncology (ASCO) and other clinical guidelines recommend using geriatric assessment to identify vulnerabilities that are not regularly captured in oncology assessments (6,7). Geriatric assessment is an extensive evaluation of multiple health domains (eg, physical function, falls, depression, cognition, nutrition, and comorbidity) using a variety of clinical tools (6). Geriatric assessment can be used to tailor interventions, and geriatric assessment can also identify frailty—a syndrome and state of increased vulnerability due to accrued impairments in multiple body systems that confers diminished ability to respond to stressors (eg, treatment, illness, and injury) (8,9). Geriatric assessment is effective in reducing chemotherapy-related toxic effects (10,11); however, successful interventions often require multidisciplinary teams including geriatric oncologists, nurse practitioners, social workers, physical/occupational therapists, nutritionists, and pharmacists. Thus, intervention on geriatric impairments requires knowledge of co-occurring impairments and coordination between healthcare providers.
To facilitate intervention planning and improve service coordination, it is important to identify impairment patterns from the multiple domains in a geriatric assessment. Our objective was to identify and describe distinct geriatric assessment impairment classes using latent class analysis (LCA) in older patients with gastrointestinal malignancies and to assess 1-year mortality by class.
Method
Data Source and Study Sample
This study included older patients (≥60 years) with gastrointestinal malignancies in the Cancer & Aging Resilience Evaluation (CARE) Registry who completed the CARE tool, a self-administered, patient-reported geriatric assessment adapted from the Cancer and Aging Research Group geriatric assessment developed by Hurria and colleagues (12–14). This self-administered geriatric assessment has high clinical utility considering the short median time for completion for CARE Registry participants (10 minutes; interquartile range: 10–16 minutes) (13). Additionally, the geriatric assessment measures and the frailty index derived from this fully patient-reported geriatric assessment have high criterion validity as they have been shown to be good predictors of overall survival and adverse treatment-related outcomes (ie, functional decline and treatment-related toxicity) (15,16).
The study sample included patients who completed the CARE tool between July 2017 and February 2022 with complete information on impairments, ≥1 reported impairment, and no prior chemotherapy in the past 6 months and before starting current chemotherapy (“prechemotherapy sample,” see Supplementary Figure 1 for inclusion). This study was exempted from institutional review board review (IRB) by the University of North Carolina at Chapel Hill Office of Human Research Ethics. The CARE registry study was approved by the University of Alabama at Birmingham IRB and written consent was obtained from each participant.
Indicators Used to Determine Impairment Profiles
We conducted LCA using 13 geriatric assessment impairments defined in the CARE tool as latent class indicators (Supplementary Table 1) (5). Indicators were dichotomized based on the presence or absence of the impairment and included recent falls (≥1 falls in the past 6 months); walking (significant limitations in walking one block); instrumental activities of daily living (IADL, ≥2 impairments); activities of daily living (ADL, ≥1 impairment); weight loss (≥3% loss within 1 month or ≥6% loss within 6 month based on the abridged version of the patient-generated subjective global assessment, abPG-SGA, thresholds defining moderate impact weight loss) (17–19); low activity (Eastern Cooperative Oncology Group Performance Status, ECOG-PS ≥3); social activity interference (reported “most” or “all of the time”); multimorbidity (≥4 comorbidities reported on the Older Americans and Services comorbidity measure (20–22); low social support (ie, someone to help at most “some of the time” on the Medical Outcomes Study Social Support Survey [23,24]); anxiety (Patient-Reported Outcomes Measurement Information System, PROMIS, Anxiety T-score >60); depression (PROMIS Depression T-score >60 [25]); cognitive impairment (PROMIS Cognitive Function T-score <40 [26,27]); and polypharmacy (≥9 daily medications [28]).
Outcomes
Vital status and date of death were identified up to October 2021 using patient name, social security number, and linkage with LexisNexis. Zip code and date of diagnosis were used for confirmation.
Patient Characteristics
Demographics were reported in the CARE tool and included race (White, Black or African American, Native American or Alaskan, Asian, Native Hawaiian, other), ethnicity (Hispanic or Latino, non-Hispanic), education level (less than high school, high school graduate, associate/Bachelors, advanced degree), and marital status (single, widowed/divorced, married). Additional information from electronic health records was extracted by a trained researcher and included age, patient-reported gender, height and weight (measured ≤2 weeks before treatment started) for calculation of body mass index (BMI), cancer type and stage, and current chemotherapy treatment line. For data cleaning purposes, patients reporting current weight of <50 pounds were excluded. BMI was categorized (underweight, normal, overweight, or obese), and incorporated Asian- (≥22.2 and ≥26.9 kg/m2) and Black-specific (≥23.4 and ≥28.1 kg/m2) cutoff points for overweight and obese categories (29). High-risk malignancies (pancreatic, hepatobiliary, and esophageal cancers) and low-risk malignancies (colorectal, gastrointestinal stromal tumors, neuroendocrine tumors, and other) were categorized based on typical estimated survival and 1-year mortality (15,30).
Frailty score was calculated based on the principles of deficit accumulation using 44 health deficit items in the CARE tool. Scores were calculated for patients who responded to ≥24 items (31–36). Items were coded as indicating the presence of deficit (“1”), absence (“0”), or intermediate responses (eg, “sometimes” or “maybe”; “0.5”), and scores were assigned to represent the overall proportion of deficits (range 0–1). Frailty scores were categorized using previously defined thresholds: robust (0–0.2), prefrail (0.2–0.35), and frail (>0.35) (14,37).
Statistical Analysis
Latent class analysis is a statistical procedure used to detect heterogeneity in a sample and to identify subgroups (38). As a form of person-centered mixture modeling, LCA uses participant responses and cross-classification of categorical indicator variables to identify latent (or unobserved) groups that share response patterns (38,39). The underlying assumption is that membership in latent classes is antecedent and explains patterns of responses, indicator variables, or scales (39,40). Responses on each indicator are assumed to be conditionally independent of each other given latent class (LC) membership (38). Models with varying number of classes were fit to the data, and posterior probabilities for membership in each class were estimated for respondents.
In the prechemotherapy impairment sample (n = 464, Supplementary Figure 1), we conducted LCA using the 13 impairment indicators to model LC probabilities. The number of latent classes used to fit the data was determined iteratively by evaluating models with 1 to 8 classes. Models were evaluated quantitatively and qualitatively using the following criteria: (1) lower values for Akaike information criteria (AIC), Bayesian information criteria (BIC), and adjusted Bayesian information criterion (aBIC); (2) bootstrapped likelihood ratio tests (BLRT, 1 000 replicates); (3) entropy; and (4) based on interpretation of resultant classes and clinical input (41). BLRT tests the null hypothesis that modeling k classes is adequate compared to modeling k + 1 classes; p values < .05 indicated that the larger model fit the sample better. Entropy assessed discrimination and values ≥0.8 indicated acceptable class separation (40). Using each patient’s posterior probabilities for the k classes, we assigned patients to 1 LC based on maximum posterior class membership probability.
To facilitate interpretation and labeling, we evaluated geriatric assessment impairment probabilities for each LC and incorporated clinical input. For the overall sample and each LC, we reported patient characteristics using counts and percentages for categorical characteristics and median, first quartile, and third quartile for continuous characteristics.
For patients enrolled in the registry before October 2021, we evaluated mortality risk using Kaplan–Meier methods for each LC. Kaplan–Meier curves were plotted for each LC, and curves based on frailty categories were plotted for comparison. Based on the Kaplan-Meier curves, multiple latent classes had few events occurring after 18 months; therefore, mortality risk estimates were provided for 6, 12, and 18 months after completion of the CARE tool—180, 365, and 540 days, respectively. Given the high expected mortality for the target older adult patient population, 12-month estimates were the focus for summarizing results in text and for exploratory analyses.
At each time point of interest, we compared risk between classes using risk differences and 95% confidence intervals for the contrasts. The LC with the largest class size was selected as the referent class. We additionally used Cox proportional hazards models to estimate hazard ratios based on patient follow-up time up to 18 months. We calculated crude estimates and estimates from models singly adjusted for demographics (age, sex), cancer characteristics (high- vs low-risk cancer, stage 4 cancer), or prognostic indices (BMI, frailty). Given our descriptive intent and the small size of some latent classes, we did not assess fully adjusted models.
In exploratory analyses, we calculated stratified 1-year mortality risks for each LC based on cancer type (high-risk vs low-risk) and stage (IV vs I–III) and evaluated the order of mortality estimates for each stratum. For each LC, we also estimated differences in 1-year mortality between strata and confidence intervals for mortality differences.
As a sensitivity analysis, we conducted single-value imputation to include patients with missing impairment items; missing indicators were recoded to “no impairment.” The imputed, impairment sample included 600 patients with mortality outcomes available for 579. Analyses were conducted using SAS statistical software version 9.4 (PROC LCA and PROC LIFETEST; SAS Institute Inc., Cary, NC) and Kaplan–Meier curves were generated using R statistical software version 4.1.1. (survminer package; Comprehensive R Archive Network, CRAN).
Results
Overall Study Sample
The analytic sample (median age 69 years, 43% women) included 464 patients that were predominately White/Caucasian (Table 1). The sample had an equal percentage of patients with high-risk and low-risk cancers, 42% had stage IV disease, and 74% were planning to receive their first chemotherapy treatment. Although all of the included patients had ≥1 impairment; 57% reported 1–3 impairments (Supplementary Figure 1). Based on the CARE frailty index, 30% of the sample was considered robust (n = 137); the remaining sample had equal percentages of patients considered prefrail (n = 163, 35%) and frail (n = 164, 35%).
Table 1.
Characteristics of the CARE Registry Study Sample and Latent Impairment Classes at Enrollment (2017–2021, N = 464)
| Characteristics, n (%) | Overall Sample (n = 464) | Mild Impairment (n = 130) | Social Support Impairment (n = 56) | Weight Loss Alone (n = 72) | Impaired, Low Anxiety/Depression (n = 105) | Impaired With Anxiety/Depression (n = 51) | Global Impairment (n = 50) |
|---|---|---|---|---|---|---|---|
| Age (median, IQR) | 69 (64–75) | 70 (65–76) | 68 (62–75) | 67 (63–73) | 70 (64–75) | 68 (64–74) | 71 (65–76) |
| Gender | |||||||
| Male | 266 (57.3) | 72 (55.4) | 35 (62.5) | 40 (55.6) | 59 (56.2) | 28 (54.9) | 32 (64.0) |
| Female | 198 (42.7) | 58 (44.6) | 21 (37.5) | 32 (44.4) | 46 (43.8) | 23 (45.1) | 18 (36.0) |
| Race | |||||||
| White/Caucasian | 359 (77.4) | 107 (82.3) | 49 (87.5) | 57 (79.2) | 72 (68.6) | 36 (70.6) | 38 (76.0) |
| Black/African American | 93 (20.0) | 21 (16.2) | 6 (10.7) | 12 (16.7) | 28 (26.7) | 15 (29.4) | 11 (22.0) |
| Asian | 6 (1.3) | 0 (0.0) | 1 (1.8) | 2 (2.8) | 2 (1.9) | 0 (0.0) | 1 (2.0) |
| American Indian/Alaska Native | 2 (0.4) | (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.9) | 0 (0.0) | 0 (0.0) |
| Other/unknown | 4 (0.9) | 2 (1.5) | 0 (0.0) | 1 (1.4) | 1 (1.0) | 0 (0.0) | 0 (0.0) |
| Education level | |||||||
| Less than high school | 69 (14.9) | 23 (17.7) | 3 (5.4) | 5 (6.9) | 20 (19.1) | 10 (19.6) | 8 (16.0) |
| High school graduate | 218 (47.0) | 60 (46.2) | 26 (46.4) | 34 (47.2) | 47 (44.8) | 23 (45.1) | 28 (56.0) |
| Associate/bachelors | 119 (25.7) | 31 (23.9) | 17 (30.4) | 26 (36.1) | 26 (24.8) | 10 (19.6) | 9 (18.0) |
| Advanced degree | 51 (11.0) | 16 (12.3) | 9 (16.1) | 7 (9.7) | 10 (9.5) | 5 (9.8) | 4 (8.0) |
| Unknown | 7 (1.5) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 2 (1.9) | 3 (5.9) | 1 (2.0) |
| Marital status | |||||||
| Single, never married | 29 (6.3) | 7 (5.4) | 3 (5.4) | 2 (2.8) | 13 (12.4) | 2 (3.9) | 2 (4.0) |
| Widowed/divorced/separated | 155 (33.4) | 43 (33.1) | 31 (55.4) | 13 (18.1) | 22 (21.0) | 28 (54.9) | 18 (36.0) |
| Married | 272 (58.6) | 78 (60.0) | 21 (37.5) | 57 (79.2) | 67 (63.8) | 19 (37.3) | 30 (60.0) |
| Unknown | 8 (1.7) | 2 (1.5) | 1 (1.8) | 0 (0.0) | 3 (2.9) | 2 (3.9) | 0 (0.0) |
| Cancer type | |||||||
| Low risk | 233 (50.2) | 76 (58.5) | 36 (64.3) | 30 (41.7) | 42 (40.0) | 27 (52.9) | 22 (44.0) |
| High risk | 231 (49.8) | 54 (41.5) | 20 (35.7) | 42 (58.3) | 63 (60.0) | 24 (47.1) | 28 (56.0) |
| Cancer stage | |||||||
| I–III | 268 (57.8) | 88 (67.7) | 41 (73.2) | 37 (51.4) | 49 (46.7) | 30 (58.8) | 23 (46.0) |
| IV | 196 (42.2) | 42 (32.3) | 15 (26.8) | 35 (48.6) | 56 (53.3) | 21 (41.2) | 27 (54.0) |
| Chemotherapy treatment (current) | |||||||
| 1 | 343 (73.9) | 102 (78.5) | 43 (76.8) | 54 (75.0) | 81 (77.1) | 31 (60.8) | 32 (64.0) |
| 2–4 | 10 (2.2) | 4 (3.1) | 2 (3.6) | 1 (1.4) | 2 (1.9) | 1 (2.0) | 0 (0.0) |
| ≥5 | 62 (13.4) | 13 (10.0) | 5 (8.9) | 9 (12.5) | 10 (9.5) | 11 (21.6) | 14 (28.0) |
| Unknown | 49 (10.6) | 11 (8.5) | 6 (10.7) | 8 (11.1) | 12 (11.4) | 8 (15.7) | 4 (8.0) |
| BMI category | |||||||
| Underweight | 21 (4.5) | 2 (1.5) | 2 (3.6) | 3 (4.2) | 5 (4.8) | 3 (5.9) | 6 (12.0) |
| Normal weight | 169 (36.4) | 34 (26.2) | 24 (42.9) | 30 (41.7) | 37 (35.2) | 29 (56.9) | 15 (30.0) |
| Overweight | 142 (30.6) | 47 (36.2) | 15 (26.8) | 25 (34.7) | 28 (26.7) | 11 (21.6) | 16 (32.0) |
| Obese | 124 (26.7) | 45 (34.6) | 14 (25.0) | 13 (18.1) | 31 (29.5) | 8 (15.7) | 13 (26.0) |
| Unknown | 8 (1.7) | 2 (1.5) | 1 (1.8) | 1 (1.4) | 4 (3.8) | 0 (0.0) | 0 (0.0) |
| Frailty | |||||||
| Robust | 137 (29.5) | 36 (27.7) | 47 (83.9) | 42 (58.3) | 10 (9.5) | 2 (3.9) | 0 (0.0) |
| Prefrail | 163 (35.1) | 68 (52.3) | 9 (16.1) | 29 (40.3) | 45 (42.9) | 12 (23.5) | 0 (0.0) |
| Frail | 164 (35.3) | 26 (20.0) | 0 (0.0) | 1 (1.4) | 50 (47.6) | 37 (72.6) | 50 (100.0) |
| Comorbidities | |||||||
| Other cancers or leukemia | 102 (22.0) | 33 (25.4) | 9 (16.1) | 16 (22.2) | 21 (20.0) | 12 (23.5) | 11 (22.0) |
| Arthritis or rheumatism | 178 (38.4) | 71 (54.6) | 13 (23.2) | 17 (23.6) | 37 (35.2) | 20 (39.2) | 20 (40.0) |
| Glaucoma | 33 (7.1) | 9 (6.9) | 1 (1.8) | 4 (5.6) | 10 (9.5) | 4 (7.8) | 5 (10.0) |
| Emphysema/chronic bronchitis | 45 (9.7) | 15 (11.5) | 2 (3.6) | 3 (4.2) | 11 (10.5) | 5 (9.8) | 9 (18.0) |
| High blood pressure | 294 (63.4) | 101 (77.7) | 28 (50.0) | 42 (58.3) | 65 (61.9) | 25 (49.0) | 33 (66.0) |
| Heart disease | 99 (21.3) | 43 (33.1) | 3 (5.4) | 7 (9.7) | 22 (21.0) | 7 (13.7) | 17 (34.0) |
| Circulation trouble in arms/legs | 99 (21.3) | 34 (26.2) | 3 (5.4) | 6 (8.3) | 22 (21.0) | 11 (21.6) | 23 (46.0) |
| Diabetes | 158 (34.1) | 61 (46.9) | 12 (21.4) | 17 (23.6) | 41 (39.1) | 8 (15.7) | 19 (38.0) |
| Stomach or intestinal disorders | 178 (38.4) | 58 (44.6) | 12 (21.4) | 20 (27.8) | 39 (37.1) | 23 (45.1) | 26 (52.0) |
| Osteoporosis | 51 (11.0) | 16 (12.3) | 5 (8.9) | 3 (4.2) | 15 (14.3) | 7 (13.7) | 5 (10.0) |
| Chronic liver or kidney disease | 87 (18.8) | 27 (20.8) | 7 (12.5) | 10 (13.9) | 22 (21.0) | 9 (17.7) | 12 (24.0) |
| Stroke | 36 (7.8) | 10 (7.7) | 1 (1.8) | 5 (6.9) | 7 (6.7) | 4 (7.8) | 9 (18.0) |
| Depression | 91 (19.6) | 22 (16.9) | 2 (3.6) | 6 (8.3) | 12 (11.4) | 29 (56.9) | 20 (40.0) |
Note: BMI = body mass index; CARE = Cancer and Aging Resilience Evaluation; IQR = interquartile range.
Latent Class Analysis—Model Fit and Class Identification
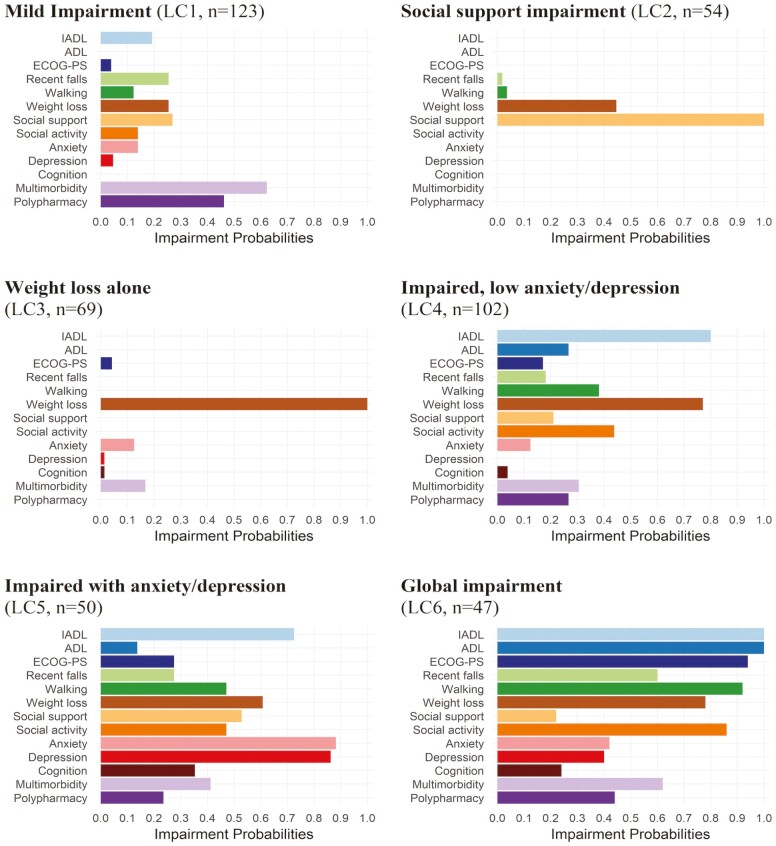
The LCA model fit criteria indicated that 6 classes fit the sample best (Table 2). ABIC was lowest for these results. BLRT p values for models with 1 to 5 latent classes were < .05 indicating that larger models were preferred; a BLRT p value > .05 indicated that 6 classes fit the data adequately relative to larger models. After assigning patients to individual classes, posterior probabilities for the 6 classes were ≥80%. The impairment latent classes were labeled based on impairment probabilities and clinical input (Figure 1, Supplementary Table 2):
Table 2.
Accuracy and Fit of Latent Class Models With 1-8 Classes (N = 464)
| No. of Classes | p Value | |||||
|---|---|---|---|---|---|---|
| LL | AIC | BIC | ABIC | BLRT | Entropy | |
| 1 | −3 300 | 2 066 | 2 120 | 2 078 | <.001 | 1.00 |
| 2 | −2 991 | 1 477 | 1 588 | 1 503 | <.001 | 0.83 |
| 3 | −2 945 | 1 411 | 1 581 | 1 451 | <.001 | 0.78 |
| 4 | −2 898 | 1 347 | 1 574 | 1 400 | .002 | 0.77 |
| 5 | −2 873 | 1 323 | 1 609 | 1 390 | .005 | 0.79 |
| 6 | −2 850 | 1 306 | 1 650 | 1 387 | .066 | 0.79 |
| 7 | −2 832 | 1 298 | 1 700 | 1 392 | .052 | 0.81 |
| 8 | −2 814 | 1 290 | 1 750 | 1 398 | .025 | 0.80 |
Note: AIC = Akaike information criterion; ABIC = adjusted Bayesian information criterion; BIC = Bayesian information criterion; BLRT = bootstrap likelihood ratio test; LL = log likelihood.
Figure 1.
Item impairment probabilities for each latent class (N = 464)a. aPosterior probability of class membership is as follows: 0.815, 0.904, 0.832, 0.800, 0.916, 0.913 for LC1–LC6, respectively. ADL = activities of daily living; ECOG-PS = Eastern Cooperative Oncology Group Performance Status; IADL = instrumental activities of daily living; LC = latent class.
LC1: Mild impairment, n = 130 (28%). Characterized by low probabilities (<30%) for functional impairments (ie, falls, walking, IADLs, and ECOG-PS), anxiety, depression, cognition, and social support; multimorbidity and polypharmacy probabilities were >60% and >40%, respectively.
LC2: Social support impairment, n = 56 (12%). Characterized by 100% social support impairment, 45% probability of weight loss, and low (<5%) probability for functional impairments, multimorbidity, anxiety, or depression.
LC3: Weight loss alone, n = 72 (16%) Characterized by 100% weight loss with low probabilities (<20%) for other impairments.
LC4: Moderate impairment with low anxiety/depression, n = 105 (23%), hereafter referred to as “impaired, low anxiety/depression” class. Characterized by higher probabilities for functional impairments, weight loss, and social activity impairments compared to LC1; and low probabilities for anxiety/depression.
LC5: Moderate impairment with anxiety/depression, n = 51 (11%), hereafter referred to as “impaired with anxiety/depression” class. Characterized by higher impairment probabilities than LC1 for functional impairments, weight loss, social activity, social support, and cognition; and high probabilities (>85%) for anxiety/depression.
LC6: Global impairment, n = 50 (11%). Characterized by higher impairment probabilities than the impaired classes (LC4 and LC5) for functional domains including >90% probability walking impairment and ECOG-PS impairment, and 100% probability of IADL and ADL impairment. Probability of social activity impairment and multimorbidity was also greater than LC5, but probabilities for anxiety/depression were <50%.
Characteristics by Latent Class
Although the 6 impairment classes had similar age distributions, the social support impairment class and global impairment class had lower percentages of women compared to the others (Table 1). The percentage of patients in non-White/non-Caucasian categories was greatest in the 2 impaired classes and the global impairment class. Patients belonging to the social support impairment and impaired with anxiety/depression classes reported greater percentages of being widowed, divorced, or separated compared to the other impairment classes. For all classes besides the social support impairment class, high-risk cancers and stage IV cancers were >40% and >30%, respectively. The impaired with anxiety/depression class and the global impairment class had the greatest percentages of patients planning to receive their 2nd chemotherapy treatment or beyond. Patients in each LC were mostly (>50%) overweight or obese except for the impaired with anxiety/depression class (37%). The global impairment class had the largest percentage of patients considered underweight (12%) compared to the other impairment classes (<6%).
The mild impairment class mostly had patients considered prefrail and 20% were frail. Patients belonging to the social support impairment and weight loss alone classes were predominately robust or prefrail. For the impaired classes, >90% of patients were prefrail or frail. All patients belonging to the global impairment class were frail. Regarding comorbidities, the social support impairment and weight loss alone classes generally had lower percentages compared to the other impairment classes. The global impairment class had the highest percentage of patients reporting stroke.
Mortality by Latent Classes
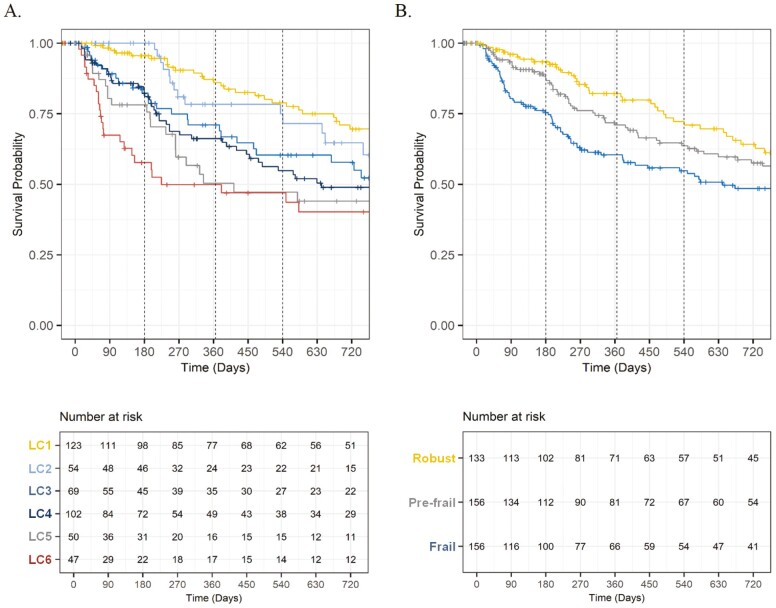
Among 445 patients enrolled in the registry before October 2021, 62 (14%), 112 (25%), and 134 (30%) deaths occurred within 6, 12, and 18 months, respectively. Kaplan–Meier curves are presented in Figure 2, and the order of 12-month mortality estimates was as follows: mild impairment class (14%); social support impairment class (22%); weight loss alone class (29%); impaired, low anxiety/depression class (34%); impaired with anxiety/depression class (50%); global impairment class (50%). At each time point of interest, the spread of mortality estimates was wider for the latent impairment classes compared to estimates based on frailty status.
Figure 2.
Kaplan–Meier curves for mortality by latent impairment class and by frailty status (CARE registry sample, 2017–2021, N = 445). CARE = Cancer and Aging Resilience Evaluation; LC = latent class.
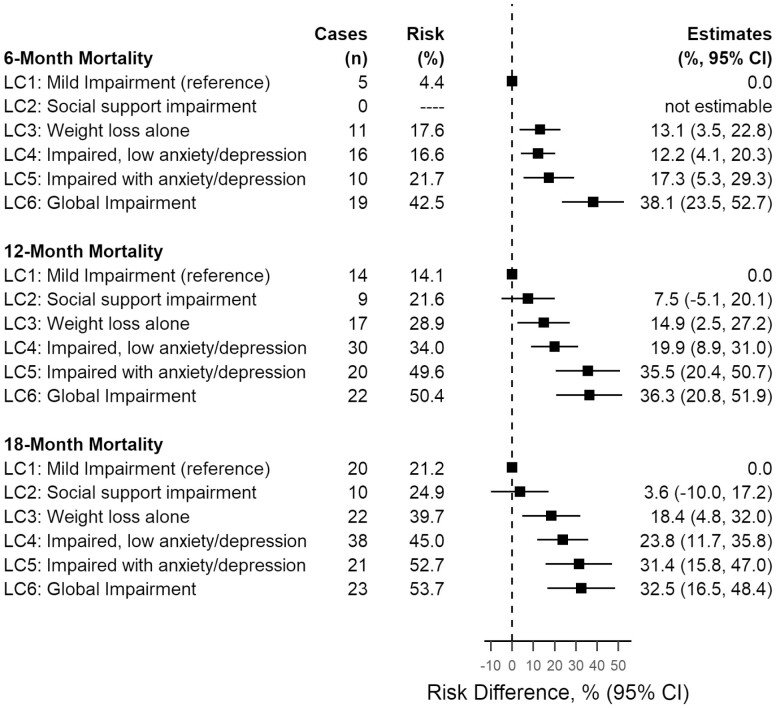
For 6-, 12-, and 18-month mortality, risk contrasts showed that estimates were greater for LC3, LC4, LC5, and LC6 compared to LC1 (Figure 3). For the 3 time points of interest, mortality estimates for LC2 (social support impairment) were either not estimable or had small differences with LC1 (ie, <10% difference). Crude and adjusted hazard ratios using data up to 18 months of follow-up showed similar results (Supplementary Figure 3). LC2 had hazard ratio point estimates ranging from 1.0 to 1.5 compared to LC1, whereas the remaining latent classes had point estimates greater than or equal to 2.0: LC3 versus LC1 (HR range: 2.0 to 2.7 across crude and adjusted models), LC4 versus LC1 (HR range: 2.3 to 2.7), LC5 versus LC1 (HR range: 3.3 to 3.9), and LC6 versus LC1 (HR range: 4.2 to 4.6).
Figure 3.
Absolute differences in risk of mortality by latent impairment class (CARE registry sample, 2017–2021, N = 445). CARE = Cancer and Aging Resilience Evaluation; CI = confidence interval; LC = latent class.
In stratified analyses, 1-year mortality estimates for the impairment classes ranged from 29% to 75% for high-risk cancers and 3% to 32% for low-risk cancers (Supplementary Table 3). For strata by cancer stage, estimates ranged from 13% to 62% for stage IV and from 15% to 42% for stage I–III (Supplementary Table 4). Across all strata, LC4, LC5, and LC6 consistently had higher-ranked mortality estimates compared to LC1.
Sensitivity Analyses
After single-value imputation for missing impairments, we included an additional 136 patients (Supplementary Table 5) and were able to replicate the 6 latent impairment classes (Supplementary Figure 4, Supplementary Table 6). Among 579 patients with follow-up data for mortality outcomes, 85 (15%), 149 (26%), and 178 (31%) deaths occurred within 6, 12, and 18 months, respectively. Compared to primary analyses, there were slight differences in the composition of the resultant latent classes; however, Kaplan–Meier plots were similar to primary analyses (Supplementary Figure 5). The order of 12-month mortality estimates differed from primary analyses: social support impairment class (21%), mild impairment class (24%); weight loss alone class (28%); global impairment class (37%); impaired, low anxiety/depression class (46%); and impaired with anxiety/depression class (50%).
Compared to patients belonging to the mild impairment class (LC1), estimated mortality risk was greater for the global impairment class (LC6) and the moderate impairment classes (LC4, LC5) at 6-, 12-, and 18-month timepoints (Supplementary Figure 6). LC4 and LC5 had the greatest estimated 12-month and 18-month mortality (range: 46%–50%). Additionally, based on risk difference and hazard ratio results, the estimated risk of mortality was similar, lower, or not estimable for patients belonging to the social support impairment class (LC2) compared to LC1 (Supplementary Figures 6 and 7). Patients characterized by weight loss alone (LC3) also had risk contrast estimates that indicated similar or only slightly elevated mortality risk compared to LC1.
Stratified analyses by cancer type and stage showed that LC4 and LC5 consistently had high 1-year mortality estimates compared to LC1 (Supplementary Tables 7 and 8). Mortality estimates for LC6 were relatively high for both strata by cancer type (1-year risk range: 48%–53%) and moderate for patients with low-risk cancer (24%). Compared to these estimates for LC6, the estimated mortality for LC6 was lower among patients with stage I–III cancer (16%).
Discussion
In this registry sample of older adults with gastrointestinal cancers, we identified 6 latent impairment classes from patient responses on a geriatric assessment: mild impairment class; social support impairment class; weight loss alone class; impaired, low anxiety/depression class; impaired with anxiety/depression class; and global impairment class. Compared to the social support impairment class and weight loss alone class, the latter 3 impairment classes were characterized by a confluence of multiple impairments. The latter 3 classes also had greater estimated mortality compared to the mild impairment class, although estimates varied across analyses and stratifications. As expected, stratified analyses by cancer type and by stage showed that high-risk gastrointestinal cancers and stage-4 disease were associated with higher estimated mortality; this was generally consistent across the 6 latent impairment classes. Within each stratum by cancer type and by stage, the latter 3 classes generally had the highest ranked mortality estimates among the impairment classes.
These results highlight the clinical value of identifying patients with multiple concurring impairments on geriatric assessment and shed light on impairment patterns, which can facilitate intervention planning. For example, cancer rehabilitation with occupational and physical therapy services for older adults with cancer could be used to address functional impairments and decrease disability caused by cancer and its treatments (42). These services are underutilized (43), which is problematic considering that unaddressed cancer- and treatment-related conditions like fatigue, lymphedema, and chemotherapy-induced peripheral neuropathy could precipitate life-long disability (44,45). Additionally, weight loss and social support impairment in older adults with cancer could be alleviated with dietitian and social worker services, respectively (46,47). Anxiety/depression can also be addressed through pharmacotherapy or psychotherapy, and psychiatry referrals may involve structured psychosocial interventions (48–50). For older patients with gastrointestinal cancers, nutrition or dental intervention may need to be coupled with cancer rehabilitation services to address weight loss, malnutrition, and functional deficits. Additionally, older patients with these impairments who also suffer from anxiety/depression or social isolation may be particularly vulnerable to adverse outcomes and require intervention to prevent further deterioration of functional capabilities (51). Our research highlights the importance of intervention packaging to support older patients with multiple impairments and distinct profiles.
This study builds off of the ELCAPA study, which previously used LCA and a limited set of geriatric assessment impairments to identify 4 health profiles in older adults with cancer: relatively healthy, malnourished, cognitive and/or mood impaired, and globally impaired (52). We used LCA on a sample of older patients with ≥1 reported impairment, and incorporated geriatric assessment impairments that were excluded in the previous study, for example, IADL, walking, falls, and anxiety. The present latent impairment classes are also focused on older patients with gastrointestinal cancers—a population in which disease course directly affects malnutrition and weight loss—and demonstrate the variable vulnerability that comes with weight loss alone versus weight loss combined with functional impairments and other impairment states, such as anxiety or depression.
Other noteworthy health profiles have been identified by Balducci and Extermann (53) and the International Society of Geriatric Oncology (54). In these categorizations, both IADL and ADL assessments were included to capture moderately impaired and strongly impaired patients. Our work also highlights the importance of including both assessments. The impaired classes and global impairment class had IADL impairment probabilities >50%, whereas only the global impairment class had high ADL impairment probability. Thus, while ADLs may identify the most vulnerable patients, the inclusion of multiple functional domains allowed for the identification of patients with moderate impairment.
Study limitations include generalizability to other populations with gastrointestinal cancers due to the CARE Registry setting, an academic site in southeastern United States. Another limitation was the restriction to patients with complete impairment information. This may exclude patients who skipped questions due to survey fatigue or patients in poor health who failed to report impairments; however, sensitivity analyses still identified high vulnerability for patients assigned to the impaired and global impairment classes. Additionally, the geriatric assessment was completed by the patient alone, which is subject to information bias. Despite this limitation, the use of a fully patient-reported geriatric assessment made it feasible to assess a large older adult and clinical population. Another study strength was in the inclusion of all available geriatric assessment impairments, which better leveraged LCA’s clustering capabilities for identifying unique impairment classes. Future studies should evaluate the validity of impairment classes in larger and more diverse samples and assess intermediate outcomes, such as treatment toxicities, modifications, and discontinuations.
Using patient responses on geriatric assessment and LCA, we identified 6 unique profiles that describe impairment patterns for older patients with gastrointestinal cancer. Knowledge on the co-occurrence of geriatric assessment impairments in this population can facilitate intervention planning and coordination of support services for older adults as they undergo cancer treatment.
Supplementary Material
Acknowledgments
The authors would like to thank the efforts of all nursing and research staff at UAB Medicine who have contributed to patient recruitment and data collection for the CARE registry.
Contributor Information
Sydney T Thai, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Jennifer L Lund, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Kelly M Kenzik, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Charles Poole, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Til Stürmer, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
John B Buse, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA.
Christian A Harmon, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Mustafa Al-Obaidi, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Grant R Williams, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, Birmingham, Alabama, USA; Division of Hematology/Oncology, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
This work was supported by the Bristol-Meyers-Squibb-University of North Carolina at Chapel Hill (UNC-CH) predoctoral Fellowship in Worldwide Health Economics and Outcomes Research and was completed as part of a dissertation thesis for the UNC-CH Department of Epidemiology. The CARE registry study is conducted with funding from the National Cancer Institute of the National Institutes of Health (K08CA234225) and the University of Rochester Clinical Trial Science Award from the National Institutes of Health (KL2 TR001999). Dr. J.L.L. also declares support from the National Institute on Aging (R21 AG068965). Dr. T.S. receives investigator-initiated research funding and support as Principal Investigator (R01AG056479) from the National Institute on Aging (NIA), and as Co-Investigator (R01CA174453, R01HL118255, R01MD011680), National Institutes of Health (NIH). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decisions to submit the manuscript for publication.
Conflict of Interests
T.S. receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Takeda, AbbVie, Boehringer Ingelheim), from pharmaceutical companies (Novo Nordisk), and from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. T.S. does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, and Novo Nordisk. All other authors declare no competing interests.
Author Contributions
Concept and design: S.T.T., J.L.L., C.P., T.S., and G.R.W. Acquisition, analysis, or interpretation of data: S.T.T., C.A.H., K.M.K., J.L.L., G.R.W., C.P., J.B.B., T.S., and M.A. Drafting of the manuscript: S.T.T. Critical revision of the manuscript for important intellectual content: S.T.T., J.L.L., G.R.W., C.P., T.S., and J.B.B.
References
- 1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 2. Muss HB, Woolf S, Berry D, et al. ; Cancer and Leukemia Group B. Adjuvant chemotherapy in older and younger women with lymph node–positive breast cancer. JAMA. 2005;293(9):1073–1081. 10.1001/jama.293.9.1073 [DOI] [PubMed] [Google Scholar]
- 3. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–1097. 10.1056/NEJMoa010957 [DOI] [PubMed] [Google Scholar]
- 4. Handforth C, Handforth C, Clegg A, Young C, Simpkins S.. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101. 10.1093/annonc/mdu540 [DOI] [PubMed] [Google Scholar]
- 5. Giri S, Al-Obaidi M, Weaver A, et al. Association between chronologic age and geriatric assessment-identified impairments: findings from the CARE registry. J Natl Compr Canc Netw. 2021;19(8):922–927. 10.6004/jnccn.2020.7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. 10.1200/JCO.2018.78.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dotan E, Walter LC, Browner IS, et al. NCCN Guidelines(R) insights: older adult oncology, version 12021. J Natl Compr Canc Netw. 2021;19(9):1006–1019. 10.6004/jnccn.2021.0043 [DOI] [PubMed] [Google Scholar]
- 8. Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc. 2005;173(5):489–495. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nadaraja S, Matzen L-E, Jørgensen TL, et al. ; Academy of Geriatric Cancer Research (AgeCare). The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: a randomized parallel-group clinical trial. J Geriatr Oncol. 2020;11(3):488–495. 10.1016/j.jgo.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 11. Li D, Sun C-L, Kim H, et al. Geriatric Assessment–Driven Intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158–e214158. 10.1001/jamaoncol.2021.4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer‐specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. 10.1002/cncr.21422 [DOI] [PubMed] [Google Scholar]
- 13. Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: the Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol. 2020;11(2):270–273. 10.1016/j.jgo.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giri S, Al‐Obaidi M, Harmon C, et al. Patient‐reported geriatric assessment‐based frailty index among older adults with gastrointestinal malignancies. J Am Geriatr Soc. 2022;71(1):136–144. 10.1111/jgs.18054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams GR, Dai C, Giri S, et al. Geriatric assessment predictors of 1-year mortality in older adults with GI malignancies: a survival tree analysis. JCO Clin Cancer Inform. 2022;6:e2200065. 10.1200/CCI.22.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giri S, Al-Obaidi M, Harmon C, et al. Patient-reported geriatric assessment-based frailty index among older adults with gastrointestinal malignancies. J Am Geriatr Soc. 2023;71(1):136–144. 10.1111/jgs.18054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams GR, Al‐Obaidi M, Dai C, et al. Association of malnutrition with geriatric assessment impairments and health‐related quality of life among older adults with gastrointestinal malignancies. Cancer. 2020;126(23):5147–5155. 10.1002/cncr.33122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabrielson DK, Scaffidi D, Leung E, et al. Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer. 2013;65(2):234–239. 10.1080/01635581.2013.755554 [DOI] [PubMed] [Google Scholar]
- 19. Jager-Wittenaar H, Ottery FD.. Assessing nutritional status in cancer: role of the Patient-Generated Subjective Global Assessment. Curr Opin Clin Nutr Metab Care. 2017;20(5):322–329. 10.1097/MCO.0000000000000389 [DOI] [PubMed] [Google Scholar]
- 20. Williams GR, Deal AM, Lund JL, et al. Patient-reported comorbidity and survival in older adults with cancer. Oncologist. April 2018;23(4):433–439. 10.1634/theoncologist.2017-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–257. 10.1016/j.jgo.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The OARS Multidimensional Functional Assessment Questionnaire. Multidimensional Functional Assessment of Older Adults. Psychology Press; 2013:16–21. 10.4324/9780203771563-7 [DOI] [Google Scholar]
- 23. Sherbourne CD, Stewart AL.. The MOS social support survey. Soc Sci Med (1982). 1991;32(6):705–714. 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- 24. Williams GR, Pisu M, Rocque GB, et al. Unmet social support needs among older adults with cancer. Cancer. 2019;125(3):473–481. 10.1002/cncr.31809 [DOI] [PubMed] [Google Scholar]
- 25. Godby RC, Dai C, Al-Obaidi M, et al. Depression among older adults with gastrointestinal malignancies. J Geriatr Oncol. 2021;12(4):599–604. 10.1016/j.jgo.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mir N, MacLennan P, Al-Obaidi M, et al. Patient-reported cognitive complaints in older adults with gastrointestinal malignancies at diagnosis—results from the Cancer & Aging Resilience Evaluation (CARE) study. J Geriatr Oncol. 2020;11(6):982–988. 10.1016/j.jgo.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fowler ME, Murdaugh D, Harmon C, et al. Longitudinal changes in patient-reported cognitive complaints among older adults with gastrointestinal malignancies—results from the Cancer and Aging Resilience Evaluation (CARE) Registry. J Cancer Surviv. 2022. 10.1007/s11764-022-01254-4 [DOI] [PMC free article] [PubMed]
- 28. Outlaw D, Dai C, Al-Obaidi M, et al. The association of polypharmacy with functional status impairments, frailty, and health-related quality of life in older adults with gastrointestinal malignancy—results from the Cancer and Aging Resilience Evaluation (CARE) registry. J Geriatr Oncol. 2022;38(29_suppl):118. 10.1016/j.jgo.2021.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caleyachetty R, Barber TM, Mohammed NI, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2021;9(7):419–426. 10.1016/S2213-8587(21)00088-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 31. Giri S, Mir N, Al-Obaidi M, et al. Use of single-item self-rated health measure to identify frailty and geriatric assessment-identified impairments among older adults with cancer. Oncologist. 2022;27(1):e45–e52. 10.1093/oncolo/oyab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rockwood K, Mitnitski A, Song X, Steen B, Skoog I.. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–979. 10.1111/j.1532-5415.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 33. Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K.. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2(1):1–8. 10.1186/1471-2318-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53(12):2184–2189. 10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 35. Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Cancer Netw. 2017;15(7):894–902. 10.6004/jnccn.2017.0122 [DOI] [PubMed] [Google Scholar]
- 36. Obaidi MA, Giri S, Mir N, et al. Use of self-rated health to identify frailty and predict mortality in older adults with cancer results from the care study. J Clin Oncol. 2020;38(15_suppl):12046–12046. 10.1200/JCO.2020.38.15_suppl.12046 [DOI] [Google Scholar]
- 37. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K.. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hagenaars JA, McCutcheon AL.. Applied Latent Class Analysis. Cambridge University Press; 2002. 10.1017/cbo9780511499531.004 [DOI] [Google Scholar]
- 39. Muthén B, Muthén LK.. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24(6):882–891. 10.1111/j.1530-0277.2000.tb02070.x [DOI] [PubMed] [Google Scholar]
- 40. Weller BE, Bowen NK, Faubert SJ.. Latent class analysis: a guide to best practice. J Black Psychol. 2020;46(4):287–311. 10.1177/0095798420930932 [DOI] [Google Scholar]
- 41. Lanza ST, Rhoades BL.. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci. 2013;14(2):157–168. 10.1007/s11121-011-0201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pergolotti M, Lyons KD, Williams GR.. Moving beyond symptom management towards cancer rehabilitation for older adults: answering the 5W’s. J Geriatr Oncol. 2018;9(6):543–549. 10.1016/j.jgo.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 43. Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB.. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J Geriatr Oncol. 2015;6(3):194–201. 10.1016/j.jgo.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35(23):2604–2612. 10.1200/JCO.2016.71.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrington SE, Stout NL, Hile E, et al. Cancer rehabilitation publications (2008–2018) with a focus on physical function: a scoping review. Phys Ther. 2020;100(3):363–415. 10.1093/ptj/pzz184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kleckner AS, Magnuson A.. The nutritional needs of older cancer survivors. J Geriatr Oncol. 2022;13(5):738–741. 10.1016/j.jgo.2021.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kadambi S, Soto-Perez-de-Celis E, Garg T, et al. Social support for older adults with cancer: Young International Society of Geriatric Oncology review paper. J Geriatr Oncol. 2020;11(2):217–224. 10.1016/j.jgo.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koll T, Pergolotti M, Holmes HM, et al. Supportive care in older adults with cancer: across the continuum. Curr Oncol Rep. 2016;18(8):51. 10.1007/s11912-016-0535-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fann JR, Fan MY, Unützer J.. Improving primary care for older adults with cancer and depression. J Gen Intern Med. 2009;24(Suppl 2):S417–S424. 10.1007/s11606-009-0999-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stubblefield MD. Cancer Rehabilitation: Principles and Practice. Springer Publishing Company; 2018. 10.1891/9780826121646 [DOI] [Google Scholar]
- 51. Jarach CM, Tettamanti M, Nobili A, D’Avanzo B.. Social isolation and loneliness as related to progression and reversion of frailty in the Survey of Health Aging Retirement in Europe (SHARE). Age Ageing. 2021;50(1):258–262. 10.1093/ageing/afaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrat E, Audureau E, Paillaud E, et al. ; ELCAPA Study Group. Four distinct health profiles in older patients with cancer: latent class analysis of the prospective ELCAPA cohort. J Gerontol A Biol Sci Med Sci. 2016;71(12):1653–1660. 10.1093/gerona/glw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balducci L, Extermann M.. Management of cancer in the older person: a practical approach. Oncologist. 2000;5(3):224–237. 10.1634/theoncologist.5-3-224 [DOI] [PubMed] [Google Scholar]
- 54. Droz J-P, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462–469. 10.1111/j.1464-410X.2010.09334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.