Abstract
Background
Pneumonic-type lung adenocarcinoma (P-ADC) is a rare and challenging subtype of primary lung cancer that can be difficult to distinguish from pneumonia based on radiological images. Furthermore, no drugs are currently available that specifically target KRAS G12V.
Case presentation
Here we report a case of P-ADC with typical and informative imaging features throughout the course of the disease, including patchy shadows, high-density lesions with aerated bronchus, diffuse ground-glass opacities, and nodular shadows from computed tomography (CT) scan. The KRAS G12V mutation was detected using Next-generation sequencing (NGS). An individualized Afatinib-based therapeutic schedule was prescribed and achieved sustained response after multiple lines of treatment had failed.
Conclusion
Our case highlights the typical and dynamic changes in imaging features of P-ADC and provides an indicative treatment strategy for KRAS G12V-mutated lung adenocarcinoma.
Keywords: Pneumonic-type lung adenocarcinoma, Pneumonia, Non-small cell lung cancer, KRAS mutation, Afatinib, Next-generation sequencing (NGS)
Introduction
Pneumonic-type lung adenocarcinoma (P-ADC) is a rare disease that typically presents with non-specific symptoms such as coughing and sputum production, occasionally accompanied by fever [1]. Laboratory examinations do not reveal any characteristic manifestations, and the imaging features often mimic the patchy shadows or consolidation of pneumonia [2]. Consequently, the diagnosis of P-ADC is frequently delayed and confused with pneumonia, especially during the Covid-19 pandemic [3, 4].
The management of P-ADC is usually similar to that of lung adenocarcinoma. For metastatic P-ADC without actionable genetic variants, chemotherapy, immunotherapy, and antiangiogenic therapy are recommended [5, 6]. Despite the recent breakthrough of Sotorasib (AMG510) targeting KRAS G12C mutation, there are currently no effective targeted therapies for KRAS G12V mutation. In this report, we present a case of typical P-ADC with KRAS G12V mutation that was sensitive to Afatinib, in order to provide insight for clinicians.
Case Presentation
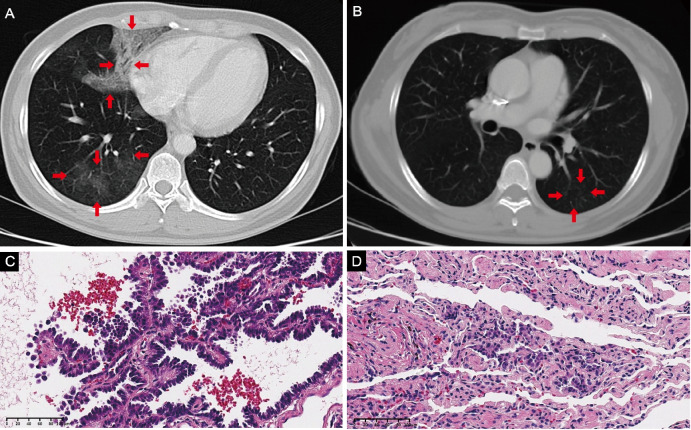
A 49-year-old woman with no smoking history presented to the clinic with patchy shadows in her lung, but she had no respiratory symptoms. On examination, laboratory tests showed a modestly elevated level of carcinoembryonic antigen (CEA) at 6.98 ng/ml, and no signs of active infection were found. A computed tomography (CT) scan showed pneumonia-like opacities and high-density lesions with aerated bronchus in the right middle and lower lobes (Fig. 1a), as well as a few pneumonia-like opacities in the left lower lobe (Fig. 1b). The percutaneous biopsy of the right middle lobe revealed lepidic adenocarcinoma. The patient then underwent a right middle and lower lobectomy, a right upper lobe wedge resection, and lymph node dissection. Pathological examination of the specimens from the right middle and lower lobes revealed a moderately to poorly differentiated adenocarcinoma with predominant lepidic growth and a small area of micropapillary pattern (Fig. 1c). The specimen from the right upper lobe showed adenocarcinoma in situ (Fig. 1d). The surgical margins were negative, and no lymph node involvement was observed (0/14). The diagnosis was primary lung adenocarcinoma (P-ADC), pT4(m)N0. Next-generation sequencing (NGS) identified KRAS G12V and PTCH1 E340V mutations, and immunohistochemical (IHC) staining indicated negative PD-L1 expression (tumor proportion score, TPS < 1%).
Fig. 1.
CT scan of lung-mediastinal window and pathology test. A, B Axial CT images of the chest showing pneumonia-like opacities and high-density lesions with aerated bronchus. H&E of the surgical specimens of right middle and lower lobes (C) and right upper lobe (D) confirm an adenocarcinoma
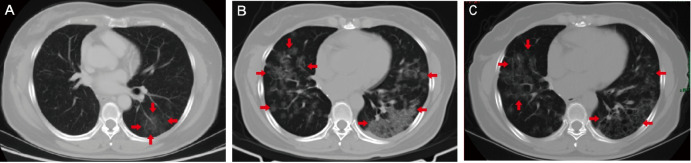
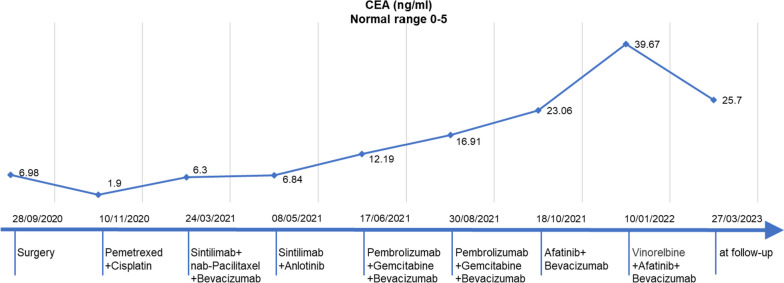
Following surgery, the patient received four cycles of adjuvant chemotherapy with pemetrexed plus cisplatin. However, after treatment, her CEA levels increased from 1.9 to 6.3 ng/ml, and CT scan revealed increased ground glass opacities (GGO) in the left lower lobe (Fig. 2a). Percutaneous biopsy of the left lung confirmed the presence of well-differentiated adenocarcinoma with predominant papillary morphology. NGS also revealed the presence of KRAS G12V and PTCH1 E340V mutations, and PD-L1 expression was positive (TPS 2%). Despite receiving multiple lines of treatment with chemotherapy, immunotherapy, and antiangiogenic therapy (Fig. 3), the disease continued to progress, as evidenced by markedly elevated serum CEA levels (Fig. 3) and increasing diffuse GGO, patches of high density, and nodular shadows on CT scan (Fig. 2b).
Fig. 2.
CT scan of lung-mediastinal window after four-cycles of adjuvant chemotherapy (A), at disease progression (B), and achieving lesion aera shrinkage (C)
Fig. 3.
Treatment course and dynamic monitoring of CEA with time line
Finally, the therapeutic schedule with Afatinib, Bevacizumab plus Vinorelbine was prescribed. This new regimen resulted in shrinkage of the lesion area (improved stable disease) (Fig. 2c) and simultaneous decrease in serum CEA levels (Fig. 3). At the time of the manuscript preparation, the patient was still taking Afatinib and had achieved a progression-free survival over 14 months, with no apparent adverse effects observed.
Discussion
The differential diagnosis between P-ADC and pneumonia is challenging, and definitive diagnosis often relies on pathological examination [7]. To date, case series of histologically confirmed P-ADC have helped summarize the imaging features. However, typical CT images of P-ADC are rarely observed in clinical practice [3]. Our case presents dynamic CT images across the entire disease stage, providing valuable insights into the imaging characteristics of P-ADC. Moreover, multiple P-ADC lesions are often histologically proven to be multi-primary lung cancers [8]. However, similar genotyping results across different lesions in this case support the conclusion of metastasis.
Targeted therapy is a well-established treatment strategy for lung adenocarcinoma with driver mutations [9]. However, drugs specifically targeting KRAS G12V are not yet available. Moll HP et al. [10] found that ERBB signaling was activated in human KRAS-mutated lung adenocarcinoma. Further, Afatinib, an approved pan-ERBB inhibitor, was shown to reduce KRAS-driven tumor growth in multiple mouse models, whereas erlotinib or gefitinib did not. In this case, facing failure of multiple lines of treatment and rapid disease progression, an individualized Afatinib-based therapeutic regimen achieved the best response of stable disease lasting 14 months. To our knowledge, this is the first case report of the real-world use of Afatinib for KRAS G12V-mutated lung adenocarcinoma, indicating a potential treatment strategy that warrants further investigation.
Conclusions
This case presents educational CT images of P-ADC and suggests the importance of considering pan-ERBB inhibitors in clinical trials for the treatment of KRAS-mutated lung cancer.
Acknowledgements
The authors thank to all members of the study team, the patient and his family.
Abbreviations
- P-ADC
pneumonic-type lung adenocarcinoma
- CT
computed tomography
- NGS
Next-generation sequencing
- CEA
carcinoembryonic antigen
- IHC
immunohistochemical
Authors’ contributions
Jie Zhao: Data curation, Writing - original draft preparation.Jiachen Xu: Writing- Reviewing and Editing.Tian Qiu: Data curation, Interpreting histological samples.Jie Wang: Conceptualization, Writing- Reviewing and Editing, Supervision.Zhijie Wang: Conceptualization, Writing- Reviewing and Editing, Supervision.
Funding
The study was supported by National key research and development project (2022YFC2505004 to Z.W.,2022YFC2505000 to J.W.), CAMS Innovation Fund for Medical Sciences (2021-1-I2M-012 to Z.W.), CAMS Key lab of translational research on lung cancer (2018PT31035 to J.W.), National Natural Sciences Foundation of China (81871889 and 82072586 to Z.W.), Beijing Natural Science Foundation (7212084 to Z.W.), National Natural Sciences Foundation Key Program (81630071 to J.W.).
Availability of data and materials
Data sharing is not applicable as no datasets were generated during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The patient consented to participate.
Consent for publication
Written informed consent for research and publication was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Zhao and Jiachen Xu contributed equally to this work.
Jie Zhao and Jiachen Xu are co-first authors.
Contributor Information
Jie Wang, Email: zlhuxi@163.com.
Zhijie Wang, Email: jie_969@163.com.
References
- 1.Huo JW, Huang XT, Li X, Gong JW, Luo TY, Li Q. Pneumonic-type lung adenocarcinoma with different ranges exhibiting different clinical, imaging, and pathological characteristics. Insights Imaging. 2021;12(1):169. doi: 10.1186/s13244-021-01114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daoud A, Laktineh A, El Zein S, Soubani AO. Unusual presentation of primary lung adenocarcinoma mimicking pneumonia: case report and literature review. Respir Med Case Rep. 2019;28:100881. doi: 10.1016/j.rmcr.2019.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Yu X, Huang Y, Nie P, Deng Y, Mao N, et al. Pneumonic-type invasive mucinous adenocarcinoma and infectious pneumonia: clinical and CT imaging analysis from multiple centers. BMC Pulm Med. 2022;22(1):460. doi: 10.1186/s12890-022-02268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Fan X, Huo JW, Luo TY, Huang XT, Gong JW. Differential diagnosis of localized pneumonic-type lung adenocarcinoma and pulmonary inflammatory lesion. Insights Imaging. 2022;13(1):49. doi: 10.1186/s13244-022-01200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei J, Tang D, Nie Y, Chen J, Peng L. Clinical characteristics and prognosis of nonsurgically treated patients with pneumonic-type adenocarcinoma. Med (Baltim) 2019;98(18):e15420. doi: 10.1097/MD.0000000000015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garfield DH, Franklin W. Dramatic response to pemetrexed in a patient with pneumonic-type mucinous bronchioloalveolar carcinoma. J Thorac Oncol. 2011;6(2):397–398. doi: 10.1097/JTO.0b013e3182011f59. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Han X, Peng L. A case report of pneumonic-type adenocarcinoma diagnosed by transbronchial cryobiopsy after the patient’s death. Med (Baltim) 2021;100(5):e24296. doi: 10.1097/MD.0000000000024296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Franklin WA, Nicholson AG, Girard N, Arenberg DA, Travis WD, et al. The IASLC lung cancer staging project: background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(5):651–665. doi: 10.1016/j.jtho.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039–1049. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moll HP, Pranz K, Musteanu M, Grabner B, Hruschka N, Mohrherr J, et al. Afatinib restrains K-RAS-driven lung tumorigenesis. Sci Transl Med. 2018;10(446):eaao2301. doi: 10.1126/scitranslmed.aao2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as no datasets were generated during the current study.