Abstract
Background:
Tapering long-term opioid therapy is an increasingly common practice, yet rapid opioid dose reductions may increase the risk of overdose. The objective of this study was to compare overdose risk following opioid dose reduction rates of ≤10%, 11% to 20%, 21% to 30%, and >30% per month to stable dosing.
Methods:
We conducted a retrospective cohort study in three health systems in Colorado and Wisconsin. Participants were patients ≥18 years of age prescribed long-term opioid therapy between January 1, 2006, and June 30, 2019. Five opioid dosing patterns and drug overdoses (fatal and nonfatal) were identified using electronic health records, pharmacy records, and the National Death Index. Cox proportional hazard regression was conducted on a propensity score-weighted cohort to estimate adjusted hazard ratios (aHRs) for follow-up periods of 1, 3, 6, 9, and 12 months after a dose reduction.
Results:
In a cohort of 17 540 patients receiving long-term opioid therapy, 42.7% of patients experienced a dose reduction. Relative to stable dosing, a dose reduction rate of >30% was associated with an increased risk of overdose and the aHR estimates decreased as the follow-up increased; the aHRs for the 1-, 6- and 12-month follow-ups were 5.33 (95% CI, 1.98–14.34), 1.81 (95% CI,1.08–3.03), and 1.49 (95% CI, 0.97–2.27), respectively. The slower tapering rates were not associated with overdose risk.
Conclusions:
Patients receiving long-term opioid therapy exposed to dose reduction rates of >30% per month had increased overdose risk relative to patients exposed to stable dosing. Results support the use of slow dose reductions to minimize the risk of overdose.
Keywords: long-term opioid therapy, opioid tapering, overdose
Introduction
In response to the opioid crisis, the Centers for Disease Control and Prevention (CDC) and other organizations issued guidelines to encourage safer opioid prescribing practices.1–4 The CDC Guidelines recommend that clinicians closely monitor patients prescribed opioids to re-evaluate whether the benefits of opioid therapy outweigh the harms, which may include opioid use disorder and overdose.1,2 When harms exceed benefits, guidelines1–5 encourage clinicians to consider slowly tapering the patient’s opioid dose, beginning with a 10% dose reduction per month for patients taking opioids for years, and 10% per week for shorter opioid therapy durations. These tapering rate recommendations, however, were based on limited evidence,1,2 and it has been suggested that inappropriately rapid tapers increase the risk for heroin use, suicide, and, paradoxically, overdose.5–10
To help guide clinicians, it is important to identify the rate at which patients prescribed long-term opioid therapy (LTOT) can safely reduce their opioid dosages. This could be evaluated with a randomized clinical trial involving patients who consent to a possible dose reduction, but such patients may not represent the patient population that experiences dose reductions in practice.11,12 Observational studies demonstrated that opioid dose reductions of ≥10% per month were associated with a modest, increased overdose risk for up to 48 months after a completed taper.13,14 However, studies have not examined the risk of overdoses occurring during or soon after a dose reduction. Such events are more likely to be causally linked to the dose reduction than overdoses occurring long after a completed dose reduction since withdrawal symptoms, pain, and the desire to use non-prescribed opioids, other drugs, and alcohol may be strongest while the dose is being reduced.15,16
Using electronic health record (EHR) data from three diverse health systems, we identified patients prescribed LTOT and applied a scan statistic methodology to identify opioid dose patterns across the follow-up, including stable doses and dose reduction rates of ≤10%, 11 to 20%, 21 to 30%, and >30% per month.17–19 We conducted a retrospective cohort study to examine the effect of the different dose reduction rates on the incidence of overdoses occurring at 1, 3, 6, 9, and 12 months after initiation of a dose reduction. We hypothesized that faster dose reductions would be associated with higher overdose risks.
Methods
Study Sites, Data, and Design
The study was conducted with data from three health systems in Colorado and Wisconsin, representing safety-net, managed care, and private medical group practices. Kaiser Permanente Colorado (KPCO) is an integrated insurance provider and health care delivery organization with medical offices in urban and suburban Colorado regions. Denver Health (DH) is an urban, integrated, safety-net health system serving Denver, Colorado. Marshfield Clinic Health System (MCHS) serves predominantly rural populations across Wisconsin.
These sites maintain secure “virtual data warehouse” research databases with harmonized member/patient data collected at the point of care through EHRs and automated pharmacy records. These virtual data warehouses use a common data model20 and include the following: membership and demographic information, procedures (Current Procedural Terminology-4 codes), diagnoses coded at each visit (International Classification of Diseases, Ninth Revision and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM] codes), pharmacy dispensings (National Drug Codes), external service and medication claims, and vital status. For all sites, vital status was confirmed with patient data linked to the National Death Index (NDI)-Plus, which identifies all deaths occurring in the United States and provides consistently coded cause-of-death data.21
Study Cohort
Across sites, we identified a cohort of patients ≥18 years of age who received LTOT between January 1, 2006, and June 30, 2019. Using electronic pharmacy dispensing data, we calculated milligram morphine equivalents (MME) in 30-day intervals using established methods.22,23 LTOT was defined as ≥3 opioid dispensings of ≥10 daily MME in 90 days.23–25 Patients were required to have ≥80 days of opioid coverage during the 90 days with no more than a 5-day gap between dispensings.6,24–26 These stringent dose and coverage criteria helped to ensure that patients were receiving a sufficient dose to be tapered and opioids for chronic pain over the 90-day period, as opposed to three short-term prescriptions for acute pain. Buprenorphine-containing products and methadone to treat opioid use disorder were excluded from the definition of LTOT and MME calculations because treatment guidelines do not recommend tapering opioid agonist treatment due to the risk of overdose after tapers.27,28 Patients in hospice or a nursing home before LTOT or who had <90 days of health plan enrollment after meeting criteria for LTOT were ineligible. For each patient, time in the cohort was censored when any of the following occurred: fatal or non-fatal overdose, other cause of death, other hospital admission for ≥32 days, hospice or nursing home admission, health plan disenrollment (KPCO or MCHS) or no longer empaneled (DH, no primary care visits in 18 months), or September 30, 2019 (study end). Patients receiving LTOT who disenrolled from the health plan but reenrolled 32 or more days later and started LTOT again in the subsequent enrollment period were eligible to re-enter the cohort as new observations. We censored at the first overdose event prior to identifying opioid dose patterns (described below) to minimize event-dependent exposures, since overdoses could prompt clinicians to reduce patients’ dosages, introducing reverse causation bias into the analysis.
Opioid Dosing Patterns
To identify opioid dosing rates, we applied scan statistic methodology to the patient cohort’s pharmacy dispensing data.18,19 Scan statistic methodology has been used in vaccine safety pharmacoepidemiologic studies to identify distinct periods of risk following immunization.29,30 The method partitioned each patient’s time into 30-day intervals and scanned through these periods to identify opioid dose patterns based on the variability of the patient’s MME using the coefficient of variation (CV)24 and trends in MME. The CV (CV = standard deviation / mean MME) permitted us to assess the change in MME over the follow-up time while accounting for each patient’s mean MME and provided a standardized measure that could be compared across patients with widely different mean MMEs. First, we identified mutually exclusive periods of ≥90 days with a CV ≤ 0.15 as “stable” dose patterns, indicating the pattern had minimal dose variability.31,32 We then fit linear trends of dose levels in the remaining periods with a CV > 0.15. Periods with negative trends were considered “dose reduction” patterns, while periods with positive trends were considered “increasing dose” patterns, and periods with MME = 0 were considered “dose discontinuations.” Patients could be exposed to ≥1 dose pattern over time. For each dose pattern, the first 30 days represented the baseline period (i.e., starting dose). Within each dose reduction pattern, we then calculated a dose reduction rate (100 × slope/initial dose within the pattern), which measured the percentage of dose reduction per 30-day period relative to the 30-day baseline period. Based on prior opioid tapering studies and guidelines, we compared four dose reduction rates to stable dosing by increments of 10%: ≤10%, 11 to 20%, 21 to 30%, and >30% per month.1,3,4,13,33 Stable dosing was the comparator of interest because of research showing that dose variability is associated with an increased odds of opioid overdose when compared to dose stability.24 (See eMethods and eFigures 1 and 2 for details.)
Comparison Groups and Follow-up
We first identified cohort members with at least one dose reduction pattern preceded by a ≥3-month stable pattern (Figure 1).34 We focused on the first dose reduction pattern, and follow-up started at the index date, which was immediately after the 30-day baseline period of the dose reduction pattern. The comparison, control group comprised patients with ≥1 stable pattern of ≥3 months not followed by a dose reduction pattern. Thus, the control group included patients whose entire follow-up was stable, and patients whose stable patterns ended with either an increasing dose pattern, dose discontinuation pattern, or the end of the study. We allowed the latter patients whose entire follow-up was not stable in the control group because using future events to define exposure status at the beginning of the follow-up (i.e., the index date) can create bias in an observational cohort study. In addition, patients in the control group could have had a dose reduction prior to the start of the stable pattern if the dose reduction was not preceded by a ≥ 3-month stable period, because excluding such patients could have reduced generalizability. For all control patients, the index date was randomly assigned based on the distribution of the lengths of stable patterns occurring before the dose reduction patterns, to ensure that the comparison and dose reduction groups had comparable stable pattern lengths preceding the index date (start of follow-up). The unit of analysis was the patient within an enrollment period; patients could be included in the analysis more than once if they had multiple enrollment periods. Patients in all groups were excluded if they had a cancer diagnosis in the 6 months prior to the start of the baseline period.
Figure 1.
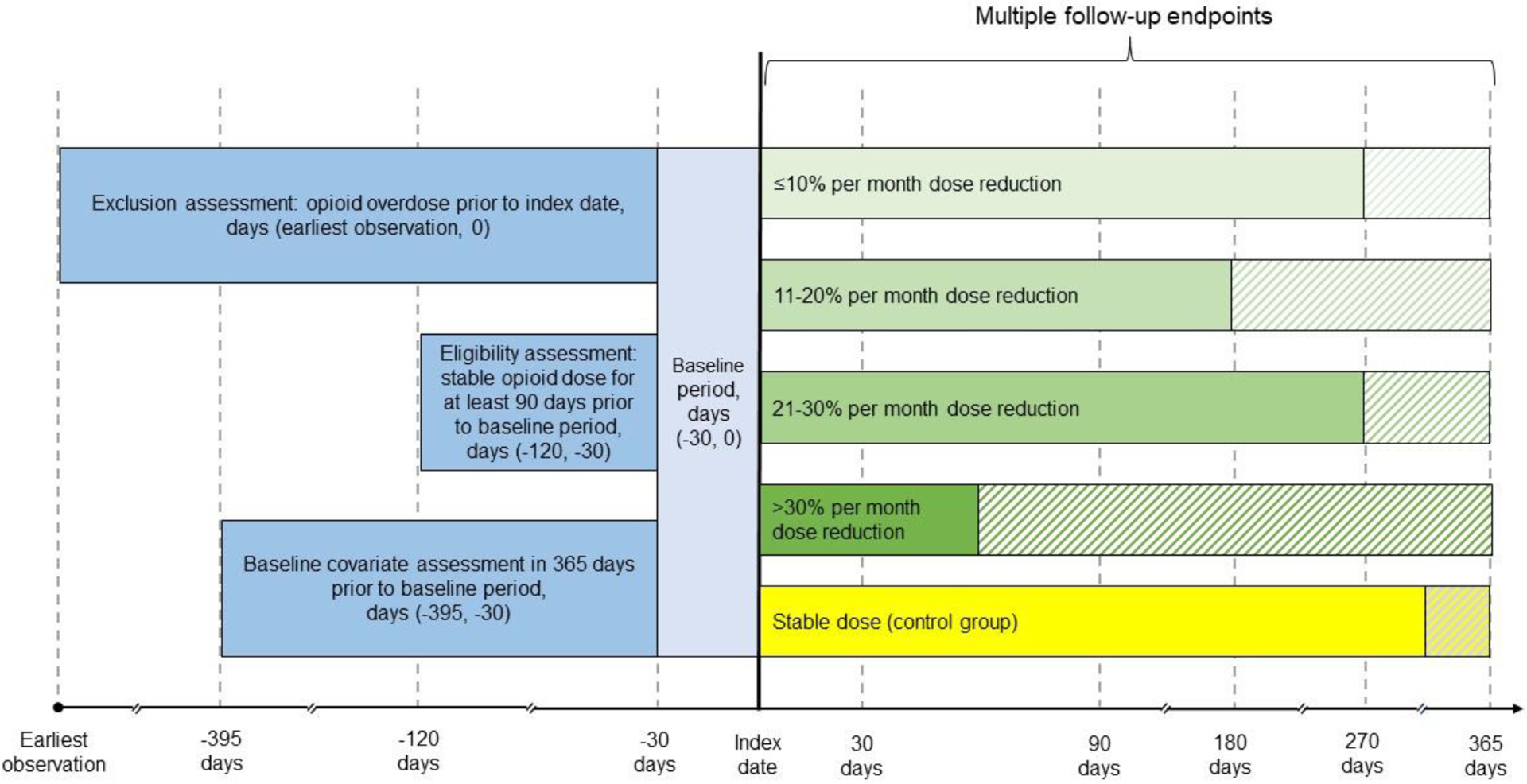
Schematic Diagram of the Study Designa
aA baseline period of 30 days was needed to determine opioid dose patterns. Patients were required to have at least 90 days of stable dosing prior to the beginning of the baseline period to be eligible for inclusion in the analysis. Patients with one or more opioid overdoses during the baseline period were excluded from the analysis. Baseline covariates were assessed in the year before the baseline period. Dose patterns are shown with varying lengths of follow-up time to illustrate variability in duration. The shaded areas represent follow-up after the index dose pattern. Patients may experience any dose pattern(s) after the index dose, except for a dose reduction preceded by ≥3 months of a stable dose (i.e., the qualifying condition to be included in the exposure group) in the control group.
Patients were followed from the index date until an overdose, another censoring event, or a maximum of 12 months, whichever came first. We chose a 12-month follow-up to provide time for an intentional slow dose reduction to occur. To examine how overdose risk differed across the follow-up, we analyzed follow-up periods of 1, 3, 6, 9, and 12 months after the index date.
Overdose Outcomes
Incident overdoses treated in emergency department and inpatient settings were identified using ICD-9/10 codes in the EHR and claims data (eTable 1). Non-specific codes and codes from ambulatory visits were excluded.35 Fatal overdoses occurring in any setting were identified using cause-of-death ICD-10 codes provided by the NDI-Plus.36
Covariate Measurement
The following were measured in the year before the 30-day baseline period: mental health diagnoses (i.e., psychotic, anxiety and stress-related, mood, attention deficit, and personality disorders); opioid use, alcohol use, tobacco use or use disorder, other substance use disorder (i.e., stimulant, marijuana, sedative, and hallucinogen); and the Quan-Deyo Modified Charlson Comorbidity Index.37,38 (See eTable 1 for the ICD-9/10 codes used.) Mean daily dose was measured during the stable period preceding the baseline period. Age, Medicaid coverage, calendar month, and year were measured at the beginning of the baseline period. Length of time on stable dosing prior to the baseline period was assessed as a continuous variable. Benzodiazepine exposure was assessed as any dispensing in the 30 days before the start of the baseline period or any dispensing with a days’ supply that ended at or crossed the baseline period. Gender and race/ethnicity were included as fixed covariates, assessed at cohort entry.
Propensity Score Methods
We conducted a propensity score analysis and applied inverse probability of treatment weighting to account for the imbalance of covariates across the five treatment groups (four dose reduction rates and stable dosing). To calculate propensity scores and inverse probability of treatment weights, we used multinomial logistic regression with treatment group as the dependent variable and the covariates described above as the independent variables. The weights were applied to the pooled data, and covariate balance was assessed with population standardized mean differences (PSDs) before and after the propensity score weighting. PSDs were calculated by setting up separate bivariate comparisons for each covariate within each of the five comparison groups (e.g., dose reduction < 10% versus the pooled population), resulting in five comparisons for each covariate.39 A PSD < 0.10 was considered adequate balance.40
Statistical Analysis
We fit Cox proportional hazards models on the pooled, propensity-weighted data to estimate hazards ratios (HRs) and 95% CIs. Site was included in the models as a strata variable. Separate models were conducted for follow-up periods of 1, 3, 6, 9, and 12 months after the index date. The proportional hazards assumption was assessed by testing the interaction between the comparison groups and time. We used 2-sided statistical tests with a P < 0.05 cut-off for statistical significance.
To assess the robustness of the results, we conducted a sensitivity analysis of patients with a prior mental health disorder diagnosis because physicians may tend to attempt dose reduction on patients with overdose risk factors, leading to confounding by indication.
To measure the potential effect of unmeasured confounding, we calculated E-values for the primary analyses.41 The E-value measures the level of unmeasured confounding that would explain the study’s observed association between dose reduction rates and overdose.
Analyses were conducted with SAS, version 9.4 software (SAS Institute, Cary NC).
Results
Cohort
After exclusions, 31 865 patients were in the LTOT patient cohort (Figure 2), with a mean follow-up of 4.43 years (SD, 3.36) and a mean time prescribed opioids over the follow-up of 3.21 years (SD, 2.89). As a result of the eligibility criteria applied, patients in the cohort were younger (55.4 vs. 61.5 years), more likely to be male (41.7 vs. 35.5%), had a higher mean opioid dose at cohort entry (48.5 vs. 24.7 MME), and had a lower comorbidity score (1.4 vs. 1.7) than excluded patients.
Figure 2.
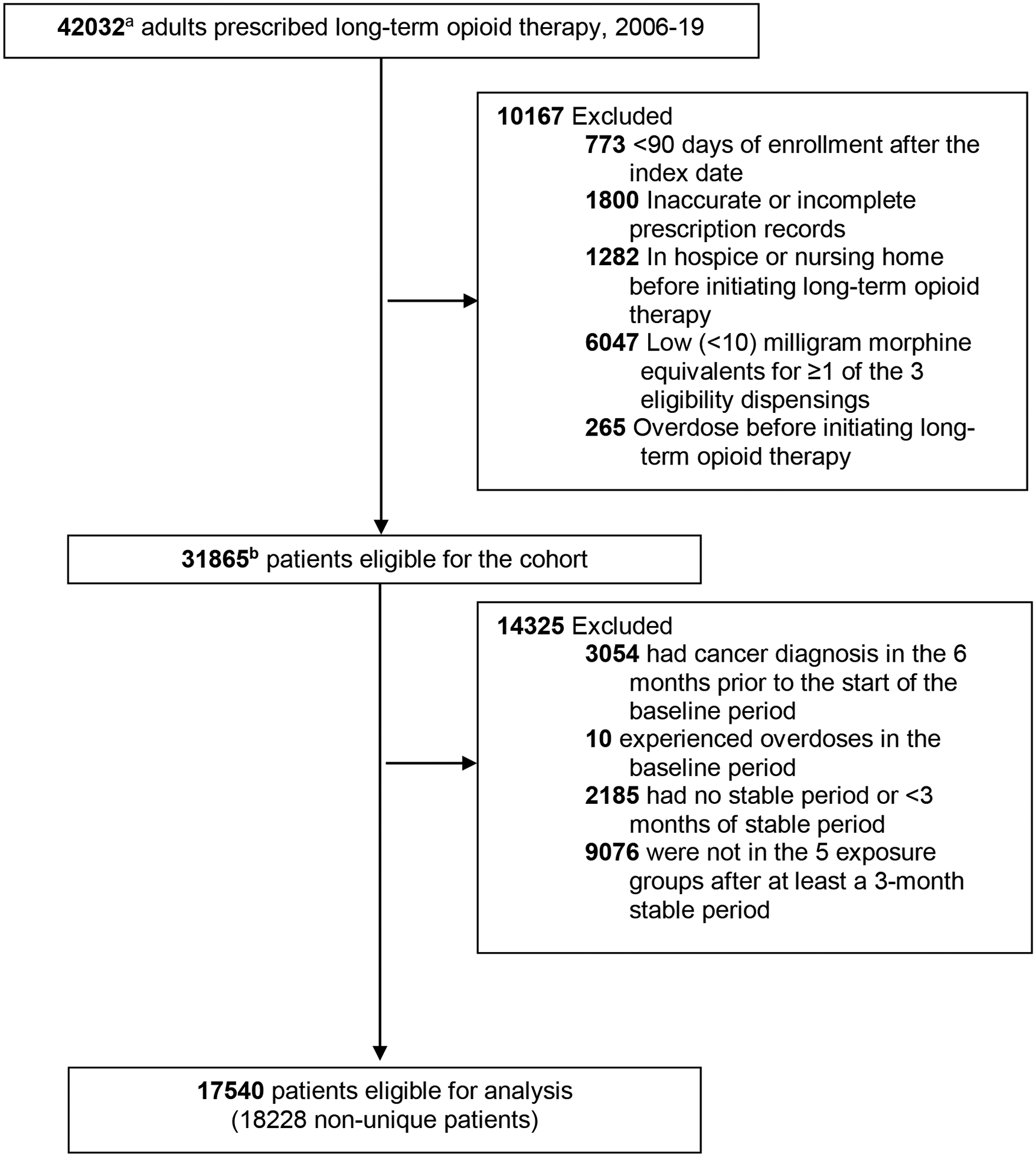
Study Flow Diagram
a44598 non-unique patients - study allowed multiple enrollments in the health system for a patient. If a patient on long-term opioid therapy disenrolled from the health system, reenrolled, and was on/started long-term opioid therapy, that person was eligible for inclusion again. b33618 non-unique patients
Among cohort patients, 123 603 opioid dose patterns were identified, of which 71.8% (n = 88 756) were categorized as stable and 28.2% (n = 34 847) were categorized as dose reductions. Approximately 63.1% of cohort members had ≥1 dose reductions across their follow-up (mean, 1.72; SD, 1.05; range 1–10).
There were 17 540 patients eligible for the analysis (Table 1). Among these patients, there were 18 228 observations accounting for multiple enrollment periods, 57.8% (n = 10 535) of which represented stable dose patterns. Among observations with a dose reduction (n = 7693; 42.2%), 36.2% (n = 2786) represented a monthly reduction rate of ≤ 10%, 20.5% (n = 1579) a rate of 11% to 20%, 13.9% (n = 1070) a rate of 21 to 30%, and 29.4% (n = 2258) a rate > 30%.
Table 1.
Patient Demographic and Clinical Characteristics of the Study Population Included in the Analyses, by Opioid dose Pattern (n = 17 540 Patients; 18 228 Observations)*.
| Characteristic | Stable (n= 10535) | ≤ 10% dose reduction/month (n = 2786) | 11 %−20% dose reduction/month (n = 1579) | 21 %−30% dose reduction/month (n= 1070) | >30% dose reduction/month (n = 2258) |
|---|---|---|---|---|---|
| Age, mean (SD), years† | 54.3 (14.5) | 56.9 (16.0) | 54.6 (15.9) | 54.1 (15.0) | 54.3 (15.6) |
| Female | 6104 (57.9) | 1701 (61.1) | 949 (60.1) | 628 (58.7) | 1304 (57.8) |
| Race/ethnicity | |||||
| Hispanic | 1930 (18.3) | 452 (16.2) | 314 (19.9) | 195 (18.2) | 386 (17.1) |
| White | 6984 (66.3) | 1939 (69.6) | 1052 (66.6) | 717 (67.0) | 1512 (67.0) |
| Black | 808 (7.7) | 206 (7.4) | 102 (6.5) | 77 (7.2) | 169 (7.5) |
| Other racial/ethnic groups | 344 (3.3) | 63 (2.3) | 43 (2.7) | 30 (2.8) | 76 (3.4) |
| Missing | 469 (4.5) | 126 (4.5) | 68 (4.3) | 51 (4.8) | 115 (5.1) |
| Medicaid coverage† | 2516 (23.9) | 555 (19.9) | 373 (23.6) | 278 (26.0) | 565 (25.0) |
| Diagnoses | |||||
| Mental health‡ | 4906 (46.6) | 1316 (47.2) | 831 (52.6) | 581 (54.3) | 1115 (49.4) |
| Opioid use disorder‡ | 430 (4.1) | 102 (3.7) | 79 (5.0) | 62 (5.8) | 121 (5.4) |
| Other substance use disorder‡ | 853 (8.1) | 230 (8.3) | 157 (9.9) | 126 (11.8) | 261 (11.6) |
| Alcohol use disorder‡ | 593 (5.6) | 151 (5.4) | 116 (7.4) | 89 (8.3) | 163 (7.2) |
| Tobacco use or nicotine use disorder‡ | 4115 (39.1) | 945 (33.9) | 599 (37.9) | 432 (40.4) | 876 (38.8) |
| Benzodiazepine dispensing§ | 1782 (16.9) | 488 (17.5) | 315 (20.0) | 214 (20.0) | 402 (17.8) |
| Modified Charlson Comorbidity Index, mean (SD)‡ | 1.1 (1.6) | 1.3 (1.7) | 1.3 (1.7) | 1.2 (1.7) | 1.2 (1.7) |
| Average daily morphine equivalents, mg, mean (SD)|| | 53.2 (79.7) | 43.0 (56.6) | 50.7 (71.9) | 59.1 (115.9) | 49.3 (66.8) |
Data are presented as number (percentage) unless otherwise indicated;
Assessed at the start of the baseline period;
Assessed in the year prior to the baseline period;
Any dispensing in the 30 days before the start of the baseline period or any dispensing prior to the baseline period with days’ supply that ended at or crossed the baseline period;
Assessed for the stable period prior to the baseline period.
After applying weights from the propensity score models and calculating the PSDs, length of time on stable dosing and month at the beginning of the baseline period had a PSD ≥ 0.10 in one of the five treatment group comparisons, and year at the beginning of the baseline period had PSD ≥ 0.10 in two of the five comparisons and were therefore included as covariates in the Cox regression models (eFigures 3a–3e). All other covariates had a PSD < 0.10 after the weighting.
Overdose
The overdose incidence rate in the cohort was 1022.96 overdoses per 100 000 person-years. In the cohort analysis, 11.0% (n = 20) of the 181 overdoses were fatal and 32.0% involved opioids (Table 2). The majority of non-fatal overdoses involved multiple substances, including alcohol, psychotropic agents, sedatives/hypnotics, and other analgesics. Among the fatal overdoses, 12 (60%) were attributed to opioids. Other contributors to fatal overdose were cocaine, alcohol, and benzodiazepines. The mean age at overdose was 50.2 (SD, 14.8), and 55.8% of the patients who experienced an overdose were female.
Table 2.
Demographic and Clinical Characteristics of Patients Who Experienced an Overdose in the 12 Months Following the Baseline Period Compared to Those Who Did Not (n = 17 540 Patients; 18 228 Observations)*.
| Characteristic | Patients who experienced an overdose (n = 181) | Patients who did not experience an overdose (n = 18047) |
|---|---|---|
| n (%) | n (%) | |
| Age, mean (SD), years† | 50.2 (14.8) | 54.7 (15.0) |
| Female | 101 (55.8) | 10585 (58.7) |
| Race/ethnicity | ||
| Hispanic | 23 (12.7) | 3254 (18.0) |
| White | 133 (73.5) | 12071 (66.9) |
| Black | 4 (2.2) | 1358 (7.5) |
| All other racial and ethnic groups | 6 (3.3) | 550 (3.1) |
| Missing | 15 (8.3) | 814 (4.5) |
| Insurance† | ||
| Medicaid | 52 (28.7) | 4235 (23.5) |
| Mental health disorder diagnosis‡ | 126 (69.6) | 8623 (47.8) |
| Opioid use disorder diagnosis‡ | 35 (19.3) | 759 (4.2) |
| Other substance use disorder diagnosis‡ | 40 (22.1) | 1587 (8.8) |
| Alcohol use disorder‡ | 32 (17.7) | 1080 (6.0) |
| Tobacco use or use disorder‡ | 96 (53.0) | 6871 (38.1) |
| Benzodiazepine dispensing§ | 68 (37.6) | 3133 (17.4) |
| Modified Charlson Comorbidity Index, mean (SD)‡ | 1.5 (1.8) | 1.2 (1.6) |
| Daily morphine equivalents, mg, mean (SD)|| | 73.5 (86.5) | 51.0 (77.1) |
| Overdose was opioid-related | 58 (32.0) | N/A |
| Overdose was fatal | 20 (11.0) | N/A |
| Opioid dose pattern | ||
| Stable | 95 (52.5) | 10 440 (57.9) |
| ≤ 10% dose reduction/month | 17 (9.4) | 2769 (15.3) |
| 11%−20% dose reduction/month | 19 (10.5) | 1560 (8.6) |
| 21%−30% dose reduction/month | 18 (9.9) | 1052 (5.8) |
| >30% dose reduction/month | 32 (17.7) | 2226 (12.3) |
Data are presented as number (percentage) unless otherwise indicated;
Assessed at the start of the baseline period;
Assessed in the year prior to the baseline period;
Any dispensing in the 30 days before the start of the baseline period or any dispensing prior to the baseline period with days supply that ended at or crossed the baseline period;
Assessed for the stable period prior to the baseline period.
We observed significantly increased risks of overdose following a >30% dose reduction, and the aHR estimates decreased as the follow-up increased (Table 3, eFigure 4, eTable 2). The aHRs for the 1- and 12-month follow-ups were 5.33 (95% CI, 1.98–14.34) and 1.49 (95% CI, 0.97–2.27; Table 3), respectively. Dose reduction rates of ≤10%, 11 to 20%, and 21 to 30% were not significantly associated with overdose across follow-up lengths.
Table 3.
Adjusted Associations Between Opioid Dose Patterns and Overdose, by Follow-up Period.
| Follow-up (months) | Adjusted hazard ratio (95% CI)a | |||
|---|---|---|---|---|
| ≤ 10% dose reduction/month | 11%−20% dose reduction/month | 21%−30% dose reduction/month | >30% dose reduction/month | |
| 1 | 1.35 (0.25, 7.42) | 1.54 (0.33, 7.26) | 1.27 (0.24, 6.62) | 5.33 (1.98, 14.34) |
| 3 | 0.45 (0.12, 1.75) | 1.19 (0.42, 3.38) | 0.99 (0.36, 2.67) | 3.39 (1.88, 6.12) |
| 6 | 0.61 (0.27, 1.38) | 0.95 (0.43, 2.12) | 1.62 (0.81, 3.22) | 1.81 (1.08, 3.03) |
| 9 | 0.75 (0.40, 1.42) | 1.31 (0.74, 2.34) | 1.71 (0.95, 3.08) | 1.61 (1.02, 2.56) |
| 12 | 0.72 (0.41, 1.2k7) | 1.30 (0.78, 2.16) | 1.57 (0.91, 2.68) | 1.49 (0.97, 2.27) |
Models fitted using Cox proportional hazards models on the propensity-weighted data. Length of time on stable dosing prior to the baseline period and month at the beginning of the baseline period had a population standardized mean differences ≥0.10 in one of the five treatment group comparisons, and year at the beginning of the baseline period had a population standardized mean differences ≥0.10 in two of the five treatment group comparisons—these three variables were included as covariates in the IPTW-weighted Cox proportional hazard models.
For the sensitivity analysis of patients with a prior mental health disorder diagnosis (eTable 3), a >30% dose reduction was associated with an increased risk of overdose that demonstrated a similar pattern to the primary analysis. The aHRs for the 1- and 12-month follow-ups were 6.72 (95% CI, 2.06–21.88) and 1.37 (95% CI, 0.83–2.28), respectively.
The E-values (lower confidence limit) for a dose reduction rate of >30% were 10.13 (3.37), 6.24 (3.17), 3.0 (1.37), 2.60 (1.16), and 2.34 (1.0) for 1-, 3-, 6-, 9-, and 12-month follow-ups, respectively. Substantial unmeasured confounding would therefore be needed to negate the associations between faster dose reduction rates and overdose.
Conclusions
In this multi-site cohort study of patients prescribed LTOT, an opioid dose reduction rate of >30% per month was associated with an approximate fivefold increased risk of overdose in the first month after initiating a dose reduction compared to stable dosing, and the magnitude of the risk attenuated as the follow-up increased. Dose reduction rates less than 30% per month were not associated with an increased risk for overdose. Among patients on stable opioid doses for whom the harms of opioid therapy outweigh the benefits, the findings of this study support the use of slow monthly dose reductions to minimize the short-term risk of overdose.
Although our results support the use of slow tapers when the benefits of opioid therapy do not outweigh the harms, they should be considered in the context of the updated CDC guideline.2 For example, it may not be appropriate to slowly taper certain high-risk patients who are screened for and diagnosed with an OUD. The Guideline recommends that such patients be offered appropriate treatment for their OUD. The Guideline also encourages physicians to consider buprenorphine, a partial opioid agonist, as a less risky option for pain management among patients who exhibit opioid misuse behaviors but do not meet criteria for an OUD and have difficultly tapering. Our results therefore suggest that, when considering a dose reduction, clinicians should be aware of the overdose risk associated with a rapid taper and refer to the Guideline to determine whether a slow taper is appropriate for their patient.
Rapid dose reduction, intended or unintended, could increase the risk of overdose through several mechanisms. Reducing the strength of the opioid medication or increasing the interval between doses could increase pain, precipitate withdrawal or induce opioid craving, potentially prompting patients to use other substances with prescribed opioids or resort to non-prescribed opioids.6,42,43 Opioid tapering has also been associated with increased anxiety,15 emotional distress, and mental health crises.13,44 In our sensitivity analysis, the associations between rapid dose reductions and overdose appeared to be greater among patients with a risk factor for overdose. This result aligns with the CDC guideline, which encourages physicians to consider nonopioid pain therapies and treatment of comorbid mental health conditions before and during a dose reduction.2
Prior observational studies have identified harms associated with opioid dose reduction and discontinuation, including increased risks of overdose,13,14,45 suicide,10,42 health plan disenrollment,26 substance use disorder,46,47 and opioid use disorder.14 The objectives of our study most closely align with those of an investigation by Agnoli and colleagues, which examined the long-term effects of tapering on overdose risk.13 While we and Agnoli and colleagues used different approaches to identify dose reductions using automated pharmacy dispensing data, we both found modest associations between dose reductions of ≥15% and long-term risk of overdose. However, because our analyses also included overdoses that occurred during dose reductions, we identified significantly elevated risks for overdoses occurring shortly after the initiation of a dose reduction of >30% per month.
In a study of national pharmacy data from 2017 to 2019, approximately half of patients receiving LTOT experienced at least one 90-day period of opioid tapering.33 Among these patients, approximately 36% experienced at least one 90-day period in which their opioid dose was reduced by ≥ 40% per month. Similarly, in our study, approximately 63.0% of patients had at least one dose reduction, and 25.5% of the dose reductions had a rate of > 30% per month. Although these studies used different datasets and methods to identify opioid dose reduction, both suggest that a substantial proportion of patients receiving LTOT are exposed to dose reduction rates that exceed recommendations.1,3
This study had limitations. First, our definition of LTOT was more stringent than many other studies,48,49 as we required a minimum dose and no more than a 10-day gap in coverage over 90 days. If this definition resulted in exclusion of patients with higher risk characteristics, we may have underestimated the risk of rapid tapering. Second, given that the method we employed for identifying dose reduction patterns was predicated on differentiating periods of dose stability from periods of dose instability using a CV threshold of 0.15, it likely missed gradual transitions between stable and slow dose reduction periods. Third, in automated pharmacy dispensing data, opioid dose reductions may result from prescribing lower strength formulations, switching medications (e.g., from oxycodone to tramadol), reducing quantity, or increasing the time between dispensings. However, we could not verify how patients were taking their medications or ascertain the reasons for dose reductions. For example, if a patient’s dose was reduced, the patient or clinician could have initiated the dose reduction with an intent to taper, or the dose reduction could have been unintentional due to missed or delayed refills. If any of the reasons for a dose reduction were also associated with overdose incidence, it could have confounded the results. In particular, it is possible that physicians tended to initiate rapid tapers when the patient exhibited risk factors for overdose, such as a diagnosis for opioid use disorder, pre-existing mental health condition or benzodiazepine co-prescription. We attempted to control for this potential source of confounding by conducting a rigorous propensity score analysis with inverse probability of treatment weighting, as well as conducting a sensitivity analysis limited to patients with a mental health disorder diagnosis. Fourth, for the longer follow-up periods, dose changes might have occurred after the first observed dose pattern had ended; such changes may have impacted the risk of overdoses attributed to the index pattern, creating misclassification bias in the analysis. Fifth, the study cohort was population-based with an approximate average age of 55 years, mean MME of 51, mean Charlson Comorbidity Index of 1.2, and OUD prevalence of 4%. This implies that the cohort was relatively young and healthy, and we lacked the statistical power to examine effects in particularly high-risk groups, such as patients with an OUD and patients older than 75 years of age with multiple chronic conditions who had been taking 90 MME or more for years.
Carefully managing opioid dose adjustments is an essential component of clinical care in the context of an opioid crisis. Clinicians should consider whether their patients benefit from a stable dose or slow dose reduction and, to the extent possible, refrain from rapid dose decreases. These results suggest that reducing patients’ opioid dosages at a rate greater than 30% per month poses significant overdose risk as the dose is being reduced.
Supplementary Material
Highlights.
An opioid dose reduction rate of >30% per month was associated with an approximate fivefold increased risk of overdose in the first month after initiating a dose reduction compared to stable dosing, and the magnitude of the risk attenuated as the follow-up increased.
Slower tapering rates were not associated with overdose risk.
These findings support the use of slow monthly opioid dose reductions to minimize the risk of short-term overdose.
Acknowledgments
We wish to thank LeeAnn Quintana, MSW (Kaiser Permanente Colorado [KPCO]), Jennifer Sawyer, BA (KPCO), Melanie Stowell, MSc (Denver Health [DH]), Judith Hase, BS (Marshfield Clinic Research Institute [MCRI]), and Linda Heeren, BS (MCRI) for project coordination, chart abstraction, and data collection assistance; and Kris Wain, MS (KPCO), M. Joshua Durfee, MSPH (DH), and Sai Sudha Medabalimi, MS, MPharm (MCRI) for data collection, management, and quality assurance. Drs. Stanley Xu (Kaiser Permanente Southern California) and Komal Narwaney (KPCO) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01DA047537. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Binswanger receives royalties for educational content on the health of incarcerated persons from UpToDate. The authors have disclosed no other conflicts of interest.
Footnotes
Compliance, Ethical Standards, and Ethical Approval
The KPCO Institutional Review Board (IRB) approved this study with a waiver of informed consent, with IRBs at DH and MCHS ceding oversight to KPCO.
Supplemental Material
Supplemental material for this article is available online at the SAJ website http://journals.sagepub.com/doi/suppl/10.1177/08897077231186216
References
- 1.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 2.Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid. 2019. Accessed May 24, 2023. https://public3.pagefreezer.com/browse/HHS.gov/16-09-2020T14:35/https://www.hhs.gov/opioids/treatment/clinicians-guide-opioid-dosage-reduction/index.html
- 4.U.S. Department of Veterans Affairs. Pain management opioid taper decision tool. A VA Clinician’s Guide. 2016. Accessed May 24, 2023. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/Pain_Opioid_Taper_Tool_IB_10_939_P96820.pdf [Google Scholar]
- 5.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binswanger IA, Glanz JM, Faul M, et al. The association between opioid discontinuation and heroin use: a nested case-control study. Drug Alcohol Depend. 2020;217:108248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnall BD, Juurlink D, Kerns RD, et al. International stakeholder community of pain experts and leaders call for an urgent action on forced opioid tapering. Pain Med. 2019;20(3):429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroenke K, Alford DP, Argoff C, et al. Challenges with implementing the Centers for Disease Control and Prevention opioid guideline: a consensus panel report. Pain Med. 2019;20(4):724–735. [DOI] [PubMed] [Google Scholar]
- 9.Mackey K, Anderson J, Bourne D, Chen E, Peterson K. Evidence brief: benefits and harms of long-term opioid dose reduction or discontinuation in patients with chronic pain. VA Evidence-based Synthesis Program Reports; 2019. [PubMed] [Google Scholar]
- 10.Oliva EM, Bowe T, Manhapra A, et al. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ. 2020;368:m283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kertesz SG, Manhapra A. The drive to taper opioids: mind the evidence, and the ethics. Spinal Cord Ser Cases. 2018;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieder TN. Is nonconsensual tapering of high-dose opioid therapy justifiable? AMA J Ethics. 2020;22(1):E651–E657. [DOI] [PubMed] [Google Scholar]
- 13.Agnoli A, Xing G, Tancredi DJ, Magnan E, Jerant A, Fenton JJ. Association of dose tapering with overdose or mental health crisis among patients prescribed long-term opioids. JAMA. 2021;326(5):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPrete BL, Ranapurwala SI, Maierhofer CN, et al. Association of opioid dose reduction with opioid overdose and opioid use disorder among patients receiving high-dose, long-term opioid therapy in North Carolina. JAMA Netw Open. 2022;5(4):e229191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank JW, Levy C, Matlock DD, et al. Patients’ perspectives on tapering of chronic opioid therapy: a qualitative study. Pain Med. 2016;17(10):1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturgeon JA, Sullivan MD, Parker-Shames S, Tauben D, Coelho P. Outcomes in long-term opioid tapering and buprenorphine transition: a retrospective clinical data analysis. Pain Med. 2020;21(12):3635–3644. [DOI] [PubMed] [Google Scholar]
- 17.Everitt BS. The Cambridge Dictionary of Statistics. Cambridge University Press; 1998. [Google Scholar]
- 18.Glaz J, Pozdnyakov V, Wallenstein S, eds. Scan Statistics: Methods and Applications. Springer Science + Business Media; 2009. [Google Scholar]
- 19.Naus JI. The distribution of the size of the maximum cluster of points on a line. J Am Stat Assoc. 1965;60(310):532–538. [Google Scholar]
- 20.Ross TR, Ng D, Brown JS, et al. The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS. 2014;2(1):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. National Death Index. 2023. Accessed May 24, 2023. https://www.cdc.gov/nchs/ndi/index.htm
- 22.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu S. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Netw Open. 2019;2(4):e192613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glanz JM, Narwaney KJ, Mueller SR, et al. Prediction model for two-year risk of opioid overdose among patients prescribed chronic opioid therapy. J Gen Intern Med. 2018;33(10):1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binswanger IA, Shetterly SM, Xu S, et al. Opioid dose trajectories and associations with mortality, opioid use disorder, continued opioid therapy, and health plan disenrollment. JAMA Netw Open. 2022;5(10):e2234671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Society of Addiction Medicine. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1–91. [DOI] [PubMed] [Google Scholar]
- 28.Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder - For Healthcare and Addiction Professionals, Policymakers, Patients, and Families. TIP 63.: U.S. Department of Health and Human Services; 2021. [PubMed] [Google Scholar]
- 29.Nagarwalla N A scan statistic with a variable window. Stat Med. 1996;15(7–9):845–850. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, Hambidge SJ, McClure DL, Daley MF, Glanz JM. A scan statistic for identifying optimal risk windows in vaccine safety studies using self-controlled case series design. Stat Med. 2013;32(19):3290–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko H, Kim HK, Chung C, et al. Association between medication adherence and intrapatient variability in tacrolimus concentration among stable kidney transplant recipients. Sci Rep. 2021;11(1):5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leino AD, King EC, Jiang W, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am J Transplant. 2019;19(5):1410–1420. [DOI] [PubMed] [Google Scholar]
- 33.Nataraj N, Strahan AE, Guy GP Jr., Losby JL, Dowell D. Dose tapering, increases, and discontinuity among patients on long-term high-dose opioid therapy in the United States, 2017–2019. Drug Alcohol Depend. 2022;234:109392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173(7):516–526. [DOI] [PubMed] [Google Scholar]
- 35.Binswanger IA, Narwaney KJ, Gardner EM, Gabella BA, Calcaterra SL, Glanz JM. Development and evaluation of a standardized research definition for opioid overdose outcomes. Subst Abus. 2019;40(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Prescription drug overdose data & statistics - guide to ICD-9-CM and ICD-10 codes related to poisoning and pain. 2013. Accessed May 24, 2023. https://stacks.cdc.gov/view/cdc/59394
- 37.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 38.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Li F. Propensity score weighting for causal inference with multiple treatments. Ann Appl Stat. 2019;13(4):2389–2415. [Google Scholar]
- 40.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the e-value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 42.Hallvik SE, El Ibrahimi S, Johnston K, et al. Patient outcomes after opioid dose reduction among patients with chronic opioid therapy. Pain. 2022;163(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller SR, Glanz JM, Nguyen AP, et al. Restrictive opioid prescribing policies and evolving risk environments: a qualitative study of the perspectives of patients who experienced an accidental opioid overdose. Int J Drug Policy. 2021;92:103077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosakowski S, Benintendi A, Lagisetty P, Larochelle MR, Bohnert ASB, Bazzi AR. Patient perspectives on improving patient-provider relationships and provider communication during opioid tapering. J Gen Intern Med. 2022;37(7):1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenton JJ, Magnan E, Tseregounis IE, Xing G, Agnoli AL, Tancredi DJ. Long-term risk of overdose or mental health crisis after opioid dose tapering. JAMA Netw Open. 2022;5(6):e2216726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mark TL, Parish W. Opioid medication discontinuation and risk of adverse opioid-related health care events. J Subst Abuse Treat. 2019;103:58–63. [DOI] [PubMed] [Google Scholar]
- 47.Quinn PD, Chang Z, Bair MJ, et al. Associations of opioid prescription dose and discontinuation with risk of substance-related morbidity in long-term opioid therapy. Pain. 2022;163(4):e588–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karmali RN, Bush C, Raman SR, Campbell CI, Skinner AC, Roberts AW. Long-term opioid therapy definitions and predictors: a systematic review. Pharmacoepidemiol Drug Saf. 2020;29(3):252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Oliveira Costa J, Bruno C, Baranwal N, et al. Variations in long-term opioid therapy definitions: a systematic review of observational studies using routinely collected data (2000–2019). Br J Clin Pharmacol. 2021;87(10):3706–3720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


