Abstract
Objective:
Mild traumatic brain injuries (mTBI) are considered self-limiting and full recovery is expected. Recent studies identify deficits persisting years after mTBI. Large-scale prospective data permit testing the hypothesis that mTBI increases incidence of affective and behavioral symptoms after new, past, or new and past mTBI.
Setting:
The study involved secondary analyses of survey responses from the Adolescent Brain Cognitive Development (ABCD) Study.
Participants:
Adolescents in the ABCD Study (n=11,869; Wave 1, ages 9–10; Wave 2, ages 11–12) whose parents reported a new (n=157), past (n=1,318) or new and past (n=50) mTBI on the Ohio State University Injury Identification Method short form were compared to controls who had no history of mTBI (n=9,667).
Design:
Multivariable binary logistic regression models examined associations between a new, past, or new and past mTBI, current affective (aggression, depression, anxiety) and behavioral (somatic, thought, social, attention, ADHD, conduct) disorders while controlling for demographic factors and baseline symptoms.
Main Measures:
The primary measure was parental reports of psychiatric and behavioral symptoms on the Child Behavior Checklist (CBCL).
Results:
Girls exhibited no significant effects after a new mTBI, although a past mTBI increased anxiety (aOR=1.83, 95% CI [1.15, 2.90]) and attention (1.89, [1.09, 3.28]) problems. Girls with new and past mTBIs reported elevated anxiety (17.90, [4.67, 68.7]), aggression (7.37, [1.49, 36.3]), social (9.07, [2.47, 33.30]), thought (7.58, [2.24, 25.60]) and conduct (6.39, [1.25, 32.50]) disorders. In boys, new mTBI increased aggression (aOR=3.83, 95% CI [1.42, 10.30]), whereas past mTBI heightened anxiety (1.91, 1.42, 2.95]), but new and past mTBIs had no significant effects.
Conclusion:
Adolescents are at greater risk of affective and behavioral symptoms after an mTBI. These effects differ as a function of gender and time of injury. Extended screening for mTBI history and monitoring of affective and behavioral disorders after mTBI in adolescents is warranted.
Keywords: mTBI, adolescence, anxiety, aggression, attention, conduct disorder
Introduction
Mild traumatic brain injury (mTBI) is a major health concern in pediatric and adolescent populations, accounting for >750,000 emergency room visits annually.1 Findings from regional and national samples identify a lifetime prevalence >20% in adolescent athletes,2–4 and ~20% in adolescents overall2,5,6 with approximately 1% experiencing mTBI each year.7 Predictors of who will experience an mTBI identify several at-risk groups including males,8 athletes,3 and people diagnosed with ADHD.9 In adolescent athletes, 25% report one or more mTBIs in their athletic careers.3 Adolescents with ADHD are more than twice as likely to experience an mTBI before age 117 and an ADHD diagnosis is associated with prolonged recovery.10 Adolescents, especially males, athletes and those with ADHD are at elevated risk of experiencing mTBI, and male athletes are less likely to report an mTBI.11,12
Recovery from mTBI is gaining recognition as a clinically important issue as research identifies persistent deficits, even in uncomplicated cases. This counters the prevailing view that mTBI is a self-limiting condition leading to full recovery. For example, undergraduates exhibit cognitive deficits years post-mTBI13 as do adults.14,15 Sex also plays a role in recovery for adolescents,16 with worse outcomes observed in older females,17,18 and dissociable likelihoods of subsequent epilepsy or schizophrenia diagnoses.19 In adults, affective disorders are elevated post-mTBI, with a meta-analysis confirming greater risks of developing depression,20 aggression,21 and mental health problems.22,23 Recovery is prolonged by affective symptoms,24,25 and social-behavioral factors (e.g., genetics, injury etiology, low socioeconomic status).26 In children, mTBI is a significant risk factor for children to develop psychiatric diagnoses27–29 or conduct disorder.30 Pediatric mTBI is also associated with higher rates of later substance use disorder.16,31 Adolescents who experience mTBI are at elevated risk of developing clinically relevant affective and behavioral symptoms.
Despite some progress understanding mTBI sequelae, our understanding is limited by reliance on retrospective studies with insufficient power to control for premorbid affective and behavioral symptoms.32 We address this by using data from the Adolescent Brain Cognitive Development (ABCD) Study. The ABCD Study prospectively tracks a national sample of adolescents’ health, including mTBI, affect, cognition, thought, behavior and social disorders. Leveraging these data permitted us to control for demographics and baseline symptomatology to probe whether boys and girls experience the same affective and behavioral impact of mTBI when compared to population controls (i.e., peers who have no hmTBI). Accordingly, the purpose of this study was to tease apart the timeline to disentangle the impact of a new mTBI, a past mTBI, or both a new and past mTBI among a national sample of boys and girls to assess patterns of affective symptoms post-mTBI.
Methods
The ABCD Study is a 10-year longitudinal study of brain and cognitive development in children beginning at ages 9−10 years across 21 U.S. sites with IRB approval provided by each site. The cohort was recruited to reflect the demographic and geographic diversity of American adolescents. Measures of behavioral and mental health functioning, substance use, familial and environmental characteristics are collected annually or biannually.33 The ABCD protocol is a comprehensive set of in-person physical, cognitive, social, emotional, environmental, behavioral, and academic assessments, including neuroimaging and biospecimen collection. In-person assessments are completed annually (6–7 hours for the child, 3 hours for the parent) or biannually (imaging, bioassays) for 10 years. Participants complete a brief, mid-year phone interview (https://nda.nih.gov/abcd). This work involves secondary data analysis and is considered exempt from IRB review.
Sample
The baseline ABCD sample includes 11,875 children aged 9–10 years. The sample is 52% female, 48% male; 52% white, 15% black/African American, 20% Hispanic, 12% other racial groups; and family income 15.1% $0 to $24,999, 42.9% $25,000 to $99,999, 42.0% $100,000 or more. This study includes all participants (n = 11,192) who completed the baseline and first one-year follow-up and responded to the Ohio State University Traumatic Brain Injury Identification Method (OSU TBI-ID) short form.
Key Independent Variable: Baseline and past-year history of mild traumatic brain injury (hmTBI)
Parents reported on two types of head injury analogs using the OSU TBI-ID short form questionnaire at baseline and first follow-up. Lifetime history and past year mTBI was defined as a “Yes” response to the following: Baseline, “Has your child ever been hospitalized or treated in an emergency room following an injury to his/her head or neck?”; follow-up “Since we last saw you on [insert date], has your child been hospitalized or treated in an emergency room following an injury to their head or neck?” Based on these two questions from the longitudinal data, a mutually exclusive hmTBI was defined with the following groups: (1) never (n=9667), (2) new mTBI (n=157), (3) mTBI in the past but not in the past year (n=1318), and (4) mTBI in the past and in the past year (n=50). Roughly 6% of these respondents indicated an mTBI with loss of consciousness.
Key Dependent Variables: Past six-month psychiatric disorders
At baseline and follow-up sessions parents reported on symptoms of adolescent anxiety, depression, somatic complaints, social problems, thought problems, attention problems, aggression, attention deficit hyperactivity disorder (ADHD) and conduct problems using the Child Behavior Checklist CBCL.34 The CBCL has excellent reliability and validity.34 Following guidelines,34 clinically significant psychiatric disorders for each of nine scales were defined as T-scores (ranging from 50 – 100) that were 70 and higher (relative to a non-clinical sample). A binary summary variable was computed to indicate clinically significant anxiety, depression, somatic complaints, social problems, thought problems, attention problems, aggression, ADHD, and conduct disorders at both baseline and first follow-up: 1 (clinically significant) T-Score greater or equal to 70 and 0 (not clinically significant) for T-Score less than or equal to 69.
Control variables
Control variables included sex (Boy, Girl), age at baseline (ages 9 or 10), race (White, Black, Other), Hispanic ethnicity (Hispanic, non-Hispanic), parental education (< high school degree, high school degree, some college, college degree or higher) and household income ($0-$24,999, $25,000-$99,999, $100,000+).
Analysis
Descriptive statistics, odds ratios (OR), and adjusted odds ratios (aOR) were calculated to assess the association between hmTBI and clinically significant psychiatric disorders during the past six months. We estimated two sets of binary logistic regression models: (1) a model that assesses the association between hmTBI and clinically significant psychiatric disorders at the first follow-up (adjusting for control variables - but not adjusting for clinically significant psychiatric disorders at baseline), and (2) a model that assesses the association between hmTBI and clinically significant psychiatric disorders at the second follow-up (adjusting for both control variables and the specific clinically significant psychiatric disorders at baseline; e.g., control for clinically significant levels of anxiety at baseline when predicting clinically significant levels of anxiety at first year follow-up). Accordingly, the second set of models provides a conservative analysis to determine if newly exposed adolescents with mTBI have greater odds of developing clinically significant psychiatric disorders at the first follow-up. Additional analyses stratify these models by sex to explore potential differences in the association between hmTBI and clinically significant psychiatric disorders among boys and girls. Moreover, models that include interaction effects between hmTBI and sex were conducted to see if there were statistically significant differences between boys and girls with respect tohmTBI and clinically significant psychiatric disorders. Analyses use Stata 17.0 and account for clustering across the 21 research sites and within families using the ‘svyset’ commands (i.e., svyset familyID, strata (siteID)); “svy, subpop’ commands are used when running all analyses. Missing data were handled using listwise deletion given that only 1.5% of the sample was lost due to missing data on the items used for this study (imputation of missing data would be inconsequential and would not change the overall results for this study).35 Finally, given the exploratory nature of the study and number of comparisons across outcomes, results at the .01 alpha level or lower are considered statistically significant (nine outcomes in total; .05/9 = .0055). While results at the .05 alpha level should be interpreted with caution, these results will be discussed in order to flag these findings with the intention for further replication in future studies using the ABCD data set.
Results
Table 1 provides the sample characteristics among respondents. 1.4% of the adolescents were classified as new mTBI (no hmTBI at baseline but indicated a mTBI in the past-year at follow-up), with 11.8% indicating a mTBI in the past (but not within the past year) and .4% indicating a mTBI in the past and within the past year. More boys (16.1%) indicated a mTBI compared to girls (10.9%).
Table 1.
Sample characteristics among respondents (n= 11,192)
| Total | Boys | Girls | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | %/mean | n | %/mean | p-valuex | |
| Sex (baseline) | |||||||
| Male | (5851) | 52.3% | NA | NA | NA | NA | |
| Female | (5337) | 47.7% | NA | NA | NA | NA | |
| Race (baseline) | |||||||
| White | (7942) | 71.0% | (4250) | 71.9% | (3734) | 70.0% | p=.086 |
| Black | (2258) | 20.2% | (1143) | 19.5% | (1114) | 20.9% | |
| Other | (992) | 8.9% | (503) | 8.6% | (489) | 9.2% | |
| Hispanic (baseline) | |||||||
| Hispanic | (2214) | 20.0% | (1156) | 20.0% | (1057) | 20.0% | p=.988 |
| Non-Hispanic | (8841) | 80.0% | (4617) | 80.0% | (4221) | 80.0% | |
| Age (baseline) | |||||||
| 9 years old | (6322) | 56.5% | (3236) | 55.3% | (3085) | 57.8% | p=.008 |
| 10 years old | (4867) | 43.5% | (2613) | 44.7% | (2251) | 42.2% | |
| Parental Level of Education (baseline) | |||||||
| Highest level of education is less than a high school degree | (521) | 4.7% | (251) | 4.3% | (270) | 5.1% | p=.249 |
| Highest level of education is a high school degree only | (997) | 8.9% | (518) | 8.9% | (479) | 9.0% | |
| Highest level of education is some college | (2837) | 25.4% | (1502) | 25.7% | (1333) | 25.0% | |
| Highest level of education is a college degree or higher | (6821) | 61.0% | (3568) | 61.1% | (3251) | 61.0% | |
| Income (baseline) | |||||||
| $0 to $24,999 | (1444) | 14.0% | (770) | 14.4% | (674) | 13.7% | p=.491 |
| $25,00 to $99,999 | (4408) | 42.9% | (2274) | 42.4% | (2133) | 43.4% | |
| $100,000 or more | (4431) | 43.1% | (2320) | 43.3% | (2109) | 42.9% | |
| Psychiatric Disorders at Baseline | |||||||
| CBCL Anxiety (T-scores greater or equal to 70) | (314) | 2.8% | (157) | 2.7% | (156) | 2.9% | p=.411 |
| CBCL Depression (T-scores greater or equal to 70) | (292) | 2.6% | (200) | 3.4% | (92) | 1.7% | p<.001 |
| CBCL Somatic (T-scores greater or equal to 70) | (324) | 2.9% | (161) | 2.8% | (162) | 3.0% | p=.370 |
| CBCL Social problems (T-scores greater or equal to 70) | (184) | 1.6% | (102) | 1.7% | (81) | 1.5% | p=.348 |
| CBCL Thought problems (T-scores greater or equal to 70) | (468) | 4.2% | (320) | 5.5% | (148) | 2.8% | p<.001 |
| CBCL Attention problems (T-scores greater or equal to 70) | (316) | 2.8% | (174) | 3.0% | (142) | 2.7% | p=.319 |
| CBCL Aggression (T-scores greater or equal to 70) | (251) | 2.2% | (165) | 2.8% | (86) | 1.6% | p<.001 |
| CBCL ADHD (T-scores greater or equal to 70) | (322) | 2.9% | (197) | 3.4% | (125) | 2.3% | p<.001 |
| CBCL Conduct problems (T-scores greater or equal to 70) | (298) | 2.7% | (161) | 2.8% | (137) | 2.6% | p=.546 |
| Psychiatric Disorders at first One Year Follow-up | |||||||
| CBCL Anxiety (T-scores greater or equal to 70) | (325) | 2.9% | (172) | 2.9% | (152) | 2.8% | p=.771 |
| CBCL Depression (T-scores greater or equal to 70) | (333) | 3.0% | (222) | 3.8% | (111) | 2.1% | p<.001 |
| CBCL Somatic (T-scores greater or equal to 70) | (331) | 3.0% | (164) | 2.8% | (166) | 3.1% | p=.339 |
| CBCL Social problems (T-scores greater or equal to 70) | (162) | 1.4% | (77) | 1.3% | (85) | 1.6% | p=.222 |
| CBCL Thought problems (T-scores greater or equal to 70) | (491) | 4.4% | (312) | 5.3% | (179) | 3.4% | p<.001 |
| CBCL Attention problems (T-scores greater or equal to 70) | (324) | 2.9% | (172) | 2.9% | (152) | 2.8% | p=.771 |
| CBCL Aggression (T-scores greater or equal to 70) | (246) | 2.2% | (153) | 2.6% | (93) | 1.7% | p=.002 |
| CBCL ADHD (T-scores greater or equal to 70) | (289) | 2.6% | (184) | 3.1% | (105) | 2.0% | p<.001 |
| CBCL Conduct problems (T-scores greater or equal to 70) | (282) | 2.5% | (148) | 2.5% | (134) | 2.5% | .948 |
| Reported a Concussion (Lifetime) – 686 removed missing | |||||||
| Never had a TBI | (9667) | 86.4% | (4908) | 83.9% | (4757) | 89.1% | p<.001 |
| New incident TBI from baseline to first follow-up | (157) | 1.4% | (97) | 1.7% | (60) | 1.1% | |
| TBI in the past but not in the past year | (1318) | 11.8% | (810) | 13.8% | (506) | 9.5% | |
| TBI in the past and in the past year | (50) | 0.4% | (36) | 0.6% | (14) | 0.3% | |
Chi-square tests of independence were used to assess bivariate differences between boys and girls.
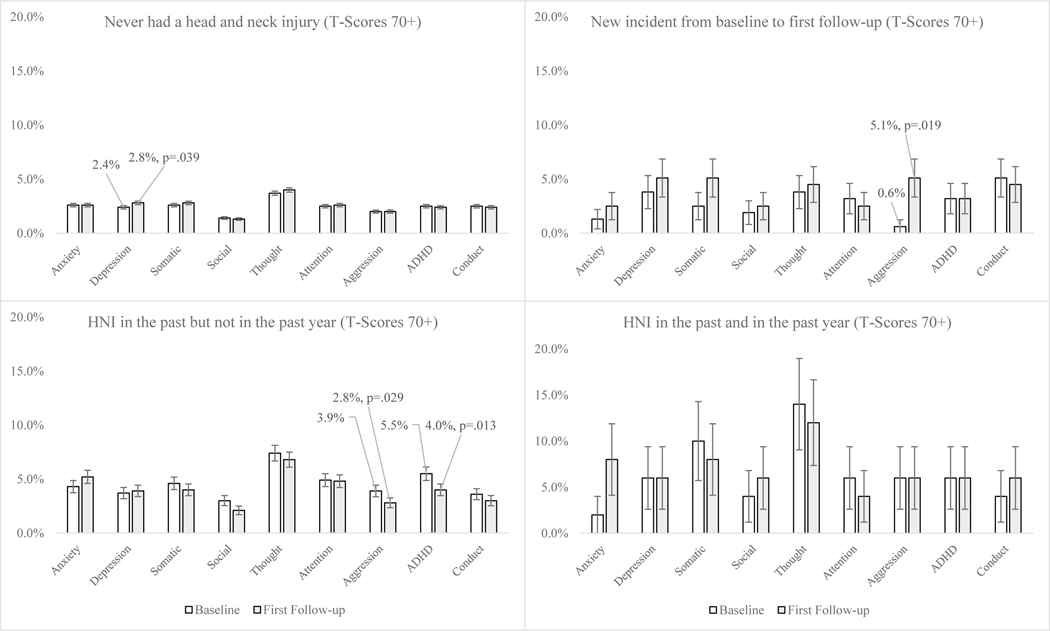
Figure 1 provides the bivariate differences in clinically significant psychiatric disorders (T-score of 70 or higher) between baseline and first follow-up by hmTBI. Several differences between baseline and first follow-up were found within each group based on mTBI history. Among adolescents who never had a mTBI, the percent experiencing clinically significant levels of depression increased from baseline to first one-year follow-up (baseline 2.4% versus 2.8% follow-up, p=.039). Adolescents with new mTBI showed an increase in clinically significant levels of aggression from baseline to first one-year follow-up (baseline .6% versus 5.1% follow-up, p=.019). Additionally, adolescents who experienced mTBI in the past saw a decline in clinically significant levels of both aggression (baseline 3.9% versus 2.8% follow-up, p=.029) and ADHD (baseline 5.5% versus 4.0% follow-up, p=.013) from baseline to first one-year follow-up.
Figure 1:
Assessing clinically significant psychiatric disorders at baseline and first one year follow-up by history of HNI
Table 2 shows the results of the analysis assessing the association between hmTBI and clinically significant psychiatric disorders. The first set of models assess the association between hmTBI and clinically significant psychiatric disorders at the first one-year follow-up without adjusting for prior history of clinically significant psychiatric disorders at baseline. Adolescents who had a mTBI in the past (but not the past year) and adolescents who had a mTBI in the past (and in the past year) had higher odds of clinically significant levels of anxiety, depression, somatic disorders, social disorders and thought disorders when compared to peers who never experienced a mTBI. For instance, adolescents who experienced a mTBI in the past and in the past year had nearly three times greater odds of reporting a clinically significant thought disorder (aOR=2.76, 95% CI=1.13,6.73) at the first one-year follow-up when compared to adolescents who have never experienced a mTBI. Further, adolescents who had a mTBI in the past (but not in the past year) had higher odds of reporting clinically significant levels of depression at the first one-year follow-up when compared to adolescents who have never had a mTBI.
Table 2.
Assessing the association between history of TBI and clinically significant psychiatric disorders (n= 11,192)
| Psychiatric Disorders at first One Year Follow-up | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 |
|---|---|---|---|---|---|---|---|---|---|
| Anxiety1 (T-score>70) | Depression1 (T-score>70) | Somatic1 (T-score>70) | Social1 (T-score>70) | Thought1 (T-score>70) | Attention1 (T-score>70) | Aggression1 (T-score>70) | ADHD1 (T-score>70) | Conduct1 (T-score>70) | |
| First set of models | (n=11,035) | (n=11,035) | (n=11,035) | (n=11,035) | (n=11,035) | (n=11,035) | (n=11,035) | (n=11,035) | (n=11,035) |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| History of TBI | |||||||||
| Never had TBI | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| New incident TBI | 1.03 (.379,2.80) | 1.85 (.893,3.86) | 1.97 (.943,4.12) | 1.93 (.700,5.32) | 1.06 (.495,2.26) | .937 (.342,2.56) | 2.02 (.903,4.54) | 1.23 (.492,3.11) | 1.50 (.646,3.48) |
| TBI in the past (not past year) | 2.07 (1.56,2.75)*** | 1.43 (1.05,1.96)* | 1.54 (1.13,2.10)** | 1.72 (1.12,2.65)* | 1.68 (1.31,2.15)*** | 1.90 (1.42,2.55) | 1.38 (.956,2.00) | 1.69 (1.24,2.42) | 1.40 (.893,1.99) |
| TBI in the past (and past year) | 3.10 (1.08,8.83)* | 1.99 (.598,6.65) | 3.21 (1.15,8.93)* | 4.25 (1.27,14.3)* | 2.76 (1.13,6.73)* | 1.36 (.340,5.46) | 2.41 (.752,7.76) | 2.11 (.655,6.81) | 2.42 (.774,7.62) |
| 3Pseudo R2 | R2 = .024 | R2 = .048 | R2 = .025 | R2 = .055 | R2 = .030 | R2 = .032 | R2 = .053 | R2 = .036 | R2 = .071 |
| Psychiatric Disorders at first One Year Follow-up | Model 10 | Model 11 | Model 12 | Model 13 | Model 14 | Model 15 | Model 16 | Model 17 | Model 18 |
| Anxiety2 (T-score>70) | Depression2 (T-score>70) | Somatic2 (T-score>70) | Social2 (T-score>70) | Thought2 (T-score>70) | Attention2 (T-score>70) | Aggression2 (T-score>70) | ADHD2 (T-score>70) | Conduct2 (T-score>70) | |
| Second set of models | (n=11,033) | (n=11,033) | (n=11,033) | (n=11,033) | (n=11,033) | (n=11,033) | (n=11,033) | (n=11,033) | (n=11,033) |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| History of TBI | |||||||||
| Never had TBI | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| New incident TBI | 1.29 (.439,3.82) | 1.75 (.775,3.94) | 2.10 (.842,5.25) | 1.92 (.467,7.95) | 1.07 (.486,2.37) | .823 (.289,2.34) | 3.14 (3.30,7.48)** | 1.27 (.467,3.47) | 1.23 (.427,3.59) |
| TBI in the past (not past year) | 1.88 (1.36,2.58)*** | 1.25 (.889,1.77) | 1.26 (.910,1.75) | 1.36 (.863,2.14) | 1.29 (.960,1.74) | 1.55 (1.10,2.19)* | 1.04 (.688,1.58) | 1.20 (.838,1.72) | 1.19 (.809,1.77) |
| TBI in the past (and past year) | 4.36 (1.26,15.0)* | 1.76 (.479,6.51) | 1.86 (.671,5.21) | 3.62 (1.27,10.3)* | 1.56 (.550,4.42) | .810 (.287,2.28) | 1.82 (.530,6.24) | 1.72 (.471,6.29) | 2.32 (.741,7.28) |
| Specific clinically significant psychiatric disorders at baseline 2 | |||||||||
| No clinically significant disorder | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Clinically significant disorder | 42.3 (32.1,55.6)*** | 26.1 (19.7,34.7)*** | 29.0 (22.1,38.3)*** | 48.6 (32.8,72.2)*** | 36.8 (29.1,46.4)*** | 36.8 (27.8,48.7)*** | 46.8 (33.5,65.5)*** | 45.1 (33.7,60.3)*** | 51.3 (37.7,69.9)*** |
| 3Pseudo R2 | R2 = .225 | R2 = .185 | R2 = .183 | R2 = .223 | R2 = .248 | R2 = .214 | R2 = .246 | R2 = .254 | R2 = .297 |
Notes:
p<.05,
p<.01,
p<.001
Each model controls for sex, age at baseline, race, Hispanic ethnicity, highest level of parental education and total household income.
Each model controls for sex, age at baseline, race, Hispanic ethnicity, highest level of parental education and total household income. Additionally, these models adjust for the specific psychiatric disorder at baseline. For instance, when predicating a clinically significant anxiety disorder at the first follow-up, the model also controls for whether the respondent had a clinically significant anxiety disorder at baseline.
Pseudo R2 in logistic regression (McFadden’s) is an analog of R2 in ordinary least squares regression.
The second set of models presented in Table 2 assessed the association between hmTBI and clinically significant psychiatric disorders at the first one-year follow-up when adjusting for prior history of clinically significant psychiatric disorders at baseline. Adolescents who had a mTBI in the past (but not the past year) had higher odds of indicating clinically significant levels of anxiety (aOR=1.88, 95% CI=1.36,2.58) and attention disorders (aOR=1.55, 95% CI=1.10,2.19) when compared to their peers with no hmTBI (when adjusting for these clinically significant disorders at baseline). Adolescents who had a mTBI in the past (and in the past year) had higher odds of indicating clinically significant levels of anxiety (aOR=4.36, 95% CI=1.26,15.0) and social disorders (aOR=3.26, 95% CI=1.27,10.3) when compared to their peers with no hmTBI (when adjusting for these clinically significant disorders at baseline). Further, adolescents who experienced a new mTBI had higher odds of indicating clinically significant aggression (aOR=3.14, 95% CI=3.30,7.48) when compared to their peers with no hmTBI (when adjusting for this clinically significant disorder at baseline).
Tables 3 and 4 provide the stratified results for boys and girls, respectively. Focusing on the models assessing the association between hmTBI and psychiatric disorders (i.e., the second set of models), the analyses show that boys who had a mTBI in the past (but not the past year) had higher odds of indicating a clinically significant anxiety disorder (aOR=1.91, 95% CI=1.23,2.95) at the first one year follow-up when compared to boys who never had a mTBI (when adjusting for this clinically significant disorder at baseline). Moreover, boys who experienced a new incident mTBI had higher odds of clinically significant aggression disorder (aOR=3.83, 95% CI=1.42,10.3) at the first one-year follow-up when compared to boys who never had a mTBI (when adjusting for this clinically significant disorder at baseline). The interaction effect models did not find any statistically significant differences in these associations mentioned above when compared to girls – these associations were similar for boys and girls (see Supplemental Table 1).
Table 3.
Assessing the association between history of TBI and clinically significant psychiatric disorders among boys (Boys; n= 5851)
| Psychiatric Disorders at first One Year Follow-up | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 |
|---|---|---|---|---|---|---|---|---|---|
| Anxiety1 (T-score>70) | Depression1 (T-score>70) | Somatic1 (T-score>70) | Social1 (T-score>70) | Thought1 (T-score>70) | Attention1 (T-score>70) | Aggression1 (T-score>70) | ADHD1 (T-score>70) | Conduct1 (T-score>70) | |
| First set of models | (n=5,760) | (n=5,760) | (n=5,760) | (n=5,238) | (n=5,760) | (n= 5,723) | (n=5,760) | (n=5,760) | (n=5,760) |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| History of TBI | |||||||||
| Never had TBI | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| New incident TBI | .801 (.196,3.27) | 1.52 (.618,3.73) | 2.20 (.849,5.69) | 1.59 (.378,6.67) | .802 (.299,2.15) | .756 (.184,3.09) | 2.12 (.777,5.78) | .680 (.160,2.87) | 1.24 (.369,4.20) |
| TBI in the past (not past year) | 1.92 (1.32,2.79)*** | 1.25 (.858,1.82) | 1.56 (1.03,2.35)* | 1.67 (.932,3.00)* | 1.53 (1.12,2.07)** | 1.95 (1.33,2.84) | 1.25 (.787,2.01) | 1.73 (1.19,2.53) | 1.37 (.855,2.19) |
| TBI in the past (and past year) | .946 (.123,7.27) | .682 (.097,4.79) | 1.09 (.147,8.18) | -- | 1.02 (.232,4.48) | -- | .933 (.134,6.48) | 1.62 (.375,7.04) | 1.09 (.160,7.52) |
| 3Pseudo R2 | R2 = .028 | R2 = .045 | R2 = .029 | R2 = .062 | R2 = .032 | R2 = .042 | R2 = .059 | R2 = .044 | R2 = .096 |
| Psychiatric Disorders at first One Year Follow-up | Model 10 | Model 11 | Model 12 | Model 13 | Model 14 | Model 15 | Model 16 | Model 17 | Model 18 |
| Anxiety2 (T-score>70) | Depression2 (T-score>70) | Somatic2 (T-score>70) | Social2 (T-score>70) | Thought2 (T-score>70) | Attention2 (T-score>70) | Aggression2 (T-score>70) | ADHD2 (T-score>70) | Conduct2 (T-score>70) | |
| Second set of models | (n=5,760) | (n=5,760) | (n=5,760) | (n=5,238) | (n=5,760) | (n=5,723) | (n=5,760) | (n=5,760) | (n=5,760) |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| History of TBI | |||||||||
| Never had TBI | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| New incident TBI | .993 (.271,3.63) | 1.63 (.632,4.23) | 2.48 (.724,8.52) | 1.72 (.200,14.7) | .689 (.319,1.48) | .524 (.119,2.30) | 3.83 (1.42,10.3)** | .712 (.169,3.00) | 1.52 (.412,5.63) |
| TBI in the past (not past year) | 1.91 (1.23,2.95)** | 1.14 (.754,1.75) | 1.24 (.790,1.94) | 1.33 (.709,2.49) | 1.18 (.808,1.74) | 1.40 (.907,2.16) | 1.01 (.600,1.71) | 1.16 (.744,1.81) | 1.22 (.731,2.03) |
| TBI in the past (and past year) | 1.00 (.078,12.8) | .857 (.107,6.81) | .755 (.150,3.78) | -- | .541 (.110,2.64) | -- | .582 (.132,2.56) | 2.20 (.169,3.00) | .790 (.220,2.83) |
| Specific clinically significant psychiatric disorders at baseline 2 | |||||||||
| No clinically significant disorder | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Clinically significant disorder | 45.7 (30.6,68.2)*** | 24.8 (17.5,35.0)*** | 31.4 (22.1,46.7)*** | 53.2 (29.9,94.9)*** | 32.6 (24.4,43.6)*** | 31.7 (21.6,46.6)*** | 45.8 (30.0,70.0)*** | 46.2 (31.8,67.2)*** | 44.2 (28.3,69.2)*** |
| 3Pseudo R2 | R2 = .228 | R2 = .190 | R2 = .192 | R2 = .249 | R2 = .261 | R2 = .209 | R2 = .270 | R2 = .277 | R2 = .303 |
Notes:
p<.05,
p<.01,
p<.001; ‘—’ indicates that this category had to be dropped due to no respondents indicating the outcome of interest.
Each model controls for sex, age at baseline, race, Hispanic ethnicity, highest level of parental education and total household income.
Each model controls for sex, age at baseline, race, Hispanic ethnicity, highest level of parental education and total household income. Additionally, these models adjust for the specific psychiatric disorder at baseline. For instance, when predicating a clinically significant anxiety disorder at the first follow-up, the model also controls for whether the respondent had a clinically significant anxiety disorder at baseline.
Pseudo R2 in logistic regression (McFadden’s) is an analog of R2 in ordinary least squares regression.
Table 4.
Assessing the association between history of TBI and clinically significant psychiatric disorders among girls (Girls; n= 5337)
| Psychiatric Disorders at first One Year Follow-up | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 |
|---|---|---|---|---|---|---|---|---|---|
| Anxiety1 (T-score>70) | Depression1 (T-score>70) | Somatic1 (T-score>70) | Social1 (T-score>70) | Thought1 (T-score>70) | Attention1 (T-score>70) | Aggression1 (T-score>70) | ADHD1 (T-score>70) | Conduct1 (T-score>70) | |
| First set of models | (n=5,273) | (n=5,273) | (n=5,273) | (n=5,273) | (n= 5,273) | (n= 5,273) | (n=5,273) | (n=5,273) | (n=5,273) |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| History of TBI | |||||||||
| Never had TBI | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| New incident TBI | 1.30 (.307,5.50) | 2.74 (.807,9.35) | 1.66 (.516,5.36) | 2.25 (.527,9.62) | 1.58 (.480,5.24) | 1.19 (.288,4.94) | 1.76 (.410,7.57) | 2.51 (.752,8.39) | 1.85 (.560,6.11) |
| TBI in the past (not past year) | 2.24 (1.45,3.44)*** | 1.94 (1.14,3.31)* | 1.50 (.945,2.40) | 1.77 (.940,3.35) | 1.98 (1.31,3.00)** | 1.83 (1.15,2.90)* | 1.66 (.908,3.04) | 1.58 (.885,2.82) | 1.45 (.844,2.50) |
| TBI in the past (and past year) | 9.82 (2.65,36.4)*** | 8.56 (1.64,44.6)* | 8.33 (2.26,30.6)*** | 15.7 (3.64,68.2)*** | 12.2 (3.93,37.9)*** | 5.31 (1.32,21.3)* | 8.37 (1.92,36.3)** | 3.53 (.487,25.6) | 5.86 (1.33,25.7)* |
| 3Pseudo R2 | R2 = .032 | R2 = .053 | R2 = .028 | R2 = .060 | R2 = .030 | R2 = .030 | R2 = .049 | R2 = .027 | R2 = .056 |
| Psychiatric Disorders at first One Year Follow-up | Model 10 | Model 11 | Model 12 | Model 13 | Model 14 | Model 15 | Model 16 | Model 17 | Model 18 |
| Anxiety2 (T-score>70) | Depression2 (T-score>70) | Somatic2 (T-score>70) | Social2 (T-score>70) | Thought2 (T-score>70) | Attention2 (T-score>70) | Aggression2 (T-score>70) | ADHD2 (T-score>70) | Conduct2 (T-score>70) | |
| Second set of models | (n=5,271) | (n=5,271) | (n=5,271) | (n=5,271) | (n=5,271) | (n=5,271) | (n=5,271) | (n=5,271) | (n=5,271) |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| History of TBI | |||||||||
| Never had TBI | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| New incident TBI | 1.59 (.240,10.6) | 1.69 (.323,8.91) | 1.61 (.402,6.47) | 2.21 (.316,15.5) | 2.03 (.420,9.83) | 1.38 (.360,5.35) | 1.99 (.337,11.7) | 2.57 (.602,10.9) | .965 (.207,4.50) |
| TBI in the past (not past year) | 1.83 (1.15,2.90)** | 1.45 (.794,2.65) | 1.27 (.788,2.07) | 1.38 (.710,2.72) | 1.54 (.978,2.42) | 1.89 (1.09,3.28)* | 1.12 (.563,2.25) | 1.27 (.675,2.42) | 1.23 (.653,2.31) |
| TBI in the past (and past year) | 17.9 (4.67,68.7)*** | 3.98 (.382,41.4) | 4.06 (.860,19.1) | 9.07 (2.47,33.3)*** | 7.58 (2.24,25.6)*** | 2.32 (.906,5.94) | 7.37 (1.49,36.3)* | .979 (.104,9.16) | 6.39 (1.25,32.5)* |
| Specific clinically significant psychiatric disorders at baseline 2 | |||||||||
| No clinically significant disorder | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Clinically significant disorder | 45.7 (29.4,63.6)*** | 28.2 (16.9,47.1)*** | 27.1 (18.3,39.9)*** | 49.8 (27.8,89.5)*** | 49.0 (32.9,72.8)*** | 45.5 (29.8,69.4)*** | 51.3 (29.3,89.6)*** | 46.0 (28.8,73.7)*** | 62.8 (40.5,97.3)*** |
| 3Pseudo R2 | R2 = .238 | R2 = .173 | R2 = .178 | R2 = .212 | R2 = .233 | R2 = .229 | R2 = .217 | R2 = .224 | R2 = .302 |
Notes:
p<.05,
p<.01,
p<.001
Each model controls for sex, age at baseline, race, Hispanic ethnicity, highest level of parental education and total household income.
Each model controls for sex, age at baseline, race, Hispanic ethnicity, highest level of parental education and total household income. Additionally, these models adjust for the specific psychiatric disorder at baseline. For instance, when predicating a clinically significant anxiety disorder at the first follow-up, the model also controls for whether the respondent had a clinically significant anxiety disorder at baseline.
Pseudo R2 in logistic regression (McFadden’s) is an analog of R2 in ordinary least squares regression.
Girls who had a mTBI in the past (but not the past year) had higher odds of indicating a clinically significant anxiety disorder (aOR=1.83, 95% CI=1.15,2.90) and attention disorder (aOR=1.89, 95% CI=1.09,3.28) at the first one-year follow-up when compared to girls who never had a mTBI (when adjusting for these clinically significant disorders at baseline). The interaction effect models did not find statistically significant differences with these specific associations when compared to boys (see Supplemental Table 1). Finally, girls who had a mTBI in the past (and in the past year) had higher odds of indicating a clinically significant anxiety disorder (aOR=17.9, 95% CI=4.67,68.7), social disorder (aOR=9.07, 95% CI=2.47,33.3), thought disorder (aOR=7.58, 95% CI=2.24,25.6), aggression disorder (aOR=7.37, 95% CI=1.49,36.3) and conduct disorder (aOR=6.39, 95% CI=1.25,32.5) at the first one year follow-up when compared to girls who never had a mTBI (when adjusting for these clinically significant disorders at baseline). The interaction effect models found each of these specific associations to be significantly different when compared to boys, except for conduct disorder (see Supplemental Table 1).
Discussion
Adolescents who experience mTBI have higher rates of clinically relevant affective symptoms and somatic disorders. The pattern of symptoms depended on gender, and the timing of their mTBI(s). Importantly, even in uncomplicated cases of mTBI there is increased affective, and behavioral symptoms. Using data from the ABCD study provided a prospectively collected sample and permitted conservative analyses controlling for socioeconomic factors and baseline affective and behavioral symptoms. Adolescents, particularly boys, who experienced a new mTBI within the last year are more likely to experience elevated aggression. Anxiety is elevated in those with a past mTBI. In other words, mTBI has a series of clinically relevant affective and behavioral consequences in boys. Furthermore, striking patterns were observed in girls. Girls with a new mTBI had no increased likelihood of symptoms, although those with a past mTBI reported higher rates of anxiety and attention problems. Surprisingly, girls who had a new and past mTBI reported a broad and sharp increase across widespread symptoms measuring aggression, anxiety, social problems, thought and conduct disorders. Indeed, these effects were the most robust.
These data are of high clinical relevance because they show that adolescents, especially girls, are especially vulnerable after a second mTBI. In adolescents who experience mTBI it is essential to continue to monitor affective, as well as somatic, symptoms to identify and address problems early. These data support the use of careful screening of mTBI history, including probable mTBIs that were medically untreated. A strength of the current findings is that these findings derive from conservative analyses controlling for premorbid symptoms and socioeconomic factors using a large, national sample of adolescent participants. Finally, these data highlight the need for evidence-based mTBI rehabilitation treatments well beyond the current time frame of medical treatment.
The observations of generally increased anxiety and aggression after an mTBI are consistent with recent findings from single sample studies.36 As these authors suggest, it is essential to screen adolescents for mTBI to understand factors contributing to their affective and behavioral problems. It is also important to understand the neural mechanism(s) that make adolescents particularly vulnerable because of their incomplete development, and the length of lifetime over which symptoms would exert their consequences.36 There are few consistent findings evaluating longitudinal neural changes in human adolescents. In adults, there are neuroanatomical changes associated with mTBI including cortical volume loss, changed patterns of network connectivity, and alterations to key subcortical regions involved in mediating threat and emotional responses.37 Effects of mTBI on the amygdalae are relevant to the current findings because of its role in emotional control, including aggression and anxiety. For instance, several studies in college athletes with a hmTBI show more psychological symptoms and reduced volumes of subcortical areas including the amygdala.38 A single mTBI in college athletes reveals lasting neural volume differences in the amygdalae.39 However, the heterogeneous nature of mTBI makes it difficult to identify neuroanatomical signatures that are either specific or sensitive.37,40,41 Despite difficulties tracking neuroanatomical changes associated with mTBI, the current results demonstrate the urgency of developing effective interventions.
Several limitations of the study are noteworthy. The data were drawn from the ABCD Study. Despite many strengths it relies on parental and self-report measures. The ABCD questions used to select cases are not perfectly aligned with mTBI diagnostic criteria. Our inclusion criteria only included those who reported an Emergency Room visit for a head or neck injury. The goal was to obtain a defensible sample of those who had a diagnosed mTBI, and without medical treatment there is no diagnosis. Admittedly, this metric it is imperfect. This criteria may retain individuals with a past (untreated, undiagnosed) mTBI in the control population. The large sample would minimize the added noise to the comparison pool. There are also known gender-related discrepancies in reporting mTBI, with reduced reporting for boys.11,12 However, our more liberal criteria may have diluted the effect sizes. The longitudinal releases from the ABCD will permit tracking these observed effects of mTBI over time. Finer-grained measures are needed to more comprehensively characterize the nature of the affective and behavioral consequences associated with mTBI. Another concern is that some of the most striking statistics were from a very small number of adolescent girls who reported both past and recent mTBI. To mitigate the effects of small N, a series of controlling factors were included in the models and the analyses are thus very conservative. Moreover, given the relatively small sample sizes for key groups based on history of concussion (i.e., new incident and TBI in the past and in the past year), interaction effect modeling was not adequately powered to precisely detect differences between boys and girls. While some difference were found between boys and girls with respect to the association between hmTBI (particularly those with past and the past year mTBI) and several psychiatric disorders in the interaction effect models, these results should be interpreted with caution and need to be replicated in future studies using the ABCD data (these differences were found in the smallest hmTBI group [n=50]). Significant deficits were associated with the much larger number of adolescents who reported a past mTBI. The conclusion that a second mTBI provokes a greater number of symptoms builds on a broader set of observations from those who had a past mTBI. Importantly, the issue of power will be mitigated as the ABCD Study continues to release data, more participants will be available with a past and recent mTBI to revisit the findings and will allow for better precision to detect significant differences between boys and girls. Despite these limitations, the data provide insight regarding the potential vulnerability of adolescents to mTBI.
Conclusion
Adolescents who experience an mTBI are significantly more likely to experience symptoms of aggression and anxiety. Whereas boys experience anxiety and aggression, girls experience a range of affective and behavioral consequences, particularly after more than one mTBI. Clinicians should continually monitor adolescents for psychological, and behavioral symptoms long after mTBI.
Supplementary Material
Funding/Support:
No funding was secured for this study.
Footnotes
Conflict of Interest Disclosures: The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Zonfrillo MR, Kim KH, Arbogast KB. Emergency Department Visits and Head Computed Tomography Utilization for Concussion Patients From 2006 to 2011. Acad Emerg Med. Jul 2015;22(7):872–7. doi: 10.1111/acem.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veliz P, McCabe SE, Eckner JT, Schulenberg JE. Trends in the Prevalence of Concussion Reported by US Adolescents, 2016–2020. JAMA. May 4 2021;325(17):1789–1791. doi: 10.1001/jama.2021.1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veliz P, McCabe SE, Eckner JT, Schulenberg JE. Prevalence of Concussion Among US Adolescents and Correlated Factors. JAMA. Sep 26 2017;318(12):1180–1182. doi: 10.1001/jama.2017.9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veliz P, Eckner JT, Zdroik J, Schulenberg JE. Lifetime Prevalence of Self-Reported Concussion Among Adolescents Involved in Competitive Sports: A National U.S. Study. J Adolesc Health. Feb 2019;64(2):272–275. doi: 10.1016/j.jadohealth.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilie G, Boak A, Adlaf EM, Asbridge M, Cusimano MD. Prevalence and correlates of traumatic brain injuries among adolescents. JAMA. Jun 26 2013;309(24):2550–2. doi: 10.1001/jama.2013.6750 [DOI] [PubMed] [Google Scholar]
- 6.Ilie G, Trenholm M, Boak A, et al. Adolescent traumatic brain injuries: Onset, mechanism and links with current academic performance and physical injuries. PLoS One. 2020;15(3):e0229489. doi: 10.1371/journal.pone.0229489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook NE, Iverson GL. Concussion Among Children in the United States General Population: Incidence and Risk Factors. Front Neurol. 2021;12:773927. doi: 10.3389/fneur.2021.773927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour SC, Adams RS, Brody DL, Puente AN, Gray JC. Prevalence and correlates of concussion in children: Data from the Adolescent Brain Cognitive Development study. Cortex. Oct 2020;131:237–250. doi: 10.1016/j.cortex.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 9.Cook NE, Karr JE, Iverson GL. Children with ADHD Have a Greater Lifetime History of Concussion: Results from the ABCD Study. J Neurotrauma. Mar 30 2021;doi: 10.1089/neu.2021.0019 [DOI] [PubMed] [Google Scholar]
- 10.Martin AK, Petersen AJ, Sesma HW, et al. Learning and Attention Deficit/Hyperactivity Disorders as Risk Factors for Prolonged Concussion Recovery in Children and Adolescents. J Int Neuropsychol Soc. Mar 22 2021:1–14. doi: 10.1017/S1355617721000229 [DOI] [PubMed] [Google Scholar]
- 11.Kroshus E, Baugh CM, Stein CJ, Austin SB, Calzo JP. Concussion reporting, sex, and conformity to traditional gender norms in young adults. J Adolesc. Jan 2017;54:110–119. doi: 10.1016/j.adolescence.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 12.Miyashita TL, Diakogeorgiou E, VanderVegt C. Gender Differences in Concussion Reporting Among High School Athletes. Sports Health. Jul 2016;8(4):359–63. doi: 10.1177/1941738116651856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arciniega H, Shires J, Furlong S, et al. Impaired visual working memory and reduced connectivity in undergraduates with a history of mild traumatic brain injury. Sci Rep. Feb 2 2021;11(1):2789. doi: 10.1038/s41598-021-80995-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailie JM, Kennedy JE, French LM, et al. Profile Analysis of the Neurobehavioral and Psychiatric Symptoms Following Combat-Related Mild Traumatic Brain Injury: Identification of Subtypes. J Head Trauma Rehabil. Jan-Feb 2016;31(1):2–12. doi: 10.1097/HTR.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 15.Carroll EL, Outtrim JG, Forsyth F, et al. Mild traumatic brain injury recovery: a growth curve modelling analysis over 2 years. J Neurol. Nov 2020;267(11):3223–3234. doi: 10.1007/s00415-020-09979-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilie G, Adlaf EM, Mann RE, et al. The moderating effects of sex and age on the association between traumatic brain injury and harmful psychological correlates among adolescents. PLoS One. 2014;9(9):e108167. doi: 10.1371/journal.pone.0108167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin HS, Temkin NR, Barber J, et al. Association of Sex and Age With Mild Traumatic Brain Injury-Related Symptoms: A TRACK-TBI Study. JAMA Netw Open. Apr 1 2021;4(4):e213046. doi: 10.1001/jamanetworkopen.2021.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinowitz AR, Li X, McCauley SR, et al. Prevalence and Predictors of Poor Recovery from Mild Traumatic Brain Injury. J Neurotrauma. Oct 1 2015;32(19):1488–96. doi: 10.1089/neu.2014.3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancelliere C, Donovan J, Cassidy JD. Is Sex an Indicator of Prognosis After Mild Traumatic Brain Injury: A Systematic Analysis of the Findings of the World Health Organization Collaborating Centre Task Force on Mild Traumatic Brain Injury and the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. Feb 2016;97(2 Suppl):S5–18. doi: 10.1016/j.apmr.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 20.Hellewell SC, Beaton CS, Welton T, Grieve SM. Characterizing the Risk of Depression Following Mild Traumatic Brain Injury: A Meta-Analysis of the Literature Comparing Chronic mTBI to Non-mTBI Populations. Front Neurol. 2020;11:350. doi: 10.3389/fneur.2020.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosti C, Coccaro EF. Mild Traumatic Brain Injury and Aggression, Impulsivity, and History of Other- and Self-Directed Aggression. J Neuropsychiatry Clin Neurosci. Summer 2018;30(3):220–227. doi: 10.1176/appi.neuropsych.17070141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughn MG, Salas-Wright CP, John R, Holzer KJ, Qian Z, Veeh C. Traumatic Brain Injury and Psychiatric Co-Morbidity in the United States. Psychiatr Q. Mar 2019;90(1):151–158. doi: 10.1007/s11126-018-9617-0 [DOI] [PubMed] [Google Scholar]
- 23.Delmonico RL, Theodore BR, Sandel ME, Armstrong MA, Camicia M. Prevalence of depression and anxiety disorders following mild traumatic brain injury. PM R. Jun 22 2021;doi: 10.1002/pmrj.12657 [DOI] [PubMed] [Google Scholar]
- 24.Zahniser E, Nelson LD, Dikmen SS, et al. The Temporal Relationship of Mental Health Problems and Functional Limitations following mTBI: A TRACK-TBI and TED Study. J Neurotrauma. Jun 2019;36(11):1786–1793. doi: 10.1089/neu.2018.6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright B, Wilmoth K, Juengst SB, Didehbani N, Maize R, Cullum CM. Perceived Recovery and Self-Reported Functioning in Adolescents with Mild Traumatic Brain Injury: The Role of Sleep, Mood, and Physical Symptoms. Dev Neurorehabil. May 2021;24(4):237–243. doi: 10.1080/17518423.2020.1858456 [DOI] [PubMed] [Google Scholar]
- 26.Zamani A, Mychasiuk R, Semple BD. Determinants of social behavior deficits and recovery after pediatric traumatic brain injury. Exp Neurol. Apr 2019;314:34–45. doi: 10.1016/j.expneurol.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 27.Max JE, Friedman K, Wilde EA, et al. Psychiatric disorders in children and adolescents 24 months after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2015;27(2):112–20. doi: 10.1176/appi.neuropsych.13080190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick BF, Connolly EJ, Nelson DV. Mild Traumatic Brain Injury as a Predictor of Classes of Youth Internalizing and Externalizing Psychopathology. Child Psychiatry Hum Dev. Feb 2021;52(1):166–178. doi: 10.1007/s10578-020-00992-9 [DOI] [PubMed] [Google Scholar]
- 29.Ilie G, Mann RE, Boak A, et al. Suicidality, bullying and other conduct and mental health correlates of traumatic brain injury in adolescents. PLoS One. 2014;9(4):e94936. doi: 10.1371/journal.pone.0094936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowet DS, Kolan A, Vaida F, et al. Novel Oppositional Defiant Disorder 6 Months After Traumatic Brain Injury in Children and Adolescents. J Neuropsychiatry Clin Neurosci. Nov 12 2021:appineuropsych21020052. doi: 10.1176/appi.neuropsych.21020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannella LA, McGary H, Ramirez SH. Brain interrupted: Early life traumatic brain injury and addiction vulnerability. Exp Neurol. Jul 2019;317:191–201. doi: 10.1016/j.expneurol.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emery CA, Barlow KM, Brooks BL, et al. A Systematic Review of Psychiatric, Psychological, and Behavioural Outcomes following Mild Traumatic Brain Injury in Children and Adolescents. Can J Psychiatry. May 2016;61(5):259–69. doi: 10.1177/0706743716643741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci. Aug 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achenbach TM, Rescorla LA. The manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 35.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. Mar 1999;8(1):3–15. doi: 10.1177/096228029900800102 [DOI] [PubMed] [Google Scholar]
- 36.Connolly EJ, McCormick BF. Mild Traumatic Brain Injury and Psychopathology in Adolescence: Evidence From the Project on Human Development in Chicago Neighborhoods. J Adolesc Health. Jul 2019;65(1):79–85. doi: 10.1016/j.jadohealth.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 37.Bigler ED. Volumetric MRI Findings in Mild Traumatic Brain Injury (mTBI) and Neuropsychological Outcome. Neuropsychol Rev. Mar 3 2021;doi: 10.1007/s11065-020-09474-0 [DOI] [PubMed] [Google Scholar]
- 38.Brett BL, Bobholz SA, Espana LY, et al. Cumulative Effects of Prior Concussion and Primary Sport Participation on Brain Morphometry in Collegiate Athletes: A Study From the NCAA-DoD CARE Consortium. Front Neurol. 2020;11:673. doi: 10.3389/fneur.2020.00673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobholz SA, Brett BL, Espana LY, et al. Prospective study of the association between sport-related concussion and brain morphometry (3T-MRI) in collegiate athletes: study from the NCAA-DoD CARE Consortium. Br J Sports Med. Feb 2021;55(3):169–174. doi: 10.1136/bjsports-2020-102002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dailey NS, Smith R, Vanuk JR, Raikes AC, Killgore WDS. Resting-state functional connectivity as a biomarker of aggression in mild traumatic brain injury. Neuroreport. Nov 7 2018;29(16):1413–1417. doi: 10.1097/WNR.0000000000001127 [DOI] [PubMed] [Google Scholar]
- 41.Smith LGF, Milliron E, Ho ML, et al. Advanced neuroimaging in traumatic brain injury: an overview. Neurosurg Focus. Dec 1 2019;47(6):E17. doi: 10.3171/2019.9.FOCUS19652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.