Abstract
Introduction
Andexanet alfa (AA) - zhzo, recombinant coagulation factor Xa, is an approved antidote for oral Xa inhibitors (apixaban and rivaroxaban). Unfractionated heparin (UFH) is commonly used for therapeutic, interventional, and surgical indications. Protamine sulfate (PrSO4) is frequently used to neutralize UFH. This study aimed to investigate the comparative neutralization profiles of AA and PrSO4 for heparins of bovine, ovine, and porcine origin.
Materials and Methods
The neutralization effect of PrSO4 at 25 µg/ml and AA at 100 µg/ml was studied on an approximate surgical/interventional concentration of heparin by supplementing whole blood with each of the heparins at 25 µg/ml. For the clotting profile (activated partial thromboplastin time: aPTT), amidolytic (anti-Xa and anti-IIa), and thrombin generation assay each of the heparin were supplemented from –10–0.62 µg/ml.
Results
In the whole blood ACT studies, all three heparins produced strong anti-coagulant effects (400–450 seconds) compared to saline (130–150 seconds). Both AA and PrSO4 almost fully neutralized the anti-coagulant effects of heparins (140–160 seconds). Both antidotes completely reversed the anticoagulant effects of all three heparins in the aPTT and thrombin generation assay. However, PrSO4 was more effective in neutralizing the anti-Xa, and anti-IIa effects than AA, which only partially neutralized these effects.
Conclusion
Andexanet alfa at 100 µg/ml effectively neutralizes the therapeutic and surgical/interventional concentrations of heparins in in-vitro settings. While differences in the anti-Xa, and anti-IIa effects between heparins were noted, anti-coagulant effect of these agents in the aPTT assay were comparable. A similar neutralization profile was observed in the ACT and thrombin generation assays by both agents.
Keywords: heparin, protamine sulfate, andexanet alfa, clot-based assays, chromogenic assays, thrombin generation assay
Introduction
Unfractionated heparin (UFH) was discovered in 1916 and used as an anticoagulant and antithrombin drug for over seven decades. Heparin is used primarily for cardiovascular disorders including acute myocardial infarction (MI), treatment of unstable angina, angioplasty, prevention and treatment of arterial and venous thromboembolism (deep venous thromboembolism: DVT or pulmonary embolism: PE), cardiopulmonary intervention and as an adjunctive agent during thrombolysis. 1 Despite the development of newer classes of inhibitors including low molecular weight heparins (LMWH's), pentasaccharide and direct oral anticoagulants (DOAC's) such as factor Xa (FXa) and factor IIa (FIIa) inhibitors, UFH has remained the anticoagulant of choice for interventional and surgical indications and hemodialysis. Protamine Sulfate is the only available effective antidote to heparin in case of hemorrhages related to UFH. 2
Protamine sulfate (PrSO4) is a 5-kDa cationic polypeptide composed of poly(l-arginine) derived from the head of the sperm of Rhine Salmon. Protamine sulfate is a primary protein, and UFH is acidic in nature. The mechanism of action of PrSO4 is still not clear. Yet, it involves binding to the negatively charged heparin molecules, which form a stable complex, displacing anti-thrombin (AT) from the heparin–AT complex. 3 The reticuloendothelial system rapidly clears the resultant protamine-heparin complex. Protamine sulfate is routinely administered postoperatively to reverse the effects of UFH required for patients undergoing cardiac surgery, cardiopulmonary bypass (CPB), orthopedic surgery, or even after dialysis. 4
Andexanet alfa (AA) – a recombinant coagulation human factor Xa (Portola Pharmaceuticals, South San Francisco, CA) is an FXa decoy protein and is approved for reversal of direct and antithrombin dependent FXa inhibitors.5,6 Andexanet alfa is also reported to neutralize the effects of heparin-related drugs. 7 Due to a global shortage of swine heparin, regulatory agencies have encouraged the development of heparin from alternative sources, such as bovine and ovine, and these preparations are already approved in some countries for clinical use. 8
Protamine sulfate produces a reversal complex with heparins and renders these agents inactive towards the inhibition of anti-Xa and IIa. Protamine sulfate is a heterogenous poly-component agents, and its mechanism of action is entirely different than andexanet alfa for the inhibition of heparins. Andexanet alfa binds with FXa inhibitors directly and reversibly block the active site.4,9 Indirect FXa inhibitors such as heparin, enoxaparin and fondaparinux binds with anti-thrombin forming a complex; heparin-AT, enoxaparin-AT and fondaparinux-AT, conformationally extrude the shape of AT and increases its affinity. Following complex formation, andexanet alfa competes with FXa and binds with heparin-AT, enoxaparin-AT and fondaparinux-AT complex reconditioning the generation of thrombin from prothrombin.4,9,10 Moreover, direct binding of andexanet alfa to heparin, enoxaparin and fondaparinux in the absence of AT has been shown to be of low affinity (Kd = 34–110 mM), suggesting that this interaction is not the physiological mechanism of action for the reversal of AT-dependent anticoagulation by andexanet alfa. 11 Several manuscripts have described the efficacy of andexanet alfa for the reversal of factor Xa inhibitors with reference to bleeding complications,12,13 dosage considerations are important, moreover the mechanism involving thrombin generation is relevant. 12 Off-note 4F-PCC's have a narrow range of FXa inhibitors neutralizability profile. Thrombin generation may be considered as a measure of relative neutralizability of Xa inhibitors by andexanet. 14
Previous reports have shown partial neutralization of heparins from different origins by andexanet alfa. A detailed mechanistic approach of the inhibitory effects of anti-Xa and anti-IIa by AA has not been addressed fully. This study aimed to investigate the comparative neutralization profiles of andexanet alfa and protamine sulfate for heparins of bovine, ovine and porcine origin.
Methods
Testing Agents
Powdered forms of pharmaceutical-grade heparins of bovine (140 U/mg), ovine (200 U/mg) and porcine (190 U/mg) origin were obtained from commercial vendors. Andexanet alfa was obtained from the hospital pharmacy (Skokie Hospital, Skokie, IL, USA) and reconstituted at 10 mg/ml according to the manufacturer's instructions. Powdered protamine sulfate (USP grade) was obtained from Choay Institute (Paris, France) and reconstituted at 1.0 mg/ml in saline.
Global Clotting Assay
Whole Blood Analysis: Hemochron whole blood coagulation system (Accriva Diagnostics, Inc. CA, USA) was used to measure whole blood activated clotting time (ACT). Whole blood was supplemented with each heparin at a final concentration of 2.5 µg/ml as control and transferred into a celite ACT tube. For reversal studies, blood was supplemented with the heparins at a final concentration of 2.5 µg/ml were either supplemented with AA at a final concentration of 100 µg/ml or PrSO4 at a final concentration of 25 µg/ml and transferred into celite ACT tubes. Results were recorded in seconds and compiled in terms of means ± SD.
Plasma Supplemented System: Each of the heparins were supplemented in citrated plasma over a concentration range of 10 - 0.62 µg/ml. Saline as a control, AA at a final concentration of 100 µg/ml, and PrSO4 at 10 µg/ml were added to individual aliquots of plasma supplemented with each drug. The TriniCLOT - aPTT reagent was obtained from Diagnostica Stago (Parsippany, NJ, USA) and used to analyze activated partial thromboplastin time (aPTT) by using an ACL-Elite (Instrumentation Laboratory, Bedford, MA, USA). Results were compiled in terms of mean SD.
Chromogenic Assay
Anti-factor Xa and anti-factor IIa activity were measured using a kinetic amidolytic method on the ACL-Elite instrument (Instrumentation Laboratory, Bedford, MA, USA). Alfa human thrombin (Enzyme Research Laboratories, South Bend, IN, USA) was diluted in 50 nM Tris buffer (pH = 8.4) to a 1.25 IU/ml concentration. Factor IIa substrate (BioMedica Diagnostics, CT, USA) used in this assay was reconstituted in sterile water to make 1 µM. Bovine factor-Xa (Enzyme Research Laboratories, South Bend, IN, USA) was diluted in 50 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/ml and factor Xa substrate 2.5 µM (BioMedica Diagnostics, CT, USA) was used in this assay and reconstituted in sterile water. Each of the heparins were supplemented in citrated normal human plasma over a concentration range of 10 - 0.62 µg/ml. Saline was used as a control while reversal profiles by AA and PrSO4 were studied by adding AA at a final concentration of 100 µg/ml and PrSO4 at 10 µg/ml to individual aliquots of plasma-supplemented drugs. The extent of reversal by AA and PrSO4 were calculated.
Inhibition of Thrombin Generation
Inhibition of thrombin generation was measured using a Fluoroskan Ascent Fluorimeter, Calibrated Automated Thrombogram (CAT, Diagnostica Stago, Parsippany, NJ, USA). Reagents used in this assay included the fluo-substrate, fluo-buffer, tissue factor high reagent (mixture of tissue factor and phospholipids), and a thrombin calibrator. Heparins were supplemented in normal pooled plasma to obtain a concentration range from 2.5 - 0.062 µg/ml. Saline, andexanet alfa at a final concentration of 100 µg/ml, and PrS04 at 10 µg/ml were added to individual aliquots of plasma supplemented with each bovine, ovine, and porcine heparin. The thrombin generation assay was performed in 96-well Immulon 2HB transparent, round bottom plates. The thrombin generation potential was measured with respect to the peak thrombin concentration, lag time, and endogenous thrombin potential (ETP) / area under the curve (AUC). Results were compiled in terms of mean SD.
Results
Global Clotting Assays
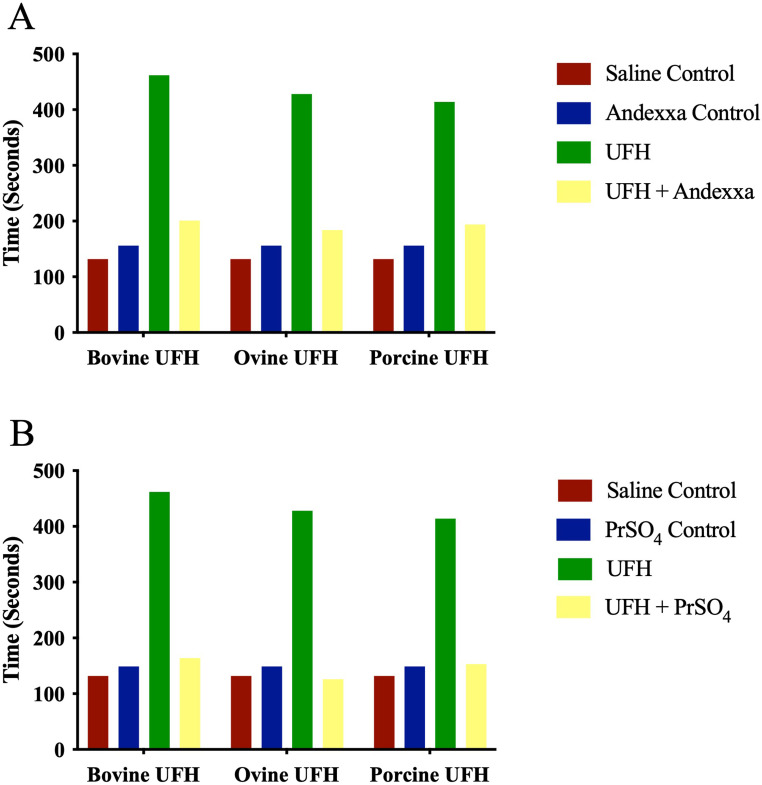
Whole Blood Analysis: The relative neutralization of heparins by AA and PrSO4 in whole blood ACT is depicted in Figure 1A-B. In the blood-activated clotting time, all three heparins at a final concentration of 2.5 µg/ml produced comparable and maximal responses compared to saline control. Protamine sulfate at a final concentration of 25 µg/ml and AA at 100 µg/ml completely reversed the anticoagulant effects of each of the heparins.
Figure 1.
A comparison of bovine, ovine and porcine UFH's and their neutralization by andexanet alfa and protamine sulfate in whole blood activated clotting time (ACT). Graph representing neutralization of UFH's at a final concentration of 2.5 μg/ml by (A) andexanet alfa and (B) protamine sulfate.
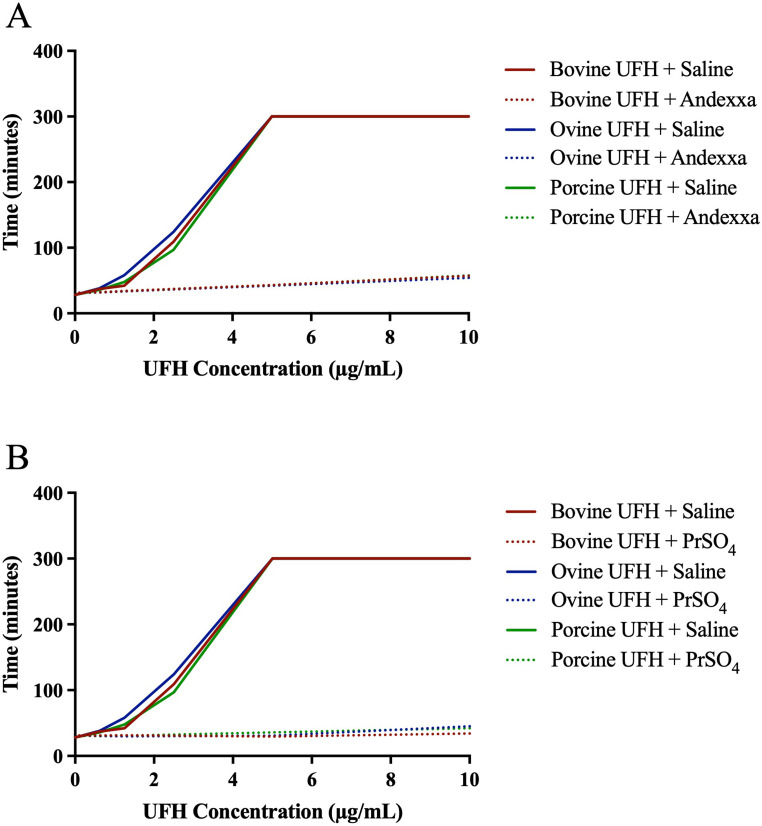
Plasma Supplemented System: Figure 2A-B shows the neutralization of bovine, ovine, and porcine heparins by AA and PrSO4 in activated partial thromboplastin time. Both AA (100 µg/ml) and PrSO4 (10 µg/ml) completely neutralized bovine, ovine and porcine heparin comparably.
Figure 2.
A comparison of bovine, ovine and porcine UFH's and their relative neutralization by andexanet alfa and protamine sulfate in activated partial thromboplastin time (aPTT). Graph representing neutralization of UFH's by (A) andexanet alfa and (B) protamine sulfate.
Chromogenic Assay
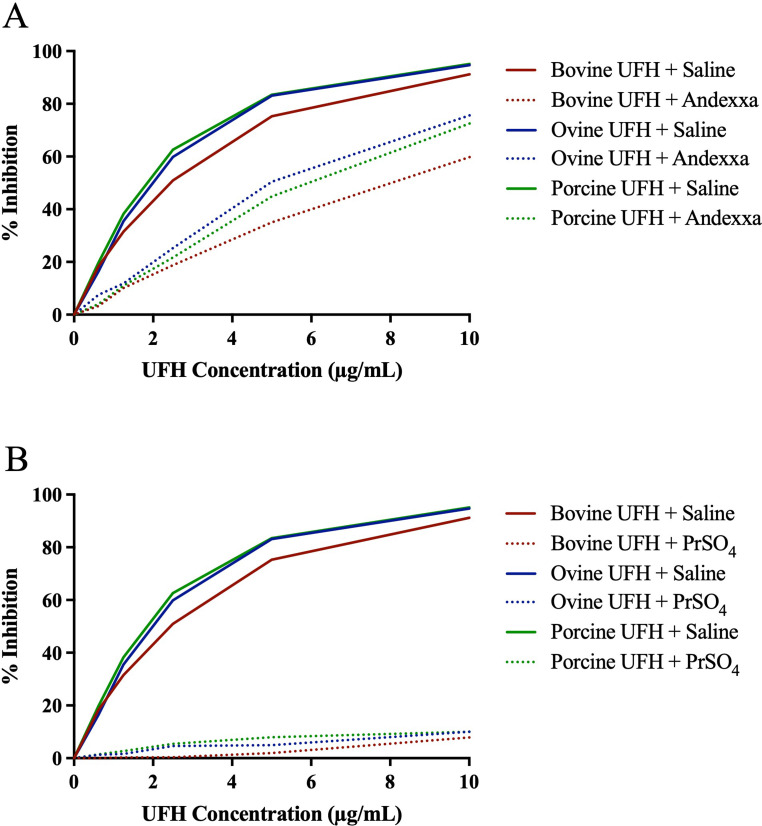
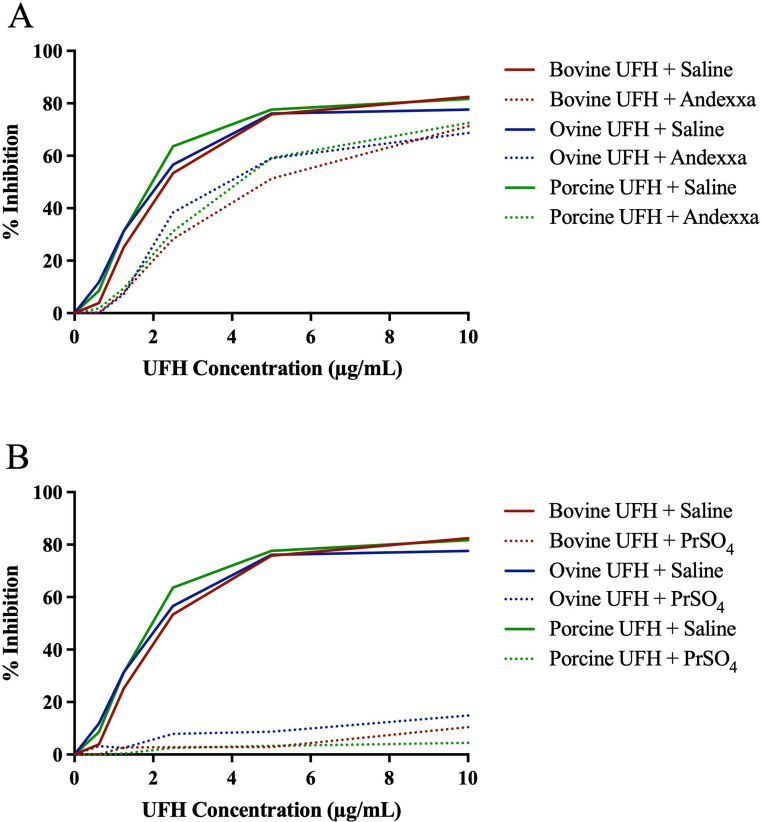
Relative neutralization of heparins by AA and PrSO4 in amidolytic anti-Xa assay are depicted in Figure 3A-B and anti-IIa assay in Figure 4A-B. Andexanet alfa at a 100 µg/ml concentration only partially neutralized all drugs in both the anti-Xa and anti-IIa assay. Protamine sulfate at a final concentration of 10 µg/ml completely and effectively neutralized heparin from all sources in anti-Xa and anti-IIa assay.
Figure 3.
A comparison of bovine, ovine and porcine UFH's and their relative neutralization by andexanet alfa and protamine sulfate in amidolytic anti-Xa assay. Graph representing neutralization of UFH's by (A) andexanet alfa and (B) protamine sulfate.
Figure 4.
A comparison of bovine, ovine and porcine UFH's and their relative neutralization by andexanet alfa and protamine sulfate in amidolytic anti-IIa assay. Graph representing neutralization of UFH's by (A) andexanet alfa and (B) protamine sulfate.
Inhibition of Thrombin Generation
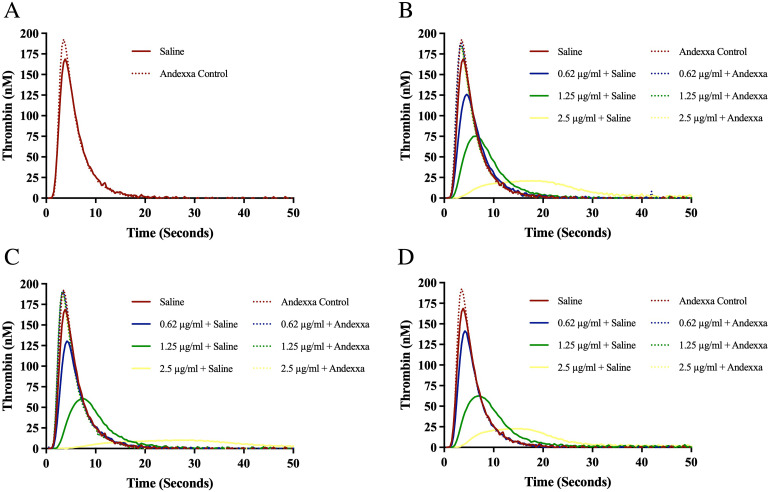
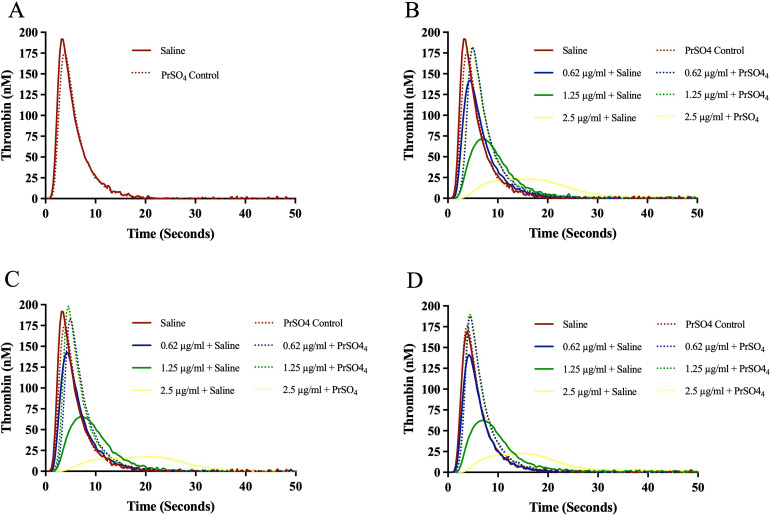
The effect of bovine, ovine and porcine heparins on the inhibition of thrombin generation (TG) and their neutralization by AA and PrSO4 are shown in Figures 5A-D and Figures 6A-D. All preparations produced a concentration-dependent effect. Ovine heparin showed relatively stronger inhibition of thrombin generation. Andexanet alfa at a concentration of 100 µg/ml and PrSO4 at 10 µg/ml completely reversed the anticoagulant effects of all heparins, even at a higher concentrations. Andexanet alfa produced more thrombin generation in comparison to PrSO4.
Figure 5.
Effect of bovine, ovine and porcine UFH's on inhibition of thrombin generation (TG) and their neutralization by andexanet alfa. Graph representing neutralization effect of andexanet alfa on (A) control, (B) bovine UFH, (C) ovine UFH and (D) porcine UFH.
Figure 6.
Effect of bovine, ovine and porcine UFH's on inhibition of thrombin generation (TG) and their neutralization by protamine sulfate. Graph representing neutralization effect of protamine sulfate on (A) control, (B) bovine UFH, (C) ovine UFH and (D) porcine UFH.
Discussion
Andexanet alfa was effective in neutralizing the anticoagulant effects of UFH of different sources as measured by various laboratory assays, such as the whole blood ACT and aPTT in a comparable manner to PrSO4. In our previous work, andexanet alfa partially neutralized UFH and enoxaparin in several TEG parameters, but it was not as effective as PrSO4 in any of the assays used. 15 Consistent with our previous observation in the current study, PrSO4 was more effective than AA in reversing Xa and IIa. Still, andexanet alfa also showed some reversal of such parameters in an UFH concentration-dependent inverse manner.
Bovine, ovine and porcine heparins produced a concentration-dependent effect on the inhibition of TG. Ovine heparin showed more potent inhibition of thrombin generation. Andexanet alfa at a concentration of 100 µg/ml and PrSO4 at 10 µg/ml completely reversed the anticoagulant effects of all heparins, even at a higher concentrations. Addition of AA on normal plasma produced an increase in thrombin generation greater than PrSO4. 16
It is interesting that andexanet alfa results in the partial neutralization of the amidolytic anti-Xa and anti-IIa effects of heparins whereas it completely neutralizes the heparin in the clot-based assay; ACT aPTT and thrombin generation assay which could be due to the assay conditions and differences in the binding sites.10,11,17 Slight binding differences can potentially lead to the observed differences. The anticoagulant and antiprotease activities of heparin and related drugs are predominantly mediated by AT. The interplay of heparin with antithrombin is primarily based on molecular weight and oligosaccharide composition of these agents which contributes to the anti-Xa and anti-IIa effects. Therefore, in the case of UFH, it restores the anti-Xa activity. Since andexanet alfa does not reverse anti-IIa activity, it is considered to be a partial antidote of heparin. 18 In circulating blood, andexanet alfa competes with FXa to bind with direct inhibitors of FXa and the pentasaccharide consensus binding oligosaccharide sequence in heparin-AT, enoxaparin-AT and fondaparinux-AT activated complexes, thus restoring the capacity of prothrombinase to generate thrombin. The anticoagulant effects of these agents are usually proportional to the anti-IIa effects. Excessive anticoagulation with heparins can lead to bleeding. Such excess anticoagulation can be reversed by the administration of protamine, which binds to heparin in a charge-dependent manner and prevents the heparin from interacting with AT.
Protamine sulfate seems more effective than AA in reversing UFH from different sources, mainly driven by its higher ability to reverse Xa and IIa activities. Intravenous PrSO4 can rapidly reverse the anticoagulant effects of heparin. Protamine sulfate is a primary protein from fish sperm that binds to heparin to form a stable complex. One milligram of PrSO4 neutralizes approximately 100 units of heparin. 19
Protamine sulfate is not free of complications. However, given its fish origin, protamine sulfate can cause hypotension, bradycardia, transient flushing and a feeling of warmth when administered too rapidly. These non-IgE-mediated anaphylactic reactions are not severe, provided the injection is given slowly (50 mg, the maximum recommended dose, over 10 min). Some responses to PrSO4 are complement-mediated and can be severe including death.20,21
Given the recent shortage of PrSO4 reported due to manufacturing delays, alternative anticoagulants such as bivalirudin were recommended. 22 In some countries, these agents are not available, and our data might be helpful for massive bleeding due to heparin use in the case of a lack of PrSO4 and AA availability. The U.S. Food and Drug Administration (FDA) approved andexanet alfa, also known as coagulation factor Xa (recombinant), inactivated-zhzo, to reverse the anticoagulant effects of oral rivaroxaban and apixaban. It is not currently approved for the reversal of heparin or other agents.
Study Limitations
This study is an in-vitro assay. The relevance of the in-vitro neutralization to the in-vivo is not clear. However, the use of whole blood is more relevant to in-vivo settings than plasma or other artificial systems. This study did not consider the complete blood profile and the effect of cellular components such as red cells. Since whole blood activated clotting time and thromboelastographic methods are used in the surgical procedures, these studies will benefit from whole blood assay for the neutralization profile of these agents. Nevertheless, the utility of these reversal parameters in potential future studies in clinical settings can validate some of these observations. Moreover, these studies were conducted at fixed concentrations of andexanet alfa and protamine sulfate. It would have been useful for these studies to be conducted over a wide range of concentrations.
Conclusion
These results suggest that AA at 100 µg/ml effectively neutralizes the therapeutic and surgical/interventional concentrations of heparins from different sources in in-vitro settings. While differences in the anti-Xa and anti-IIa effects between heparins were noted, the anti-coagulant effect of these agents in the aPTT assay were comparable. A similar neutralization profile was observed in the ACT assay by both agents. The pharmacologic implication of these observations requires further investigations.
Acknowledgments
The authors are thankful to the staff of the Hemostasis Research Laboratory for their expert assistance in completing this study. We are thankful to the Cardiovascular Institute for partial support of these studies. A special thanks to Ms. Erin Healy-Erickson for assisting in the preparation of this manuscript.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Fakiha Siddiqui https://orcid.org/0000-0002-2219-7049
Debra Hoppensteadt https://orcid.org/0000-0001-9342-4213
Eduardo Ramacciotti https://orcid.org/0000-0002-5735-1333
Jawed Fareed https://orcid.org/0000-0003-3465-2499
References
- 1.Oduah EI, Linhardt RJ, Sharfstein ST. Heparin: Past, present, and future. Pharmaceuticals (Basel). 2016;9(3):38. doi: 10.3390/ph9030038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danek BA, Kearney KE, Chung CJet al. et al. The contemporary role of protamine in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2023;102(1):111-120. doi: 10.1002/ccd.30679 [DOI] [PubMed] [Google Scholar]
- 3.Carr JA, Silverman N. The heparin-protamine interaction. A review. J Cardiovasc Surg (Torino). 1999;40(4):659-666. [PubMed] [Google Scholar]
- 4.Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446-451. doi: 10.1038/nm.3102 [DOI] [PubMed] [Google Scholar]
- 5.Pharmaceuticals. P. U.S. FDA approves Portola Pharmaceuticals Andexxa®, the first and only antidote for the reversal of factor Xa inhibitors. In; 2018.
- 6.Heo YA. Andexanet alfa: First global approval. Drugs. 2018;78(10):1049-1055. doi: 10.1007/s40265-018-0940-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui F, Tafur A, Bontekoe Eet al. Assay-based differentiation in the neutralization profile of unfractionated heparin, enoxaparin, and fondaparinux by Andexanet Alfa. Clin Appl Thromb Hemost. 2020;26:1076029619895120. doi: 10.1177/1076029619895120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson G, Jeske W, Iqbal Oet al. Potency adjusted blended heparin of bovine, ovine, and porcine heparin exhibit comparable biologic effects to referenced single-sourced porcine heparin. Clin Appl Thromb Hemost. 2023;29:10760296231163251. doi: 10.1177/10760296231163251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh CH, Fredenburgh JC, Weitz JI. The real decoy: An antidote for factor Xa-directed anticoagulants. Circ Res. 2013;113(8):954-957. doi: 10.1161/circresaha.113.302297 [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Lin J, Curnutte JT, Conley PB. Reversal of heparin-induced anticoagulation by Andexanet Alfa, a universal antidote for factor Xa inhibitors. Blood. 2015;126(23):2329-2329. doi: 10.1182/blood.V126.23.2329.232926359437 [DOI] [Google Scholar]
- 11.Kalathottukaren MT, Creagh AL, Abbina Set al. Comparison of reversal activity and mechanism of action of UHRA, andexanet, and PER977 on heparin and oral FXa inhibitors. Blood Adv. 2018;2(16):2104-2114. doi: 10.1182/bloodadvances.2016003616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly SJ, Milling TJ, Eikelboom JW, et al. Andexanet Alfa for acute Major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-1141. doi: 10.1056/NEJMoa1607887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of Andexanet Alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. doi: 10.1056/NEJMoa1814051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu G, Lin J, Bui K, Curnutte JT, Conley PB. Andexanet versus prothrombin complex concentrates: Differences in reversal of factor Xa inhibitors in in vitro thrombin generation. Res Pract Thromb Haemost. 2020;4(8):1282-1294. doi: 10.1002/rth2.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis J, Iqbal O, Jeske W, Hoppensteadt D, Siddiqui F, Fareed J. Differential neutralization of unfractionated heparin and enoxaparin by Andexanet Alfa. Clin Appl Thromb Hemost. 2022;28:10760296221099934. doi: 10.1177/10760296221099934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui F, Tafur A, Ramacciotti LSet al. Reversal of factor Xa inhibitors by Andexanet Alfa may increase thrombogenesis compared to pretreatment values. Clin Appl Thromb Hemost. 2019;25:1076029619863493. doi: 10.1177/1076029619863493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui F, Tafur A, Bontekoe Eet al. Assay-Based differentiation in the neutralization profile of unfractionated heparin, enoxaparin, and fondaparinux by Andexanet Alfa. Clin Appl Thromb Hemost. 2020;26:1076029619895120. doi: 10.1177/1076029619895120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: An overview of current developments. Thromb Haemost. 2015;113(5):931-942. doi: 10.1160/th14-11-0982 [DOI] [PubMed] [Google Scholar]
- 19.Levy JH, Ghadimi K, Kizhakkedathu JN, Iba T. What's fishy about protamine? Clinical use, adverse reactions, and potential alternatives. J Thromb Haemost. 2023;21(7):1714-1723. doi: 10.1016/j.jtha.2023.04.005 [DOI] [PubMed] [Google Scholar]
- 20.Stone ME, Vespe MW. Heparin rebound: An in-depth review. J Cardiothorac Vasc Anesth. 2023;37(4):601-612. doi: 10.1053/j.jvca.2022.12.019 [DOI] [PubMed] [Google Scholar]
- 21.Nybo M, Madsen JS. Serious anaphylactic reactions due to protamine sulfate: A systematic literature review. Basic Clin Pharmacol Toxicol. 2008;103(2):192-196. doi: 10.1111/j.1742-7843.2008.00274.x [DOI] [PubMed] [Google Scholar]
- 22.Letter. F. http://editor.fresenius-kabi.us/PIUs/Protamine_Sulfate_Supply_Update_04_16_2021.pdf .