Abstract
Background:
Thoracic aortic aneurysms (TAA) are a progressive disease characterized by inflammation, smooth muscle cell activation and matrix degradation. We hypothesized that mesenchymal stem cells (MSCs) can immunomodulate vascular inflammation and remodeling via altered microRNA (miRNAs) expression profile to attenuate TAA formation.
Materials and methods:
C57BL/6 mice underwent topical elastase application to form descending TAAs. Mice were also treated with MSCs on days 1 and 5 and aortas were analyzed on day 14 for aortic diameter. Cytokine array was performed in aortic tissue and total RNA was tagged and hybridized for miRNAs microarray analysis. Immunohistochemistry was performed for elastin degradation and leukocyte infiltration.
Results:
Treatment with MSCs significantly attenuated aortic diameter and TAA formation compared to untreated mice. MSC administration also attenuated T-cell, neutrophil and macrophage infiltration and prevented elastic degradation to mitigate vascular remodeling. MSC treatment also attenuated aortic inflammation by decreasing proinflammatory cytokines (CXCL13, IL-27, CXCL12 and RANTES) and upregulating anti-inflammatory interleukin-10 expression in aortic tissue of elastase-treated mice. TAA formation demonstrated activation of specific miRNAs that are associated with aortic inflammation and vascular remodeling. Our results also demonstrated that MSCs modulate a different set of miRNAs that are associated with decrease leukocyte infiltration and vascular inflammation to attenuate the aortic diameter and TAA formation.
Conclusions:
These results indicate that MSCs immunomodulate specific miRNAs that are associated with modulating hallmarks of aortic inflammation and vascular remodeling of aortic aneurysms. Targeted therapies designed using MSCs and miRNAs have the potential to regulate the growth and development of TAAs.
Keywords: Mesenchymal stem cells, Extracellular matrix, Thoracic aortic aneurysm, Vascular smooth muscle cell, Micro ribonucleic acids
Introduction
Aortic aneurysms are the 17th leading cause of death for those over age 65, with rupture resulting in over an 80% mortality rate.1,2 Aneurysms are normally classified according to their anatomical location, that is, abdominal aortic aneurysms (AAA) and thoracic aortic aneurysms (TAA). Recently, aneurysms have been suggested to have different pathologies based on their anatomical location despite demonstrating similar physical characteristics. These pathology remain largely unknown, but could be linked to the embryological origin of the endothelial and vascular smooth muscle cells (SMCs) that compose them. Although the incidence of descending TAA is difficult to estimate due to its asymptomatic nature, recent data estimates a prevalence of 16.3 per 100,000 cases in 2002 compared to 10.7 in 1987, results likely due to increased use of high resolution imaging to diagnose and monitor the disease.3,4 In current clinical practice, there remains no proven medical treatment therapy to inhibit progression or prevent TAA rupture.
Mesenchymal stem cells (MSCs) have been implicated in the treatment of numerous cardiovascular diseases, including AAA formation.6,7 Our group has seen that MSCs and their derivatives, microvesicles, were both able to attenuate AAA formation by targeting inflammatory pathways.7 With their ability to regulate multiple inflammatory pathways, MSC administration may represent a possible strategy for TAA inhibition as well. We have previously shown that MSCs modulate aortic inflammation by decreasing the expression of pro-inflammatory cytokine that is, IL-17, IL-1β, TNF-α and HMGB1.6–8 In previous studies of myocardial infarction, atheroloscerosis,9–11 and other diseases,12–14 MSCs have been associated with activation of the anti-inflammatory cytokine interleukin-10 (IL-10). Recent studies indicate that the crosstalk between immune cells with MSCs can occur via microRNAs (miRNAs) to modulate communication with tissue-injured cells.15–17 MSCs modulate miRNAs to alter pathophysiological function and cytokine secretion, which opens a new dimension on the fate and behavior of MSCs and on their potential application in regenerative medicine.
Recent studies have demonstrated that MSC-derived extracellular vesicles (EVs) can be used as effective platforms to enrich specific miRNAs that can repress translation of specific mRNAs, and determine the fate of tissue inflammation.18,19 MSCs and MSC-derived EVs have been shown to harbor a variety of mRNAs and miRNAs. MSCs can preferentially secrete miRNA in the precursor instead of the mature form and these pre-miRNAs can be enriched in EVs, which are readily up taken by inflammatory immune cells, suggesting a potential mechanism in regulation of tissue inflammation. Therefore, MSCs can potentially exert miRNA-mediated biological effects by directly affecting other cells or through secretion of pre-miRNA in EVs.
There is a growing recognition of the role of miRNAs in the pathophysiology of vascular diseases, including atherosclerosis and aneurysm formation.20,21 miRNAs are short, noncoding, single stranded RNA segments that play a critical role in gene expression by binding to the untranslated region of messenger RNA (mRNA). By binding to the mRNA, miRNAs can post-transcriptionally control mRNA function and promote mRNA degradation before translation. The role of miRNAs in vascular disease formation has focused predominantly on atherosclerosis and AAA formation with few studies investigating the role of miRNAs in TAA formation.20–24 It is hypothesized that administration of MSCs inhibits TAA formation through its broad anti-inflammatory effects.
Methods
Elastase TAA model
This experiment was approved by the University of Virginia Institutional Animal Care and Use Committee (protocol #3634). The topical elastase TAA model has been previously described in detail.5 Briefly, 8–12 wk old male C57Bl/6 wild type (WT) mice (Jackson Laboratory, Bar Harbor, ME) mice were induced with isoflurane, intubated, and maintained under general anesthesia. They underwent left thoracotomy exposure of the descending thoracic aorta. The pleura was dissected off the aorta and a purified porcine elastase (Sigma Aldrich, St. Louis, MO) soaked sponge was placed on the exposed aorta for 4 min. The sponge was removed and the thoracic cavity was rinsed with 0.5 mL sterile saline. The lung was then re-expanded and the chest was closed in three layers. After a 2 wk interval mice were re-anesthetized, intubated and the aorta was exposed. After careful dissection, the aorta was measured by photomicrometry at the maximal aneurysmal level and a distal control segment that was not exposed to elastase. Maximal aortic dilation (%) was calculated as [maximal aortic diameter – internal control diameter] ÷ internal control diameter * 100%. The internal control was a small segment of normal distal thoracic aorta. Aortic tissue was harvested on day 14 for further analyses.
Stem cell treatment
A sham group underwent thoracotomy with utilization of a saline soaked sponge. The elastase group underwent TAA surgery as described above and vehicle tail vein injections with 0.1 mL phosphate buffered saline on postoperative days 1 and 5. Finally, the MSC group underwent TAA surgery and tail vein injection of 1 × 106 first-passage MSCs suspended in 0.1 mL phosphate buffered saline on postoperative days 1 and 5. The process for MSC collection has been previously described.7 Briefly, human umbilical cord-derived MSCs were isolated from Wharton’s jelly. Umbilical cord derived MSCs were differentiated with StemPro differentiation kits for chondrogenesis, adipogenesis and osteogenesis following the protocols included with the kits (Life Technologies, Grand Island, NY). Further characterization of MSCs done by flow cytometry confirms a pattern consistent with MSC population showing an expression of CD90, CD73, CD105 and CD44. MSCs lacked expression of CD45, CD34, CD11b, CD19, and HLA-DR.
Cytokine quantification
Mouse cytokine array (R&D Systems, Minneapolis, MN) was performed per the manufacturer’s instructions. Murine aortic tissue was analyzed for expression of CXCL13, C5a (Complement component 5a), G-CSF, GM-CSF, CCL1, Eotaxin, CD54, IFN-γ, IL-1α, IL-1β, IL-1rα, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12(p70), IL-13, IL-16, IL-17, IL-23, IL-27, CXCL10, CXCL11, CXCL1, M-CSF, MCP-1, MCP-5, CXCL9, CCL3, CCL4, MIP-2, RANTES, CXCL12, CCL17, TIMP-1, TNF-α, and TREM-1and expressed as pg/mL.
Histology
Murine aortas were harvested at sacrifice for histology analysis after undergoing left ventricular puncture and 4% paraformaldehyde antegrade perfusion at physiologic pressure. Further fixation was achieved by overnight incubation in 4% paraformaldehyde at 4°C followed by paraffin embedding and sectioning at 5 μm. After microwave antigen retrieval, antibodies were bound and detected using VectaStain Elite Kit (Vector Laboratories Inc., Burlingame, California). Antibodies for IHC staining were anti-rat Mac2/LGAL3 for macrophages (Cedarlane Laboratories, Burlington, Canada), anti-mouse anti-Neutrophil (Ly 6B.2) for neutrophils (AbD Serotec, Oxford, United Kingdom), and goat anti-mouse CD3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Visualization color development was completed using diaminobenzidine (Dako Corporation, Carpinteria, California) and images were acquired using AxioCam Software version 4.6 via 40X, objectives and an AxioCam MRc camera (Carl Zeiss Inc., Thornwood, New York). Threshold gated positive signal was detected within the AOI and quantified using Image-Pro Plus version 7.0 (Media Cybernetics Inc., Bethesda, Maryland). Elastin depletion was quantified by counting the number of breaks per vessel and then averaged and graphed. Images were quantified and counted using two independent observers and are graphed as the mean ± standard deviation.
MicroRNA array
Murine aortic tissue was harvested and collected in RNA-later solution on day 14 and total RNA was isolated using the Trizol reagent and following the manufacturer’s protocol. RNA purity analysis was performed in accordance with previous established parameters.25 Total RNA was labeled using FlashTag Biotin HSR RNA Labeling Kit (Affymetrix, CA) and used for Affymetrix GeneChip miRNAs v4.0 microarray hybridization. After hybridization each chip was scanned on an Affymetrix GeneChip Scanner 3000 G7 according to the GeneChip Expression Analysis Technical Manual procedures (Affymetrix, Santa Clara, CA). Raw intensities for every probe were stored in electronic files (.DAT and .CEL formats) by the GeneChip Operating Software (Affymetrix, Santa Clara, CA). The detection of individual intensity values above background for each probe set from the microarray raw data was performed using Wilcoxon Test. Data normalization and expression summaries were obtained using the Robust Multiarray Analysis algorithm. Two-sample t test analysis was used for pairwise comparison analysis in R programming environment. A P value <0.01 was considered significant by controlling a False Discovery Rate below 10% calculated from estimated q-values for each miRNA probe set ID.
Statistical methods
Statistical analysis of aortic diameters was performed using GraphPad Prism 7 software (GraphPad Software, La Jolla, CA). Values are presented as the mean ± standard deviation of the mean (SD). One-way ANOVA after Tukey’s statistical test was used to determine the differences among multiple groups and Mann-Whitney test was used for pair-wise comparisons of groups. A value of P < 0.05 was considered statistically significant.
Results
MSC administration attenuates TAA formation
Using the elastase TAA model (Fig. 1A), aortic diameter was measured in WT mice with or without MSC administration. There was a significant increase in aortic diameter in the elastase-treated WT mice (i.e., TAA formation) on day 14 compared to saline-treated controls (102.2 ± 17.7% versus 9.5 ± 10.6%, respectively) (Fig. 1B). Importantly, TAA formation was significantly attenuated in MSC-treated mice (TAA + MSCs) compared to elastase-treated (TAA) mice (78.5 ± 17.9% versus 102.2% ± 17.7%, respectively).
Fig. 1 –
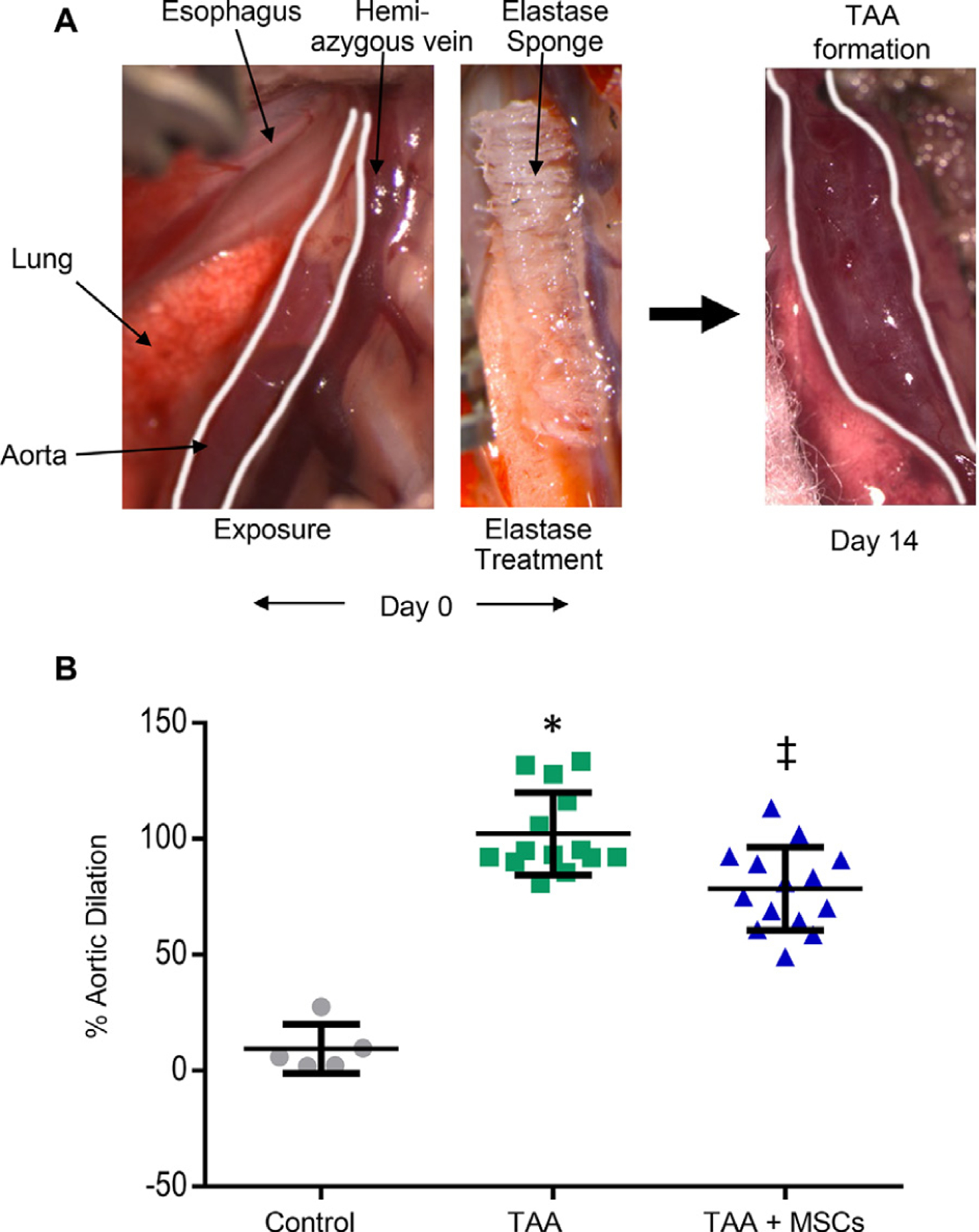
MSC treatment attenuates TAA formation. (A) Representative depiction of the experimental murine TAA model. (B) A significant increase in thoracic aortic diameter was observed in elastase-treated mice compared to sham (saline treatment) surgeries. TAA was significantly attenuated in MSC treated mice compared to elastase controls. n = 5–14 mice/group; (*P < 0.0001; Control versus TAA and ‡P = 0.0006; TAA + MSCs versus TAA). MSC = mesenchymal stem cell; TAA = thoracic aortic aneurysms.
Also, comparative histology and immunohistological analysis of aortic tissue revealed a significant attenuation of inflammatory cell infiltration (CD3 + T cells, macrophages and neutrophils), and decrease in elastic fiber disruption in MSC-treated elastase-treated WT mice compared to elastase-treated mice alone (Fig. 2).
Fig. 2 –
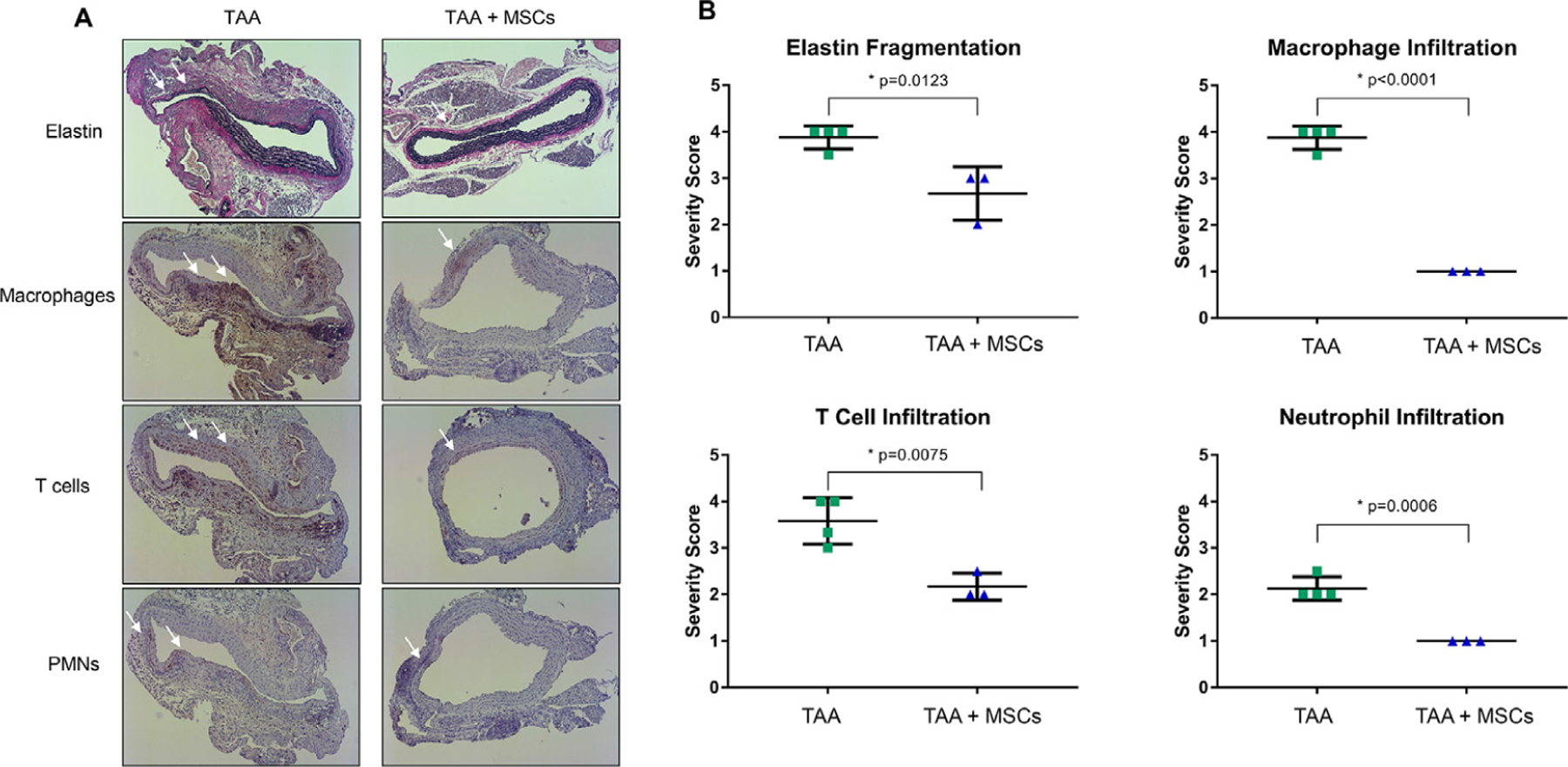
MSC treatment mitigates leukocyte infiltration and elastin degradation. Comparative histology (A) and quantification (B) performed on aortic tissue at day 14 demonstrates decreased elastin fragmentation, as well as mitigation of macrophage, T-cell and neutrophil infiltration after MSC treatment compared to elastase controls. n = 3–4/group; arrows show areas of immunostaining. MSC = mesenchymal stem cell.
MSCs mitigate aortic inflammation during TAA
Cytokine array on aortic tissue demonstrated significant attenuation of pro-inflammatory cytokines (CXCL13, CXCL10, IL-27, CXCL12 and RANTES) and complement component 5a in elastase-treated mice after MSC-administration mice compared to elastase-treated mice alone. A significant increase in anti-inflammatory cytokine (IL-10) was also observed in MSC-treated mice compared to untreated mice with TAAs (Fig. 3). No significant differences were observed for the other analyzed cytokines (described in Methods) between the comparative groups.
Fig. 3 –
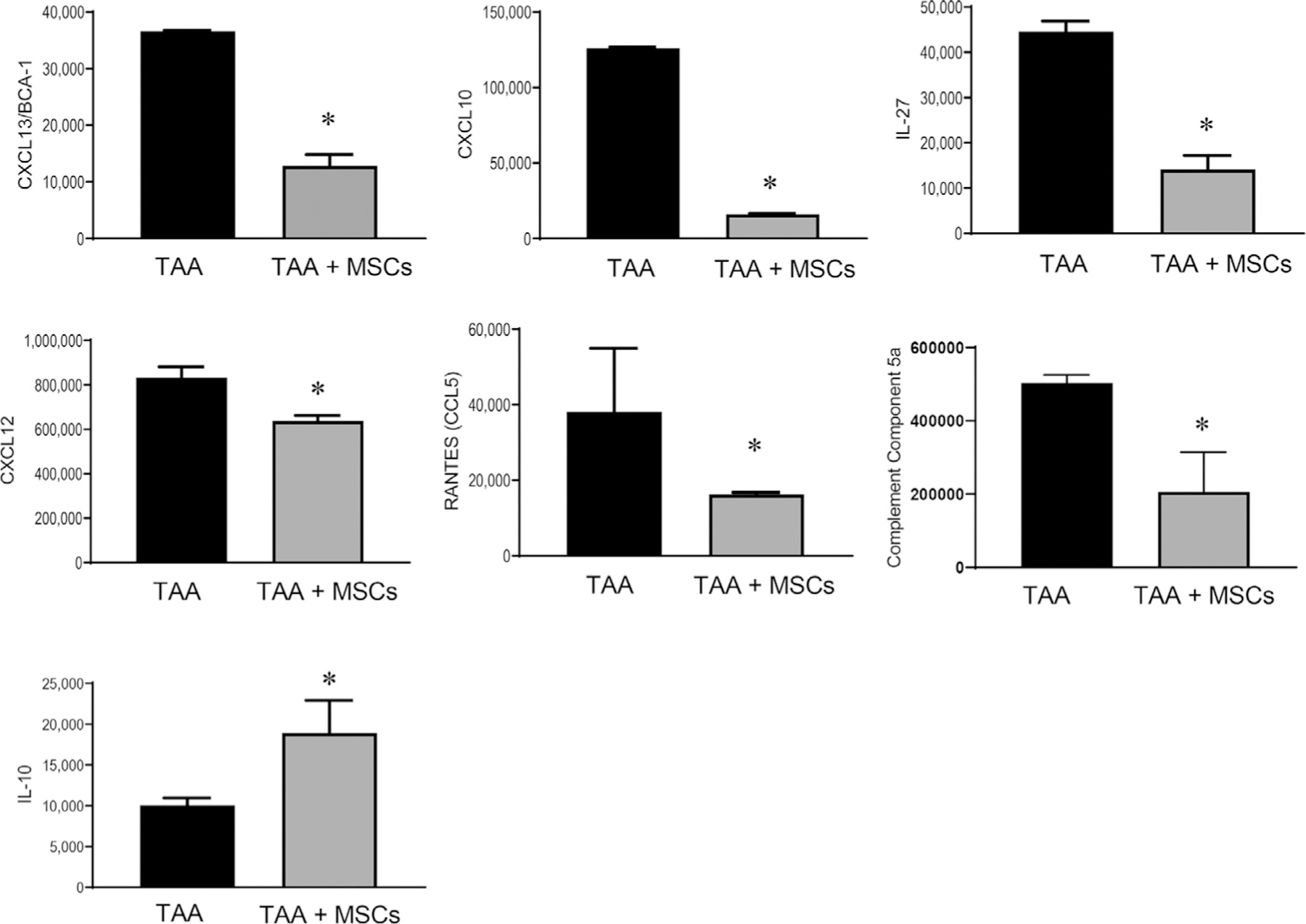
MSC treatment significantly attenuates aortic inflammation during TAA formation. Pro-inflammatory cytokine expression in aortic tissue on day 14 is significantly attenuated by MSC treatment compared to untreated elastase controls (TAA) in WT mice. Simultaneously, the expression of anti-inflammatory cytokine IL-10 was significantly increased in MSC-treated mice compared to control TAAs. *P < 0.05; n = 4/group. IL-10 = interleukin-10; MSC = mesenchymal stem cell; TAA = thoracic aortic aneurysms; WT = wild type.
Differentially expressed microRNAs after TAA formation
Pairwise comparison analyses of elastase-treated mice compared with controls identified significantly differentially expressed miRNA profiles associated with TAA formation on day 14. Intensity value analysis of hybridized microarrays detected 449 miRNA probe sets in the murine aortic tissue. Briefly, 53 miRNA present probe sets demonstrated to be significantly differentially expressed between study groups in accordance with established statistical parameters (Table 1). Of those, 25 miRNAs were found up-regulated and 28 miRNAs downregulated after elastase treatment compared to control (Fig. 4A and C).
Table 1 –
MicroRNA profile change associated with development of TAAs.
| MicroRNA | Elastase (TAA) | Sham | Fold change | P value |
|---|---|---|---|---|
| mmu-miR-466j | 4.27 | 1.37 | 7.5 | 0.000321 |
| mmu-miR-669m-5p | 4.2 | 1.75 | 5.47 | 0.048789 |
| mmu-miR-466m-5p | 4.2 | 1.75 | 5.47 | 0.048789 |
| mmu-miR-7030-5p | 5.78 | 3.37 | 5.3 | 0.002533 |
| mmu-miR-16-1-3p | 5.13 | 2.86 | 4.81 | 0.015729 |
| mmu-miR-466f-5p | 3.77 | 1.83 | 3.83 | 0.013445 |
| mmu-miR-669f-5p | 4.79 | 2.85 | 3.82 | 0.005678 |
| mmu-miR-146a-5p | 10.36 | 8.43 | 3.81 | 0.001597 |
| mmu-miR-297a-5p | 3.56 | 1.95 | 3.04 | 0.014395 |
| mmu-miR-184-3p | 2.61 | 1.06 | 2.92 | 0.034604 |
| mmu-miR-298-5p | 3.82 | 2.27 | 2.91 | 0.026738 |
| mmu-miR-675-3p | 2.36 | 0.95 | 2.67 | 0.000908 |
| mmu-miR-21a-3p | 2.83 | 1.48 | 2.54 | 0.024625 |
| mmu-miR-466c-5p | 2.59 | 1.36 | 2.36 | 0.000456 |
| mmu-miR-7242-5p | 2.55 | 1.32 | 2.34 | 0.006377 |
| mmu-mir-466f-1 | 3.01 | 1.8 | 2.32 | 0.023617 |
| mmu-miR-466i-5p | 3.38 | 2.18 | 2.29 | 0.014127 |
| mmu-miR-3082-5p | 2.66 | 1.51 | 2.22 | 0.000485 |
| mmu-miR-3113-5p | 2.09 | 0.96 | 2.19 | 0.027791 |
| mmu-miR-6366 | 10.69 | 9.59 | 2.15 | 0.047585 |
| mmu-miR-3470b | 5.13 | 4.03 | 2.14 | 0.024054 |
| mmu-miR-296-3p | 4.38 | 3.29 | 2.13 | 0.012443 |
| mmu-miR-211-3p | 8.63 | 7.59 | 2.05 | 0.022572 |
| mmu-miR-431-3p | 2.05 | 1.03 | 2.03 | 0.004913 |
| mmu-mir-7017 | 2.66 | 1.65 | 2.01 | 0.049597 |
| mmu-miR-27b-3p | 10.73 | 11.74 | −2.01 | 0.017931 |
| mmu-miR-135a-1-3p | 5.32 | 6.33 | −2.01 | 0.029722 |
| mmu-miR-29c-5p | 1.39 | 2.43 | −2.06 | 0.025172 |
| mmu-miR-30e-5p | 6.41 | 7.46 | −2.07 | 0.035031 |
| mmu-miR-193b-3p | 9.98 | 11.06 | −2.11 | 0.034595 |
| mmu-miR-30b-5p | 8.97 | 10.07 | −2.15 | 0.037288 |
| mmu-miR-6402 | 1.07 | 2.18 | −2.17 | 0.00896 |
| mmu-miR-29b-2-5p | 3.74 | 4.87 | −2.18 | 0.037587 |
| mmu-miR-28c | 4.39 | 5.57 | −2.28 | 0.009741 |
| mmu-miR-330-5p | 1.06 | 2.27 | −2.32 | 0.009426 |
| mmu-let-7g-5p | 8.67 | 9.89 | −2.33 | 0.047137 |
| mmu-miR-143-5p | 6.22 | 7.46 | −2.36 | 0.035235 |
| mmu-miR-30c-5p | 10.08 | 11.33 | −2.39 | 0.016775 |
| mmu-miR-30c-2-3p | 5.04 | 6.32 | −2.42 | 0.030918 |
| mmu-miR-193b-5p | 4.62 | 5.93 | −2.48 | 0.046162 |
| mmu-miR-224-3p | 2.03 | 3.35 | −2.49 | 0.011135 |
| mmu-miR-140-5p | 8.54 | 10 | −2.75 | 0.00921 |
| mmu-miR-26b-5p | 4.27 | 5.77 | −2.83 | 0.009411 |
| mmu-miR-486-5p | 8.51 | 10.13 | −3.08 | 0.008965 |
| mmu-miR-3107-5p | 8.51 | 10.13 | −3.08 | 0.008965 |
| mmu-miR-224-5p | 1.39 | 3.05 | −3.16 | 0.034512 |
| mmu-miR-187-3p | 6.44 | 8.17 | −3.33 | 0.0044 |
| mmu-miR-486-3p | 3.11 | 4.94 | −3.57 | 0.021334 |
| mmu-miR-451a | 6.09 | 8.43 | −5.06 | 0.04488 |
| mmu-miR-133b-3p | 4.9 | 7.32 | −5.35 | 0.007351 |
| mmu-miR-29c-3p | 4.52 | 6.98 | −5.5 | 0.005186 |
| mmu-miR-24-1-5p | 2.41 | 4.98 | −5.92 | 0.002812 |
| mmu-miR-133a-3p | 5.4 | 8.17 | −6.78 | 0.004342 |
Highlighted miRNAs represent the important miRs that regulate vascular inflammation.
Fig. 4 –
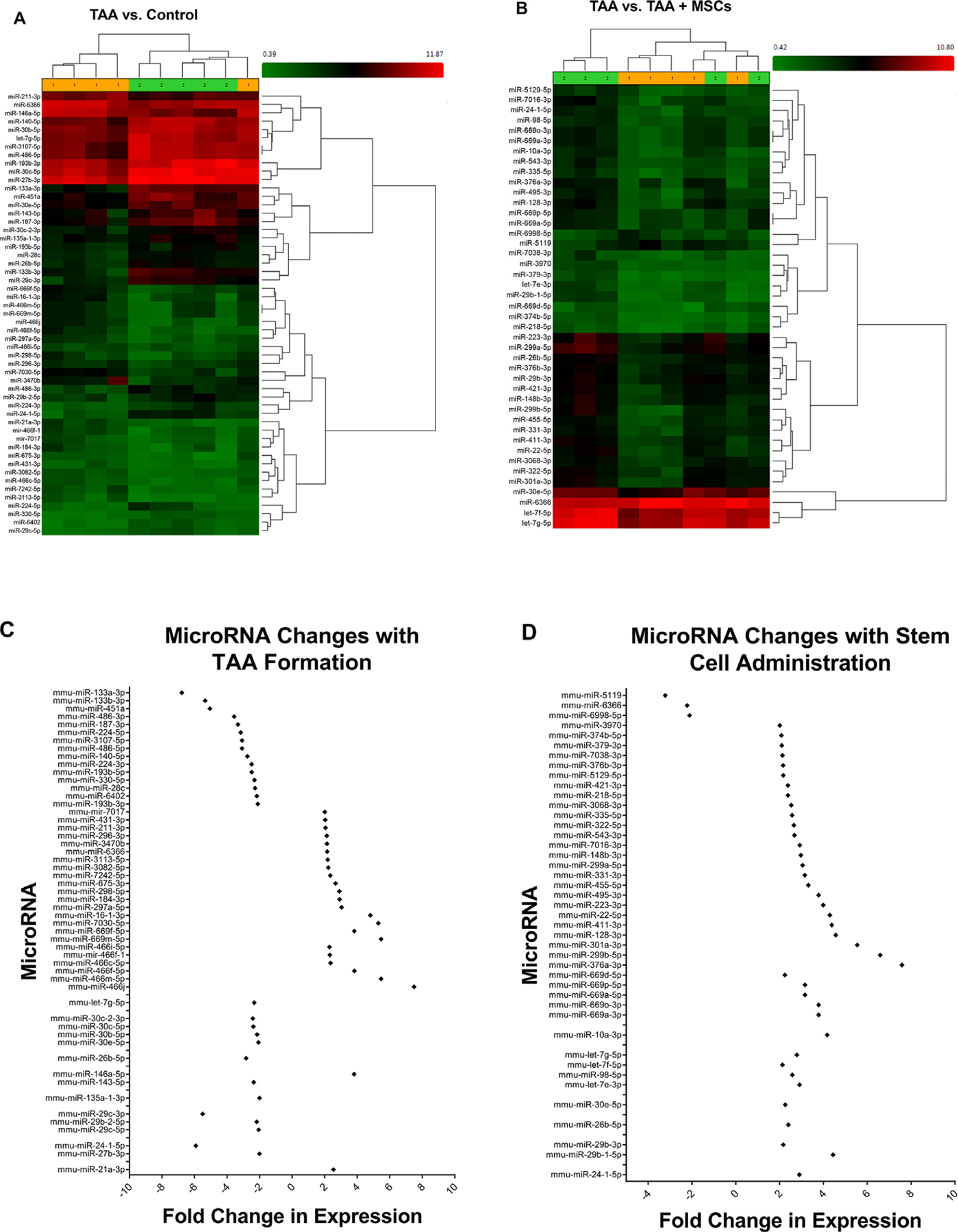
miRNA expression is significantly altered after MSC administration in TAA tissue. (A) Heat map of significant differences in miRNAs expression between murine aortic tissue from controls (saline treated) and elastase treated mice (TAA) demonstrating significantly different miRNAs profiles in the development of TAA. (B) Heat map of significantly altered miRNAs profile of mice aortic tissue between TAA and TAA + MSCs. (C) Differential expression of miRNAs that are significantly altered during TAA formation between elastase-treated mice and saline controls. (D) Differential expression of miRNAs that are significantly altered after MSC administration in elastase-treated mice compared to untreated elastase controls (TAA). n = 5 mice/group. miRNA = microRNAs; MSC = mesenchymal stem cell; TAA = thoracic aortic aneurysms;
Differentially expressed microRNAs after TAA formation and stem cell attenuation
Pairwise analyses of aortic tissue was performed after MSC treatment compared to untreated TAA that identified significantly differentially expressed miRNA profiles. Intensity value analysis of hybridized microarrays detected 449 miRNA probe sets in the murine aortic tissue. Briefly, 43 miRNA present probe sets demonstrated to be significantly differentially expressed between study groups in accordance with established statistical parameters (Table 2). Of those, 40 miRNAs were found up-regulated and 3 miRNAs down-regulated after stem cell treatment compared to elastase-treated controls (Fig. 4B and D). The highest upregulation was observed in miR-376a-3p, which showed an approximately 7.6-fold increase in elastase-treated aortic tissue compared to controls on day 14 (Table 2).
Table 2 –
MicroRNA profile change in TAA tissue after MSC treatment.
| MicroRNA | Elastase + MSC signal (log2) | Elastase signal (log2) | Fold change | P value |
|---|---|---|---|---|
| mmu-miR-376a-3p | 4.76 | 1.84 | 7.58 | 0.044013 |
| mmu-miR-299b-5p | 5.3 | 2.58 | 6.59 | 0.004059 |
| mmu-miR-301a-3p | 5.39 | 2.92 | 5.54 | 0.037528 |
| mmu-miR-128-3p | 4.87 | 2.68 | 4.56 | 0.009085 |
| mmu-miR-29b-1-5p | 3.37 | 1.22 | 4.44 | 0.02805 |
| mmu-miR-411-3p | 5.3 | 3.17 | 4.38 | 0.013038 |
| mmu-miR-22-5p | 5.12 | 3.02 | 4.29 | 0.023967 |
| mmu-miR-10a-3p | 3.8 | 1.74 | 4.17 | 0.037689 |
| mmu-miR-223-3p | 6.25 | 4.25 | 3.99 | 0.0016 |
| mmu-miR-669a-3p | 4.26 | 2.34 | 3.78 | 0.003122 |
| mmu-miR-669o-3p | 4.26 | 2.34 | 3.78 | 0.003122 |
| mmu-miR-495-3p | 4.52 | 2.6 | 3.78 | 0.008263 |
| mmu-miR-455-5p | 4.73 | 3.01 | 3.31 | 0.013662 |
| mmu-miR-669a-5p | 4.39 | 2.73 | 3.16 | 0.002839 |
| mmu-miR-669p-5p | 4.39 | 2.73 | 3.16 | 0.002839 |
| mmu-miR-331-3p | 4.88 | 3.22 | 3.15 | 0.017655 |
| mmu-miR-299a-5p | 6.56 | 4.95 | 3.05 | 0.006338 |
| mmu-miR-148b-3p | 5.3 | 3.73 | 2.97 | 0.028573 |
| mmu-miR-7016-3p | 4.25 | 2.71 | 2.92 | 0.038342 |
| mmu-let-7e-3p | 2.79 | 1.25 | 2.91 | 0.01945 |
| mmu-miR-24-1-5p | 3.95 | 2.41 | 2.9 | 0.044265 |
| mmu-let-7g-5p | 10.15 | 8.67 | 2.79 | 0.006384 |
| mmu-miR-543-3p | 3.63 | 2.21 | 2.68 | 0.013444 |
| mmu-miR-322-5p | 5.42 | 4.01 | 2.65 | 0.03431 |
| mmu-miR-98-5p | 3.9 | 2.53 | 2.58 | 0.010727 |
| mmu-miR-335-5p | 3.29 | 1.93 | 2.57 | 0.012811 |
| mmu-miR-3068-3p | 5.04 | 3.7 | 2.53 | 0.023827 |
| mmu-miR-26b-5p | 5.53 | 4.27 | 2.4 | 0.003901 |
| mmu-miR-218-5p | 2.41 | 1.17 | 2.38 | 0.000283 |
| mmu-miR-421-3p | 5.4 | 4.15 | 2.38 | 0.035859 |
| mmu-miR-30e-5p | 7.58 | 6.41 | 2.26 | 0.046385 |
| mmu-miR-669d-5p | 2.59 | 1.42 | 2.24 | 0.026746 |
| mmu-miR-29b-3p | 5.67 | 4.55 | 2.17 | 0.02868 |
| mmu-miR-5129-5p | 2.98 | 1.86 | 2.17 | 0.024048 |
| mmu-miR-376b-3p | 5.41 | 4.3 | 2.16 | 0.025656 |
| mmu-let-7f-5p | 9.85 | 8.76 | 2.13 | 0.017774 |
| mmu-miR-7038-3p | 2.71 | 1.62 | 2.13 | 0.04768 |
| mmu-miR-379-3p | 2.28 | 1.21 | 2.1 | 0.025117 |
| mmu-miR-374b-5p | 2.18 | 1.12 | 2.08 | 0.000251 |
| mmu-miR-3970 | 2.71 | 1.7 | 2.01 | 0.025751 |
| mmu-miR-6998-5p | 2.25 | 3.33 | −2.11 | 0.04866 |
| mmu-miR-6366 | 9.54 | 10.69 | −2.22 | 0.036116 |
| mmu-miR-5119 | 2.84 | 4.52 | −3.22 | 0.043782 |
Highlighted miRNAs represent the important miRs known to be associated with inflammation during aortic aneurysm formation.
Discussion
This study demonstrates that administration of MSCs attenuates descending TAA formation in a topical elastase murine model. The present results demonstrate that MSCs have the ability to immunomodulate aortic inflammation and mitigate vascular remodeling observed during TAA formation. Additionally, this study establishes that changes in miRNA expression are seen with formation of descending TAA in a topical elastase murine model. The miRNA profile of descending TAA formation is only partially similar to AAA formation and includes significantly increased miR-21, as well as decreased miR-27, −135, −143, and −29 expressions in the thoracic aortic tissue. In addition, MSC administration attenuated TAA formation via molecular signaling pathways that were distinct as compared to mechanistic pathways involved in pathogenesis of TAA formation, as seen by a miRNA profile with minimal overlap to TAA formation and with upregulation of miR-24, −10 and −29 pathways (Fig. 5).
Fig. 5 –
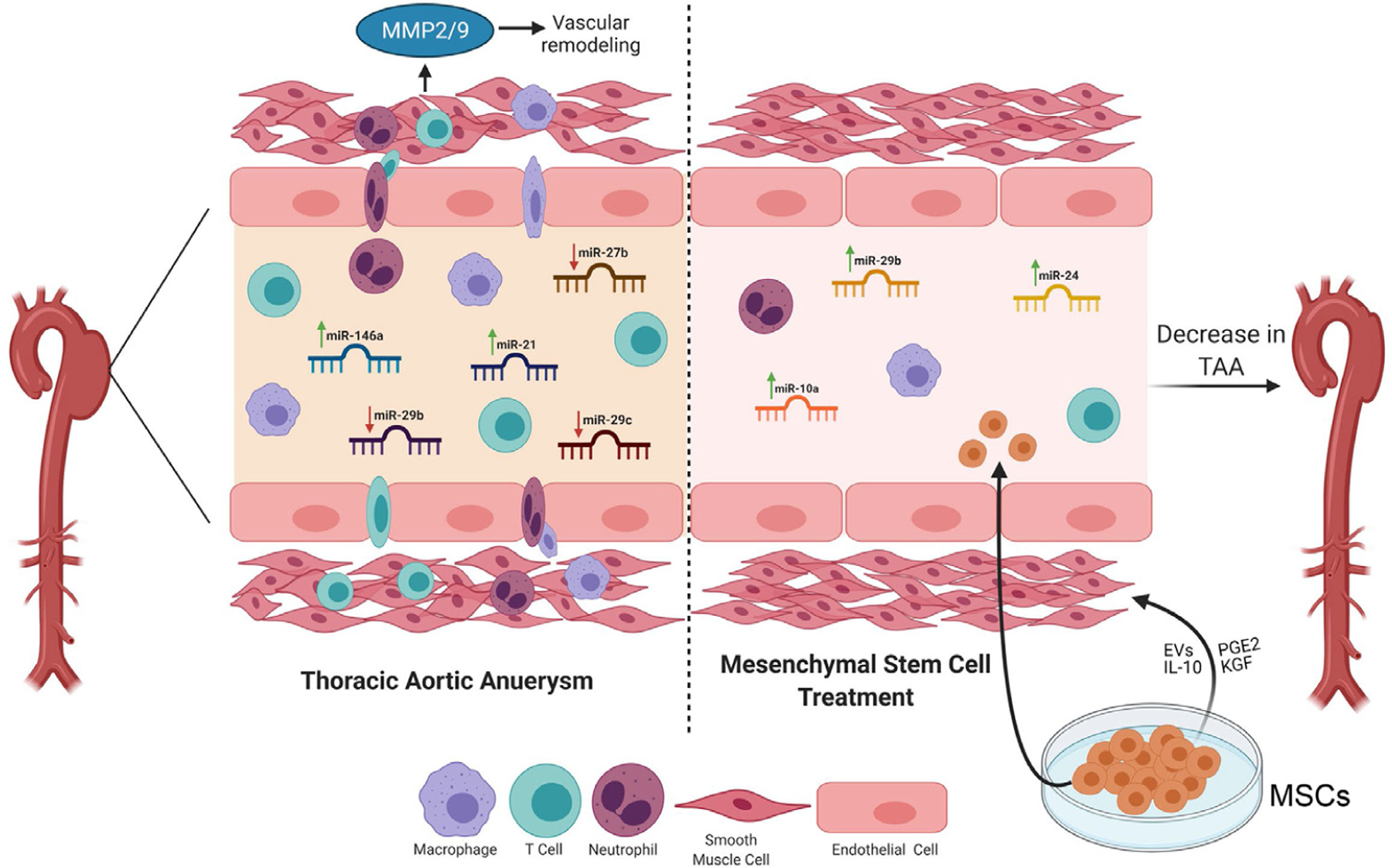
Schematic description of MSC-mediated attenuation of TAA. Upregulation of specific miRNAs that is, miR-146a and miR-21, as well as downregulation of miR-29b, miR-29c and miR-27b, is associated with leukocyte infiltration, aortic inflammation and loss of smooth muscle cell activation leading to vascular remodeling and TAA formation. Treatment with MSCs can lead to upregulation of miR-24, miR-29b and miR-10a that mitigates endothelial permeability and leukocyte transmigration, thereby preventing elastin degradation and preserving smooth muscle cell integrity. MSCs can exert anti-inflammatory effects via direct cell-cell interactions and/or via paracrine secretions to immunomodulate aortic inflammation and vascular remodeling and prevent TAA formation. EVs = extracellular vesicles; MSC = mesenchymal stem cell; PGE2 = prostaglandin E2; KGF = keratinocyte growth factor; MMP = matrix metalloproteinases.
The interplay between miRNAs and their targets is complex, with both single miRNAs influence multiple targets, and multiple miRNAs being involved in the regulation of pathophysiologic processes. In this case, the regulation of TAA formation in a murine model appears to be regulation by a multitude of miRNA families. For example, clusters of miRNA are associated with regulation of SMCs via different downstream and upstream pathways of TGF-ß and thus miRNAs may help clarify the sometimes contradictory roles TGF-ß has in aortic aneurysm formation. The identification of multiple miRNA clusters that regulate the vascular endothelium suggests that the media layer may not be the only aortic segment responsible for aneurysm formation. The modulation of specific miRNAs by MSCs in the context of aortic inflammation and vascular remodeling represents a novel mechanistic signaling aspect in TAAs.
One of the most commonly associated miRNAs with cardiovascular disease is miR-21. This key modulator of proliferation and apoptosis of vascular SMCs is upregulated during formation of ascending thoracic and abdominal aortic aneurysm.26,27 Similarly, TAA formation was also associated with increased miR-21a-3p expression. Previous studies have shown that both human and murine AAA demonstrate downregulation of the miR-23b/24/27b cluster.28 These miRNA regulate regulates SMC proliferation and migration via chitinase 3-like 1 and NF-κB. Interestingly, TAA formation was also associated with downregulation of miR-27b-3p and miR-24-1-5p while MSC treatment led to miR-24-1-5p upregulation. Moreover, MSC treatment resulted in upregulation of miR-24 which was associated with mitigation of aortic inflammation, vascular remodeling as well as a decrease in aortic diameter formation. In contrast to miR-24, miR-29 downregulation has been shown to reduce AAA in a murine model and is associated with pro-fibrotic changes mediated via TGF-β.29 In contrast, TAA formation was associated with downregulation of miR-29c-5p, −29b-2-5p and −29c-3p expressions, which was reversed by treatment with MSCs that resulted in upregulation of miR-29b-3p and miR-29b-1-5p.
We have previously demonstrated that KLF4 plays a critical role in abdominal aortic aneurysm formation with regulation of SMC phenotypic switching.30 It is likely that MSC administration may lead to the reversal of SMC phenotypic switching via regulation of KLF4. Aneurysm formation was associated with decreased expression of miR-135a-1-3p, and is associated with TGF-ß signaling with overlap of miR-21 and −145. TGF-ß is known to downregulate KLF4 by activating miR-135a-5p, potentially suggesting the miR-135 family overall has a role in KLF4 regulation.31 The role for TGF-β in aneurysm development largely originated from research into hereditary causes of TAA formation.32 The role and downstream mechanisms of TGF-β in nonsyndromic TAA cases is less well understood, but there is a clear separation of canonical (SMAD based) and noncanonical (ERK/JNK) pathways. Canonical SMAD based signaling appears protective with the inverse true of noncanonical signaling.33 The impact of TGF-β appears to depend on the cell type, concentration, and overall context of signaling. Further analysis of the impact of miRNAs on TGF-β may help clarify some of these discrepancies.
Additionally associated with KLF4 is miR-143/145, where again via TGF-ß these miRNAs downregulate KLF4. TAA aneurysm formation was associated with decreased expression of miR-143-5p, thus enabling upregulation of KLF4 and VSMC phenotypic switching.34 Finally, increased KLF4 decreases miR-146a, which promotes VSMC proliferation.35 This relationship of KLF4 regulating miR-146a insinuates that the decreased levels of miR-146a-5p seen with TAA formation indicate elevated levels of KLF4. Finally, we observe a significant change in miR-26a expression, which is associated with the TGF-ß signaling pathway and VSMC proliferation and inhibition of apoptosis.36 Again utilizing the DIANA mirPath software miR-26b can be expected to have similar targets and associated effects as miR-26a. Reinforcing the role of the KLF4, SMAD and TGF-ß pathways, there was downregulation of miR-26b-5p with aneurysm formation, which was reversed by stem cell administration resulting in increased expression compared to controls.
The miR-30-5p family (miR-30a/b/c/d/e) has been investigated for a number of cardiovascular conditions including left ventricular hypertrophy and myocyte apoptosis, sickle cell anemia, and angiogenesis. Recently KLF2 and sheer stress were found to upregulate expression of the miR-30-5p family.37 This miRNA family have anti-inflammatory properties via reduction of inflammatory cell adhesion molecules. In addition, KLF2 appears to play a critical role in AAA formation.38 The miRNA profile in aneurysmal mice was consistent with the miR-30-5p family having regulatory interactions with KLF2 and aneurysm formation. Specifically, aneurysm formation was associated with decreased expression of miR-30b/c/e-5p while stem cell administration correlated with increased levels of miR-30e-5p. Finally, KLF2 downregulates inflammatory cytokines including IL-1ß, which is known to be a potent inhibitor of AAA formation and was reduced with stem cell administration, which supports the role of KLF2 and miR-30-5p in TAA formation.5,39
The let-7 family has also been linked to TGF-ß by Fibroblast Growth Factor in the regulation of vascular endothelium. An additional role for let-7 may relate to VSMC autophagy and apoptosis in the setting of oxidized LDL.40 This may extend to regulation of VSMCs during TAA formation, as let-7g-5p was downregulated with development of TAA. However, the role of let-7 may relate more to immunomodulation and regulation of MSCs.41 With the administration of MSCs, let-7e-3p, −7g-5p, let-7f-5p and miR-98-5p were all significantly upregulated. With the predominance of let-7 family changes occurring with the administration of MSCs, further research is required to differentiate its vascular and stem cell regulatory control mechanisms in TAA.
MSC treatment was also associated with upregulation of miR-10a in the attenuation of TAA. The role of miR-10a in vascular regulation was identified because of its downregulation in athero-susceptible regions of the swine aorta as previously described.42 The regulatory roles of miR-10a include Homeobox A1 transcription, and recently IκB/NF-κB–mediated inflammation via phosphorylation of IκBα. This role of endothelial phenotypic regulation by MSCs deserves further investigation as a potential target for TAA mitigation with potential roles for miR-10a and let-7. Finally, there were a few miRNA clusters identified that have not been evaluated in the setting of aortic aneurysms. This includes miR-466 and 669 families that had the highest levels of increased expression with TAA development. Additionally, a large cluster of miR-669 were upregulated with MSC administration. These, among the many other miRNAs without known potential links to vascular regulation represent a field both ripe for research and in need of further clarification.
One of the limitations of this study is that the long term effects of MSCs may not be completely recapitulated in this murine model, and remain to be determined in a chronic aneurysm and rupture model. In the context of aortic aneurysms, intravenous administration of MSCs have been shown to localize in the aortic media/adventitia up to 4 wk after treatment.43 However, significant concerns still remain pertinent to requirement of multiple doses for a chronic inflammatory condition, possibility of low targeting ability to the site of aortic remodeling and trapping of MSCs in other tissues. The safety and efficacy of multiple doses of MSCs or MSC-derived EVs remains to be determined in a chronic TAA model which is likely to decipher a clinically translational strategy for a relevant therapeutic potential.
In summary, our study demonstrates the mediatory role of specific miRNAs in regulating signaling pathways related to inflammation and vascular remodeling. Furthermore, the multifaceted impact of MSCs on miRNA expression helps to explain how they attenuate TAA formation by affecting specific miRNAs related to vascular inflammation. These results further our understanding of the distinct mechanisms involved in the formation of TAA, and delineates the protective role of MSCs in aortic aneurysm formation thereby offering a potential therapeutic approach. Future studies are required to harness the protective influence of miRNA antagonists against pro-inflammatory pathways, in conjunction with MSC-derived EVs, to further accentuate the immunomodulatory capacities of MSCs in protection against aortic aneurysms.
Supplementary Material
Acknowledgment
This work was supported by AHA Scientist Development Grant 14SDG18730000 (M.S.), NIH R01 HL126668 (G.A.), RO1 HL081629 (G.R.U.) grants. The schematic figure was created using biorender.com.
Footnotes
Disclosure
Dr Ailawadi discloses unrelated personal fees from Edwards Lifesciences, Medtronic, Abbott and Atricure. All other authors have nothing to disclose with regard to commercial support.
Supplementary Materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jss.2021.06.057.
REFERENCES
- 1.WISQARS leading causes of death reports, national and regional, 1999 – 2014: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Davis FM, Rateri DL, Daugherty A. Mechanisms of aortic aneurysm formation: translating preclinical studies into clinical therapies. Heart. 2014;100:1498–1505. [DOI] [PubMed] [Google Scholar]
- 3.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. [DOI] [PubMed] [Google Scholar]
- 4.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55:841–857. [DOI] [PubMed] [Google Scholar]
- 5.Johnston WF, Salmon M, Pope NH, et al. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130:S51–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma AK, Lu G, Jester A, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126:S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma AK, Salmon MD, Lu G, et al. Mesenchymal stem cells attenuate NADPH oxidase-dependent high mobility group box 1 production and inhibit abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2016;36:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JP, Salmon M, Pope NH, et al. Attenuation of aortic aneurysms with stem cells from different genders. J Surg Res. 2015;199:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchfield JS, Iwasaki M, Koyanagi M, et al. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. 2008;103:203–211. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Guo X, Chen SY. Function and therapeutic potential of mesenchymal stem cells in atherosclerosis. Front Cardiovasc Med. 2017;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du YY, Zhou SH, Zhou T, et al. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy. 2008;10:469–478. [DOI] [PubMed] [Google Scholar]
- 12.Choi JJ, Yoo SA, Park SJ, et al. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima M, Nito C, Sowa K, et al. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol Ther Methods Clin Dev. 2017;6:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Ren H, Yuan X, Ma H, Shi X, Ding Y. Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res. 2018;48:E194–E202. [DOI] [PubMed] [Google Scholar]
- 15.Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. [DOI] [PubMed] [Google Scholar]
- 17.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinosa M, Lu G, Su G, et al. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. 2018. fj201701138RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asgarpour K, Shojaei Z, Amiri F, et al. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maegdefessel L, Dalman RL, Tsao PS. Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu Rev Med. 2014;65:49–62. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg MW, Moore KJ. MicroRNA regulation of atherosclerosis. Circ Res. 2016;118:703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busch A, Busch M, Scholz CJ, et al. Aneurysm miRNA signature differs, depending on disease localization and morphology. Int J Mol Sci. 2016;17(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milewicz DM. MicroRNAs, fibrotic remodeling, and aortic aneurysms. J Clin Invest. 2012;122:490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluijter JP, Pasterkamp G. MicroRNAs: the swing voters in vascular disease waiting for a program. Circ Res. 2017;120:5–7. [DOI] [PubMed] [Google Scholar]
- 25.Mas VR, Maluf DG, Archer KJ, et al. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med. 2009;15:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maegdefessel L, Azuma J, Toh R, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012;4:122ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanzler I, Rolle K, El-Sayed Ahmad A, et al. MiRNA modulation in ascending aortic aneurysms. Thorac cardiovasc Surg. 2013;61:OP189. [Google Scholar]
- 28.Maegdefessel L, Spin JM, Raaz U, et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maegdefessel L, Azuma J, Toh R, et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmon M, Johnston WF, Woo A, et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013;128:S163–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao S, Tian C, Ding Y, et al. Down-regulation of Kruppel-like factor-4 by microRNA-135a-5p promotes proliferation and metastasis in hepatocellular carcinoma by transforming growth factor-beta1. Oncotarget. 2016;7:42566–42578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillis E, Van Laer L, Loeys BL. Genetics of thoracic aortic aneurysm: at the crossroad of transforming growth factor-beta signaling and vascular smooth muscle cell contractility. Circ Res. 2013;113:327–340. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Krishna S, Walker PJ, Norman P, Golledge J. Transforming growth factor-beta and abdominal aortic aneurysms. Cardiovasc Pathol. 2013;22:126–132. [DOI] [PubMed] [Google Scholar]
- 34.Davis-Dusenbery BN, Chan MC, Reno KE, et al. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun SG, Zheng B, Han M, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leeper NJ, Raiesdana A, Kojima Y, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demolli S, Doebele C, Doddaballapur A, et al. MicroRNA-30 mediates anti-inflammatory effects of shear stress and KLF2 via repression of angiopoietin 2. J Mol Cell Cardiol. 2015;88:111–119. [DOI] [PubMed] [Google Scholar]
- 38.Salmon M, Spinosa M, Zehner ZE, Upchurch GR, Ailawadi G. Klf4, Klf2, and Zfp148 activate autophagy-related genes in smooth muscle cells during aortic aneurysm formation. Physiol Rep. 2019;7:e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das H, Kumar A, Lin Z, et al. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding Z, Wang X, Schnackenberg L, et al. Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7 g. Int J Cardiol. 2013;168:1378–1385. [DOI] [PubMed] [Google Scholar]
- 41.Gaeta X, Le L, Lin Y, Xie Y, Lowry WE. Defining transcriptional regulatory mechanisms for primary let-7 miRNAs. PLoS One. 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu XM, Yamawaki-Ogata A, Oshima H, Ueda Y, Usui A, Narita Y. Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J Transl Med. 2013;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


