Abstract
Bioengineered tissues have become increasingly more sophisticated owing to recent advancements in the fields of biomaterials, microfabrication, microfluidics, genetic engineering, and stem cell and developmental biology. In the coming years, the ability to engineer artificial constructs that accurately mimic the compositional, architectural, and functional properties of human tissues, will profoundly impact the therapeutic and diagnostic aspects of the healthcare industry. In this regard, bioengineered cardiac tissues are of particular importance due to the extremely limited ability of the myocardium to self-regenerate, as well as the remarkably high mortality associated with cardiovascular diseases worldwide. As novel microphysiological systems make the transition from bench to bedside, their implementation in high throughput drug screening, personalized diagnostics, disease modeling, and targeted therapy validation will bring forth a paradigm shift in the clinical management of cardiovascular diseases. Here, we will review the current state of the art in experimental in vitro platforms for next generation diagnostics and therapy validation. We will describe recent advancements in the development of smart biomaterials, biofabrication techniques, and stem cell engineering, aimed at recapitulating cardiovascular function at the tissue- and organ levels. In addition, integrative and multidisciplinary approaches to engineer biomimetic cardiovascular constructs with unprecedented human and clinical relevance will be discussed. We will comment on the implementation of these platforms in high throughput drug screening, in vitro disease modeling and therapy validation. Lastly, future perspectives will be provided on how these biomimetic platforms will aid in the transition towards patient centered diagnostics, and the development of personalized targeted therapeutics.
1. Introduction
Cardiovascular diseases (CVDs) constitute the number one cause of mortality worldwide, accounting for approximately 31% of all reported deaths in 2015 [1]. In the US alone, annual direct medical costs associated with CVDs are estimated to rise to more than USD $818 billion by 2030, while costs associated with loss of productivity could exceed USD $275 billion [2]. Despite significant scientific and technological advancements in the engineering of biomedical devices and surgical solutions for the treatment of CVDs, the development of new therapeutic strategies has been hindered by the lack of appropriate models for high-throughput drug screening and treatment validation. Although preclinical drug screening is almost universally carried out in vivo using murine models, cardiovascular pathophysiology and drug response in humans are often radically different [3]. This in turn has led to relatively high failure rates during clinical trials, raising the overall cost of drug development and increasing the risk of unforeseen side effects. For instance, a number of Food and Drug Administration (FDA) approved drugs for the treatment of CVDs, and even for other pathologies such as diabetes and cancer, have been found to exert cardiotoxic effects and have thus been withdrawn from the market [4,5]. In addition, the high costs and ethical concerns associated with the use of live animals have brought forth the need to develop more accurate in vitro models, which could better recapitulate cardiovascular pathophysiology and pharmacological responses in humans.
Conventional in vitro studies are traditionally carried out using relatively cheap and simple two-dimensional (2D) cultures, based on primary cells isolated from different animal species. More recently, cells derived from cardiac progenitor cells (CPCs) [6], human embryonic stem cells (ESCs) [7,8], and human induced pluripotent stem cells (iPSCs) [9,10] have been used as pharmacological models for the evaluation of various cardioactive drugs. Furthermore, recent advancements in genome-editing technologies have allowed the engineering of pathological cell types for in vitro disease modeling [11]. The remarkable ability to generate patient- and disease-specific cardiovascular phenotypes holds great potential for the development of high-throughput drug screening platforms and personalized medicine [12,13]. However, 2D models fail to reproduce critical phenotypic characteristics of cardiovascular tissues such as their structural and compositional features, as well as relevant cell–cell and cell–extracellular matrix (ECM) interactions [14]. More recently, three-dimensional (3D) approaches such as cardiosphere-derived cells and cell sheets have been used to better mimic the spatial arrangements in which cells grow in the native tissues [15,16]. These 3D systems allow cells to actively interact with each other and the ECM in relevant spatial arrangements, which provides biochemical and biophysical stimuli that heavily influence cell fate. However, despite their enhanced physiological relevance, conventional 3D cultures still lack many of the architectural [17], mechanical [18] and electroconductive [19] features of cardiovascular tissues [20]. This in turn greatly limits their potential to evaluate the safety and efficacy of new drug candidates, since they are not representative of the elaborate crosstalk between different cell types, the ECM, and the cardiovascular microenvironments.
In recent years, remarkable advancements in biomaterials science and the development of powerful biofabrication techniques, have enabled the engineering of artificial human tissues that possess an unprecedented level of physiological relevance [21–23]. Tissue engineering (TE) has enabled the development of custom biomimetic tissues, which have served as models for a plethora of applications in pharmaceutics [24–26], regenerative medicine [27,28], diagnostics [29], as well as fundamental research to elucidate the mechanisms that underlie disease onset and progression [4,30,31]. Moreover, the combination of TE with microfabrication techniques and microfluidics have enabled the development of microphysiological systems, also termed organs-on-chips, which can serve as systemic models of human physiology and disease [32,33]. Organs-on-chips platforms offer the unique ability to host bioengineered 3D constructs in a controlled microenvironment, while allowing the delivery of mechanical and electrophysiological stimuli [34]. This is particularly important, since cardiovascular tissues are comprised of a multitude of cell types (e.g., cardiomyocytes (CMs), cardiac fibroblasts, endothelial cells, Purkinje cells, etc.) arranged in an anisotropic and hierarchical organization [32]. Furthermore, the intrinsic control system that regulates the contractile function of the myocardium can respond to both external and systemic cues, while its complex architecture is supported by a 3D framework of various ECM proteins [35]. Therefore, the remarkable versatility of microphysiological systems is optimal for the engineering of in vitro models that mimic the complex structure, composition, heterocellularity, dynamism, and highly regulated function of cardiovascular tissues (Fig. 1).
Fig. 1. Bioengineered in vitro models of cardiovascular function and disease.
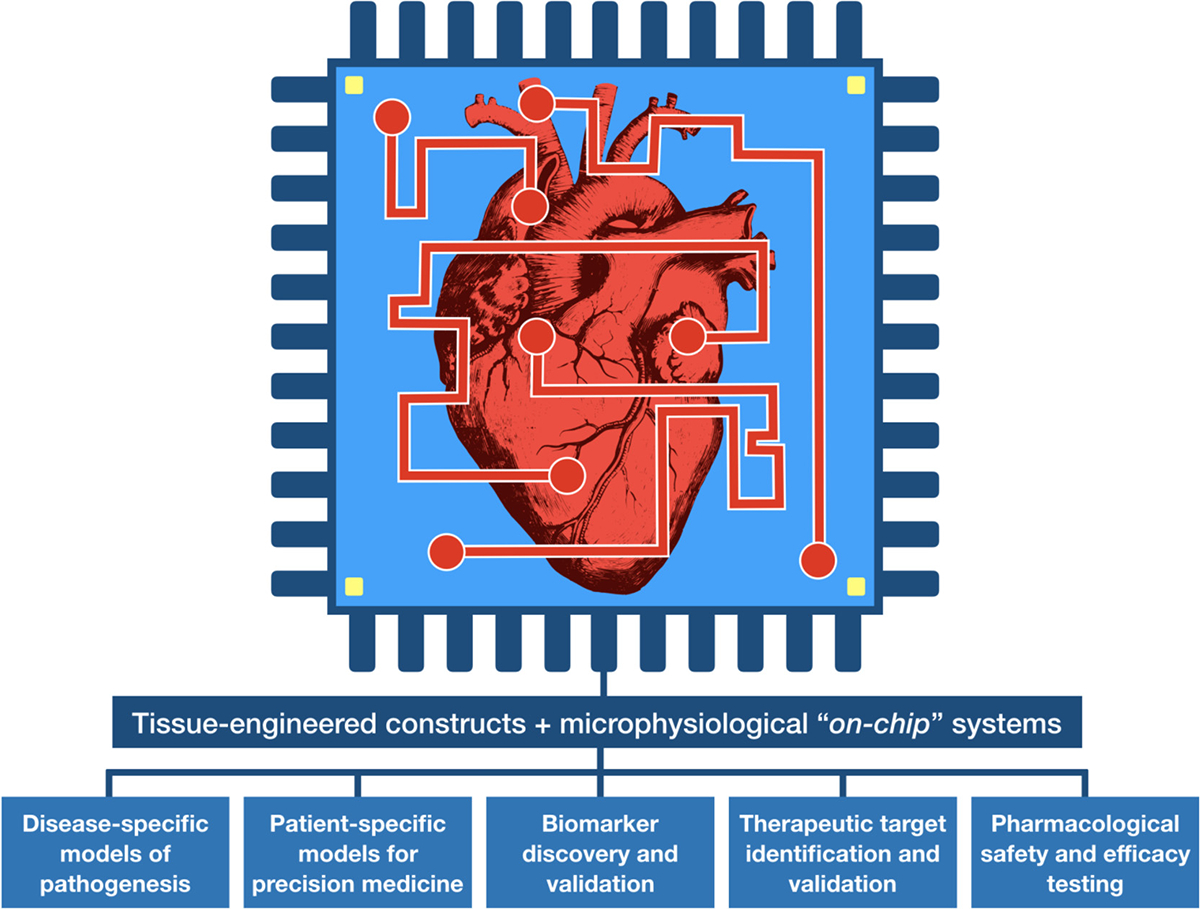
Tissue engineered constructs offer several advantages over conventional 2D cultures such as enhanced physiological relevance, establishment of cell-cell and cell-ECM interactions, delivery of physicochemical cues, biomimetic microarchitecture, and 3D gradients of stiffness, nutrients, biochemical factors, oxygen, etc. In the context of cardiovascular studies, the incorporation of bioengineered tissues into microfluidic-based platforms allows the recapitulation and precise control over critical physiological parameters such as flow, and electrical and mechanical stimuli. Furthermore, the generation of disease- and even patient-specific phenotypes through stem cell technology has enabled the development of highly representative models of cardiovascular diseases. These sophisticated in vitro platforms have emerged as powerful tools for several diagnostic (biomarker discovery and validation), and therapeutic (target identification and validation) applications, as well as high-throughput drug safety and efficacy testing.
The sustained and rapid progress in the fields of TE and microphysiological systems has led to the development of artificial tissues that can be used as platforms to assess drug efficacy and safety before pre-clinical assessment. Moreover, the classic paradigm of engineering replacement tissues that could be used as grafts for therapeutic applications is rapidly evolving towards the development of sophisticated biomimetic systems for in vitro high-throughput screening. Here, we will review recent advancements in the development of bioengineered in vitro models of cardiovascular tissues (i.e., blood vessels, cardiac valves, and myocardial tissue), with an emphasis on high-throughput drug screening and therapeutic validation. We will start with an overview of the different types of smart and multifunctional biomaterials that have been developed to support the growth and function of cardiovascular phenotypes in vitro. We will then discuss modern biofabrication methods that have enabled the manufacture of artificial cardiovascular tissue constructs with biomimetic microarchitectures and enhanced functionality. We will also review state of the art organ-on-a-chip models of cardiovascular function, which have emerged as testing platforms for drug and device testing, disease modeling, and precision medicine. Lastly, we will provide an outlook and future perspectives, and comment on the most significant technological and scientific challenges that remain.
2. Biomimetic design of biomaterials for cardiovascular TE
Although some strategies for scaffold-free fabrication of cardiovascular tissues have been reported [36–39], the development of bioengineered cardiovascular constructs often requires biomaterials with defined physicochemical properties. This is mainly because the tissues that comprise the cardiovascular system possess several unique and diverse properties that determine their functionality (Fig. 2a). For instance, the myocardium exhibits an anisotropic contractile structure, where cells are aligned to provide increased mechanical support and propagate electrical conductivity along the axis, parallel to the muscle fibers (Fig. 2b) [40,41]. On the other hand, adult healthy blood vessels are multi-layered structures of varying thickness and mechanical properties, which are comprised of elastic muscle fibers in characteristic arrangements that withstand physiologic burst pressures (Fig. 2c) [42]. Lastly, cardiac valves possess distinct structural and functional features, owing to stratified ECM layers that confer distinct properties to the leaflets and supporting structures that undergo constant shear stress (Fig. 2d) [42]. Therefore, biomaterials used for cardiovascular TE should provide a biocompatible 3D framework with electrical, mechanical, compositional, biological, and microarchitectural features that mimic the complexity of the native tissues.
Fig. 2. Biomimetic design criteria for the development of scaffolds for cardiovascular tissue engineering.
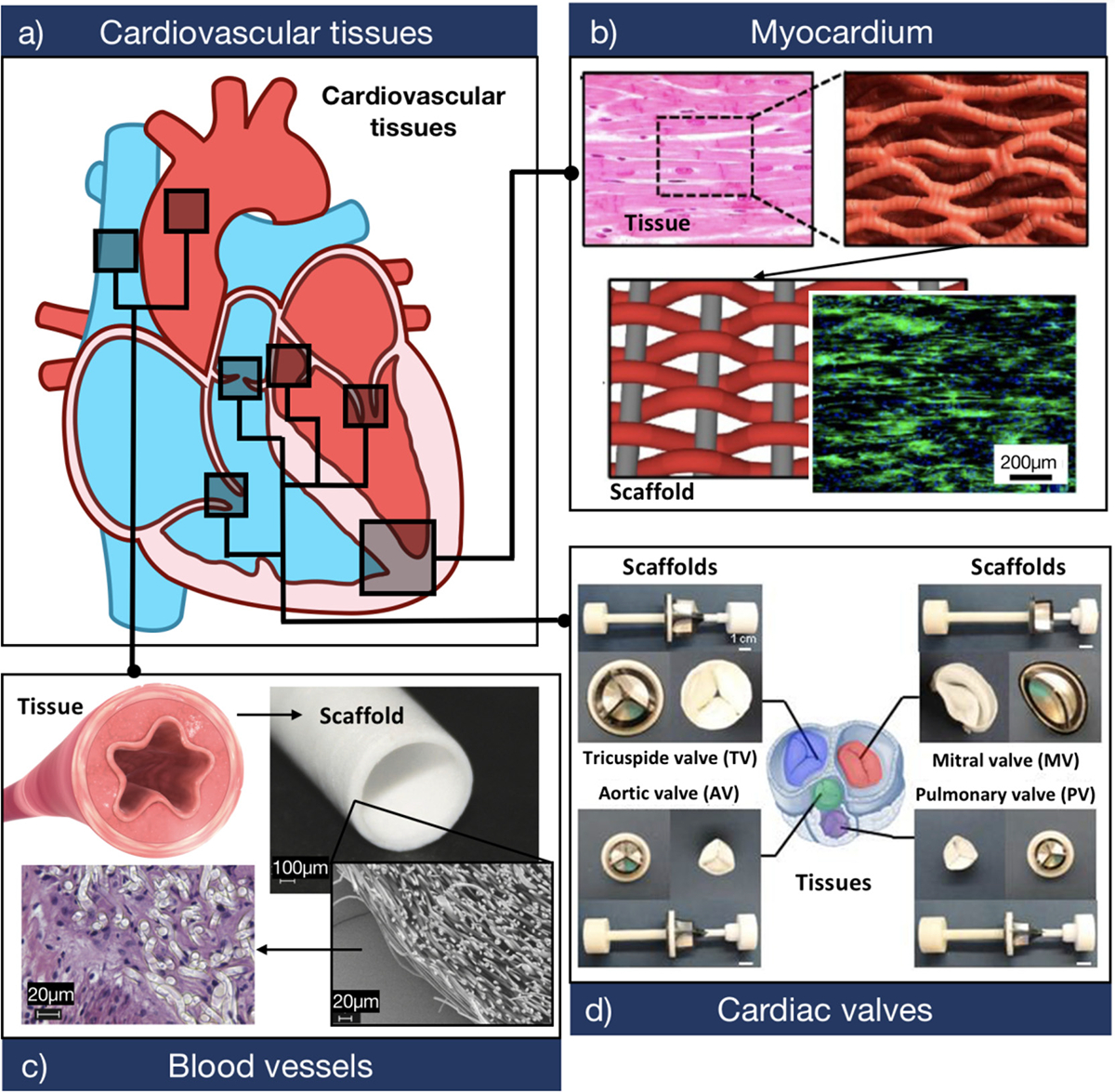
a) Schematic of a sagittal plane of the human heart showing the three main tissues that comprise the cardiovascular system. b) Biomaterials used to develop myocardial tissues should provide a highly biocompatible and electroconductive matrix that supports CM function. The panel shows a representative F-actin (green)/DAPI (blue) fluorescent image of CMs growing on a biologically inspired interwoven scaffold. The scaffold was comprised of aligned conductive nanofiber yarns, synthesized from PCL, silk fibroin, and CNTs (Adapted from Ref. [85]). c) Biomaterials used to develop TEBVs should provide adequate burst pressure and mechanical compliance, while also allowing the attachment and proliferation of endothelial cells. The panel shows the histological assessment of endothelial cells growing on a tubular scaffold. The scaffold was comprised of electrospun poly (L-lactide) microfibers (Adapted from Ref. [86]). d) Biomaterials used to develop TEHVs should be able to withstand high trans-valvular pressures while also maintaining low flexural stiffness. The panel shows four types of cardiac valve scaffolds synthesized through double component deposition (DCD) of poly (ester urethane) urea (PEUU) microfibers (Adapted from Ref. [87]).
Many research strategies have focused on the development of 3D hydrogel scaffolds for cardiovascular TE using a variety of natural and/or synthetic polymers. In contrast to naturally-derived polymers, synthetic biomaterials often allow greater control over physical properties such as mechanical stiffness, degradation rate, and porosity [43,44]. More recently, smart synthetic biomaterials with enhanced functionality such as self-assembly, self-healing, thermo-responsiveness, and pH-responsiveness have also been developed for TE applications [45–48]. Some examples of synthetic biomaterials that have been extensively used for the engineering of cardiovascular constructs include poly (ethylene glycol) (PEG) [49], poly (ε-caprolactone) (PCL) [50], poly (l-lactic acid) (PLA) [51], poly (glycolic acid) (PGA) [52], and biodegradable polyurethanes (PUs) [53]. On the other hand, collagen, gelatin, fibrin, and alginate represent some of the most widely used naturally-derived biomaterials for cardiovascular TE [54–57]. In general, although natural polymers are known to possess high bioactivity and biocompatibility, they often lack mechanical stiffness and require the addition of other components for enhanced structural stability. In addition, one of the key advantages of natural polymers over synthetic materials is the presence of intrinsic peptide sequences, which can promote cell adhesion and proliferation in vitro [58]. However, although synthetic and even some naturally-derived polymers do not exhibit intrinsic cell supporting capabilities, they can be readily modified through the incorporation of bioactive peptides such as RGD, CAG, REDV, and YIGSR [59,60]. The different types of naturally-derived and synthetic-based biomaterials used in the development of tissue engineered myocardium [61–63], cardiac valves [64–67], and blood vessels [68–72] have been extensively reviewed in the literature and will not be discussed here.
Despite the wide variety of biomaterials currently reported in the literature, there is still a need for more sophisticated alternatives that can enhance the physiological relevance of bioengineered constructs. Thus, the development of new biomaterials for cardiovascular TE should incorporate biomimetic design criteria, based on the specific requirements of the different types of tissues that comprise the cardiovascular system (Fig. 2). For instance, biomaterials used to develop bioengineered myocardial constructs should provide a substrate that promotes the electromechanical coupling of CMs. This is mainly because the contractile function of the heart is powered by the synergistic motion of CMs, which in turn is triggered by electrical signals that are propagated across the myocardium [73]. In addition, previous studies have shown that electrical stimuli can trigger different cellular responses such as increased cardiomyogenesis of embryonic stem cells and maintenance of the CM phenotype in vitro [74]. Despite the remarkable biocompatibility and versatility of hydrogel-based scaffolds, the insulating nature of polymeric hydrogels limits their application for the propagation of electroactive phenotypes such as CMs. To address this limitation, previous groups have explored the incorporation of conductive nanomaterials such as gold, graphene oxide (GO), and reduced graphene oxide (rGO)nanoparticles, carbon nanotubes (CNTs), and conductive polymers such as polyaniline (PANi), and polypyrrole (PPy) into polymer-based hydrogels (Fig. 2b) [75–80]. These electroconductive hydrogels (ECHs) have been shown to promote cell-cell interactions, and the synchronous contraction of CMs in vitro [81,82]. However, they are often hindered by several limitations, such as cytotoxic responses triggered by the addition of conductive components to the polymeric network [83]. To address this, our group recently reported a novel method for the development of highly biocompatible ECHs based on the incorporation of a choline-based bio-ionic liquid (Bio-IL) into different polymers such as gelatin methacryloyl (GelMA) and poly (ethylene glycol) diacrylate (PEGDA) [84]. This approach offered several technical advantages over conventional approaches, such as high electrical conductivity and biocompatibility, as well as tunable mechanical and microstructural properties. Similar to this approach, future strategies should also be aimed toward the development of highly biocompatible biomaterials that can support the phenotype and electromechanical function of CMs in vitro. This in turn will lead to bioengineered myocardial tissues with synchronous and strong contractility that can not only be used as functional grafts for therapy, but also as models for in vitro research.
Another type of cardiovascular tissue with high clinical significance is blood vessels. Native vessels are comprised of multiple layers of smooth muscle cells that provide biomechanical support to withstand high cyclic pressures. In addition, the ECM of these tissues is rich in elastin and collagen fibers, which are arranged circularly around the vessel. Due to these characteristic properties, native blood vessels are highly elastic tissues with the ability to extend and retract against the pressures of blood flow, while also maintaining mechanical integrity. Therefore, the design of tissue engineered blood vessels (TEBVs) should incorporate biomaterials that provide adequate burst pressure, fatigueresistance, and mechanical compliance [88–91]. In addition, these biomaterials should also be able to support endothelial cell attachment and proliferation for the proper colonization of the scaffold (Fig. 2c). Several groups have reported different functionalization strategies via the use of coatings and chemical and protein modifications to promote the endothelialization of the scaffold [59,71,72,92]. For instance, different molecules such as fucoidan, heparin, chondroitin sulfate, hyaluronic acid (HA), as well as antioxidant compounds and ECM proteins including fibronectin, laminin, and collagen have been used to improve cell-material interactions to promote endothelialization [93]. These studies have demonstrated that surface modification often leads to increased endothelial cell attachment and proliferation, as well as overexpression of endothelial markers such as smooth muscle myosin heavy chain (SM-MHC) [94]. Therefore, future strategies should aim towards the development of highly biocompatible elastomeric materials that promote endothelial cell infiltration and ECM deposition, while also minimizing negative responses at the blood–material interface.
Heart valves are structures that mechanically control the unidirectional blood flow in the heart through the opening and closing of its leaflets. Engineering heart valve constructs remains highly challenging due to the complex geometry of their native structures, their remarkable ability to withstand high trans-valvular pressures, and their low flexural stiffness [95]. In addition, valve leaflets possess an intricate ECM that is comprised of three distinct layers comprised mainly of collagen fibers, proteoglycans, and elastin [95,96]. Biomaterials used to develop tissue engineered heart valves (TEHVs) should exhibit a fine balance of stiffness and elasticity to mimic the mechanical property of the native tissues [97]. Conventional approaches for the fabrication of TEHVs are based on the use of decellularized allografts from donor heart valves, or xenografts from animal-derived small intestine submucosa [98]. More recently, synthetic biodegradable elastomers have emerged as attractive alternatives to allografts due to their high mechanical compliance, as well as their controllable chemical structure and degradability (Fig. 2d) [67]. Furthermore, current strategies for the development of TEHVs have shifted from the use of inert substrates towards bioactive materials that can instruct cell behavior in vitro and modulate tissue integration in vivo [66]. However, due to their complex biomechanical properties, the accurate recapitulation of the biological features of cardiac valves with a single biomaterial remains highly challenging. Because of this, composite scaffolds based on cell supportive materials reinforced with biocompatible elastomers, and incorporated with bioactive proteins/peptides/polysaccharides could be more suitable for the development of TEHVs [67].
Despite all the significant advancements described above, the use of biomaterials alone is not enough to develop biomimetic constructs that can mimic the complex microarchitecture and functionality of the native tissues. As a result, a variety of biofabrication techniques have been developed, which allow the accurate reproduction of native physiological structures at the micro- and nano-scale.
3. Biofabrication of physiologically relevant cardiovascular constructs
Biofabrication refers to the combination of cells, biomaterials, and bioactive factors with advanced fabrication techniques to generate functional tissue constructs, with a level of complexity exceeding simple 2D or 3D cultures. Current fabrication techniques enable precise control over the spatial arrangement of cells and/or materials to accurately mimic the microstructural architecture and diverse composition of native tissues. Artificial tissues constructed from currently available biomaterials and cell types can be used for a myriad of applications, including platforms to study healthy and diseased tissue microenvironments [99,100], regenerative scaffolds [101], and drug screening [102]. In the context of cardiovascular tissues, biofabrication can be used to control the alignment of CMs to recapitulate cardiac anisotropy, and to introduce vascularization, a feature that is essential to cell survival in large cell-laden constructs. In this section, we will focus on advanced biofabrication techniques to manufacture constructs with a degree of complexity that specifically resembles native cardiovascular tissues.
3.1. Fiber-based methods
Fiber fabrication techniques, such as microfluidic spinning and electrospinning, have the potential to generate fibrous structures with highly tunable properties, including fiber size, mechanical stiffness and elasticity, topography, porosity, and composition [103–116]. Microfluidic spinning is the process of flowing a liquid precursor or prepolymer solution within a microchannel, followed by rapid polymerization or crosslinking to yield a continuous solid fiber. Polymerization can occur within the channel, using co-axial flow systems, or at the exit of the channel, as is accomplished in wet-spinning. Examples of materials used for microfluidic spinning of fibers for TE include poly (lactic-co-glycolic) acid (PLGA) [105,107], alginate [106,108,114–116], gelatin/GelMA [109,114], PEGDA [117], collagen [115,116], and fibrin [115]. The main benefit of microfluidic fiber spinning is that it allows the direct encapsulation of cells within the fibrous structures (Fig. 3a) [118]. In addition, multiple cell types and/or materials can be apposed together in a single or adjacent fiber to fabricate tissue-like structures with diverse cellular, chemical, and mechanical composition [110,114,119]. In the context of cardiac TE, fibrous structures can be generated to mimic cardiomuscular fibers or vasculature structures found in the native heart. For example, core-shell fibers laden with endothelial cells were fabricated using a specialized dual co-axial microfluidic chip [115]. This dual co-axial chip was fabricated by inserting a series of round pulled glass capillaries into rectangular glass tubes, which were housed in plastic chambers connected to the sheath inlets. A mixture of cells and ECM proteins was flowed through the core capillaries, and subsequently contained as a solution of sodium alginate in the shell was crosslinked with aqueous calcium chloride [115]. Using this method, encapsulation of human umbilical vein endothelial cells (HUVECs) in a type-I collagen hydrogel fiber led to the formation of a monolayer along the inner diameter of the fiber (Fig. 3b). After cell maturation, the calcium-alginate shell could be selectively removed with alginate lyase. This type of structure could ultimately lead to the formation of a mature lumen. In the same study, primary rat CMs were encapsulated in a type-I collagen/fibrin hybrid hydrogel fiber. By day 3 of culture, spontaneous contraction of the entire fiber at a frequency of 0.5–1 Hz was observed [115]. Despite the diversity in material and cellular composition, achievable with microfluidic fiber fabrication technology, the extruded fibers are discrete and lack secondary structure. Therefore, in many cases post-fabrication assembly of fibers, such as weaving [119,120], braiding or crocheting [119], or knitting [121] is required to generate a structure that mimics that of native tissues. In addition, cellular activity and function, such as CM contraction, is highly dependent on matrix stiffness [122]. Thus, post-processing of softer, compliant materials might be impractical for constructs that lack structural integrity.
Fig. 3. Biofabrication strategies to develop physiologically relevant cardiovascular tissue constructs.

a) Microfluidic spinning enables direct encapsulation of cells in a hydrogel fiber. Various types of tissues can be matured in the fibrous constructs (reproduced from Ref. [118]). b) Mature blood vessel lumens can be generated by encapsulation of HUVECs in a type-I collagen fiber (reproduced from Ref. [115]). c) Electrospinning onto a cylindrical rotating mandrel can be used to generate highly aligned fibers. d) ECM-based scaffolds can be formed by first growing fibroblasts on the mats followed by decellularization, which leaves behind a highly proteinaceous matrix of fibronectin and collagen. e) This coating method leads to cellular alignment and maintenance of the cardiac phenotype (reproduced from Ref. [123]). f) Patterning methods, such as micromolding, can be used to align cardiac cells and measure their contractile activity in vitro. g) Combined with microfabrication of electrical units, this platform can measure changes in cardiac contractility in response to drugs (adapted from Ref. [124]). h) A similar method can be used to form high aspect ratio PDMS pillars. i) These pillars direct organization of cardiac cells around the PDMS pillars to generate aligned cardiac constructs (adapted from Ref. [125]). j) Bioprinting techniques can be used to generate endothelialized tissue constructs that mimic the highly vascularized myocardium. k) Perfusion of cell culture media in a custom-made bioreactor maintains cell viability and activity for long periods of time. l) When drugs are perfused through the bioreactor, the tissue responds in a dose-dependent manner (reproduced from Ref. [99]). m) The use of support baths for bioprinting provides opportunities to print complex hydrogel structures. n) An embryonic chick heart was printed with this method, using an alginate hydrogel (reproduced from Ref. [126]).
Electrospinning is another technique that is used to rapidly fabricate mats of fine fibers, usually on micro- or nano-meter scales’. This approach involves the application of a high voltage to a liquid solution of a polymer, causing the formation of a Taylor cone and rapid spinning of fine fibers. Layers of the fibers pack together during the spinning process to form a thin, highly porous mat. A variety of polymers are compatible with this technique, but general requirements for the fabrication are a suitable viscosity of the liquid polymer solution and rapid evaporation of the solvent during spinning [127]. Some examples of polymers that are commonly used in electrospinning for TE applications include PCL [113,128–130], gelatin/GelMA [112,131], PLA [104], poly (glycerol sebacate) PGS [129], and PLGA [132]. However, it is difficult to encapsulate cells into scaffolds using this technology, and thus it is generally used for 2D cell studies, in which cells are seeded directly on top of the fibrous mats. Highly aligned fibers can be spun using a dynamic electrospinning apparatus, in which the fibers are spun onto a rotating mandrel. The alignment is controlled by increasing the rotational velocity of the mandrel [111,133]. This technique was utilized by Suhaeri et al. for the design of a platform for cardiomyoblast differentiation and CM maturation [123]. Their approach consisted of electrospinning highly aligned poly (l-lactide-co-caprolactone) (PLCL) fibers (Fig. 3c) and seeding the fibrous mats with fibroblasts. Decellularization of the mat left behind fibroblast-derived ECM, providing a naturally-derived proteinaceous matrix containing fibronectin and collagen type I (Fig. 3d). This ECM-containing matrix supported differentiation of H9c2 cardiomyoblast cells, as well as phenotype preservation of neonatal rat CMs more effectively than fibronectin-coated fibers (Fig. 3e). Despite the ease of fabrication and the variety of materials compatible with electrospinning, the harsh conditions of the process and the nanoscale dimensions of the fibers make it difficult to encapsulate cells in the fibers for cardiovascular TE applications. An excellent review outlining the benefits and pitfalls of microfluidic spinning and electrospinning technologies for TE applications is available [127].
3.2. Micropatterning
The native myocardium is hierarchically organized into bundles of highly vascularized and aligned cardiac muscle fibers, providing the tissue with directionally dependent properties that dictate its performance [134]. In order to appropriately recapitulate the anisotropic nature of the tissue in vitro, it is often necessary to present topographical cues that will ultimately mediate complex cellular organization [135]. Common methods to generate these cues include photomasking [125,136–139], microcontact printing [140–142], and micromolding [141,143,144]. These strategies are generally used to promote alignment of cells, seeded within or on the surface of the structures, which enhances the expression of key cardiac markers and improves the bulk contractile force. For example, in a recent study, grooves were generated on the surface of alginate/fibronectin hydrogels using a micromolding technique [141]. CMs seeded on these structures exhibited significantly higher alignment of sarcomeric alpha-actinin, smooth muscle cells, and filamentous actin. In addition, the muscular thin films (MTFs) that were formed on the hydrogel surface demonstrated high contractile stresses when stimulated under an electric field. Lind et al. used a similar method to align human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) on a micropatterned polydimethylsiloxane (PDMS) sheet, but also added layers beneath the patterned substrate capable of recording electrical activity of the iPSC-CMs contracting and relaxing in real time (Fig. 3f) [124]. The extended utility of this device for high-throughput drug screening was demonstrated by collecting readings from aligned iPSC-CMs in the presence of various cardiac drugs. The introduction of different drugs to the MTFs elicited a similar response in beating properties and contractile stress for iPSC-CMs, which suggested that this platform could be used to predict drug effects in humans for pre-clinical validation (Fig. 3g). Micromolding and microcontact printing techniques are remarkably useful to produce aligned cellular constructs, but these strategies are generally considered as 2D and are limited in their ability to truly recapitulate the 3D nature of native tissues. In this regard, one group used micromolding to form high aspect ratio pillars of PDMS elastomer, followed by seeding a cardiac cell-laden fibrin hydrogel in the inter-pillar space (Fig. 3h) [125,145]. Using this approach, they observed considerable organization of muscle cells around the pillars, highly aligned sarcomeric alpha-actinin and DAPI-stained nuclei, as well as enhanced expression of the connexin-43 junction protein (Fig. 3i).
Photomasking is a technique compatible with photosensitive materials that can be used to produce quasi-3D structures. Currently, there is an abundance of biomaterials compatible with photomasking, including polymers that are formed with chemistries such as acryloyl/methacryloyl chain-growth polymerization [146], thiol-ene click chemistry [147], thiol-Michael addition, thiol-yne, and others [148]. The generation of biomolecule patterns usually requires modification of the molecule to incorporate a photosensitive functional group [149]. Some of these reactions require the addition of a photoinitiator, which is activated in the presence of light and propagates the radical reaction. Biocompatible photoinitiators include 2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959) [150], lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) [151], Eosin Y disodium salt/triethanolamine co-initiators [152], riboflavin/triethanolamine co-initiators [153], as well as other less commonly used photoinitiators such as bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide (Irgacure 819), 1,5-diphenyl-1,4-diyn-3-one (diynone), and camphorquinone (CQ)/N,N-dimethylaminobenzoic acid ethyl ester (DMAB) co-initiators [154]. These structures are termed “quasi-3D” because the height of the structures is generally much smaller than the length and width, resulting high aspect ratio hydrogels that are not truly 3D. For example, Aubin et al. generated parallel lines of 3T3 fibroblast-laden GelMA hydrogels using a photomasking technique [139]. High degree of cellular alignment was achieved by decreasing the line width from 200 μm to 50 μm over the course of 5 days, and as the cells spread within the patterned lines. In a similar study, CMs and cardiac fibroblasts were co-cultured within micropatterned GelMA constructs [155]. No significant differences in cell alignment were observed as the width of the constructs was decreased from 500 μm to 125 μm. However, constructs with diameters smaller than this were not tested, and it has been shown that a diameter smaller than 100 μm is required to induce alignment for cardiac cells [156]. The same methodology can be applied to create patterns of materials that inhibit cell attachment, thus confining the cells to regions of unexposed material. One example of this technique is the patterning of photocrosslinkable chitosan onto glass or PLL-coated polystyrene [157]. Cardiac cells cultured on these platforms preferentially attached to the chitosan-free regions, directing their alignment along thin (< 100 μm) lanes. Photopatterning can also be used to create biomimetic gradients in stiffness [158] and/or biomolecule concentration [159]. Gradients in stiffness drive durotaxis, the migration of cells across a stiffness gradient, whereas molecule gradients drive chemotaxis, cell migration over a concentration gradient. In vitro studies of durotaxis can elucidate mechanisms such as differentiation and muscle tissue innervation [160], both of which are important parameters for understanding CVDs [161,162]. For example, Tse et al. formed a polyacrylamide hydrogel with a stiffness gradient by photocrosslinking the hydrogel precursor through a mask with a gradient in transparency. Mesenchymal stem cells (MSCs) cultured on the hydrogel with a stiffness gradient of 1.06 ± 0.1 kPa/mm, similar to gradients found in both the diseased and healthy myocardium, were shown to durotax up the gradient (i.e., towards the stiffer regions of the gel), and then differentiated into a more contractile muscle-like phenotype [163]. Generally, photopatterning techniques enable precise control over the spatiotemporal arrangement of biomaterials, expanding their utility to serve as platforms for generating tissue-like patterns [148].
3.3. 3D bioprinting
Although the fabrication methods discussed in the previous sections have proven useful in recapitulating some complexities of native cardiovascular tissues, several challenges remain that limit their ability to generate large-scale, physiologically relevant tissues in vitro. 3D bioprinting is one of the most advanced biofabrication techniques available to generate complex biomimetic structures. Several types of bioprinting techniques exist, such as stereolithography [164,165], inkjet [166], extrusion [167], two-photon polymerization [168], and laser-induced forward transfer [169]. However, there are several design criteria that must be considered to determine the best printing method for a specific application. Previous reviews provide a complete overview of the advantages and disadvantages of the various forms of bioprinting and bioinks [170,171]. The ability for autonomous multi-scale layer-by-layer processing via bioprinting allows the fabrication of constructs with considerably larger size and precision in all directions. In addition, bioprinting techniques are usually amenable to the use of multiple materials and thus, multi-cell deposition in a single print. This in turn allows to more accurately replicate the diverse cellular and compositional demographic of native tissues. One example of this is the incorporation of vascular networks into large constructs, which enables fabrication of larger, vascularized tissue constructs that mimic blood perfusion in native tissues. For example, Lee et al. employed a bioprinting technique to generate a microvascular network comprised of large (~1mm diameter) pre-formed synthetic vessels [172]. Their approach involved printing large vascular channels in a parallel arrangement and culturing endothelial cells in a fibrin gel between the channels. Perfusion of endothelial cell growth media through the large channels led to spontaneous angiogenic sprouting from the large vessels, and microcapillary formation between the larger channels. In addition, the diffusion of 10 kDa dextran through the vascular system revealed that constructs with capillaries showed faster diffusion rates compared to constructs without the microcapillary network. In another study, a microfluidic approach was used to directly print perfusable vascular conduits in a single continuous printing step [173]. Constructs with perfusable endothelialized channels such as the ones developed in this work can also be used to study the pharmacokinetics of drugs in large cardiovascular constructs.
Bioprinting has yet to realize its full potential in generating functional cardiovascular constructs; however, some studies have shown promising results in fabricating cardiac-like tissues in vitro. For instance, an endothelialized myocardium was constructed in vitro with a microfluidic bioprinting strategy employed by Zhang et al. [102]. They fabricated a platform for vascularization that could later be seeded with CMs, by first printing filaments laden with endothelial cells -mimicking the highly vascularized myocardium (Fig. 3j). Endothelial cells migrated to the periphery of the printed filaments and CMs aligned parallel to the extruded filaments, which were arranged in a layered lattice pattern. Long-term viability and function were preserved by maintaining the tissue-like structure in the center of a custom-made perfusion bioreactor (Fig. 3k). They demonstrated the utility of their platform as a basic model for drug screening with a common anti-cancer model drug, doxorubicin. The cardiac tissues responded to the perfused solution of doxorubicin in a dose-dependent manner, exhibiting enhanced reduction in beating rate concomitant with an increase in drug concentration (Fig. 3l). Although these models had structural properties and cell composition similar to the native myocardium, there are several obstacles that remain. In general, one of the main issues associated with this and other bioprinting methodologies is the use of printing patterns in the shape of stacked rectilinear lattices [174]. Although this printing pattern is often chosen to improve the bulk structural integrity of the printed constructs, this grid-like order does not exist in native tissues [126]. To improve upon the extensive work that has been performed in the field of bioprinting, efforts must be made to support printing of complex 3D structures that more closely resemble native tissue architecture. One major setback is that softer biomaterials, which would allow for better spreading, fusion, and differentiation of cells in their 3D structures, are not self-supporting. Because of this, previous groups have explored approaches to stabilize printed structures through the addition of rheology modifiers such as cellulose nanofibers [175], or by printing support structures coincidently with a soft hydrogel. For instance, Kang et al. developed an integrated tissue-organ printer (ITOP) that extruded stiff PCL filaments alongside soft cell-laden hydrogels to provide structural support to the large 3D matrices [176]. Although the authors suggested that the PCL supports would degrade as the tissue grows, the degradation rate of PCL is particularly slow [177]. Since tissue morphogenesis is highly dependent upon substrate stiffness [178], the presence of PCL in soft tissues might elicit unexpected cellular responses. More recently, other groups have investigated the use of support baths as a new methodology for printing complex structures (Fig. 3m) [126,179,180]. These methods help circumvent the limitations associated with the use of softer biomaterials for 3D printing of hydrogels [171]. For instance, Hinton et al. demonstrated the ability to print soft tissue structures such as an embryonic chick heart (Fig. 3n) and a human brain scaled to 3 cm in length, by directly extruding alginate into a support bath of gelatin microparticles and calcium chloride [126]. Since gelatin melts at physiological temperatures, the printed structure could easily be released after incubation at 37 °C. Novel printing strategies such as this have great potential to expand the possibilities of using 3D printing for cardiovascular applications.
Biofabrication strategies have shown tremendous promise in recapitulating the native microarchitecture of human tissues, using strategies that involve a calculated combination of cells and biomaterials. Despite the benefits of biofabrication as a method that can generate microscale biomimetic features, the experimental implementation of bioengineered constructs in drug screening and disease modeling still presents some technical limitations. For instance, in vitro platforms for pharmacological assessment require real-time monitoring of cellular activity as external stimuli or drugs are introduced to the system. Moreover, microtissues grown under conventional culture conditions are not able to reproduce the dynamic heterocellular interactions that occur in native cardiovascular microenvironments. In this regard, microphysiological on-chip models have shown great promise as platforms for building culture systems with tissue- and organ-level functionality.
4. Cardiovascular organ-on-a-chip platforms
Due to the complexity of the cardiovascular system, there are several diseases associated with different structural components of cardiovascular tissues (e.g., valves, Purkinje fibers, ventricles, veins, coronary arteries, capillaries, etc.), as well as the various cell types that make up these tissues (e.g., cardiac fibroblasts, endothelial cells, pacemaker cells, nodal cells, epicardial cells, smooth muscle cells, CMs, etc.) (Fig. 4a). Therefore, the mechanistic investigation of CVDs, the development of new therapeutic drugs, and microphysiological and electrophysiological studies of cardiac function have always been among the most active topics in biomedical research [181]. However, due to the systemic component and the remarkable heterogeneity between the cellular and molecular mechanisms of CVDs, conventional in vitro models do not provide an adequate level of physiological relevance. Furthermore, traditional in vitro approaches are not capable of direct measurement and modulation of complex biological variables, limiting their ability to screen important clinical parameters [182]. To overcome these limitations, different groups have focused on the development of miniaturized culture platforms termed organs-on-chips [181,183]. These microphysiological models can mimic the function of complex cardiovascular tissues, while also allowing the measurement of key biological responses in a high-throughput fashion. This is achieved mainly through advanced mincroengineering technologies such as microfluidics, microfabrication, and microelectronics. Furthermore, these “heart-on-a-chip” platforms not only circumvent the limitations of conventional in vitro models, but they also enable the recapitulation of complex phenomena such as flow and electromechanical stimuli, disease physiopathology, and tissue- and organ-level responses to drugs [184].
Fig. 4. Different biomedical applications of heart-on-a-chip platforms.
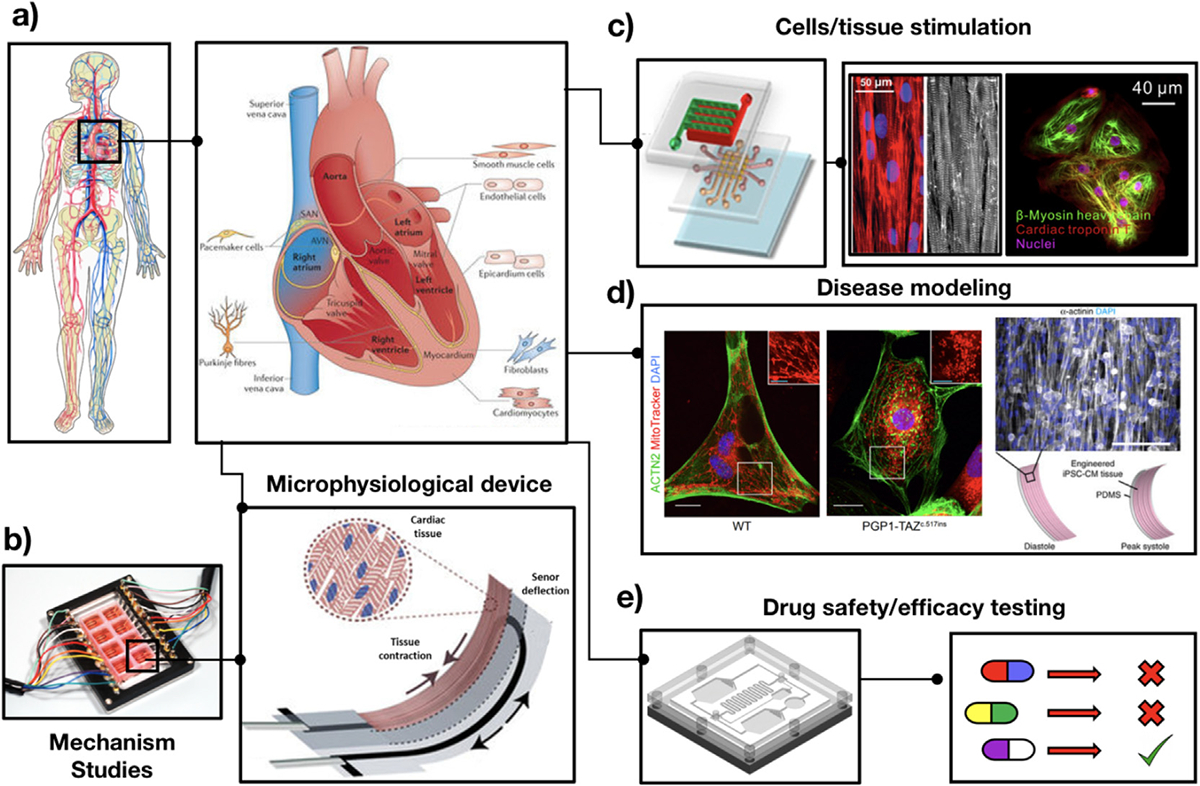
a) Schematic of a human heart, showing the different cell types contributing to structural support, and its biochemical, mechanical, electrical and functional properties (adapted from Ref. [185]). Heart-on-a-chip platforms have emerged as versatile platforms with several different applications, including: b) Mechanistic studies of cardiac function. Schematic of a microphysiological device engineered via multimaterial 3D printing and used for generating functional stem cell-derived laminar cardiac tissues (adapted from Ref. [186]); c) Delivery of physiological stimuli to cells and tissues. Schematic of an integrated microfluidic device for mechanical stimulation, and representative fluorescent images of iPSC-CMs (adapted from Refs. [187,188]); d) In vitro disease modeling. Representative fluorescent images (right) of iPSC-CMs laden elastomers with fibronectin patterned lines, self-organized into anisotropic myocardial tissues, and (left) control (WT) and BTHS iPSC-CMs (PGP1-TAZ) showing differences in the distribution and morphology of mitochondria (adapted from Ref. [189]); and e) Design of microengineered platforms for in vitro drug safety and efficacy testing, which can be used for the development of personalized targeted therapies.
Heart-on-a-chip platforms have been mainly used to investigate the spatial arrangements and functional interactions between different cardiac phenotypes (Fig. 4b), and for the targeted stimulation of cardiac cells/stem cells to yield functional cardiac microtissues (Fig. 4c) [181]. In this regard, a variety of biochemical [187,189–193], mechanical (e.g., strain and shear force) [192,194–198], physical (e.g., surface/structural features) [190,199–203], electrical [196,204–209], optical [210–212], as well as thermal and magnetic [213,214] stimuli have been shown to influence the differentiation, alignment, and physiological behavior of cardiac cells/stem cells in vitro. For instance, Marsano et al. engineered a microphysiological model to study the mechanical function of the myocardium. This microengineered device delivered homogeneous uniaxial cyclic strain to cell-laden scaffolds, which ultimately yielded mature and functional cardiac microtissues [215]. This platform could also be used to study hypertrophic changes occurring in cardiac tissues triggered by the combined action of mechanical and biochemical stimulation. In another study, Ma et al. developed an in vitro model of the myocardium through the integration of a microelectrode array-based biochip, laser-patterning, and microfabrication [207]. They used this platform to analyze the differences in electrical conductivities of different cell types within the myocardium. Their results demonstrated that stem cell-CM bridges showed stronger and more stable electrical signaling through gap junctions, when compared to myocyte-fibroblast contacts [207].
Apart from mechanical stimulation, electrical stimulation of cardiac cells is one of the most important factors to accurately mimic the contractile function of native cardiac tissues [196,204–209,216–218]. Previous groups have explored the use of electrical stimuli to modulate the rate and duration of action potentials in CMs in vitro, which in turn promotes the synchronous contraction of bioengineered tissue constructs [219–222]. This is mainly because direct electrical stimulation mediates the release and re-uptake of Ca2+ ions via the sarcoplasmic reticulum, which directly influences the contractile function of CMs. Moreover, different studies have shown that electrical stimulation influences the expression of cardiac markers and affects cell migration and alignment, as well as the formation of gap junctions and intercellular connections [223–227]. In another study, Chen et al. designed an on-chip microsystem that mimicked the pumping functionality of the heart. This microfluidic circulatory system was comprised of on-chip pressure sensors, four pumping units, and passive check valves to mimic the four cardiac chambers and the heart valves in vitro [228]. This microsystem enabled the precise monitoring and control of the circulatory pressure and the beating rate. Therefore, the engineered heart-on-a-chip system could potentially be used for physiological studies of blood circulation and also for the modeling of CVDs such as bradycardia, tachycardia, and hypotension [228].
In addition to the electromechanical stimulation of bioengineered tissues, heart-on-a-chip systems have also been used for modeling and mechanistic studies of CVDs such as myocardial infarction, arrhythmia, cardiomyopathy and ischemia (Fig. 4d) [229,230]. For instance, various research groups have investigated the differences in cellular organization and alignment using electrical fields for the in vitro modeling of cardiac arrhythmias [223,231–233]. In a recent study, Ren et al. used a microfluidic platform to develop a micropillar array-aided platform to mimic hypoxia-induced myocardial injury [229]. Using this system, different apoptotic responses of myocardial cells such as cell shrinkage, cytoskeleton disintegration, activation of caspase-3, and decrease in mitochondrial membrane potential could be monitored. Their results suggested that this platform could be used not only to study cellular responses to myocardial infarction, but also for the investigation of organ/tissue disease dynamics [229]. Furthermore, heart-on-a-chip models could play a key role in the comprehensive screening and investigation of rare diseases that lack adequate pre-clinical and clinical models [234]. For instance, Wang et al. developed a heart-on-a-chip model using patient-derived and genetically engineered iPSCs to study the pathophysiologic basis of the cardiomyopathy of Barth syndrome (BTHS), a particularly rare mitochondrial disorder [189]. They used this platform to show that the abnormal contractile function of CMs in BTHS occurs due to mutations in the Tafazzin (TAZ) gene, and to investigate potential treatment strategies against BTHS. Taken together, these studies highlight the potential of on-chip models to elucidate key disease mechanisms, which in turn could aid in the identification of novel therapeutic targets [189,235].
Microscale heart-on-a-chip platforms can be also used for screening drugs for the treatment of CVDs, evaluating cardiotoxic side effects, and identifying multiple drug interactions (Fig. 4e) [181]. Currently, several drugs (e.g., Clomacron, Astemizole, Fen-phen, Prenylamine, Dexfenfluramine, Chlorphentermine, and Terfenadine) have been withdrawn by the FDA due to various hepatotoxic, and cardiotoxic side effects, such as the development of fatal arrhythmias, ventricular tachycardia, CVD, and cardiac fibrosis [236,237]. Heart-on-a-chip platforms can provide suitable, cost-effective, and time-efficient drug testing during pre-clinical stages, due to their high degree of biomimicry and human relevance [183]. Because of these characteristics, microphysiological platforms can be remarkably useful in the determination of the safety, efficacy, and side effects of drugs for the treatment of cardiovascular and other diseases. This in turn can dramatically reduce the costs associated with preclinical assessment, development, production, and implementation of therapeutic drugs [222]. In this regard, biological variables such as cell viability, expression of cardiac markers, rate and synchronicity of CM contraction, and several other functional parameters can be evaluated to establish optimal drug dosages and regimens [181,222]. For instance, Agarwal et al. developed a microengineered heart-on-a-chip system to investigate the inotropic effect of isoproterenol (a non-selective β-adrenergic agonist used for the treatment of bradycardia) on the contractility of neonatal rat ventricular myocytes, using a 3D multicellular culture system [238]. Using this approach, they could rapidly and effectively measure the contractile function of the cells across a wide range of drug concentrations (i.e., from 1 nM to 100 mM), which demonstrated the capability of their system for pharmacological assessment. In another study, Mathur et al. developed a cardiac microphysiological system to determine the toxicity of different drugs, using both iPSCs and native tissues [239]. This platform was able to support the viability and functionality of iPSC-derived cardiac tissues for several weeks. Using this approach, they evaluated the effect of drugs on the electrophysiological behavior of the cells, following the administration of varying doses of different drugs including Verapamil, Isoproterenol, Metoprolol, and E-4031.
The remarkable versatility of on-chip platforms originates in part from the ability to incorporate different cell culture approaches depending on the requirements of each experimental design. To this date, on-chip evaluation of drugs for cardiovascular applications has been conducted using 2D [199] and 3D cultures, bioprinted tissues [186,240,241], single cells [242–244], as well as multicellular cultures [230,245–247]. Table 1 shows a summary of different types of on-chip systems for biomedical applications, including cell/tissue stimulation, CVD modeling, as well as drug safety and efficacy testing.
Table 1.
Cardiovascular organ-on-a-chip platforms and their applications in cellular behavior and mechanistic studies, drug testing, and investigation of different cardiac diseases.
| Application | Cell source | Culture conditions | Type of microdevice | Type of biopolymer/electrode | Type of drug | Cardiac marker evaluated | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Cell Stimulation | Electrical stimulation | Neonatal rat ventricular myocytes | 3D cell encapsulation Static condition | PDMS/microelectrodes | collagen/carbon graphite, titanium, stainless steel, titanium nitride-coated titanium | – | Connexin-43 (CX43), cardiac troponin-I (cTnT), β-myosin heavy chain (β-MHC), α-myosin heavy chain (α-MHC), muscle-type creatine kinase (CK-MM) and β-actin | [248] |
| Human bone marrow- MSCs | 2D culture, Static condition | PDMS | PDMS/CNTs | – | GATA4, MEF2C, MYH7, NKX2.5, TUBB, CX43, TNNT2, and OCT4 | [196] | ||
| Neonatal rat cardiomyocytes (rCMs) | 3D multicellular culture Perfusion system | Perfusion bioreactor connected to electrodes | Alginate/Carbon | – | CX43 sarcomeric α-actinin | [249] | ||
| Neonatal rat ventricular myocytes | 3D multicellular culture | Poly (N-isopropylacrylamide)/PDMS | Platinum | Isoproterenol | CX43, sarcomeric α-actinin | [238] | ||
| Neonatal rCMs Bone marrow rat MSCs | multicellular culture | PDMS/Microelectrode arrays (MEAs) | Indium tin oxide (ITO) | – | CX43, sarcomeric α-actinin | [207] | ||
| Human embryonic stem cells (hESCs) | 2D culture, Static condition | PDMS/microelectrodes bioreactor | stainless steel, titanium-nitride-coated titanium, titanium | – | cTnT | [205] | ||
| human iPSC-derived CMs | 2D culture, Static condition | electrophysiology-activated cell cytometry/microelectrodes | ITO | – | – | [209] | ||
| Mechanical stimulation | Murine ESCs (mESCs) expressing a cardiac specific α-MHC promoter human microvascular endothelial cells (hMVECs) | 2D and 3D multicellular culture, Static condition | PDMS/Motorized microfluidic platform Cyclic stretch | Gelatin/collagen | – | Myosin heavy chain, α isoform (MHC-α) ki-67 | [192] | |
| hESCs hiPSCs |
2D and 3D multicellular culture | PDMS Microfluidic Device Cyclic stress Uniaxial cyclic strain |
Type I collagen | – | cTnT, smooth muscle α-actin (SMA) | [194] | ||
| mESCs | 3D multicellular culture | Cyclic strain | Collagen and fibronectin | – | Titin, pancadherin, CX43 | [195] | ||
| Rat BMSCs | 2D culture, Static condition | Parallel plate-type device Shear Stress |
– | – | CX43, cTnT, sarcomeric α-actinin | [197] | ||
| rBMSC | 2D culture, Static condition | Parallel-plate flow chamber cyclic strain | – | – | cTnT, CX43, MEF2C | [198] | ||
| Physical stimulation | mESCs | 2D culture, Static condition | – | PCL/gelatin nanofibrous scaffold/ | – | CX43, sarcomeric α-actinin | [200] | |
| Human dermal fibroblasts (HDF) hMSC rBMSC |
3D multicellular culture | 3D bioprinted micropatterning | Gelatin | – | CD29 and GATA4 | [201] | ||
| rCMs | 2D culture, Static condition | Silicon wafers/PDMS/micropatterned laminin surfaces | PLGA membranes | – | Sarcomeric myosin heavy chain (MF20), CX43, pancadherin | [250] | ||
| rCMs NIH-3T3 fibroblasts |
2D multicellular culture, Static condition | PDMS microfluidic molds | HA | – | cTnT, CX43 | [251] | ||
| Biochemical stimulation | mESCs | 2D culture, Static condition | Integrated microfluidic culture device with micropillar arrays and microvalves | – | – | Platelet endothelial cell adhesion molecule (PECAM) SMA | [187] | |
| mESCs | 2D culture, Static condition | Multi-layer PDMS microfluidic array platform | poly-l-lysine (PLL) and laminin | – | Neurofilaments and nestin | [191] | ||
| mESCs, expressing a cardiac specific α-MHC promoter hMSCs |
2D and 3D multicellular culture, Static condition | PDMS/Motorized microfluidic platform | Gelatin, collagen | BMP-2 | ki-67 MHC-α |
[192] | ||
| Disease modeling | Myocardial infarction | hMSCs | 2D and 3D culture, Static condition | Tissue culture plate | RGD modified alginate | – | FGF1, FGF2, VEGFA165, TGFβ1 and HSPA6 | [204] |
| Hypoxia-induced myocardial injury | Rat heart myocardium H9c2 cells | 2D culture Perfusion condition | PDMS microfluidic platform | – | Carbonyl cyanide p- trifluoromethoxyphenylhy-drazone (FCCP) | Actin | [229] | |
| Hypotension Bradycardia |
HUVECs | 2D culture Perfusion condition | PDMS microfluidic platform with pressure sensor | – | – | mouse monoclonal anti-ZO-1 (1A12) | [228] | |
| Mitochondrial cardiomyopathy of Barth syndrome | Human iPSCs rCMs | 2D multicellular culture, Static condition | Elastomers micropatterned with fibronectin lines | – | – | stage-specific embryonic antigen-4 (SSEA-4), β-tubulin III SMA, TNNT2, ACTN2, α-actinin MitoTracker |
[189] | |
| Drug Testing | Proarrhythmic drug, anticancer drug | Neonatal rHCs | 3D multicellular culture, Static condition | PDMS platform/24 well-plate | Fibrinogen/Matrigel/thrombin | Chromanol, Doxorubicin | lectin, α-actinin, CX43 DRAQ5 | [252] |
| Antibradycardia drug | hiPSC-CM Neonatal rCMs | 2D and 3D multicellular culture, Perfusion condition | PDMS microfluidic device/Cyclic strain | Fibrin | Isoprenaline | α-actinin, CX43, ki-67 cTnT | [215] | |
| Causing Q-Tc interval prolongation | mouse ECMs | Single cell | on-a-chip system with an agarose microchamber | collagen type I/agarose | Haloperidol (Antipsychotic drag) | – | [243] | |
| Treatment of bradycardia and long QT syndrome | hiPSC CMs | 2D multicellular culture, Static condition | PDMS microfluidic device | Matrigel | Verapamil Isoproterenol Metoprolol E-4031 |
SMA | [239] | |
| Chronotropic drug | Neonatal rat ventricular myocytes | 2D multicellular culture, Static condition | PDMS/PIPAAm multiple chips device | – | Epinephrine | SMA | [230] |
5. Current outlook and future perspectives
The development of biomimetic in vitro models of the cardiovascular system remains particularly challenging, mainly due to the complex dynamics of relevant physiological phenomena such as blood flow, mechanical stretching, and electrical stimulation. In recent years, the development of advanced biomaterials with tailored physical, biochemical, and biological features, as well as their integration with modern biofabrication approaches has enabled the development of bioengineered constructs with tissue-level complexity. Bioengineered tissues have become increasingly more sophisticated, which has enhanced their therapeutic potential for the regeneration and repair of cardiovascular tissues. However, a major opportunity also exists to implement these biomimetic 3D models for the study of the cellular and molecular mechanisms that drive CVD onset and progression. In addition, the integration of bioengineered tissues with perfusable microfluidic networks that mimic the vasculature not only allows the delivery of nutrients and soluble biomolecules, but also contributes to the functional maturation and interaction of different cardiovascular phenotypes. In the context of CVDs, on-chip platforms provide unparalleled technical advantages, since they enable the precise manipulation of flow rate and shear stress for the recapitulation of capillary, arterial, and venous flow behavior under different pathological conditions. Furthermore, microphysiological platforms allow the rapid and reproducible measurement of relevant physiological parameters for the high throughput evaluation of drug safety and efficacy.
Drug development for CVDs remains particularly challenging since clinical trials often involve large numbers of patients that need to be monitored over extended periods of time, while the tolerance of these patients to adverse side effects is extremely low. These and other obstacles associated with the availability of experimental models and the high variability among individual patients have significantly slowed down the pace of new drug development. In this regard, on-chip platforms hold great potential to narrow the gap between conventional in vitro models and live animals used for in vivo experimentation, thus lowering the time, resources, and ethical concerns associated with current approaches. Despite this remarkable versatility, several technological and scientific challenges remain. Pioneering studies on on-chip platforms have relied mainly on animal-derived cells due to their ease of accessibility and relatively simple manipulation. However, these cells are not representative of human physiology and exhibit marked differences in drug response and disease mechanisms, which highlights the need for the use of cells of human origin. Recent developments in iPSC technologies and recombinant DNA techniques have enabled the differentiation of functional human cardiovascular phenotypes in the laboratory (Fig. 5a) [253,254]. Moreover, the fact that iPSCs can be generated on a patient by patient basis is remarkably advantageous for the development of in vitro models for personalized drug screening (Fig. 5b) and for the understanding of patient specific fundamentals related to CVDs (Fig. 5c). However, although research on cardiovascular genetics has made significant progress in the last decade, personalized therapeutic strategies based on the genetic makeup of individual patients are scarce (Fig. 5d) [255].
Fig. 5. Implementation of bioengineered tissues and on-chip platforms for high-throughput screening and precision medicine.

a) Patient-specific cells, both terminally mature and undifferentiated progenitors, can be used as cell sources for personalized medicine applications, which include: b) Microscale biomimetic tissue constructs for the rapid and high throughput assessment of drug safety and efficacy; c) Development of organ-on-a-chip platforms for disease modeling, based on the modulation of physiological determinants, such as abnormal flow, electrical stimulation, and tissue mechanics; d) Design of organ-on-a-chip platforms to facilitate the development of personalized therapeutic regimes that take into account the genetic make-up and the remarkable heterogeneity of each individual patient.
In future years, the efficient translation of technological advancements in the fields of device miniaturization, additive 3D manufacturing, and microelectronics will allow the development of fully instrumented microphysiological systems that can be readily integrated into cost-effective and automated platforms. In addition, future developments in the fields of TE, iPSC engineering, and DNA editing will enable the efficient translation of organ- and disease complexity into microscale tissues for personalized therapeutics and diagnostics. However, the implementation of iPSC-based TE strategies will also require the establishment of standardized protocols for the high-throughput evaluation of functional cardiovascular phenotypes, and the accurate recapitulation of disease states [253]. Moreover, the development of platforms based on the integration of healthy and diseased tissues, as well as multi-organ experimental set ups are required to accurately mimic cardiovascular pathophysiology. However, the integration of multiple organs-on-chips has led to new technical challenges related to organ scaling based on size and function, the development of universal culture media, as well as the determination of the smallest functional units that can still recapitulate drug responses in the native organs. Despite these and other challenges, future scientific and technological developments stemming from the collaboration between clinicians, engineers, and industry experts will certainly facilitate the validation and clinical translation of biomimetic in vitro models. This in turn will lead to more efficient, safe, and ethical strategies for the widespread commercialization of biomedical devices, drugs, and personalized theranostic solutions.
Acknowledgements
NA acknowledges the support from the American Heart Association (AHA, 16SDG31280010), NA also acknowledges the support from the National Institutes of Health (NIH), R01EB023052 and R01HL140618. RPL gratefully acknowledges the institutional funding received from the Escuela de Ingenieria y Ciencias at Tecnológico de Monterrey, México, and funding provided from the Consejo Nacional de Ciencia y Tecnología, CONACyT.
References
- [1].World Health Organization, Cardiovascular Diseases (CVDs) FAQ Sheet, (2018) http://www.who.int/mediacentre/factsheets/fs317/en/, Accessed date: 24 January 2018.
- [2].C.D.C. Foundation, Heart Disease and Stroke Cost America Nearly $1 Billion a Day in Medical Costs, Lost Productivity, (2015), Accessed date: 28 February 2018.
- [3].Kaese S, Verheule S, Cardiac electrophysiology in mice: a matter of size, Front. Physiol 3 (2012) 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ryan AJ, Brougham CM, Garciarena CD, Kerrigan SW, O’Brien FJ, Towards 3D in vitro models for the study of cardiovascular tissues and disease, Drug Discov. Today 21 (9) (2016) 1437–1445. [DOI] [PubMed] [Google Scholar]
- [5].Shah RR, Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics 7 (6) (2006) 889–908. [DOI] [PubMed] [Google Scholar]
- [6].Amini H, Rezaie J, Vosoughi A, Rahbarghazi R, Nouri M, Cardiac progenitor cells application in cardiovascular disease, J. Cardiovasc. Thorac. Res 9 (3) (2017) 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barad L, Schick R, Zeevi-Levin N, Itskovitz-Eldor J, Binah O, Human embryonic stem cells vs human induced pluripotent stem cells for cardiac repair, Can. J. Cardiol 30 (11) (2014) 1279–1287. [DOI] [PubMed] [Google Scholar]
- [8].Siu CW, Moore JC, Li RA, Human embryonic stem cell-derived cardiomyocytes for heart therapies, Cardiovasc. Haematol. Disord. - Drug Targets 7 (2) (2007) 145–152. [DOI] [PubMed] [Google Scholar]
- [9].Brandao KO, Tabel VA, Atsma DE, Mummery CL, Davis RP, Human pluripotent stem cell models of cardiac disease: from mechanisms to therapies, Dis. Model. Mech 10 (9) (2017) 1039–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang W, Lan F, Zhang H, Human induced pluripotent stem cells for inherited cardiovascular diseases modeling, Curr. Stem Cell Res. Ther 11 (7) (2016) 533–541. [DOI] [PubMed] [Google Scholar]
- [11].Shaheen N, Shiti A, Gepstein L, Pluripotent stem cell-based platforms in cardiac disease modeling and drug testing, Clin. Pharmacol. Ther 102 (2) (2017) 203–208. [DOI] [PubMed] [Google Scholar]
- [12].Del Alamo JC, Lemons D, Serrano R, Savchenko A, Cerignoli F, Bodmer R, Mercola M, High throughput physiological screening of iPSC-derived cardiomyocytes for drug development, Biochim. Biophys. Acta 1863 (7 Pt B) (2016) 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mercola M, Colas A, Willems E, Induced pluripotent stem cells in cardiovascular drug discovery, Circ. Res 112 (3) (2013) 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wolf F, Vogt F, Schmitz-Rode T, Jockenhoevel S, Mela P, Bioengineered vascular constructs as living models for in vitro cardiovascular research, Drug Discov. Today 21 (9) (2016) 1446–1455. [DOI] [PubMed] [Google Scholar]
- [15].Zuppinger C, 3D culture for cardiac cells, Biochim. Biophys. Acta 1863 (7 Pt B) (2016) 1873–1881. [DOI] [PubMed] [Google Scholar]
- [16].Marban E, Cingolani E, Heart to heart: cardiospheres for myocardial regeneration, Heart Rhythm 9 (10) (2012) 1727–1731. [DOI] [PubMed] [Google Scholar]
- [17].LeGrice I, Pope A, Smaill B, The architecture of the heart: myocyte organization and the cardiac extracellular matrix, in: Villarreal FJ (Ed.), Interstitial Fibrosis in Heart Failure, Springer New York, New York, NY, 2005, pp. 3–21. [Google Scholar]
- [18].Ammar KA, Paterick TE, Khandheria BK, Jan MF, Kramer C, Umland MM, Tercius AJ, Baratta L, Tajik AJ, Myocardial mechanics: understanding and applying three-dimensional speckle tracking echocardiography in clinical practice, Echocardiography 29 (7) (2012) 861–872. [DOI] [PubMed] [Google Scholar]
- [19].McArthur L, Chilton L, Smith GL, Nicklin SA, Electrical consequences of cardiac myocyte: fibroblast coupling, Biochem. Soc. Trans 43 (3) (2015) 513–518. [DOI] [PubMed] [Google Scholar]
- [20].Cimetta E, Godier-Furnemont A, Vunjak-Novakovic G, Bioengineering heart tissue for in vitro testing, Curr. Opin. Biotechnol 24 (5) (2013) 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sachs PC, Mollica PA, Bruno RD, Tissue specific microenvironments: a key tool for tissue engineering and regenerative medicine, J. Biol. Eng 11 (2017) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fujita B, Zimmermann WH, Myocardial tissue engineering for regenerative applications, Curr. Cardiol. Rep 19 (9) (2017) 78. [DOI] [PubMed] [Google Scholar]
- [23].Fleischer S, Feiner R, Dvir T, Cutting-edge platforms in cardiac tissue engineering, Curr. Opin. Biotechnol 47 (2017) 23–29. [DOI] [PubMed] [Google Scholar]
- [24].Pacheco DP, Reis RL, Correlo VM, Marques AP, The crosstalk between tissue engineering and pharmaceutical biotechnology: recent advances and future directions, Curr. Pharmaceut. Biotechnol 16 (11) (2015) 1012–1023. [DOI] [PubMed] [Google Scholar]
- [25].Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT, 3D bioprinting for drug discovery and development in pharmaceutics, Acta Biomater 57 (2017) 26–46. [DOI] [PubMed] [Google Scholar]
- [26].Goole J, Amighi K, 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems, Int. J. Pharm 499 (1–2) (2016) 376–394. [DOI] [PubMed] [Google Scholar]
- [27].Aljohani W, Ullah MW, Zhang X, Yang G, Bioprinting and its applications in tissue engineering and regenerative medicine, Int. J. Biol. Macromol 107 (Pt A) (2018) 261–275. [DOI] [PubMed] [Google Scholar]
- [28].Foyt DA, Norman MDA, Yu TTL, Gentleman E, Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine, Adv. Healthc. Mater 7 (8) (2018) e1700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jang J, Park JY, Gao G, Cho DW, Biomaterials-based 3D cell printing for nextgeneration therapeutics and diagnostics, Biomaterials 156 (2018) 88–106. [DOI] [PubMed] [Google Scholar]
- [30].Liaw CY, Ji S, Guvendiren M, Engineering 3D hydrogels for personalized in vitro human tissue models, Adv. Healthc. Mater 7 (4) (2018). [DOI] [PubMed] [Google Scholar]
- [31].Low LA, Tagle DA, Tissue chips - innovative tools for drug development and disease modeling, Lab a Chip 17 (18) (2017) 3026–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caddeo S, Boffito M, Sartori S, Tissue engineering approaches in the design of healthy and pathological in vitro tissue models, Front. Bioeng. Biotechnol 5 (2017) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE, Microengineered physiological biomimicry: organs-on-chips, Lab a Chip 12 (12) (2012) 2156–2164. [DOI] [PubMed] [Google Scholar]
- [34].Dehne EM, Hasenberg T, Marx U, The ascendance of microphysiological systems to solve the drug testing dilemma, Future Sci. OA 3 (2) (2017) FSO185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurokawa YK, George SC, Tissue engineering the cardiac microenvironment: multicellular microphysiological systems for drug screening, Adv. Drug Deliv. Rev 96 (2016) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rogozhnikov D, O’Brien PJ, Elahipanah S, Yousaf MN, Scaffold free bio-orthogonal assembly of 3-dimensional cardiac tissue via cell surface engineering, Sci. Rep 6 (2016) 39806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Noguchi R, Nakayama K, Itoh M, Kamohara K, Furukawa K, Oyama J, Node K, Morita S, Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease, J. Heart Lung Transplant 35 (1) (2016) 137–145. [DOI] [PubMed] [Google Scholar]
- [38].Stevens KR, Pabon L, Muskheli V, Murry CE, Scaffold-free human cardiac tissue patch created from embryonic stem cells, Tissue Eng. Part A 15 (6) (2009) 1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li Y, Zhang D, Artificial cardiac muscle with or without the use of scaffolds, BioMed Res. Int 2017 (2017) 8473465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kitsara M, Agbulut O, Kontziampasis D, Chen Y, Menasche P, Fibers for hearts: a critical review on electrospinning for cardiac tissue engineering, Acta Biomater 48 (2017) 20–40. [DOI] [PubMed] [Google Scholar]
- [41].Clayton RH, Bernus O, Cherry EM, Dierckx H, Fenton FH, Mirabella L, Panfilov AV, Sachse FB, Seemann G, Zhang H, Models of cardiac tissue electrophysiology: progress, challenges and open questions, Prog. Biophys. Mol. Biol 104 (1–3) (2011) 22–48. [DOI] [PubMed] [Google Scholar]
- [42].Hinton RB, Yutzey KE, Heart valve structure and function in development and disease, Annu. Rev. Physiol 73 (2011) 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Miyamoto S, Shoji T, Miyachi H, Shinoka T, CHAPTER 9 smart biomaterials for cardiovascular tissue engineering, smart materials for tissue engineering: applications, The Royal Society of Chemistry, 2017, pp. 230–257. [Google Scholar]
- [44].Naderi H, Matin MM, Bahrami AR, Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems, J. Biomater. Appl 26 (4) (2011) 383–417. [DOI] [PubMed] [Google Scholar]
- [45].Nguyen PQ, Courchesne ND, Duraj-Thatte A, Praveschotinunt P, Joshi NS, Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials, Adv. Mater 30 (19) (2018) e1704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chang J, He J, Mao M, Zhou W, Lei Q, Li X, Li D, Chua CK, Zhao X, Advanced material strategies for next-generation additive manufacturing, Materials 11 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ippel BD, Dankers PYW, Introduction of nature’s complexity in engineered blood-compatible biomaterials, Adv. Healthc. Mater 7 (1) (2018). [DOI] [PubMed] [Google Scholar]
- [48].Hollingshead S, Lin CY, Liu JC, Designing smart materials with recombinant proteins, Macromol. Biosci 17 (7) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim YS, Cho K, Lee HJ, Chang S, Lee H, Kim JH, Koh W-G, Highly conductive and hydrated PEG-based hydrogels for the potential application of a tissue engineering scaffold, React. Funct. Polym 109 (2016) 15–22. [Google Scholar]
- [50].Crowder SW, Liang Y, Rath R, Park AM, Maltais S, Pintauro PN, Hofmeister W, Lim CC, Wang X, Sung HJ, Poly(epsilon-caprolactone)-carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells, Nanomedicine 8 (11) (2013) 1763–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Santoro M, Shah SR, Walker JL, Mikos AG, Poly(lactic acid) nanofibrous scaffolds for tissue engineering, Adv. Drug Deliv. Rev 107 (2016) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rai R, Tallawi M, Frati C, Falco A, Gervasi A, Quaini F, Roether JA, Hochburger T, Schubert DW, Seik L, Barbani N, Lazzeri L, Rosellini E, Boccaccini AR, Bioactive electrospun fibers of poly(glycerol sebacate) and poly (epsilon-caprolactone) for cardiac patch application, Adv. Healthc. Mater 4 (13) (2015) 2012–2025. [DOI] [PubMed] [Google Scholar]
- [53].Chen J, Dong R, Ge J, Guo B, Ma PX, Biocompatible, biodegradable, and electroactive polyurethane-urea elastomers with tunable hydrophilicity for skeletal muscle tissue engineering, ACS Appl. Mater. Interfaces 7 (51) (2015) 28273–28285. [DOI] [PubMed] [Google Scholar]
- [54].Best C, Onwuka E, Pepper V, Sams M, Breuer J, Breuer C, Cardiovascular tissue engineering: preclinical validation to bedside application, Physiology 31 (1) (2016) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shaikh FM, Callanan A, Kavanagh EG, Burke PE, Grace PA, McGloughlin TM, Fibrin: a natural biodegradable scaffold in vascular tissue engineering, Cells Tissues Organs 188 (4) (2008) 333–346. [DOI] [PubMed] [Google Scholar]
- [56].Perea-Gil I, Prat-Vidal C, Bayes-Genis A, In vivo experience with natural scaffolds for myocardial infarction: the times they are a-changin’, Stem Cell Res. Ther 6 (2015) 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sreejit P, Verma RS, Natural ECM as biomaterial for scaffold based cardiac regeneration using adult bone marrow derived stem cells, Stem Cell Rev 9 (2) (2013) 158–171. [DOI] [PubMed] [Google Scholar]
- [58].Sepantafar M, Maheronnaghsh R, Mohammadi H, Rajabi-Zeleti S, Annabi N, Aghdami N, Baharvand H, Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair, Biotechnol. Adv 34 (4) (2016) 362–379. [DOI] [PubMed] [Google Scholar]
- [59].Ren X, Feng Y, Guo J, Wang H, Li Q, Yang J, Hao X, Lv J, Ma N, Li W, Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications, Chem. Soc. Rev 44 (15) (2015) 5680–5742. [DOI] [PubMed] [Google Scholar]
- [60].Li J, Zhang K, Huang N, Engineering cardiovascular implant surfaces to create a vascular endothelial growth microenvironment, Biotechnol. J 12 (12) (2017). [DOI] [PubMed] [Google Scholar]
- [61].Chaudhuri R, Ramachandran M, Moharil P, Harumalani M, Jaiswal AK, Biomaterials and cells for cardiac tissue engineering: current choices, Mater. Sci. Eng. C Mater. Biol. Appl 79 (2017) 950–957. [DOI] [PubMed] [Google Scholar]
- [62].Reis LA, Chiu LL, Feric N, Fu L, Radisic M, Biomaterials in myocardial tissue engineering, J. Tissue Eng. Regen. Med 10 (1) (2016) 11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT, Custom design of the cardiac microenvironment with biomaterials, Circ. Res 97 (1) (2005) 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nachlas ALY, Li S, Davis ME, Developing a clinically relevant tissue engineered heart valve-a review of current approaches, Adv. Healthc. Mater 6 (24) (2017). [DOI] [PubMed] [Google Scholar]
- [65].Sewell-Loftin MK, Chun YW, Khademhosseini A, Merryman WD, EMT-inducing biomaterials for heart valve engineering: taking cues from developmental biology, J. Cardiovasc. Transl. Res 4 (5) (2011) 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xue Y, Sant V, Phillippi J, Sant S, Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves, Acta Biomater 48 (2017) 2–19. [DOI] [PubMed] [Google Scholar]
- [67].Zhang X, Xu B, Puperi DS, Wu Y, West JL, Grande-Allen KJ, Application of hydrogels in heart valve tissue engineering, J. Long Term Eff. Med. Implants 25 (1–2) (2015) 105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Couet F, Rajan N, Mantovani D, Macromolecular biomaterials for scaffold-based vascular tissue engineering, Macromol. Biosci 7 (5) (2007) 701–718. [DOI] [PubMed] [Google Scholar]
- [69].Hiob MA, Crouch GW, Weiss AS, Elastomers in vascular tissue engineering, Curr. Opin. Biotechnol 40 (2016) 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Li ZK, Wu ZS, Lu T, Yuan HY, Tang H, Tang ZJ, Tan L, Wang B, Yan SM, Materials and surface modification for tissue engineered vascular scaffolds, J. Biomater. Sci. Polym. Ed 27 (15) (2016) 1534–1552. [DOI] [PubMed] [Google Scholar]
- [71].Ravi S, Chaikof EL, Biomaterials for vascular tissue engineering, Regen. Med 5 (1) (2010) 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stegemann JP, Kaszuba SN, Rowe SL, Review: advances in vascular tissue engineering using protein-based biomaterials, Tissue Eng 13 (11) (2007) 2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee J, Razu ME, Wang X, Lacerda C, Kim JJ, Biomimetic cardiac microsystems for pathophysiological studies and drug screens, J. Lab. Autom 20 (2) (2015) 96–106. [DOI] [PubMed] [Google Scholar]
- [74].Cui H, Liu Y, Cheng Y, Zhang Z, Zhang P, Chen X, Wei Y, In vitro study of electroactive tetraaniline-containing thermosensitive hydrogels for cardiac tissue engineering, Biomacromolecules 15 (4) (2014) 1115–1123. [DOI] [PubMed] [Google Scholar]
- [75].Shin SR, Zihlmann C, Akbari M, Assawes P, Cheung L, Zhang K, Manoharan V, Zhang YS, Yuksekkaya M, Wan KT, Nikkhah M, Dokmeci MR, Tang XS, Khademhosseini A, Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering, Small 12 (27) (2016) 3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Guiseppi-Elie A, Electroconductive hydrogels: synthesis, characterization and biomedical applications, Biomaterials 31 (10) (2010) 2701–2716. [DOI] [PubMed] [Google Scholar]
- [77].Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G, Electroconductive natural polymer-based hydrogels, Biomaterials 111 (2016) 40–54. [DOI] [PubMed] [Google Scholar]
- [78].Balint R, Cassidy NJ, Cartmell SH, Conductive polymers: towards a smart biomaterial for tissue engineering, Acta Biomater 10 (6) (2014) 2341–2353. [DOI] [PubMed] [Google Scholar]
- [79].Kai D, Prabhakaran MP, Jin G, Ramakrishna S, Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering, J. Biomed. Mater. Res 99 (3) (2011) 376–385. [DOI] [PubMed] [Google Scholar]
- [80].Broda CR, Lee JY, Sirivisoot S, Schmidt CE, Harrison BS, A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering, J. Biomed. Mater. Res 98 (4) (2011) 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yang B, Yao F, Hao T, Fang W, Ye L, Zhang Y, Wang Y, Li J, Wang C, Development of electrically conductive double-network hydrogels via one-step facile strategy for cardiac tissue engineering, Adv. Healthc. Mater 5 (4) (2016) 474–488. [DOI] [PubMed] [Google Scholar]
- [82].Liu Y, Lu J, Xu G, Wei J, Zhang Z, Li X, Tuning the conductivity and inner structure of electrospun fibers to promote cardiomyocyte elongation and synchronous beating, Mater. Sci. Eng. C Mater. Biol. Appl 69 (2016) 865–874. [DOI] [PubMed] [Google Scholar]
- [83].Mawad D, Lauto A, Wallace GG, Conductive polymer hydrogels, in: Kalia S (Ed.), Polymeric Hydrogels as Smart Biomaterials, Springer International Publishing, Cham, 2016, pp. 19–44. [Google Scholar]
- [84].Noshadi I, Walker BW, Portillo-Lara R, Shirzaei Sani E, Gomes N, Aziziyan MR, Annabi N, Engineering biodegradable and biocompatible bio-ionic liquid conjugated hydrogels with tunable conductivity and mechanical properties, Sci. Rep 7 (1) (2017) 4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wu Y, Wang L, Guo B, Ma PX, Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy, ACS Nano 11 (6) (2017) 5646–5659. [DOI] [PubMed] [Google Scholar]
- [86].Popryadukhin PV, Popov GI, Yukina GY, Dobrovolskaya IP, Ivan’kova EM, Vavilov VN, Yudin VE, Tissue-engineered vascular graft of small diameter based on electrospun polylactide microfibers, Int. J. Biomater 2017 (2017) 9034186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].D’Amore A, Luketich SK, Raffa GM, Olia S, Menallo G, Mazzola A, D’Accardi F, Grunberg T, Gu X, Pilato M, Kameneva MV, Badhwar V, Wagner WR, Heart valve scaffold fabrication: bioinspired control of macro-scale morphology, mechanics and micro-structure, Biomaterials 150 (2018) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tsai T-S, Pillay V, Choonara YE, Du Toit LC, Modi G, Naidoo D, Kumar P, A polyvinyl alcohol-polyaniline based electro-conductive hydrogel for controlled stimuli-actuable release of indomethacin, Polymers 3 (1) (2011). [Google Scholar]
- [89].McKee CT, Last JA, Russell P, Murphy CJ, Indentation versus tensile measurements of Young’s modulus for soft biological tissues, Tissue Eng. B Rev 17 (3) (2011) 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Benrashid E, McCoy CC, Youngwirth LM, Kim J, Manson RJ, Otto JC, Lawson JH, Tissue engineered vascular grafts: origins, development, and current strategies for clinical application, Methods 99 (2016) 13–19. [DOI] [PubMed] [Google Scholar]
- [91].Pashneh-Tala S, MacNeil S, Claeyssens F, The tissue-engineered vascular graft-past, present, and future, Tissue Eng. B Rev 22 (1) (2016) 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li J, Ding M, Fu Q, Tan H, Xie X, Zhong Y, A novel strategy to graft RGD peptide on biomaterials surfaces for endothelization of small-diamater vascular grafts and tissue engineering blood vessel, J. Mater. Sci. Mater. Med 19 (7) (2008) 2595–2603. [DOI] [PubMed] [Google Scholar]
- [93].Bedair TM, ElNaggar MA, Joung YK, Han DK, Recent advances to accelerate re-endothelialization for vascular stents, J. Tissue Eng 8 (2017) 2041731417731546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Blit PH, Battiston KG, Yang M, Paul Santerre J, Woodhouse KA, Electrospun elastin-like polypeptide enriched polyurethanes and their interactions with vascular smooth muscle cells, Acta Biomater 8 (7) (2012) 2493–2503. [DOI] [PubMed] [Google Scholar]
- [95].Hasan A, Saliba J, Pezeshgi Modarres H, Bakhaty A, Nasajpour A, Mofrad MRK, Sanati-Nezhad A, Micro and nanotechnologies in heart valve tissue engineering, Biomaterials 103 (2016) 278–292. [DOI] [PubMed] [Google Scholar]
- [96].Fallahiarezoudar E, Ahmadipourroudposht M, Idris A, Mohd Yusof N, A review of: application of synthetic scaffold in tissue engineering heart valves, Mater. Sci. Eng. C Mater. Biol. Appl 48 (2015) 556–565. [DOI] [PubMed] [Google Scholar]
- [97].Chen Q, Liang S, Thouas GA, Elastomeric biomaterials for tissue engineering, Prog. Polym. Sci 38 (3) (2013) 584–671. [Google Scholar]
- [98].Kluin J, Talacua H, Smits AIPM, Emmert MY, Brugmans MCP, Fioretta ES, Dijkman PE, Söntjens SHM, Duijvelshoff R, Dekker S, Janssen-van den Broek MWJT, Lintas V, Vink A, Hoerstrup SP, Janssen HM, Dankers PYW, Baaijens FPT, Bouten CVC, In situ heart valve tissue engineering using a bioresorbable elastomeric implant – from material design to 12 months follow-up in sheep, Biomaterials 125 (2017) 101–117. [DOI] [PubMed] [Google Scholar]
- [99].Zhang Y, Arneri A, Bersini S, Shin S-R, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci M, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials 110 (2016) 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, Conklin BR, Grigoropoulos CP, Healy KE, Three-dimensional filamentous human diseased cardiac tissue model, Biomaterials 35 (5) (2014) 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jang J, Park H-J, Kim S-W, Kim H, Park J, Na S, Kim H, Park M, Choi S, Park S, Kim S, Kwon S-M, Kim P-J, Cho D-W, 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair, Biomaterials 112 (2017) 264–274. [DOI] [PubMed] [Google Scholar]
- [102].Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials 110 (2016) 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Akbari M, Tamayol a.L.I., Annabi N, Juncker D, Khademhosseini a.L.I., Microtechnologies in the fabrication of fibers for tissue engineering, Microfluid. Med. Appl 36 (2015) 1–18. [Google Scholar]
- [104].Zhang J, Zheng T, Alarçin E, Byambaa B, Guan X, Ding J, Zhang Y, Li Z, Porous electrospun fibers with self-sealing functionality: an enabling strategy for trapping biomacromolecules, Small 13 (47) (2017) 1701949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hwang CM, Park Y, Park JY, Lee K, Sun K, Khademhosseini A, Lee SH, Controlled cellular orientation on PLGA microfibers with defined diameters, Biomed. Microdevices 11 (4) (2009) 739–746. [DOI] [PubMed] [Google Scholar]
- [106].Shin SJ, Park JY, Lee JY, Park H, Park YD, Lee KB, Whang CM, Lee SH, “On the fly” continuous generation of alginate fibers using a microfluidic device, Langmuir 23 (17) (2007) 9104–9108. [DOI] [PubMed] [Google Scholar]
- [107].Ellis MJ, Chaudhuri JB, Poly(lactic-co-glycolic acid) hollow fibre membranes for use as a tissue engineering scaffold, Biotechnol. Bioeng 96 (1) (2007) 177–187. [DOI] [PubMed] [Google Scholar]
- [108].Lee KH, Shin SJ, Park Y, Lee SH, Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications, Small 5 (11) (2009) 1264–1268. [DOI] [PubMed] [Google Scholar]
- [109].Shi X, Ostrovidov S, Zhao Y, Liang X, Kasuya M, Kurihara K, Nakajima K, Bae H, Wu H, Khademhosseini A, Microfluidic spinning of cell-responsive grooved microfibers, Adv. Funct. Mater 25 (15) (2015) 2250–2259. [Google Scholar]
- [110].Kang E, Jeong G, Choi Y, Lee K, Khademhosseini A, Lee S-H, Digitally tunable physicochemical coding of material composition and topography in continuous microfibres, Nat. Mater 10 (11) (2011) 877–883. [DOI] [PubMed] [Google Scholar]
- [111].Kai D, Prabhakaran MP, Jin G, Ramakrishna S, Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering, J. Biomed. Mater. Res. B Appl. Biomater 98 B (2) (2011) 379–386. [DOI] [PubMed] [Google Scholar]
- [112].Zhao X, Sun X, Yildirimer L, Lang Q, Lin ZY, Zheng R, Zhang Y, Cui W, Annabi N, Khademhosseini A, Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing, Acta Biomater 49 (2017) 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Masoumi N, Larson BL, Annabi N, Kharaziha M, Zamanian B, Shapero KS, Cubberley AT, Camci-Unal G, Manning KB, Mayer JE, Khademhosseini A, Electrospun PGS: PCL microfibers align human valvular interstitial cells and provide tunable scaffold anisotropy, Adv. Healthc. Mater 3 (6) (2014) 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tamayol A, Najafabadi A, Aliakbarian B, Arab-Tehrany E, Akbari M, Annabi N, Juncker D, Khademhosseini A, Hydrogel templates for rapid manufacturing of bioactive fibers and 3D constructs, Adv. Healthc. Mater 4 (14) (2015) 2146–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K, Matsunaga YT, Shimoyama Y, Takeuchi S, Metre-long cell-laden microfibres exhibit tissue morphologies and functions, Nat. Mater 12 (6) (2013) 584–590. [DOI] [PubMed] [Google Scholar]
- [116].Leng L, McAllister A, Zhang B, Radisic M, Günther A, Mosaic hydrogels: one-step formation of multiscale soft materials, Adv. Mater 24 (27) (2012) 3650–3658. [DOI] [PubMed] [Google Scholar]
- [117].Choi C-H, Yi H, Hwang S, Weitz DA, Lee C-S, Microfluidic fabrication of complex-shaped microfibers by liquid template-aided multiphase microflow, Lab a Chip 11 (8) (2011) 1477–1483. [DOI] [PubMed] [Google Scholar]
- [118].Jun Y, Kang E, Chae S, Lee S-H, Microfluidic spinning of micro- and nano-scale fibers for tissue engineering, Lab a Chip 14 (13) (2014) 2145–2160. [DOI] [PubMed] [Google Scholar]
- [119].Akbari M, Tamayol A, Laforte V, Annabi N, Najafabadi A, Khademhosseini A, Juncker D, Composite living fibers for creating tissue constructs using textile techniques, Adv. Funct. Mater 24 (26) (2014) 4060–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wu Y, Wang L, Guo B, Ma PX, Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy, ACS Nano 11 (6) (2017) 5646–5659. [DOI] [PubMed] [Google Scholar]
- [121].Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D, Fiber-based tissue engineering: progress, challenges, and opportunities, Biotechnol. Adv 31 (5) (2013) 669–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shapira-Schweitzer K, Seliktar D, Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial, Acta Biomater 3 (1) (2007) 33–41. [DOI] [PubMed] [Google Scholar]
- [123].Suhaeri M, Subbiah R, Kim S-H, Kim C-H, Oh S, Kim S-H, Park K, Novel platform of cardiomyocyte culture and coculture via fibroblast-derived matrixcoupled aligned electrospun nanofiber, ACS Appl. Mater. Interfaces 9 (1) (2016) 224–235. [DOI] [PubMed] [Google Scholar]
- [124].Lind JU, Yadid M, Perkins I, O’Connor BB, Eweje F, Chantre CO, Hemphill MA, Yuan H, Campbell PH, Vlassak JJ, Parker KK, Cardiac microphysiological devices with flexible thin-film sensors for higher-throughput drug screening, Lab a Chip 17 (21) (2017) 3692–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bian W, Liau B, Badie N, Bursac N, Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues, Nat. Protoc 4 (10) (2009) 1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hinton TJ, Jallerat Q, Palchesko RN, Park J, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW, Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels, Sci. Adv 1 (9) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Cheng J, Jun Y, Qin J, Lee S-H, Electrospinning versus microfluidic spinning of functional fibers for biomedical applications, Biomaterials 114 (2017) 121–143. [DOI] [PubMed] [Google Scholar]
- [128].Borriello A, Guarino V, Schiavo L, Alvarez-Perez MA, Ambrosio L, Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle, J. Mater. Sci. Mater. Med 22 (4) (2011) 1053–1062. [DOI] [PubMed] [Google Scholar]
- [129].Masoumi N, Annabi N, Assmann A, Larson BL, Hjortnaes J, Alemdar N, Kharaziha M, Manning KB, Mayer JE, Khademhosseini A, Tri-layered elastomeric scaffolds for engineering heart valve leaflets, Biomaterials 35 (27) (2014) 7774–7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hu J, Tian L, Prabhakaran MP, Ding X, Ramakrishna S, Fabrication of nerve growth factor encapsulated aligned poly ( -caprolactone) nanofibers and their assessment as a potential neural tissue engineering scaffold, Polymers 8 (2) (2016) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Nagarajan S, Soussan L, Bechelany M, Teyssier C, Cavaillès V, Pochat-Bohatier C, Miele P, Kalkura N, Janot J-M, Balme S, Novel biocompatible electrospun gelatin fiber mats with antibiotic drug delivery properties, J. Mater. Chem. B 4 (6) (2016) 1134–1141. [DOI] [PubMed] [Google Scholar]
- [132].Wong H, Lam C, Wen F, Chong S, Tan N, Jerry C, Pal M, Tan L, Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers, Biofabrication 8 (1) (2016) 15004. [DOI] [PubMed] [Google Scholar]
- [133].Breukers RD, Gilmore KJ, Kita M, Wagner KK, Higgins MJ, Moulton SE, Clark GM, Officer DL, Kapsa RMI, Wallace GG, Creating conductive structures for cell growth: growth and alignment of myogenic cell types on polythiophenes, J. Biomed. Mater. Res 95A (1) (2010) 256–268. [DOI] [PubMed] [Google Scholar]
- [134].Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE, Accordion-like honeycombs for tissue engineering of cardiac anisotropy, Nat. Mater 7 (12) (2008) 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kim H, Jiao A, Hwang NS, Kim M, Kang D, Kim D-H, Suh K-Y, Nanotopography-guided tissue engineering and regenerative medicine, Adv. Drug Deliv. Rev 65 (4) (2013) 536–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Trantidou T, Rao C, Barrett H, Camelliti P, Pinto K, Yacoub MH, Athanasiou T, Toumazou C, Terracciano CM, Prodromakis T, Selective hydrophilic modification of Parylene C films: a new approach to cell micro-patterning for synthetic biology applications, Biofabrication 6 (2) (2014) 25004. [DOI] [PubMed] [Google Scholar]
- [137].Ma Z, Wang J, Loskill P, Huebsch N, Koo S, Svedlund FL, Marks NC, Hua EW, Grigoropoulos CP, Conklin BR, Healy KE, Self-organizing human cardiac microchambers mediated by geometric confinement, Nat. Commun 6 (1) (2015) 7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bian W, Bursac N, Engineered skeletal muscle tissue networks with controllable architecture, Biomaterials 30 (7) (2009) 1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A, Directed 3D cell alignment and elongation in microengineered hydrogels, Biomaterials 31 (27) (2010) 6941–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Castaño AG, Hortigüela V, Lagunas A, Cortina C, Montserrat N, Samitier J, Martínez E, Protein patterning on hydrogels by direct microcontact printing: application to cardiac differentiation, RSC Adv 4 (55) (2014) 29120–29123. [Google Scholar]
- [141].Agarwal A, Farouz Y, Nesmith A, Deravi LF, McCain M, Parker K, Micropatterning alginate substrates for in vitro cardiovascular muscle on a chip, Adv. Funct. Mater 23 (30) (2013) 3738–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Badie N, Bursac N, Novel micropatterned cardiac cell cultures with realistic ventricular microstructure, Biophys. J 96 (9) (2009) 3873–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker K, Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues, Biomaterials 35 (21) (2014) 5462–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, Weiss AS, Highly elastic micropatterned hydrogel for engineering functional cardiac tissue, Adv. Funct. Mater 23 (39) (2013) 4950–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Liau B, Christoforou N, Leong KW, Bursac N, Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function, Biomaterials 32 (35) (2011) 9180–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, 25th anniversary article: rational design and applications of hydrogels in regenerative medicine, Adv. Mater 26 (1) (2014) 85–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].McCall JD, Anseth KS, Thiol–ene photopolymerizations provide a facile method to encapsulate proteins and maintain their bioactivity, Biomacromolecules 13 (8) (2012) 2410–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Brown TE, Anseth KS, Spatiotemporal hydrogel biomaterials for regenerative medicine, Chem. Soc. Rev 46 (21) (2017) 6532–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lee SH, Moon JJ, West JL, Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration, Biomaterials 29 (20) (2008) 2962–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels, Biomaterials 31 (21) (2010) 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS, Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility, Biomaterials 30 (35) (2009) 6702–6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Noshadi I, Hong S, Sullivan KE, Sani E, Portillo-Lara R, Tamayol A, Shin S, Gao AE, Stoppel WL, Iii LD, Khademhosseini A, Annabi N, In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels, Biomater. Sci 5 (10) (2017) 2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Nguyen AK, Gittard SD, Koroleva A, Schlie S, Gaidukeviciute A, Chichkov BN, Narayan RJ, Two-photon polymerization of polyethylene glycol diacrylate scaffolds with riboflavin and triethanolamine used as a water-soluble photoinitiator, Regen. Med 8 (6) (2013) 725–738. [DOI] [PubMed] [Google Scholar]
- [154].Liska R, Schuster M, Inführ R, Turecek C, Fritscher C, Seidl B, Schmidt V, Kuna L, Haase A, Varga F, Lichtenegger H, Stampfl J, Photopolymers for rapid prototyping, J. Coating Technol. Res 4 (4) (2007) 505–510. [Google Scholar]
- [155].Saini H, Navaei A, Putten A, Nikkhah M, 3D cardiac microtissues encapsulated with the Co-Culture of cardiomyocytes and cardiac fibroblasts, Adv. Healthc. Mater 4 (13) (2015) 1961–1971. [DOI] [PubMed] [Google Scholar]
- [156].Salick MR, Napiwocki BN, Sha J, Knight GT, Chindhy SA, Kamp TJ, Ashton RS, Crone WC, Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes, Biomaterials 35 (15) (2014) 4454–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Karp JM, Yeo Y, Geng W, Cannizarro C, Yan K, Kohane DS, Vunjak-Novakovic G, Langer RS, Radisic M, A photolithographic method to create cellular micropatterns, Biomaterials 27 (27) (2006) 4755–4764. [DOI] [PubMed] [Google Scholar]
- [158].Sunyer R, Jin AJ, Nossal R, Sackett DL, Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response, PLoS One 7 (10) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tong X, Jiang J, Zhu D, Yang F, Hydrogels with dual gradients of mechanical and biochemical cues for deciphering cell-niche interactions, ACS Biomater. Sci. Eng 2 (5) (2016) 845–852. [DOI] [PubMed] [Google Scholar]
- [160].Choi Y, Vincent LG, Lee AR, Kretchmer KC, Chirasatitsin S, Dobke MK, Engler AJ, The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices, Biomaterials 33 (29) (2012) 6943–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour AJ, Ardell JL, Shivkumar K, Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system, J. Physiol 594 (2) (2016) 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yong K, Li Y, Huang G, Lu T, Safwani W, Pingguan-Murphy B, Xu F, Mechanoregulation of cardiac myofibroblast differentiation: implications for cardiac fibrosis and therapy, Am. J. Physiol. Heart Circ. Physiol 309 (4) (2015). [DOI] [PubMed] [Google Scholar]
- [163].Tse JR, Engler AJ, Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate, PLoS One 6 (1) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Arcaute K, Mann BK, Wicker RB, Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells, Ann. Biomed. Eng 34 (9) (2006) 1429–1441. [DOI] [PubMed] [Google Scholar]
- [165].Gauvin R, Chen Y-C, Lee J, Soman P, Zorlutuna P, Nichol JW, Bae H, Chen S, Khademhosseini A, Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography, Biomaterials 33 (15) (2012) 3824–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Xu T, Zhao W, Zhu J-M, Albanna MZ, Yoo JJ, Atala A, Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology, Biomaterials 34 (1) (2013) 130–139. [DOI] [PubMed] [Google Scholar]
- [167].Ozbolat IT, Hospodiuk M, Current advances and future perspectives in extrusionbased bioprinting, Biomaterials 76 (2016) 321–343. [DOI] [PubMed] [Google Scholar]
- [168].Culver JC, Hoffmann JC, Poché RA, Slater JH, West JL, Dickinson ME, Three-dimensional biomimetic patterning in hydrogels to guide cellular organization, Adv. Mater 24 (17) (2012) 2344–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ali M, Pages E, Ducom A, Fontaine A, Guillemot F, Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution, Biofabrication 6 (4) (2014) 45001. [DOI] [PubMed] [Google Scholar]
- [170].Murphy SV, Atala A, 3D bioprinting of tissues and organs, Nat. Biotechnol 32 (8) (2014) 773–785. [DOI] [PubMed] [Google Scholar]
- [171].Jungst T, Smolan W, Schacht K, Scheibel T, Groll J, Strategies and molecular design criteria for 3D printable hydrogels, Chem. Rev 116 (3) (2016) 1496–1539. [DOI] [PubMed] [Google Scholar]
- [172].Lee VK, Lanzi AM, Ngo H, Yoo S-S, Vincent PA, Dai G, Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology, Cell. Mol. Bioeng 7 (3) (2014) 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials 106 (2016) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gaetani R, Doevendans PA, Metz C, Alblas J, Messina E, Giacomello A, Sluijter J, Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells, Biomaterials 33 (6) (2012) 1782–1790. [DOI] [PubMed] [Google Scholar]
- [175].Shin S, Park S, Park M, Jeong E, Na K, Youn HJ, Hyun J, Cellulose nanofibers for the enhancement of printability of low viscosity gelatin derivatives, BioResources 12 (2) (2017) 2941–2954. [Google Scholar]
- [176].Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nature Biotechnol 34 (3) (2016) 312–319. [DOI] [PubMed] [Google Scholar]
- [177].Sung H-J, Meredith C, Johnson C, Galis ZS, The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis, Biomaterials 25 (26) (2004) 5735–5742. [DOI] [PubMed] [Google Scholar]
- [178].Discher DE, Tissue cells feel and respond to the stiffness of their substrate, Science 310 (5751) (2005) 1139–1143. [DOI] [PubMed] [Google Scholar]
- [179].Bhattacharjee T, Zehnder SM, Rowe KG, Jain S, Nixon RM, Sawyer WG, Angelini TE, Writing in the granular gel medium, Sci. Adv 1 (8) (2015) e1500655–e1500655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Bhattacharjee T, Gil CJ, Marshall SL, Urueña JM, O’Bryan CS, Carstens M, Keselowsky B, Palmer GD, Ghivizzani S, Gibbs CP, Sawyer WG, Angelini TE, Liquid-like solids support cells in 3D, ACS Biomater. Sci. Eng 2 (10) (2016) 1787–1795. [DOI] [PubMed] [Google Scholar]
- [181].Jastrzebska E, Tomecka E, Jesion I, Heart-on-a-chip based on stem cell biology, Biosens. Bioelectron 75 (2016) 67–81. [DOI] [PubMed] [Google Scholar]
- [182].Zhang YS, Aleman J, Arneri A, Bersini S, Piraino F, Shin SR, Dokmeci MR, Khademhosseini A, From cardiac tissue engineering to heart-on-a-chip: beating challenges, Biomed. Mater 10 (3) (2015) 034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Selimovic S, Dokmeci MR, Khademhosseini A, Organs-on-a-chip for drug discovery, Curr. Opin. Pharmacol 13 (5) (2013) 829–833. [DOI] [PubMed] [Google Scholar]
- [184].Cho S, Yoon JY, Organ-on-a-chip for assessing environmental toxicants, Curr. Opin. Biotechnol 45 (2017) 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Xin M, Olson EN, Bassel-Duby R, Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair, Nat. Rev. Mol. Cell Biol 14 (8) (2013) 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing, Nat. Mater 16 (3) (2017) 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lee JM, Kim JE, Kang E, Lee SH, Chung BG, An integrated microfluidic culture device to regulate endothelial cell differentiation from embryonic stem cells, Electrophoresis 32 (22) (2011) 3133–3137. [DOI] [PubMed] [Google Scholar]
- [188].Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, Conklin BR, Grigoropoulos CP, Healy KE, Three-dimensional filamentous human diseased cardiac tissue model, Biomaterials 35 (5) (2014) 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT, Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies, Nat. Med 20 (6) (2014) 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Park JY, Takayama S, Lee S-H, Regulating microenvironmental stimuli for stem cells and cancer cells using microsystems, Integrat. Biol 2 (5–6) (2010) 229–240. [DOI] [PubMed] [Google Scholar]
- [191].Kang E, Choi YY, Jun Y, Chung BG, Lee S-H, Development of a multi-layer microfluidic array chip to culture and replate uniform-sized embryoid bodies without manual cell retrieval, Lab a Chip 10 (20) (2010) 2651–2654. [DOI] [PubMed] [Google Scholar]
- [192].Wan C.-r., Chung S, Kamm RD, Differentiation of embryonic stem cells into cardiomyocytes in a compliant microfluidic system, Ann. Biomed. Eng 39 (6) (2011) 1840–1847. [DOI] [PubMed] [Google Scholar]
- [193].Moya ML, Hsu YH, Lee AP, Hughes CC, George SC, In vitro perfused human capillary networks, Tissue Eng. C Meth 19 (9) (2013) 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ruan JL, Tulloch NL, Saiget M, Paige SL, Razumova MV, Regnier M, Tung KC, Keller G, Pabon L, Reinecke H, Murry CE, Mechanical stress promotes maturation of human myocardium from pluripotent stem cell-derived progenitors, Stem Cell 33 (7) (2015) 2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Shimko VF, Claycomb WC, Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes, Tissue Eng 14 (1) (2008) 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Pavesi A, Adriani G, Rasponi M, Zervantonakis IK, Fiore GB, Kamm RD, Controlled electromechanical cell stimulation on-a-chip, Sci. Rep 5 (2015) 11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Huang Y, Jia X, Bai K, Gong X, Fan Y, Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells, Arch. Med. Res 41 (7) (2010) 497–505. [DOI] [PubMed] [Google Scholar]
- [198].Huang Y, Zheng L, Gong X, Jia X, Song W, Liu M, Fan Y, Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells, PLoS One 7 (4) (2012) e34960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Xiao Y, Zhang B, Hsieh A, Thavandiran N, Martin C, Radisic M, Chapter 12-Microfluidic Cell Culture Techniques, Microfluidic Cell Culture Systems, William Andrew Publishing, Oxford, 2013, pp. 303–321. [Google Scholar]
- [200].Ghasemi-Mobarakeh L, Prabhakaran MP, Nematollahi M, Karbalaie K, Ramakrishna S, Nasr-Esfahani MH, Embryonic stem cell differentiation to cardiomyocytes on nanostructured scaffolds for myocardial tissue regeneration, Int. J. Polym. Mater. Polym. Biomater 63 (5) (2014) 240–245. [Google Scholar]
- [201].Bhuthalingam R, Lim PQ, Irvine SA, Agrawal A, Mhaisalkar PS, An J, Chua CK, Venkatraman S, A novel 3D printing method for cell alignment and differentiation, Int. J. Bioprint 1 (1) (2015) 57–65. [Google Scholar]
- [202].Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA, The effect of matrix composition of 3D constructs on embryonic stem cell differentiation, Biomaterials 26 (31) (2005) 6194–6207. [DOI] [PubMed] [Google Scholar]
- [203].Lee KH, Kwon GH, Shin SJ, Baek JY, Han DK, Park Y, Lee SH, Hydrophilic electrospun polyurethane nanofiber matrices for hMSC culture in a microfluidic cell chip, J. Biomed. Mater. Res 90 (2) (2009) 619–628. [DOI] [PubMed] [Google Scholar]
- [204].Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ, The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat, Biomaterials 31 (27) (2010) 7012–7020. [DOI] [PubMed] [Google Scholar]
- [205].Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, Vunjak-Novakovic G, Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species, Exp. Cell Res 315 (20) (2009) 3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Tandon N, Marsano A, Maidhof R, Numata K, Montouri-Sorrentino C, Cannizzaro C, Voldman J, Vunjak-Novakovic G, Surface-patterned electrode bioreactor for electrical stimulation, Lab a Chip 10 (6) (2010) 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ma Z, Liu Q, Liu H, Yang H, Yun JX, Eisenberg C, Borg TK, Xu M, Gao BZ, Laser-patterned stem-cell bridges in a cardiac muscle model for on-chip electrical conductivity analyses, Lab a Chip 12 (3) (2012) 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Takeuchi A, Shimba K, Takayama Y, Kotani K, Lee JK, Noshiro M, Jimbo Y, Microfabricated device for co-culture of sympathetic neuron and iPS-derived cardiomyocytes, 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2013, pp. 3817–3820. [DOI] [PubMed] [Google Scholar]
- [209].Myers FB, Zarins CK, Abilez OJ, Lee LP, Label-free electrophysiological cytometry for stem cell-derived cardiomyocyte clusters, Lab a Chip 13 (2) (2013) 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Sraj I, Marr DWM, Eggleton CD, Linear diode laser bar optical stretchers for cell deformation, Biomed. Opt. Express 1 (2) (2010) 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang H-Z, Rosati B, Brink PR, Cohen IS, Entcheva E, Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery, circulation, Arrhythm. Electrophysiol 4 (5) (2011) 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Zhuge Y, Patlolla B, Ramakrishnan C, Beygui RE, Zarins CK, Deisseroth K, Kuhl E, Abilez OJ, Human pluripotent stem cell tools for cardiac optogenetics, Conf. Proc. IEEE Eng. Med. Biol. Soc 2014 (2014) 6171–6174. [DOI] [PubMed] [Google Scholar]
- [213].Geuss LR, Wu DC, Ramamoorthy D, Alford CD, Suggs LJ, Paramagnetic beads and magnetically mediated strain enhance cardiomyogenesis in mouse embryoid bodies, PLoS One 9 (12) (2014) e113982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Tsai MT, Li WJ, Tuan RS, Chang WH, Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation, J. Orthop. Res 27 (9) (2009) 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M, Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues, Lab a Chip 16 (3) (2016) 599–610. [DOI] [PubMed] [Google Scholar]
- [216].Dahl KN, Kalinowski A, Pekkan K, Mechanobiology and the microcirculation: cellular, nuclear and fluid mechanics, Microcirculation 17 (3) (2010) 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Simmons CS, Petzold BC, Pruitt BL, Microsystems for biomimetic stimulation of cardiac cells, Lab a Chip 12 (18) (2012) 3235–3248. [DOI] [PubMed] [Google Scholar]
- [218].Schroer AK, Shotwell MS, Sidorov VY, Wikswo JP, Merryman WD, I-Wire Heart-on-a-Chip II: biomechanical analysis of contractile, three-dimensional cardiomyocyte tissue constructs, Acta Biomater 48 (2017) 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Börnchen C, Müller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation, J. Mol. Cell. Cardiol 74 (2014) 151–161. [DOI] [PubMed] [Google Scholar]
- [220].Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G, Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds, Proc. Natl. Acad. Sci. Unit. States Am 101 (52) (2004) 18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Dorn T, Goedel A, Lam JT, Haas J, Tian Q, Herrmann F, Bundschu K, Dobreva G, Schiemann M, Dirschinger R, Direct Nkx2-5 transcriptional repression of Isl1 controls cardiomyocyte subtype identity, Stem Cell 33 (4) (2015) 1113–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Bulka M, Jastrzebska E, Heart-on-a-chip systems, in: Brzozka Z, Jastrzebska E (Eds.), Cardiac Cell Culture Technologies: Microfluidic and On-chip Systems, Springer International Publishing, Cham, 2018, pp. 169–199. [Google Scholar]
- [223].Shin SR, Zhang YS, Kim D-J, Manbohi A, Avci H, Silvestri A, Aleman J, Hu N, Kilic T, Keung W, Aptamer-based microfluidic electrochemical biosensor for monitoring cell-secreted trace cardiac biomarkers, Anal. Chem 88 (20) (2016) 10019–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Mannhardt I, Saleem U, Benzin A, Schulze T, Klampe B, Eschenhagen T, Hansen A, Automated contraction analysis of human engineered heart tissue for cardiac drug safety screening, JoVE: JoVE 122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Yu F, Zhao Y, Gu J, Quigley KL, Chi NC, Tai Y-C, Hsiai TK, Flexible microelectrode arrays to interface epicardial electrical signals with intracardial calcium transients in zebrafish hearts, Biomed. Microdevices 14 (2) (2012) 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Zhang D, Shadrin IY, Lam J, Xian H-Q, Snodgrass HR, Bursac N, Tissueengineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes, Biomaterials 34 (23) (2013) 5813–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Natarajan A, Stancescu M, Dhir V, Armstrong C, Sommerhage F, Hickman JJ, Molnar P, Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform, Biomaterials 32 (18) (2011) 4267–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Chen Y, Chan HN, Michael SA, Shen Y, Chen Y, Tian Q, Huang L, Wu H, A microfluidic circulatory system integrated with capillary-assisted pressure sensors, Lab a Chip 17 (4) (2017) 653–662. [DOI] [PubMed] [Google Scholar]
- [229].Ren L, Liu W, Wang Y, Wang J-C, Tu Q, Xu J, Liu R, Shen S-F, Wang J, Investigation of hypoxia-induced myocardial injury dynamics in a tissue interface mimicking microfluidic device, Anal. Chem 85 (1) (2012) 235–244. [DOI] [PubMed] [Google Scholar]
- [230].Grosberg A, Alford PW, McCain ML, Parker KK, Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip, Lab a Chip 11 (24) (2011) 4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes, Nat. Methods 10 (8) (2013) 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Lasher RA, Pahnke AQ, Johnson JM, Sachse FB, Hitchcock RW, Electrical stimulation directs engineered cardiac tissue to an age-matched native phenotype, J. Tissue Eng 3 (1) (2012) 2041731412455354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Kobuszewska A, Tomecka E, Zukowski K, Jastrzebska E, Chudy M, Dybko A, Renaud P, Brzozka Z, Heart-on-a-Chip: an investigation of the influence of static and perfusion conditions on cardiac (H9C2) cell proliferation, morphology, and alignment, SLAS Technol.: Translat. Life Sci. Innovat 22 (5) (2017) 536–546. [DOI] [PubMed] [Google Scholar]
- [234].Esch EW, Bahinski A, Huh D, Organs-on-chips at the frontiers of drug discovery, Nat. Rev. Drug Discov 14 (4) (2015) 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Zweigerdt R, Gruh I, Martin U, Your heart on a chip: iPSC-based modeling of Barth-syndrome-associated cardiomyopathy, Cell Stem Cell 15 (1) (2014) 9–11. [DOI] [PubMed] [Google Scholar]
- [236].Qureshi ZP, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson KB, Szeinbach SL, Market withdrawal of new molecular entities approved in the United States from 1980 to 2009, Pharmacoepidemiol. Drug Saf 20 (7) (2011) 772–777. [DOI] [PubMed] [Google Scholar]
- [237].Onakpoya IJ, Heneghan CJ, Aronson JK, Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature, BMC Med 14 (2016) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Agarwal A, Goss JA, Cho A, McCain ML, Parker KK, Microfluidic heart on a chip for higher throughput pharmacological studies, Lab a Chip 13 (18) (2013) 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Human iPSC-based cardiac microphysiological system for drug screening applications, Sci. Rep 5 (2015) 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Yue T, Nakajima M, Takeuchi M, Hu C, Huang Q, Fukuda T, On-chip self-assembly of cell embedded microstructures to vascular-like microtubes, Lab a Chip 14 (6) (2014) 1151–1161. [DOI] [PubMed] [Google Scholar]
- [241].Nguyen M-D, Tinney JP, Yuan F, Roussel TJ, El-Baz A, Giridharan G, Keller BB, Sethu P, Cardiac cell culture model as a left ventricle mimic for cardiac tissue generation, Anal. Chem 85 (18) (2013) 8773–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Cheng W, Klauke N, Sedgwick H, Smith GL, Cooper JM, Metabolic monitoring of the electrically stimulated single heart cell within a microfluidic platform, Lab a Chip 6 (11) (2006) 1424–1431. [DOI] [PubMed] [Google Scholar]
- [243].Kaneko T, Kojima K, Yasuda K, An on-chip cardiomyocyte cell network assay for stable drug screening regarding community effect of cell network size, Analyst 132 (9) (2007) 892–898. [DOI] [PubMed] [Google Scholar]
- [244].Ges IA, Dzhura IA, Baudenbacher FJ, On-chip acidification rate measurements from single cardiac cells confined in sub-nanoliter volumes, Biomed. Microdevices 10 (3) (2008) 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Nguyen M-D, Tinney JP, Ye F, Elnakib AA, Yuan F, El-Baz A, Sethu P, Keller BB, Giridharan GA, Effects of physiologic mechanical stimulation on embryonic chick cardiomyocytes using a microfluidic cardiac cell culture model, Anal. Chem 87 (4) (2015) 2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Cheah L-T, Dou Y-H, Seymour A-ML, Dyer CE, Haswell SJ, Wadhawan JD, Greenman J, Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species, Lab a Chip 10 (20) (2010) 2720–2726. [DOI] [PubMed] [Google Scholar]
- [247].Pong T, Adams WJ, Bray M-A, Feinberg AW, Sheehy SP, Werdich AA, Parker KK, Hierarchical architecture influences calcium dynamics in engineered cardiac muscle, Exp. Biol. Med 236 (3) (2011) 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G, Optimization of electrical stimulation parameters for cardiac tissue engineering, J. Tissue Eng. Regen. Med 5 (6) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S, Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering, Tissue Eng. C Meth 16 (6) (2010) 1417–1426. [DOI] [PubMed] [Google Scholar]
- [250].McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS, In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces, J. Biomed. Mater. Res 60 (3) (2002) 472–479. [DOI] [PubMed] [Google Scholar]
- [251].Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M, Microfluidic patterning for fabrication of contractile cardiac organoids, Biomed. Microdevices 9 (2) (2007) 149–157. [DOI] [PubMed] [Google Scholar]
- [252].Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwörer A, Uebeler J, Eschenhagen T, Development of a drug screening platform based on engineered heart tissue, Circ. Res 107 (1) (2010) 35–44. [DOI] [PubMed] [Google Scholar]
- [253].Smith AS, Macadangdang J, Leung W, Laflamme MA, Kim DH, Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening, Biotechnol. Adv 35 (1) (2017) 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Mathur A, Loskill P, Hong S, Lee J, Marcus SG, Dumont L, Conklin BR, Willenbring H, Lee LP, Healy KE, Human induced pluripotent stem cell-based microphysiological tissue models of myocardium and liver for drug development, Stem Cell Res. Ther 4 (Suppl 1) (2013) S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Ribas J, Sadeghi H, Manbachi A, Leijten J, Brinegar K, Zhang YS, Ferreira L, Khademhosseini A, Cardiovascular organ-on-a-chip platforms for drug discovery and development, Appl. In Vitro Toxicol 2 (2) (2016) 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


