Abstract
Background
The purpose of this study was to compare 3-year overall survival after simultaneous portal (PVE) and hepatic vein (HVE) embolization versus PVE alone in patients undergoing liver resection for primary and secondary cancers of the liver.
Methods
In this multicentre retrospective study, all DRAGON 0 centres provided 3-year follow-up data for all patients who had PVE/HVE or PVE, and were included in DRAGON 0 between 2016 and 2019. Kaplan–Meier analysis was undertaken to assess 3-year overall and recurrence/progression-free survival. Factors affecting survival were evaluated using univariable and multivariable Cox regression analyses.
Results
In total, 199 patients were included from 7 centres, of whom 39 underwent PVE/HVE and 160 PVE alone. Groups differed in median age (P = 0.008). As reported previously, PVE/HVE resulted in a significantly higher resection rate than PVE alone (92 versus 68%; P = 0.007). Three-year overall survival was significantly higher in the PVE/HVE group (median survival not reached after 36 months versus 20 months after PVE; P = 0.004). Univariable and multivariable analyses identified PVE/HVE as an independent predictor of survival (univariable HR 0.46, 95% c.i. 0.27 to 0.76; P = 0.003).
Conclusion
Overall survival after PVE/HVE is substantially longer than that after PVE alone in patients with primary and secondary liver tumours.
The multicentre international retrospective DRAGON 0 study showed that simultaneous portal (PVE) and hepatic vein (HVE) embolization in patients with cancer in the liver increases the future liver remnant hypertrophy, kinetic growth rate, and resection rate compared with PVE alone. Mid-term oncological follow-up showed that median 3-year overall survival was significantly increased in the PVE/HVE group.
Introduction
Partial resection of the liver is central to the oncological treatment strategy to extend overall survival associated with several liver cancers, including colorectal liver metastases (CRLMs), hepatocellular carcinoma, and cholangiocarcinoma. Favourable long-term outcomes are achieved when complete resection can be accomplished. Resectability, however, is limited by the capacity of the future liver remnant (FLR), so only 30% of patients with primary or secondary cancers to the liver qualify for resection1,2.
FLR hypertrophy-inducing procedures help to overcome that limitation, and aim to improve volume and function of the FLR before major liver resection in patients with a small FLR at risk of posthepatectomy liver failure (PHLF). In general, to prevent PHLF, a FLR of 30% in patients without underlying liver disease (for example fibrosis or cirrhosis) is considered as a safe cut-off for major liver resections3–5.
Portal vein embolization (PVE) is the current standard FLR hypertrophy-inducing procedure6–8. PVE-induced hypertrophy, however, is relatively slow and often results in a long wait to gain sufficient liver growth. As a result, approximately 30–40% of patients do not undergo resection, mainly owing to tumour progression while awaiting sufficient liver growth9. Rapid FLR growth may help to prevent this failure to progress to resection10. Moreover, studies11 have shown that a high kinetic growth rate (KGR) yields low morbidity after partial hepatectomy.
FLR hypertrophy can be accelerated by the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure, in which portal vein collaterals are prevented by surgical transection (in situ split) of the liver parenchyma in addition to portal vein ligation12. Despite increased FLR hypertrophy resulting in an improved resection rate, ALPPS requires two operations, and is associated with a high morbidity and mortality rate mainly associated with PHLF13,14. Therefore, ALPPS is considered to be a high-risk alternative to PVE.
Contemporary oncological care is characterized by optimization of surgical care pathways, personalization of treatments, and a fast-growing use of percutaneous approaches to minimize the impact on the patient. Within that perspective, simultaneous embolization of the portal and hepatic veins (PVE/HVE) has been introduced. Multiple studies have shown that PVE/HVE induces rapid liver hypertrophy to a similar extent as observed in ALPPS, without the need for a highly invasive procedure and associated morbidity and mortality15. The technique, in which the hepatic outflow is occluded in addition to conventional PVE, was first described by Guiu et al.16. However, Guiu and colleagues additionally injected glue into the hepatic veins and described the procedure as liver venous deprivation (LVD). Many centres perform a simplified version of LVD, called PVE/HVE or ‘double embolization’, whereby one or two hepatic veins are occluded with vascular plugs only. The DRAGON collaborative, an international group of 70 major hepatopancreatobiliary centres set up to study PVE/HVE prospectively, decided in consensus to refrain from the additional application of glue to minimize the risk of migration of embolization material.
The recent retrospective observational DRAGON 0 study demonstrated a substantially increased resection rate, comparable to that of ALPPS, after PVE/HVE, with a safety profile similar to that of PVE alone17. These findings were confirmed in a meta-analysis of all published comparative studies comparing PVE/HVE versus PVE alone, and fit well in the contemporary development of minimization of the physical impact of oncological treatments18,19. It has not yet been determined whether this also translates into better oncological outcomes. Therefore, the aim of this study was to assess the 3-year overall survival and recurrence/progression-free survival of the retrospective DRAGON 0 cohort, currently the largest comparative series on PVE/HVE.
Methods
Ethical approval
The study was approved by the ethical review board azM/UM (approval number 2019-1375) and conducted in accordance with the Declaration of Helsinki of 1996.
Study design and settings
This study was designed as a multicentre retrospective cohort study in which seven centres of the DRAGON collaborative enrolled patients who had PVE or PVE/HVE between January 2016 and December 2019. The primary manuscript, assessing hypertrophy and resection rate, was published in 202117. For the present study, all centres were additionally requested to provided 3-year follow-up data on all enrolled patients. In the event of insufficient liver growth following PVE, a cross-over to rescue HVE did not occur in any patient. Patients in whom surgical resection failed for any reason after embolization (either PVE or PVE/HVE) were included in the overall survival analysis and recurrence/progression-free survival analysis by intention to treat. Data are reported in accordance with the STROBE reporting guidelines for cohort studies20.
Participants
All centres in the international DRAGON collaborative that undertook more than five PVE/HVE procedures between 2016 and 2019 provided patient, imaging, and follow-up data from local databases, multidisciplinary tumour board records, planning and operation logs, and embolization protocols. If follow-up data were missing, the centres’ active surveillance programmes, patients and primary-care physicians were contacted.
Variables
The primary endpoint of this analysis was 3-year overall survival. Secondary variables of interest were 3-year recurrence/progression-free survival, volumetric data, and postoperative outcomes including 90-day mortality. Volumetric data for the liver were specified as standardized FLR (sFLR) based on Vauthey's formula (18.51 × bodyweight + 191.8)21. Liver growth was provided as KGR, calculated as the difference between the sFLR before and after embolization divided by the time elapsed between the intervention and first volumetric assessment11. The surgical resection procedure was classified according to the Brisbane terminology (right hepatectomy: segments V–VIII; extended right hepatectomy: segments IV–VIII by indication including segment I; left hepatectomy: segments II–IV; extended left hepatectomy: segments II–IV + V and VIII)22. Postoperative complications and PHLF were assessed according to the Clavien–Dindo classification23 and International Study Group of Liver Surgery (ISGLS) criteria24 respectively. Tumour type and surgical resection status (R) were based on the final pathology reports of the individual hospitals.
Subgroups
A subgroup analysis was undertaken for patients with CRLM because such patients represent the largest cohort in this study. Additionally, the 3-year oncological outcome was assessed using the 1 : 1 matched subgroup from the previous DRAGON 0 publication17. The 1 : 1 match was carried out in consideration of following parameters: age, Charlson index, cirrhosis, diabetes, whether the patient received bevacizumab, and interval from intervention to first volumetric assessment.
Sensitivity analysis for time bias
Owing to the retrospective nature of the study, selection bias could not be avoided. Likewise, an era bias could not be ruled out because of the increased performance of PVE/HVE in recent years. To check the era bias, a multivariable analysis was conducted corrected for year of embolization. The decision to resect was not based on a prospectively defined FLR volume cut-off; however, all participating centres accepted a FLR volume cut-off of 30% for normal livers and 40% for patients with underlying liver disease.
Statistical analysis
Categorical variables are presented as number of patients with percentage, and continuous variables as median (i.q.r.). To compare groups, Mann–Whitney U test was used for continuous data, and χ2 test or Fisher's exact test for categorical variables. Kaplan–Meier analysis in combination with the log rank test was performed to analyse 3-year recurrence/progression-free and overall survival. If there was no evidence of survival status (deceased or still alive), the last date when the patient was seen was used as censoring date. A univariable Cox regression analysis was used to assess the group effect (PVE/HVE versus PVE alone). Based on available evidence, factors that might have an impact on survival were considered for the multivariable analysis using the all-in technique. Cox regression analysis was used for this analysis. Analyses were undertaken and graphics created using JMP® 15.0 (SAS Institute, Cary, NC, USA) and GraphPad® Prism (GraphPad Software, La Jolla, CA, USA). Two-sided P ≤ 0.050 was considered statistically significant.
Results
In 7 centres of the DRAGON collaborative that provided data for both the initial comparative series and this oncological follow-up analysis, 39 patients underwent PVE/HVE and 160 patients PVE alone (Fig. 1). There was no significant difference in demographics, except for patient age (Table 1). CRLM was the most common tumour type.
Fig. 1.
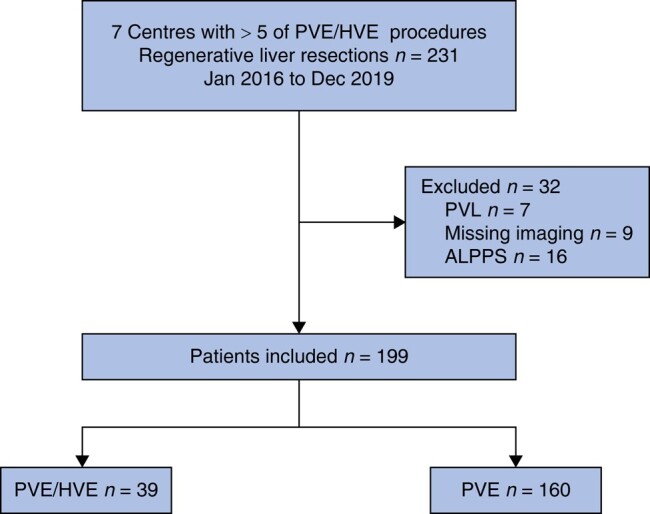
Study flow chart
PVE, portal vein embolization; HVE, hepatic vein embolization; PVL portal vein ligation; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
Table 1.
Patient demographics
| PVE/HVE (n = 39) | PVE (n = 160) | P* | |
|---|---|---|---|
| Age (years), median (i.q.r.) | 63 (52–67) | 67 (58–73) | 0.008† |
| Sex | 0.359 | ||
| Female | 18 (46) | 61 (38) | |
| Male | 21 (54) | 99 (62) | |
| BMI (kg/m2), median (i.q.r.) | 24.4 (23–27) | 25.2 (23–28) | 0.307† |
| Cirrhosis | 1 (3) | 12 (8) | 0.257 |
| Diabetes | 4 (10) | 28 (18) | 0.270 |
| Type of tumour | 0.515 | ||
| CRLM | 19 (49) | 85 (53) | |
| HCC | 4 (10) | 11 (7) | |
| IHCC | 4 (10) | 22 (14) | |
| PHCC | 5 (13) | 25 (15) | |
| GBCA | 4 (10) | 9 (6) | |
| Other | 3 (8) | 8 (5) | |
| Neoadjuvant chemotherapy | 19 (49) | 77 (48) | 0.758 |
| FOLFOX/XELOX | 14 (74) | 50 (65) | |
| FOLFIRI/XELIRI | 2 (11) | 13 (17) | |
| FOLFIRINOX | 2 (11) | 10 (13) | |
| Other | 1 (5) | 4 (5) | |
| Biological agent | 13 (68) | 60 (71) | 0.766 |
| Bevacizumab | 4 (31) | 39 (65) | |
| Cetuximab/panitumumab | 9 (69) | 21 (35) | |
| Volumetric data | |||
| sFLR1, median (i.q.r.) | 18 (16–23) | 19 (15–25) | 0.804† |
| Interval from intervention to first volumetry (days), median (i.q.r.) | 17 (13–32) | 24 (19–37) | 0.009† |
| sFLR2, median (i.q.r.) | 31 (24–39) | 28 (21–37) | 0.102† |
| % hypertrophy, median (i.q.r.) | 59 (4579) | 48 (24–69) | 0.020† |
| Kinetic growth rate during interval from intervention to first volumetry (per week), median (i.q.r.) | 3.5 (2.2–7.1) | 2.5 (1.1–3.8) | < 0.001† |
Values are n (%) unless otherwise indicated. PVE, portal vein embolization; HVE, hepatic vein embolization; CRLM, colorectal liver metastasis; HCC, hepatocellular carcinoma; IHCC, intrahepatic cholangiocarcinoma; PHCC, perihilar cholangiocarcinoma; GBCA, gallbladder cancer; FOLFOX, 5-Fluorouracil/Oxaliplatin; XELOX, Capecitabine/Oxaliplatin; FOLFIRI, 5-Fluorouracil/Irinotecan; XELIRI, Capecitabine/Irinotecan; FOLFIRINOX, 5-fluorouracil/Irinotecan/Leucovorin/Oxaliplatine; sFLR, standardized future liver remnant. *χ2 or Fisher's exact test, except †Mann–Whitney U test.
Updated follow-up data indicated that 92% of patients who underwent PVE/HVE had tumour resection. This was 2% higher than noted in the initial report as one patient was initially considered not to have undergone resection owing to insufficient liver growth in the primary study interval17. In the PVE group, the resection rate was still 68% (P = 0.007).
The KGR was significantly higher after PVE/HVE compared with PVE alone. More patients underwent extended liver resections after PVE/HVE, whereas operating times were longer in the PVE group (Table 2). There were no significant differences in postoperative complications (Clavien–Dindo), PHLF (ISGLS), and 90-day mortality between the two groups.
Table 2.
Operative clinical data and oncological outcomes
| PVE/HVE (n = 39) | PVE (n = 160) | P* | |
|---|---|---|---|
| Resection | 0.007 | ||
| Feasible | 36 (92) | 109 (68) | |
| Failed | 3 | 51 | |
| Progression of disease | 2 (67) | 31(61) | |
| Insufficient liver growth | 0 (0) | 17(33) | |
| Postinterventional complications | 1(33) | 3(6) | |
| Interval from intervention to resection (days), median (i.q.r.) | 37 (21–52) | 41 (28–61) | 0.132† |
| Type of resection | < 0.001 | ||
| Right hepatectomy | 5 (14) | 55 (50) | |
| Extended right hepatectomy | 30 (83) | 50 (46) | |
| Left hepatectomy | 0 (0) | 2 (2) | |
| Extended left hepatectomy | 1 (3) | 2 (2) | |
| Duration of operation (min), median (i.q.r.) | 321 (210–443) | 385 (311–435) | 0.044† |
| Blood loss (ml), median (i.q.r.) | 800 (500–1450) | 650 (400–1500) | 0.708† |
| Major complications (≥ Clavien–Dindo IIIA) | 9 (23) | 37 (34) | 0.546 |
| PHLF according to ISGLS criteria | 4 (10) | 27 (25) | 0.145 |
| Death within 90 days after resection | 1 (3) | 17 (15.6) | 0.065 |
| Oncological outcomes | |||
| Negative resection margin, R0 | 28 of 36 (78) | 85 of 109 (78) | 0.615 |
| Follow-up time (months), median i.q.r.) | 32 | 17 | 0.652‡ |
| Recurrence | 18/36 (50) | 56/109 (51) | 0.559 |
| Death | 12 (31) | 91 (57) | 0.003 |
| Tumour-related death | 8 (21) | 74 (47) | 0.196 |
| Further treatment | 26 (67) | 69 (43) | 0.307 |
| Adjuvant/palliative chemotherapy | 22 (56) | 51 (32) | |
| Surgery for recurrent liver disease | 6 (15) | 12 (8) | |
| Ablation of liver | 5 (13) | 17 (11) | |
| Median overall survival (months) | Not reached | 20 | 0.004‡ |
| Median progression-free survival | 19 | 11 | 0.088‡ |
Values are n (%) unless otherwise indicated. PVE, portal vein embolization; HVE, hepatic vein embolization; PHLF, posthepatectomy liver failure; ISGLS, International Study Group of Liver Surgery; *χ2 or Fisher's exact test, except †Mann–Whitney U test and ‡log rank test.
Survival
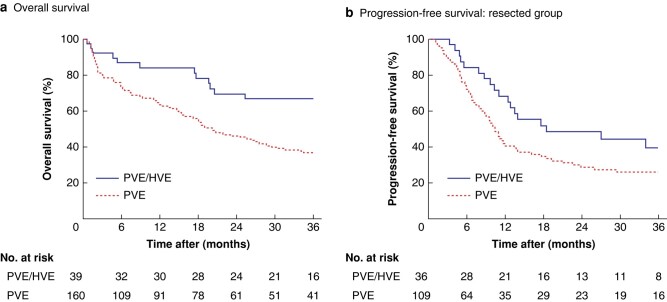
Of the 199 included patients, 19 (10%) were lost to follow-up (4 in the PVE/HVE group and 15 in the PVE-alone group) with respect to the endpoint survival before 36 months. These patients were mainly from referral centres and were marked as censored. Median follow-up was 32 and 17 months after PVE/HVE and PVE respectively (Table 2). In the PVE/HVE group, median overall survival was not reached within 36 months, compared with a median survival time of 20 months after PVE alone (P = 0.004) (Fig. 2). Recurrence/progression-free survival did not differ significantly between the groups.
Fig. 2.
Overall survival, and progression-free survival among patients who underwent resection (total group)
a Overall survival, and b progression-free survival among patients who underwent resection. PVE, portal vein embolization; HVE, hepatic vein embolization. a P = 0.004, b P = 0.088 (log rank test).
Patients who did not undergo surgical resection following PVE/HVE and PVE had significantly worse overall survival (P < 0.001) (Fig. S1). In a survival analysis excluding 90-day deaths, PVE/HVE remained superior to PVE alone (P = 0.014) (Fig. S2). Survival analysis of the 1 : 1 matched subgroup also showed improved survival for PVE/HVE compared with PVE alone (P < 0.001) (Fig. S3).
Multivariable analysis for survival
Univariable Cox regression analysis was undertaken to assess the effect of the intervention (PVE/HVE) compared with the control treatment (PVE) on the mortality rate within 36 months. The HR in this analysis was 0.46 (95% c.i. 0.27 to 0.76; P = 0.003) (Table 3). Two multivariable analyses were also undertaken for the endpoint survival (Tables 4 and 5). To correct for the time of inclusion (time of embolization), to check the era bias, another multivariable analysis was conducted (Table 6). In the first multivariable analysis, patient age, diabetes, cirrhosis, and CRLM were included in addition to the treatment group variable (PVE versus PVE/HVE), while maintaining an appropriate sample size (197 patients). All these factors might be related to survival. The HR for PVE/HVE versus PVE alone was 0.57 (0.33 to 0.99; P = 0.047). In the second multivariable analysis, major complications (at least Clavien–Dindo grade IIIA), complete tumour resection (R0), and blood loss were also included in the model. Owing to missing data in both groups, the cohort size decreased to 145 after including these additional factors. The HR for PVE/HVE versus PVE in this analysis was 0.50 (0.26 to 0.95; P = 0.034). In the two multivariable analyses, age and major complications seemed to have an independent, negative effect on survival. Finally, the HR for PVE/HVE versus PVE in the analysis corrected for year of inclusion was 0.47 (0.24 to 0.92; P = 0.027). Year of inclusion seemed to have no effect on overall survival, with an HR of 0.95 (0.78 to 1.17; P = 0.650).
Table 3.
Univariable analysis of overall survival for treatment group (portal vein embolization versus portal vein embolization/hepatic vein embolization)
| HR | P | |
|---|---|---|
| Treatment group (PVE/HVE versus PVE) | 0.46 (0.27, 0.76) | 0.003 |
Values in parentheses are 95% confidence intervals. The analysis included 197 patients. PVE, portal vein embolization; HVE, hepatic vein embolization.
Table 4.
Multivariable analysis of overall survival for five variables
| HR | P | |
|---|---|---|
| Treatment group (PVE/HVE versus PVE) | 0.57 (0.33, 0.99) | 0.047 |
| Age (years) (continuous) | 1.04 (1.02, 1.06) | < 0.001 |
| Diabetes (yes versus no) | 1.10 (0.68, 1.78) | 0.701 |
| Cirrhosis (yes versus no) | 1.08 (0.51, 2.28) | 0.840 |
| CRLM (yes versus no) | 1.11 (0.77, 1.61) | 0.575 |
Values in parentheses are 95% confidence intervals. The analysis included 197 patients. HR for continuous variables are shown per unit increase, PVE, portal vein embolization; HVE, hepatic vein embolization; CRLM, colorectal liver metastasis.
Table 5.
Multivariable analysis of overall survival for eight variables
| HR | P | |
|---|---|---|
| Treatment group (PVE/HVE versus PVE) | 0.50 (0.26, 0.95) | 0.034 |
| Ages (years) (continuous) | 1.03 (1.01, 1.06) | 0.007 |
| Diabetes (yes versus no) | 1.02 (0.54, 1.93) | 0.949 |
| Cirrhosis (yes versus no) | 1.99 (0.57, 6.89) | 0.280 |
| CRLM (yes versus no) | 0.96 (0.59, 1.54) | 0.851 |
| Major complications, Clavien–Dindo ≥ IIIa (yes versus no) | 2.79 (1.76, 4.44) | < 0.001 |
| Blood loss (ml) (continuous) | 1.00 (1.00, 1.00) | 0.467 |
| R0 (yes versus no) | 0.65 (0.38, 1.10) | 0.108 |
Values in parentheses are 95% confidence intervals. The analysis included 145 patients. HR for continuous variables are shown per unit increase, PVE, portal vein embolization; HVE, hepatic vein embolization; CRLM, colorectal liver metastasis.
Table 6.
Multivariable analysis of overall survival for treatment group (portal vein embolization versus portal vein embolization/hepatic vein embolization) with correction for year of intervention
| HR | P | |
|---|---|---|
| Treatment group (PVE versus PVE/HVE) | 0.47 (0.24, 0.92) | 0.027 |
| Year of inclusion/intervention | 0.95 (0.78, 1.17) | 0.650 |
Values in parentheses are 95% confidence intervals. The analysis included 196 patients. PVE, portal vein embolization; HVE, hepatic vein embolization.
Subgroup analysis of colorectal liver metastasis
The Charlson Co-morbidity Index score and the percentage of patients who underwent two-stage hepatectomy (TSH) were higher in the PVE group in the subgroup of patients with CRLM (Table S1). The KGR was higher after PVE/HVE, whereas the resection rate did not differ significantly between PVE/HVE and PVE alone in this subgroup analysis. After a median follow-up of 34 months (PVE/HVE) and 20 months (PVE), which did not differ statistically (P = 0.123), median overall survival was not reached in the PVE/HVE group, whereas it was 26 months in PVE-alone group (P = 0.036) (Fig. S4). There was no significant difference in recurrence/progression-free survival between the two treatments among patients with resected CRLM (median 19 months for PVE/HVE versus 11 months for PVE; P = 0.077). Patients who did not undergo surgical resection after PVE/HVE and PVE had significantly worse overall survival (P < 0.001) (Fig. S5).
Discussion
Along with increased FLR hypertrophy and resection rate, and despite a variety of potential biases owing to the retrospective design, in the present study PVE/HVE was associated with significantly better overall survival than PVE alone in patients with liver cancers and in a subgroup of patients with colorectal liver metastases. The findings support the need for the recently initiated prospective international multicentre RCTs comparing PVE/HVE versus PVE in CRLM alone (DRAGON 2 trial; NCT05428735) and in primary liver cancers (DRAGON PLC).
Patients who did not undergo liver resection after PVE/HVE or PVE alone had significantly worse survival than those who had surgical resection. Univariable and multivariable Cox regression analyses for the endpoint survival demonstrated that PVE/HVE was associated with survival with HRs of between 0.46 and 0.57.
The reason why patients who had PVE/HVE lived longer in this study is most likely multifactorial: a higher resection rate owing to the rapid hypertrophy following PVE/HVE, the lower 90-day mortality rate after resection, and an increased depth of oncological response by a higher proportion of extended resections. Although, the KGR was significantly higher in the PVE/HVE group, the sFLR2 did not differ between groups. The significance of rapid liver growth and an increased resection rate was first demonstrated in the ALPPS arm of the LIGRO trial10. In LIGRO, the resection rate and median overall survival were significantly improved in patients undergoing ALPPS compared with TSH (46 versus 26 months respectively; P = 0.028)25. ALPPS in LIGRO allowed resection in 92% versus 57% of patients in the TSH arm (P < 0.001). This increased resection rate resulted directly in improved survival on long-term follow-up. Similarly, the present study has now also shown that the previously reported feasibility of resection rate of 92% in the PVE/HVE group compared with 68% in the PVE arm translated into improved median survival time in the PVE/HVE arm. However, it needs to be emphasized that this study was retrospective, whereas LIGRO had prospectively randomized cohorts. Furthermore, the present study included a variety of tumour aetiologies, whereas only patients with CRLM and neoadjuvant chemotherapy were included in LIGRO. In contrast, 9% of patients with CRLM in the PVE group did not receive neoadjuvant chemotherapy in the present study. Although a recent meta-analysis18 revealed a pooled resectability rate of 87% after PVE/HVE, the resectability rate following PVE/HVE here (92%) was relatively high compared with that reported by other studies26,27 which described lower resection rates. Nevertheless, the general gist of the present findings in this study is very similar to the median-term survival data presented by LIGRO. The findings might also suggest that an initial more extensive reduction of cancer mass in the liver leads to improved median survival in patients with a variety of tumour aetiologies.
In contrast, patients who did not undergo surgical resection had significantly worse survival. Brouquet et al.28 were the first to show the oncological relevance of successful completion of a two-stage strategy in a retrospective analysis of patients with CRLM. Non-completion of a two-stage procedure and major complications were revealed as the only predictors of survival. LIGRO was the first study to confirm these retrospective findings in a randomized study. In line with both studies, the multivariable analysis here showed that PVE/HVE is an independent predictor for survival, and that accelerated liver growth and a higher resection rate are associated with better survival.
There was no difference in recurrence/progression-free survival between treatment groups. It may appear contradictory that overall survival differed significantly, but not recurrence/progression-free survival. However, the finding can be explained by the lower resectability rate in PVE, as these patients had significantly worse overall survival. The lack of difference in recurrence/progression-free survival can also be explained by the lower resectability rate in PVE. If significantly more patients are unable to undergo surgical resection after PVE alone, there are fewer patients to develop recurrence.
The initial enthusiasm for ALPPS neglected an important downside of the rapid hypertrophy in that procedure: its maximally invasive surgical approach in stage 1 of the TSH. Even the first ALPPS series revealed an incidence of Clavien–Dindo grade III and IV complications of 44% and an in-house mortality rate of 12%12. Later studies even reported a 90-day mortality rate of 15–48%13,14,29–31, especially in elderly patients and those with primary liver tumours14. Despite increased selection of patients and the preferential use of ALPPS in CRLM in the following years, morbidity and mortality rates have remained comparatively high10,32,33.
Despite the resection rate of 68% after PVE, which is slightly lower than in previously published studies, survival after PVE demonstrated here remained very similar to that in other studies8,25. As experience with HVE is growing, this procedure is increasingly being used as a salvage or rescue procedure after PVE in patients with insufficient FLR hypertrophy. The initiation of a high FLR hypertrophy rate by limiting the formation of intrahepatic collaterals with simultaneous PVE/HVE allows a shorter interval between embolization and resection.
The general feeling is that a simultaneous embolization procedure will be superior to a staged/sequential procedure. Whether survival after staged/sequential PVE/HVE is comparable to simultaneously performed PVE/HVE remains unknown.
This study has several limitations. First, the retrospective design and the absence of independent monitoring may have introduced reporting bias. However, by close collaboration, an attempt was made to keep these sources of bias as small as possible. Moreover, as a result of the retrospective design, there was variation in embolization techniques, and resectability was not defined homogenously among participating centres. Second, selection bias cannot be completely ruled out, but was probably minimal as all patients undergoing embolization in participating centres within the time period analysed were included. However, because of the younger age, shorter operating time, lower Charlson Co-morbidity Index score, and smaller number of two-stage resections in the CRLM subgroup, there seemed to be a selection bias for extended resections in the PVE/HVE group. Third, an era bias cannot be excluded because PVE/HVE as the newest regenerative procedure has been mainly performed in recent years. Embolization techniques have changed during the long observation period. In recent years, glue has been used increasingly for PVE (especially in the PVE/HVE group) as glue was shown to be superior to other embolic agents regarding the induction of liver hypertrophy34. However, microparticles were still used in some patients in the PVE-alone group. In addition, treatment options for intrahepatic or extrahepatic recurrence have become increasingly aggressive over time. Furthermore, the definition of technical resectability and the need for regenerative liver surgery have changed over study interval. Although a sFLR of 20% was originally accepted as threshold for safe liver resection, a cut-off of 25–30% has become accepted more in recent years.
Collaborators
DRAGON trials collaborative: L. Aldrighetti (IRCCS San Raffaele Hospital, Milano, Italy); L. van Baardewijk (Maxima Medical Centre, Eindhoven, Netherlands); L. Barbier (Auckland District Health Board, Auckland, New Zealand); C. Binkert (Kantonsspital (KSW) Winterthur, Winterthur, Switzerland); B. Björnsson (University Hospital of Linköping, Linköping, Sweden); E. Cugat Andorrà (Hospital Universitari Germans Trias i Pujol de Badalona, Barcelona, Spain); B. Arslan (Rush University Medical Center, Chicago, United States); I. Baclija (Kaiser Franz Josef Hospital, Vienna, Austria); M. H. A. Bemelmans (Maastricht University Medical Centre+, Maastricht, Netherlands); C. Bent (RBCH, Royal Bournemouth and Christchurch Hospital, Bournemouth, United Kingdom); M. T. de Boer (University Medial Centre Groningen, Groningen, Netherlands); R. P. H. Bokkers (University Medical Centre Groningen, Groningen, Netherlands); D. de Boo (Monash Health, Clayton, Australia); D. Breen (University Hospital Southampton, Southampton, United Kingdom); S. Breitenstein (Kantonsspital (KSW) Winterthur, Winterthur, Switzerland); P. Bruners (University Hospital Aachen, Aachen, Germany); A. Cappelli (Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy); U. Carling (Oslo University Hospital, Oslo, Norway); M. Casellas i Robert (University Hospital Dr. Josep Trueta, Griona, Spain); B. Chan (Univeristy Hospital Aintree, Liverpool, United Kingdom); F. De Cobelli (IRCCS Ospedale San Raffale, Milano, Italy); C. Cha (Yale School of Medicine, New Haven, United States); J. Choi (Western Health, Melbourne, Australia); M. Crawford (Royal Prince Alfred Hospital, Sydney, Australia); D. Croagh (Monash Health, Clayton, Australia); R. M. van Dam (Maastricht University Medical Centre+, Maastricht, Netherlands); F. Deprez (CHU-UcLouvain-Namur, Yvoir, Belgium); O. Detry (CHU Liege, University of Liege, Belgium); M. Dewulf (Maastricht University Medical Centre+, Maastricht, Netherlands); R. Díaz-Nieto (Aintree University Hospital, Liverpool, United Kingdom); A. Dili (CHU-UCL Namur site Godine, Namur, Belgium); J. I. Erdmann (Amsterdam University Medical Centres, Amsterdan, Netherlands); J. Codina Font (University Hospital Dr. Josep Trueta, Girona, Spain); R. Davis (Aintree University Hospital, Liverpool, United Kingdom); M. Delle (Karolinska University Hospital, Stockholm, Sweden); R. Fernando (Auckland District Health Board, Auckland, New Zealand); O. Fisher (Royal Prince Alfred Hospital, Sydney, Australia); S. Fouraschen (University Medial Centre Groningen, Groningen, Netherlands); Å. A. Fretland (Oslo University Hospital, Oslo, Norway); Y. Fundora (Clínic Barcelona, Barcelona, Spain); A. Gelabert (Parc Tauli Hospital, Sabadell, Spain); L. Gerard (CHU Liege, Liege Belgium); P. Gobardhan (Amphia, Breda, Netherlands); F. Gómez (Clínic de Barcelona, Barcelona, Spain); F. Guiliante (Catholic University Rome, Rome, Italy); T. Gruenberger (Kaiser Franz Josef Spital, Vienna, Austria); L. F. Grochola (Kantonsspital (KSW) Winterthur, Winterthur, Switzerland); D. Grünhagen (Erasmus Medical Centre, Cancer Institute, Rotterdam, Netherlands); J. Guitart Giménez (University Hospital Mútua Terassa, Spain); J. Hagendoorn (UMC Utrecht Cancer Center, Utrecht, Netherlands); J. Heil (Goethe-University Frankfurt, University Hospital Frankfurt, Germany); D. Heise (University Hospital Aachen, Aachen, Germany); E. Herrero (University Hospital Mútua Terassa, Terassa, Spain); G. Hess (Clarunis, University Centre for Gastrointestinal and Liver Diseases, Basel, Switzerland); M. Abu Hilal (Fondazione Poliambulanza Hospital Institute, Brescia, Italy); M. Hoffmann (St. Clara hospital, Basel, Switzerland); R. Iezzi (Agostino Gemelli University Hospital Rome, Rome, Italy); F. Imani (Amphia, Breda, Netherlands); N. Inmutto (Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand); S. James (Maastricht University Medical Centre+, Maastricht, Netherlands); F. Garcia Borobia (Parc Tauli Hospital, Sabadell, Spain); E. Jovine (Maggiore Hospital, Bologna, Italy); J. Kalil (McGill University Medical Centre, Montreal, Canada); P. Kingham (Memorial Sloan Kettering Cancer Center, New York, United States); O. Kollmar (Clarunis, University Centre for Gastrointestinal and Liver Diseases, Basel, Switzerland); J. Kleeff (University Hospital Halle (Saale), Halle (Saale), Germany); C. van der Leij (Maastricht University Medical Center+, Maastricht, Netherlands); S. Lopez-Ben (University Hospital Dr. Josep Trueta, Girona, Spain); A. Macdonald (Oxford University Hospitals, Oxford, United Kingdom); M. Meijerink (Amsterdam University Medical Centres Amsterdam, Netherlands); R. Korenblik (Maastricht University Medical Centre+, Maastricht, Netherlands); W. Lapisatepun (Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand); W. Leclercq (Maxima Medical Centre, Eindhoven, Netherlands); R. Lindsay (Belfast Health and Social Care Trust, Belfast, United Kingdom); V. Lucidi (Erasme University Hospital—ULB, Brussels, Belgium); D. C. Madoff (Yale School of Medicine, New Haven, United States); G. Martel (the Ottawa hospital, Ottawa, Canada); H. Mehrzad (Queen Elisabeth Hospital Birmingham, University Hospital Birmingham, Birmingham, United Kingdom); K. Menon (King's College Hospital, London, United Kingdom); P. Metrakos (McGill University Hospital, Montreal, Canada); S. Modi (University Hospital Southampton, Southampton, United Kingdom); N. Montanari (Maggiore Hospital, Bologna, Italy); J. Sampere Moragues (Hospital Universitari Germans Trias i Pujol de Badalona, Barcelona, Spain); J. Navinés López (Hospital Universitari Germans Trias i Pujol de Badalona, Barcelona, Spain); U. P. Neumann (University Hospital Aachen, Aachen, Germany); J. Nguyen (Western Health, Melbourne, Australia); P. Peddu (King's College Hospital, London, United Kingdom); J. Primrose (University Hospital Southampton, Southampton, United Kingdom); S. W. M. Olde Damink (Maastricht University Medical Centre+, Maastricht, Netherlands); X. Qu (Zhongshan hospital, Fundan University, Shanghai, China); D. A. Raptis (Royal Free Hospital, London, United Kingdom); F. Ratti (IRCCS Ospedale San Raffaele, Milan, Italy); S. Ryan (The Ottawa hospital, Ottawa, Canada); F. Ridouani (Memorial Sloan Kettering Cancer Center, New York, United States); I. H. M. Borel Rinkes (University Medical Centre Utrecht, Cancer Center, Utrecht, Netherlands); C. Rogan (Royal Prince Alfred Hospital, Sydney, Australia); U. Ronellenfitsch (University Hospital Halle (Saale), Halle (Saale), Germany); M. Serenari (Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy); A. Salik (University Hospital of Linköping, Linköping, Sweden); C. Sallemi (Fondazione Poliambulanza Hospital Institute, Brescia, Italy); P. Sandström (University Hospital of Linköping, Linköping, Sweden); E. Santos Martin (Memorial Sloan Kettering Cancer Center, New York, USA); L. Sarría (Miguel Servet University Hospital and University of Zaragoza, Zaragoza, Spain); E. Schadde (Klinik Hirslanden, Luzern and Zurich, Switzerland); A. Serrablo (Miguel Servet University Hospital Zaragoza, Zaragoza, Spain); U. Settmacher (University Hospital Jena, Jena, Germany); J. Smits (Maastricht University Medical Centre+, Maastricht, Netherlands); M. L. J. Smits (University Medical Centre Utrecht, Utrecht, Netherlands); A. Snitzbauer (Goethe-University Frankfurt, University Hospital Frankfurt, Germany); Z. Soonawalla (Oxford University Hosptials NHS Foundation Trust, Oxford, United Kingdom); E. Sparrelid (Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden); E. Spuentrup (Hospital Saarbrücken, Saarbrücken, Germany); G. Stavrou (Hospital Saarbrücken, Saarbrücken, Germany); R. Sutcliffe (University Hospital Birmingham, Birmingham, United Kingdom); I. Tancredi (Eramse University Hospital—ULB, Brussels, Belgium); J. C. Tasse (Rush University Medical Center, Chicago, United States); U. Teichgräber (University Hospital Jena, Jena, Germany); V. Udupa (Oxford University Hosptials NHS Foundation Trust, Oxford, United Kingdom); D. A. Valenti (McGill University Health Centre, Montreal, Canada); D. Vass (Belfast Health and Social Care Trust, Belfast, United Kingdom); T. Vogl (Goethe-University Frankfurt, University Hospital Frankfurt, Germany); X. Wang (Zhongshan Hospital, Fudan University, Shanghai, China); S. White (The Newcastle upon Tyne Hospitals, Newcastle, United Kingdom); J. F. De Wispelaere (CHU-UCL Namur site Godinne, Namur, Belgium); W. Wohlgemuth (University of Halle-Wittenberg, Halle (Saale)), Germany; D. YU (Royal Free Hospital London, London, United Kingdom); I. J. A. J. Zijlstra (Amsterdam University Medical Centres, Amsterdam, Netherlands).
Supplementary Material
Acknowledgements
R.K. and J.H. contributed equally as first authors; and E.S. and R.M.V.D. made an equal contribution as senior authors. The authors acknowledge the contributions of S. Kern (Department of Anaesthesiology, Group Beck Schimmer, University Hospital Zurich, Zurich, Switzerland) and C. Lambrecht (Clinical Trial Centre Liege, University Hospital Liege, Liege, Belgium). This study was not preregistered in an independent, institutional registry.
Contributor Information
Remon Korenblik, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
Jan Heil, Institute of Physiology, University of Zurich, Zurich, Switzerland; Department of General, Visceral and Transplant Surgery, University Hospital Frankfurt, Goethe-University Frankfurt, Frankfurt, Germany.
Jens Smits, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
Sinead James, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
Bram Olij, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
Wolf O Bechstein, Department of General, Visceral and Transplant Surgery, University Hospital Frankfurt, Goethe-University Frankfurt, Frankfurt, Germany.
Marc H A Bemelmans, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands; Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany.
Christoph A Binkert, Department of Radiology, Cantonal Hospital Winterthur, Winterthur, Switzerland.
Stefan Breitenstein, Department of General and Visceral Surgery, Cantonal Hospital Winterthur, Winterthur, Switzerland.
Michael Williams, Department of Surgery, Rush University Medical Center Chicago, Chicago, Illinois, USA.
Olivier Detry, Department of Abdominal Surgery and Transplantation, University of Liege, CHU Liege, Liege, Belgium.
Maxime J L Dewulf, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands; Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany.
Alexandra Dili, Department of Abdominal Surgery, CHU-UC Louvain-Namur, Yvoir, Belgium.
Lukasz F Grochola, Department of General and Visceral Surgery, Cantonal Hospital Winterthur, Winterthur, Switzerland.
Jon Grote, Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany.
Daniel Heise, Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany; Department of General, Visceral and Transplant Surgery, University Hospital Essen, Essen, Germany.
Jennifer A Kalil, Department of Surgery, Section of Hepato-pancreatico-biliary Surgery, McGill University Health Centre, Montreal, Québec, Canada.
Peter Metrakos, Department of Surgery, Section of Hepato-pancreatico-biliary Surgery, McGill University Health Centre, Montreal, Québec, Canada.
Ulf P Neumann, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands; Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany; Department of General, Visceral and Transplant Surgery, University Hospital Essen, Essen, Germany.
Sam G Pappas, Department of Surgery, Rush University Medical Center Chicago, Chicago, Illinois, USA.
Francesca Pennetta, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
Andreas A Schnitzbauer, Department of General, Visceral and Transplant Surgery, University Hospital Frankfurt, Goethe-University Frankfurt, Frankfurt, Germany.
Jordan C Tasse, Department of Radiology, Rush University Medical Center Chicago, Chicago, Illinois, USA.
Bjorn Winkens, Department of Methodology and Statistics, Care and Public Health Research Institute (CAPHRI), Maastricht University, Maastricht, the Netherlands.
Steven W M Olde Damink, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands; Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany; NUTRIM—School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, the Netherlands.
Christiaan van der Leij, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Radiology, Maastricht University Medical Centre, Maastricht, the Netherlands.
Erik Schadde, Institute of Physiology, University of Zurich, Zurich, Switzerland; Department of Surgery, Rush University Medical Center Chicago, Chicago, Illinois, USA; Surgical Centre, Hirslanden Clinic Zurich, Zurich, Switzerland; Switzerland Surgical Centre, Hirslanden Clinic St Anna Luzern, Luzern, Switzerland.
Ronald M van Dam, GROW—School for Oncology and Reproduction, Maastricht University, Maastricht, the Netherlands; Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands; Department of General, Visceral and Transplant Surgery, University Hospital Aachen, Aachen, Germany.
DRAGON trials collaborative:
L Aldrighetti, L van Baardewijk, L Barbier, C Binkert, B Björnsson, Cugat Andorrà E, B Arslan, I Baclija, M H A Bemelmans, C Bent, M T de Boer, R P H Bokkers, D de Boo, D Breen, S Breitenstein, P Bruners, A Cappelli, U Carling, M Casellas i Robert, B Chan, F De Cobelli, C Cha, J Choi, M Crawford, D Croagh, R M van Dam, F Deprez, O Detry, M Dewulf, R Díaz-Nieto, A Dili, J I Erdmann, J Codina Font, R Davis, M Delle, R Fernando, O Fisher, S Fouraschen, Å A Fretland, Y Fundora, A Gelabert, L Gerard, P Gobardhan, F Gómez, F Guiliante, T Gruenberger, L F Grochola, D Grünhagen, J Guitart Giménez, J Hagendoorn, J Heil, D Heise, E Herrero, G Hess, M Abu Hilal, M Hoffmann, R Iezzi, F Imani, N Inmutto, S James, F Garcia Borobia, E Jovine, J Kalil, P Kingham, O Kollmar, J Kleeff, C van der Leij, S Lopez-Ben, A Macdonald, M Meijerink, R Korenblik, W Lapisatepun, W Leclercq, R Lindsay, V Lucidi, D C Madoff, G Martel, H Mehrzad, K Menon, P Metrakos, S Modi, N Montanari, J Sampere Moragues, J Navinés López, U P Neumann, J Nguyen, P Peddu, J Primrose, S W M Olde Damink, X Qu, D A Raptis, F Ratti, S Ryan, F Ridouani, I H M Borel Rinkes, C Rogan, U Ronellenfitsch, M Serenari, A Salik, C Sallemi, P Sandström, E Santos Martin, L Sarría, E Schadde, A Serrablo, U Settmacher, J Smits, M L J Smits, A Snitzbauer, Z Soonawalla, E Sparrelid, E Spuentrup, G Stavrou, R Sutcliffe, I Tancredi, J C Tasse, U Teichgräber, V Udupa, D A Valenti, D Vass, T Vogl, X Wang, S White, J F De Wispelaere, W Wohlgemuth, D YU, and I J A J Zijlstra
Funding
The KWF Dutch Cancer Society provided unrestricted financial support for the DRAGON trials (Grant number 12501).
Author contributions
Remon Korenblik (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Jan Heil (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing—original draft), Jens Smits (Data curation, Project administration, Writing—original draft), Sinead James (Data curation, Formal analysis, Project administration, Writing—original draft), Wolf Bechstein (Conceptualization, Writing—review & editing), Marc Bemelmans (Conceptualization, Writing—review & editing), Christoph Binkert (Conceptualization, Investigation, Writing—review & editing), Stefan Breitenstein (Conceptualization, Writing—review & editing), Michael Williams (Data curation, Writing—review & editing), Olivier Detry (Conceptualization, Data curation, Writing—review & editing), Maxime Dewulf (Conceptualization, Writing—original draft, Writing—review & editing), Alexandra Dili (Data curation, Writing—review & editing), Lukasz Grochola (Data curation, Writing—review & editing), Jon Grote (Data curation, Writing—review & editing), Daniel Heise (Supervision, Writing—review & editing), Jennifer Kalil (Data curation, Writing—review & editing), Peter Metrakos (CRediT contribution not specified), Ulf Neumann (Conceptualization, Writing—review & editing), Sam Pappas (Conceptualization, Writing—review & editing), Francesca Pennetta (Data curation, Project administration, Writing—original draft), Andreas Schnitzbauer (Methodology, Supervision, Writing—review & editing), Jordan Tasse (Data curation, Supervision, Writing—review & editing), Bjorn Winkens (Formal analysis, Methodology, Software, Supervision, Validation), Steven W. M. Olde Damink (Conceptualization, Supervision, Writing—review & editing), Christiaan van der Leij (Conceptualization, Investigation, Methodology, Supervision, Writing—review & editing), Erik Schadde (Conceptualization, Investigation, Methodology, Supervision, Writing—review & editing), and Ronald van Dam (Conceptualization, Investigation, Methodology, Supervision, Writing—review & editing)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Deidentified data are available upon reasonable request.
References
- 1. Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol 2003;14:ii13–ii16 [DOI] [PubMed] [Google Scholar]
- 2. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13 772 patients in Japan. Ann Surg 2007;245:909–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg 2012;29:6–17 [DOI] [PubMed] [Google Scholar]
- 5. Schindl M, Redhead D, Fearon K, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER) . The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdalla E, Hicks M, Vauthey J. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88:165–175 [DOI] [PubMed] [Google Scholar]
- 7. Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Lienden K, Van Den Esschert J, De Graaf W, Bipat S, Lameris JS, van Gulik TM et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regimbeau JM, Cosse C, Kaiser G, Hubert C, Laurent C, Lapointe R et al. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB (Oxford) 2017;19:396–405 [DOI] [PubMed] [Google Scholar]
- 10. Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a scandinavian multicenter randomized controlled trial (LIGRO trial). Ann Surg 2018;267:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–414 [DOI] [PubMed] [Google Scholar]
- 13. Schadde E, Ardiles V, Slankamenac K, hernandez-Alejandro R, Lang H, de Santibaňes E et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510–1519 [DOI] [PubMed] [Google Scholar]
- 14. Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829–836; discussion 836–828 [DOI] [PubMed] [Google Scholar]
- 15. Schadde E, Guiu B, Deal R, Kalil J, Arslan B, Tasse J et al. Simultaneous hepatic and portal vein ligation induces rapid liver hypertrophy: a study in pigs. Surgery 2019;165:525–533 [DOI] [PubMed] [Google Scholar]
- 16. Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259–4267 [DOI] [PubMed] [Google Scholar]
- 17. Heil J, Korenblik R, Heid F, Bechstein WO, Bemelmans M, Binkert C et al. Preoperative portal vein or portal and hepatic vein embolization: DRAGON collaborative group analysis. Br J Surg 2021;108:834–842 [DOI] [PubMed] [Google Scholar]
- 18. Korenblik R, Van Zon JF, Olij B, Heil J, Dewulf MJL, Neumann UP et al. Resectability of bilobar liver tumours after simultaneous portal and hepatic vein embolization versus portal vein embolization alone: meta-analysis. BJS Open 2022;6:zrac141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell RJ, Hakeem AR, Pandanaboyana S, Davidson BR, Prasad RK, Dasari BVM. Portal vein embolization versus dual vein embolization for management of the future liver remnant in patients undergoing major hepatectomy: meta-analysis. BJS Open 2022;6:zrac131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349 [DOI] [PubMed] [Google Scholar]
- 21. Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002;8:233–240 [DOI] [PubMed] [Google Scholar]
- 22. Strasberg S, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford) 2000;2:333–339 [Google Scholar]
- 23. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–724 [DOI] [PubMed] [Google Scholar]
- 25. Hasselgren K, Røsok BI, Larsen PN, Sparrelid E, Lindell G, Schultz NA et al. ALPPS improves survival compared with TSH in patients affected of CRLM: survival analysis from the randomized controlled trial LIGRO. Ann Surg 2021;273:442–448 [DOI] [PubMed] [Google Scholar]
- 26. Chebaro A, Buc E, Durin T, Chiche L, Brustia R, Didier A et al. Liver venous deprivation or associating liver partition and portal vein ligation for staged hepatectomy? A retrospective multicentric study. Ann Surg 2021;274:874–880 [DOI] [PubMed] [Google Scholar]
- 27. Ghosn M, Kingham TP, Ridouani F, Santos E, Yarmohammadi H, Boas FE et al. Percutaneous liver venous deprivation: outcomes in heavily pretreated metastatic colorectal cancer patients. HPB (Oxford) 2022;24:404–412 [DOI] [PubMed] [Google Scholar]
- 28. Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olthof PB, Coelen RJ, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case–control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serenari M, Zanello M, Schadde E, Toschi E, Ratti F, Gringeri E et al. Importance of primary indication and liver function between stages: results of a multicenter Italian audit of ALPPS 2012–2014. HPB (Oxford) 2016;18:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons learned from 15 cases at a single centre. Zeitschrift für Gastroenterologie 2014;52:35–42 [DOI] [PubMed] [Google Scholar]
- 32. Linecker M, Björnsson B, Stavrou GA, Oldhafer KJ, Lurje G, Neumann U et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann Surg 2017;266:779–786 [DOI] [PubMed] [Google Scholar]
- 33. Moris D, Ronnekleiv-Kelly S, Kostakis ID, Tsilimigras DI, Beal EW, Papalampros A et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg 2018;42:806–815 [DOI] [PubMed] [Google Scholar]
- 34. Luz JHM, Veloso Gomes F, Costa NV, Vasco I, Coimbra E, Luz PM et al. BestFLR trial: liver regeneration at CT before major hepatectomies for liver cancer—a randomized controlled trial comparing portal vein embolization with N-butyl-cyanoacrylate plus iodized oil versus polyvinyl alcohol particles plus coils. Radiology 2021;299:715–724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data are available upon reasonable request.
References
- 1. Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol 2003;14:ii13–ii16 [DOI] [PubMed] [Google Scholar]
- 2. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13 772 patients in Japan. Ann Surg 2007;245:909–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg 2012;29:6–17 [DOI] [PubMed] [Google Scholar]
- 5. Schindl M, Redhead D, Fearon K, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER) . The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdalla E, Hicks M, Vauthey J. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88:165–175 [DOI] [PubMed] [Google Scholar]
- 7. Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Lienden K, Van Den Esschert J, De Graaf W, Bipat S, Lameris JS, van Gulik TM et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regimbeau JM, Cosse C, Kaiser G, Hubert C, Laurent C, Lapointe R et al. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB (Oxford) 2017;19:396–405 [DOI] [PubMed] [Google Scholar]
- 10. Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a scandinavian multicenter randomized controlled trial (LIGRO trial). Ann Surg 2018;267:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–414 [DOI] [PubMed] [Google Scholar]
- 13. Schadde E, Ardiles V, Slankamenac K, hernandez-Alejandro R, Lang H, de Santibaňes E et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510–1519 [DOI] [PubMed] [Google Scholar]
- 14. Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829–836; discussion 836–828 [DOI] [PubMed] [Google Scholar]
- 15. Schadde E, Guiu B, Deal R, Kalil J, Arslan B, Tasse J et al. Simultaneous hepatic and portal vein ligation induces rapid liver hypertrophy: a study in pigs. Surgery 2019;165:525–533 [DOI] [PubMed] [Google Scholar]
- 16. Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259–4267 [DOI] [PubMed] [Google Scholar]
- 17. Heil J, Korenblik R, Heid F, Bechstein WO, Bemelmans M, Binkert C et al. Preoperative portal vein or portal and hepatic vein embolization: DRAGON collaborative group analysis. Br J Surg 2021;108:834–842 [DOI] [PubMed] [Google Scholar]
- 18. Korenblik R, Van Zon JF, Olij B, Heil J, Dewulf MJL, Neumann UP et al. Resectability of bilobar liver tumours after simultaneous portal and hepatic vein embolization versus portal vein embolization alone: meta-analysis. BJS Open 2022;6:zrac141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell RJ, Hakeem AR, Pandanaboyana S, Davidson BR, Prasad RK, Dasari BVM. Portal vein embolization versus dual vein embolization for management of the future liver remnant in patients undergoing major hepatectomy: meta-analysis. BJS Open 2022;6:zrac131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349 [DOI] [PubMed] [Google Scholar]
- 21. Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002;8:233–240 [DOI] [PubMed] [Google Scholar]
- 22. Strasberg S, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford) 2000;2:333–339 [Google Scholar]
- 23. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–724 [DOI] [PubMed] [Google Scholar]
- 25. Hasselgren K, Røsok BI, Larsen PN, Sparrelid E, Lindell G, Schultz NA et al. ALPPS improves survival compared with TSH in patients affected of CRLM: survival analysis from the randomized controlled trial LIGRO. Ann Surg 2021;273:442–448 [DOI] [PubMed] [Google Scholar]
- 26. Chebaro A, Buc E, Durin T, Chiche L, Brustia R, Didier A et al. Liver venous deprivation or associating liver partition and portal vein ligation for staged hepatectomy? A retrospective multicentric study. Ann Surg 2021;274:874–880 [DOI] [PubMed] [Google Scholar]
- 27. Ghosn M, Kingham TP, Ridouani F, Santos E, Yarmohammadi H, Boas FE et al. Percutaneous liver venous deprivation: outcomes in heavily pretreated metastatic colorectal cancer patients. HPB (Oxford) 2022;24:404–412 [DOI] [PubMed] [Google Scholar]
- 28. Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olthof PB, Coelen RJ, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case–control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serenari M, Zanello M, Schadde E, Toschi E, Ratti F, Gringeri E et al. Importance of primary indication and liver function between stages: results of a multicenter Italian audit of ALPPS 2012–2014. HPB (Oxford) 2016;18:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons learned from 15 cases at a single centre. Zeitschrift für Gastroenterologie 2014;52:35–42 [DOI] [PubMed] [Google Scholar]
- 32. Linecker M, Björnsson B, Stavrou GA, Oldhafer KJ, Lurje G, Neumann U et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann Surg 2017;266:779–786 [DOI] [PubMed] [Google Scholar]
- 33. Moris D, Ronnekleiv-Kelly S, Kostakis ID, Tsilimigras DI, Beal EW, Papalampros A et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg 2018;42:806–815 [DOI] [PubMed] [Google Scholar]
- 34. Luz JHM, Veloso Gomes F, Costa NV, Vasco I, Coimbra E, Luz PM et al. BestFLR trial: liver regeneration at CT before major hepatectomies for liver cancer—a randomized controlled trial comparing portal vein embolization with N-butyl-cyanoacrylate plus iodized oil versus polyvinyl alcohol particles plus coils. Radiology 2021;299:715–724 [DOI] [PubMed] [Google Scholar]