Abstract
Substitution of granulin from the Trichoplusia ni granulosis virus (TnGV) for polyhedrin of the Autographa californica multinucleocapsid nuclear polyhedrosis virus (AcMNPV) yielded a few very large (2 to 5 μm) cuboidal inclusions in the cytoplasm and nucleus of infected cells. These polyhedra lacked the beveled edges characteristic of wild-type AcMNPV polyhedra, contained fractures, and occluded few virions. Placing a nuclear localization signal (KRKK) in granulin directed more granulin to the nucleus and resulted in more structurally uniform cuboidal inclusions in which no virions were observed. A granulin-polyhedrin chimera produced tetrahedral occlusions with more virions than granulin inclusions but many fewer than wild-type polyhedra. Despite the unusual structure of the granulin and granulin-polyhedrin inclusions, they interacted with AcMNPV p10 fibrillar structures and electron-dense spacers that are precursors of the polyhedral calyx. The change in inclusion shape obtained with the granulin-polyhedrin chimera demonstrates that the primary amino acid sequence affects occlusion body shape, but the large cuboidal inclusions formed by granulin indicate that the amino acid sequence is not the only determinant. The failure of granulin or the granulin-polyhedrin chimera to properly occlude AcMNPV virions suggests that specific interactions occur between polyhedrin and other viral proteins which facilitate normal virion occlusion and occlusion body assembly and shape in baculoviruses.
Most baculoviruses produce occlusions of a characteristic size and shape, but little is known about the specific interactions between the occlusion matrix protein and other viral components that control virion occlusion and occlusion body morphology. In nuclear polyhedrosis viruses (NPVs), it has been hypothesized that virion occlusion and polyhedral growth are initiated by specific interactions between polyhedrin molecules and the virion envelope (17). Ultrastructural evidence suggests that polyhedron formation is initiated by nucleation of polyhedrin on the virion surface (9, 10, 16), after which polyhedra grow by the addition of more virions and polyhedrin. In granulosis viruses (GVs), granulin deposition begins at one end or one side of the virion envelope and proceeds around the particle (2). It has been suggested that the size and shape of mature occlusion bodies are determined to a large extent by the sequence or secondary structure of the occlusion protein (4, 6), and a number of polyhedrin mutations have been described for which this is the case (3–8), including a mutant of the Autographa californica multinucleocapsid NPV (AcMNPV), in which leucine rather than proline occurs at residue 58, producing large cuboidal polyhedra that often lack virions (4). Information on potential functional domains of AcMNPV polyhedrin has been provided by fusion of different polyhedrin regions to β-galactosidase (20). Polyhedrin contains a nuclear localization signal at residues 32 to 35 (KRKK). Mutation of this signal to NGNN abolished nuclear localization, and large cuboidal polyhedrin crystals formed in the cytoplasm. Additionally, amino acids 19 to 130 were shown to be required for formation of polyhedron-like crystals in the nucleus, and amino acids 30 to 130 were shown to be required for stable localization of fusion proteins to the nucleus, though the latter fusion did not form crystals (20).
The studies described above suggest that specific regions or amino acids of polyhedrin are critical to interactions with polyhedrin and other viral molecules. Substitution of a heterologous occlusion protein for AcMNPV polyhedrin separates the effects of interactions between occlusion matrix proteins from the effects of virion interactions with polyhedrin, assisting identification of the molecular determinants of these traits. Expression of Spodoptera frugiperda MNPV (SfMNPV) polyhedrin, which has 93% similarity to AcMNPV polyhedrin, in an occlusion-negative (occ mutant) AcMNPV strain (14) resulted in small polyhedra that contained fewer virions than wild-type polyhedra. SfMNPV polyhedrin was expressed at a low level in the recombinant compared with expression in wild-type AcMNPV, and the recombinant virus had reduced expression of the 25K gene, which also could have affected the polyhedron phenotype. However, interactions between virions and the heterologous polyhedrin occurred, even if at a reduced level.
The possibility of functional conservation between granulins and polyhedrins has not previously been explored, however. To determine whether the protein domains conserved between the Trichoplusia ni GV (TnGV) granulin (1) and AcMNPV polyhedrin (18) included those regions important to virion occlusion, we expressed the granulin gene in occ mutant AcMNPV and studied virion occlusion and occlusion body formation. We selected TnGV granulin because its amino acid similarity to AcMNPV polyhedrin is only 72% and because, in wild-type TnGV infection, occlusions are markedly different in size and shape from AcMNPV polyhedra. Since wild-type TnGV granulin has no nuclear localization signal and therefore might not reach as high a concentration in the nucleus as does AcMNPV polyhedrin, and since a low concentration of occlusion protein in the nucleus has been suggested as the proximal cause of the few-polyhedra (FP) mutant phenotype (21), we constructed a granulin mutant that contained the AcMNPV polyhedrin nuclear localization signal (KRKK). A hybrid granulin-polyhedrin gene was also constructed and expressed to determine whether the highly conserved C-terminal region of polyhedrin plays a role in virion occlusion.
Viruses and cells.
Viruses were grown in BTI-TN-5B1-4 (Tn5) cells (Invitrogen, Carlsbad, Calif.) maintained as monolayer cultures in TC-100 (12) supplemented with 10% fetal bovine serum (Gibco BRL, Gaithersburg, Md.). Recombinant strains of AcMNPV were constructed with the Bac-to-Bac system (Gibco BRL) and maintained in Escherichia coli as described in the Bac-to-Bac manual. The TnGV was obtained from John D. Paschke (Purdue University, West Lafayette, Ind.) and propagated in T. ni larvae.
Cloning of polyhedrin and granulin open reading frames (ORFs).
TnGV DNA was digested with EcoRV and ligated into the EcoRV site of pcDNAII (Invitrogen) to produce a genomic library. This library was screened with a digoxigenin-labeled probe (Genius system; Boehringer Mannheim, Indianapolis, Ind.) made from a 444-bp HindIII-EcoRI fragment of the granulin gene (1). The probe fragment was purified from an EcoRI digest of a HindIII genomic clone by agarose gel electrophoresis and glass bead purification (Geneclean; BIO 101, La Jolla, Calif.). An EcoRV clone of about 7 kb was identified and digested with NheI and AseI to yield a 918-bp fragment containing the granulin gene. The ends of this fragment were filled with Klenow fragment (Boehringer Mannheim), and the fragment was purified by gel electrophoresis and recovered with a Qiaquick column (Qiagen, Chatsworth, Calif.) and then ligated (Fastlink kit; Epicentre, Madison, Wis.) into a modified pFASTBAC (Bac-to-Bac kit; Gibco BRL) that lacked the AcMNPV polyhedrin promoter and that had been digested with StuI. The polyhedrin promoter was deleted from the modified pFASTBAC by digestion with BamHI and SnaBI, followed by blunting with Klenow fragment and self-ligation to produce the plasmid pFBd. All restriction enzymes were from New England Biolabs (Beverly, Mass.) unless otherwise specified.
The granulin ORF was amplified from pFBd-granulin by PCR using AmpliTaq polymerase (Perkin-Elmer, Norwalk, Conn.) and the following primers: forward, ATGGGATACAACAAATCATTGAGAT; and reverse, CCTAATAGTGATTGGAGTAATTAATGGT, employing conditions recommended by the manufacturer. All primers were obtained from Genosys Biotechnologies (The Woodlands, Tex.). This yielded a product with the expected size of 818 bp. The AcMNPV polyhedrin ORF was amplified from a cloned EcoRI-I fragment of AcMNPV (strain E2) by a similar PCR procedure with the following primers: forward, ATGCCGGATTATTCATACCGTCCC; reverse, AAATCTACAACGCACAGAATCTAGCG. This yielded a product with the expected size of 797 bp.
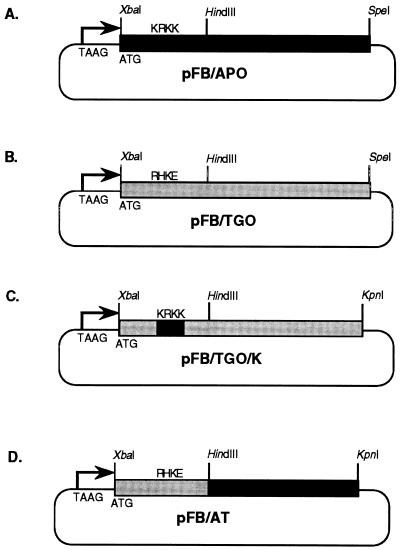
For cloning, PCR products were treated with T4 DNA polymerase to remove any non-template nucleotides, followed by treatment with T4 polynucleotide kinase to add phosphate groups. The DNA was then purified by Geneclean and ligated into the EcoRV site of pcDNAII. The resulting constructs, pc/TGO and pc/APO, were each digested with SpeI and XbaI. The occlusion gene fragment was purified by gel electrophoresis and ligated into the XbaI and SpeI sites of pFASTBAC (Gibco BRL), under the control of the AcMNPV polyhedrin promoter in pFASTBAC, to create pFB/TGO and pFB/APO (Fig. 1).
FIG. 1.
AcMNPV transfer plasmids constructed for expression of granulin, granulin-polyhedrin chimera, and polyhedrin genes. (A) pFB/APO, for expression of the AcMNPV polyhedrin gene, under control of the polyhedrin promoter. (B) pFB/TGO, for expression of the TnGV granulin gene, under control of the polyhedrin promoter. (C) pFB/TGO/K, for expression of a modified TnGV granulin gene with a nuclear localization signal. (D) pFB/AT, for expression of a hybrid granulin-polyhedrin gene. pFB/AT contains the 5′ end of the granulin ORF from pFB/TGO and the 3′ end of the polyhedrin ORF from pFB/APO.
Construction of granulin with a nuclear localization signal.
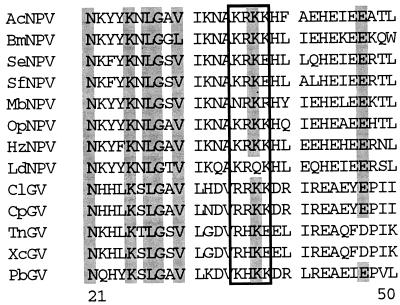
Alignment of the predicted amino acid sequences of selected polyhedrin and granulin genes available from GenBank (Fig. 2) showed that most of these contain a motif at amino acids 30 to 33 similar to the AcMNPV polyhedrin basic amino acid sequence KRKK, which has been demonstrated to function as a nuclear localization signal (20). The homologous region of the predicted TnGV granulin protein, however, is the non-consensus sequence RHKE. Accordingly, to determine whether the presence of this sequence affected granulin nuclear localization and occlusion body formation, RHKE was replaced by mutagenesis with the equivalent sequence, KRKK, that occurs in AcMNPV polyhedrin.
FIG. 2.
Alignment of predicted sequences for amino acids 21 to 50 of selected granulin and polyhedrin genes. Sequences for the following viruses were obtained from GenBank and aligned with Clustal: AcNPV, AcMNPV; BmNPV, Bombyx mori MNPV; SeNPV, SeMNPV; SfNPV, SfMNPV; MbNPV, Mamestra brassicae MNPV; OpNPV, OpMNPV; HzNPV, Helicoverpa zea SNPV; LdNPV, Lymantria dispar MNPV; ClGV, Cryptophlebia leucotrieta GV; CpGV, Cydia pomonella GV; XcGV, Xestia c-nigrans GV; and PbGV, Pieris brassicae GV. The nuclear localization signal of AcMNPV polyhedrin and homologous regions of other occlusion proteins are boxed. Conserved amino acids are shaded gray.
Mutagenesis of the granulin ORF to introduce a nuclear localization signal was done by the splicing overlap extension method (19). Forward and reverse primers identical to the ones described above, except for the addition of a full XbaI restriction site to the forward primer and of a KpnI site to the reverse primer, were synthesized. The primer sequences were, respectively, GCTCTAGAATGGGATACAACAAATCATTGAGAT and GGGGTACCCCTAATATGATTGGAGTAATTAATGGT. Forward and reverse mutagenic primers were also designed in which three bases were changed so that the sequence AGG CAC AAG GAG, coding for the amino acids RHKE (amino acids 35 to 38), was mutated to AAG CGC AAG AAG, coding for the amino acids KRKK (primer sequences, respectively, CTGGGTGATGTGAAGCGCAAGAAGGAATTGATTCGCGAAG and CGAATCAATTCCTTCTTGCGCTTCACATCACCCAGTAC). For the first round of PCR, the granulin ORF forward primer and the mutagenic reverse primer were paired to produce the first product. The mutagenic forward primer and the granulin ORF reverse primer were paired to produce the second product. Both reactions were conducted at an annealing temperature of 55°C. In the second round, the first and second PCR products were purified by gel electrophoresis and combined with the granulin ORF forward and reverse primers at a higher annealing temperature (65°C) to produce a full-length mutagenized product. This product was purified with a spin column (Qiaquick), digested with XbaI and KpnI, and ligated into pFASTBAC digested with XbaI and KpnI to produce the plasmid pFB/TGO/K (Fig. 1). The presence of the mutation was confirmed by DNA sequencing.
Construction of a hybrid granulin-polyhedrin ORF.
The AcMNPV polyhedrin and TnGV granulin genes contain a conserved HindIII site in predicted amino acid 84 of polyhedrin and amino acid 87 of granulin. To construct a hybrid gene, pFB/APO was digested with HindIII, and a 0.6-kb fragment was purified by agarose gel electrophoresis and ligated into the gel-purified vector fragment of HindIII-digested pFB/TGO. Clones were screened for orientation by restriction enzyme digestion. The resulting construct, pFB/AT, contained amino acids 1 to 87 of granulin in frame with amino acids 84 to 245 of polyhedrin (Fig. 1).
Each construct was transferred into a polyhedrin-negative AcMNPV strain with the Bac-to-Bac kit, following the recommendations of the manufacturer (Gibco BRL). The final viral constructs were as follows: Ac/FB/TGO, with the TnGV granulin gene; Ac/FB/TGO/K, with the modified TnGV granulin gene, encoding a nuclear localization signal; Ac/FB/AT, with the chimeric granulin-polyhedrin; and Ac/FB/APO, with the AcMNPV polyhedrin gene. All occlusion protein genes were expressed under control of the AcMNPV polyhedrin promoter to ensure equivalent expression. All constructs were confirmed by sequencing, phenotype of infected cells, and, in the case of the granulin constructs, dot blotting of viral DNA with the granulin probe described above.
Transfection of insect cells and T. ni larvae.
Viral DNA for the recombinant constructs was isolated from E. coli by alkaline lysis miniprep and transfected into Tn5 cells (Invitrogen) in 75-cm2 tissue culture flasks with Insectin (Invitrogen) or Cellfectin (Gibco BRL) according to the manufacturers’ instructions. Recombinant viruses were maintained in Tn5 cells. T. ni larvae were infected by puncture with a pin that had been dipped in virus-containing cell culture medium and were examined at 6 days postinfection.
Light and electron microscopy.
After transfection, Tn5 cells were monitored in 75-cm2 tissue culture flasks with phase microscopy involving a Zeiss Invertoscope D or as wet mounts involving a Zeiss Photomicroscope III. Infected cells to be embedded for electron microscopy were rinsed from the bottom of the flask with a Pasteur pipette and pelleted at approximately 500 × g. They were then transferred to a microcentrifuge tube, prefixed for 15 to 30 min in 1.5% glutaraldehyde–0.25% sucrose, resuspended in 3% glutaraldehyde–0.25% sucrose, fixed for at least 2 h, and processed for light and electron microscopy as described previously (9).
Expression of the TnGV granulin gene by AcMNPV.
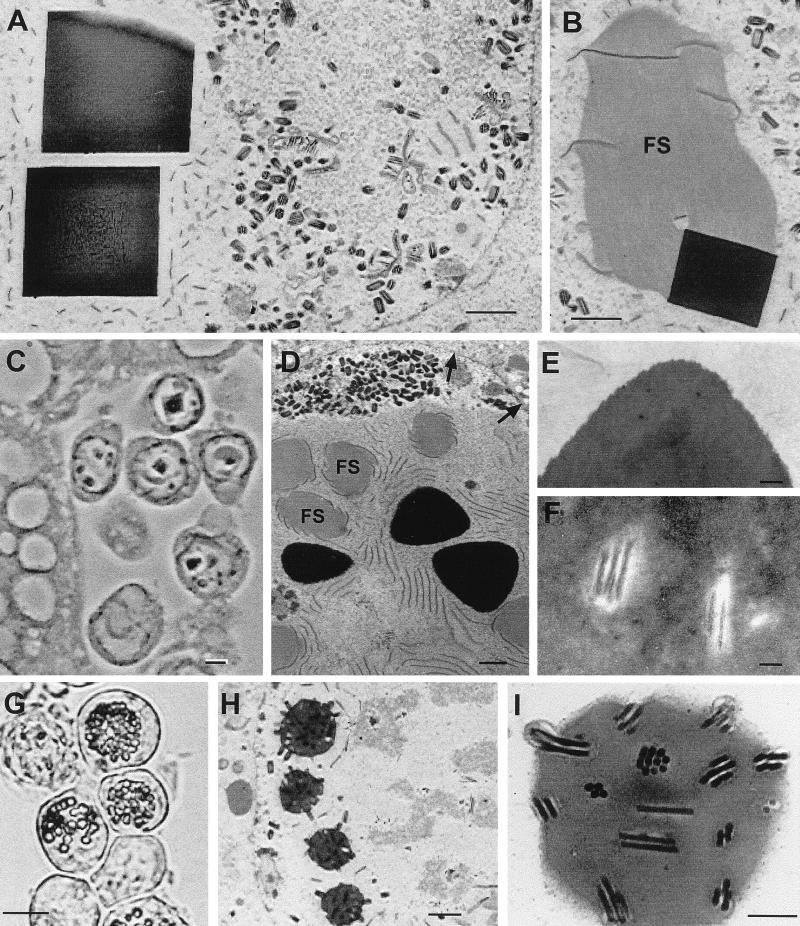
The recombinant AcMNPV (Ac/FB/TGO) expressing the TnGV granulin gene typically produced a few large cuboidal “polyhedra,” averaging 2 to 5 μm on edge, in each infected cell, although in some cells only a single large inclusion was produced (Fig. 3A). The granulin inclusions occurred in both the cytoplasm and the nucleus. In a sample of 30 cells, the mean number of granulin inclusions in the nucleus was 2.5 (range, 0 to 12); in the cytoplasm, it was 2.0 (range, 0 to 6).
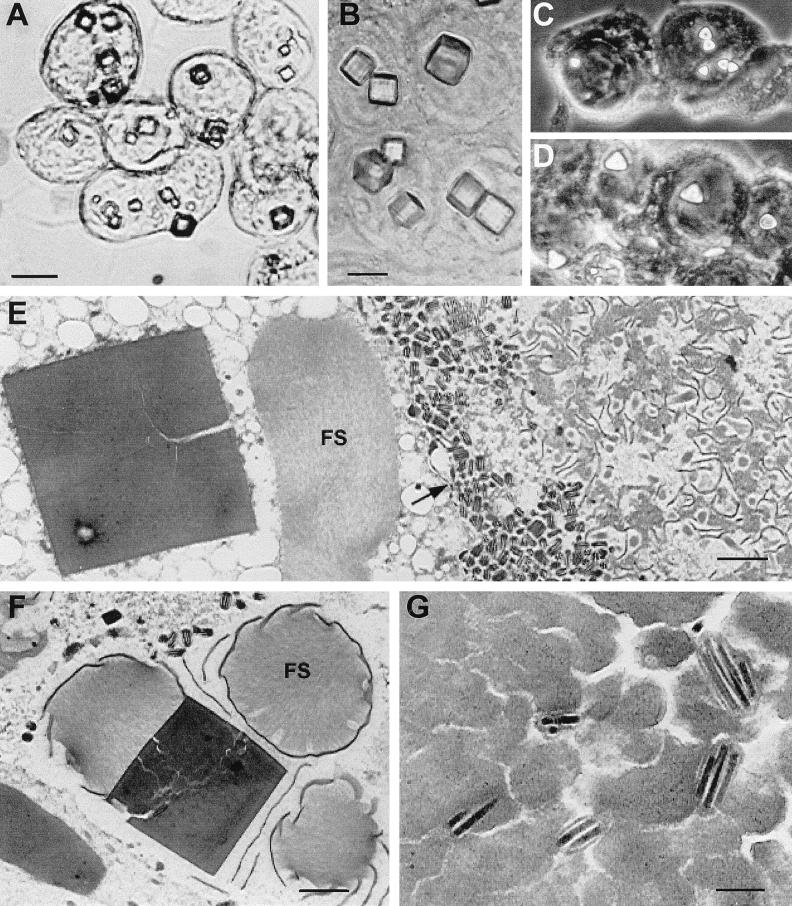
FIG. 3.
Light and electron micrographs of granulin and granulin-polyhedrin chimera crystals produced in Tn5 cells by recombinant AcMNPVs at 4 days postinfection. (A to D) Light micrographs of infected cells with large cuboidal crystals formed by wild-type granulin in the cytoplasm and nucleus (A), crystals formed in the nucleus by granulin containing the nuclear localization signal, KRKK (B), and tetrahedral crystals formed in the nucleus by the granulin-polyhedrin chimera (C and D). (E and F) Electron micrographs showing a large, fractured granulin crystal in the cytoplasm (E) and in the nucleus (F). In panel E, fibrillar structures (FS) are located in the cytoplasm adjacent to the crystal, and numerous unoccluded AcMNPV virions are present just inside the nuclear membrane (arrow). In panel F, fibrillar structures and electron-dense spacers surround a nuclear granulin crystal. (G) AcMNPV virions embedded in a heavily fissured intranuclear granulin crystal. The bars equal 6 μm in panels A to D, 1 μm in panels E and F, and 200 nm in panel G.
Examination by electron microscopy of cells infected with Ac/FB/TGO revealed aggregations of large numbers of virions concentrated along the periphery of the nucleus, adjacent to the nuclear membrane (Fig. 3E). Virions were not observed in most of the intranuclear granulin crystals observed in these cells but did occur in some, at a much lower concentration than that typical of wild-type AcMNPV polyhedra (Fig. 3E to G). No small occlusions containing a single virion, similar to TnGV granules, were observed.
The nuclear granulin inclusions were disrupted by numerous fractures but did not contain obvious cellular or viral components except for virions. The fractured appearance of the granulin polyhedra was not likely to be an artifact of sample preparation, as samples of the virus expressing the AcMNPV polyhedrin gene (Ac/FB/APO) prepared at the same time were normal in appearance. Fibrillar structures were often associated with the granulin crystals, whether or not the crystal contained virions, similar to the p10-containing structures associated with developing polyhedra in wild-type virus infections. Electron-dense spacers occurred adjacent to and aligned with the outer surface of nuclear granulin crystals. The granulin inclusions that developed in the cytoplasm were also fractured but contained no virions (Fig. 3E).
Expression of the TnGV granulin gene with a nuclear localization signal.
In cells infected with Ac/FB/TGO/K (producing KRKK-granulin), cuboidal inclusions were evident in the cell nucleus by three days postinfection. Most nuclei contained only one or a few large cuboidal granulin inclusions, whereas others contained many smaller inclusions (Fig. 3B). Occasionally, two inclusions appeared to be in the process of combining to form a large crystal.
When examined by electron microscopy, the inclusions produced by Ac/FB/TGO/K were homogeneous, contained no virions, and did not appear fractured (Fig. 4A and B). As in Ac/FB/TGO, large numbers of virions accumulated at the periphery of the nucleus. The granulin inclusions were fewer, larger, and less evenly spaced around the periphery of the nucleus than typical polyhedra in cells infected with wild-type AcMNPV. Association of the granulin inclusions with fibrillar networks was common, and electron-dense spacers could be seen aligned with the outer surface. Although more than 95% of the KRKK-granulin inclusions occurred in the nucleus, an occasional inclusion, also homogeneous and unfractured, was observed in the cytoplasm.
FIG. 4.
Micrographs of Tn5 cells infected with recombinant AcMNPVs that produce granulin with the KRKK nuclear localization signal (A to C), the granulin-polyhedrin chimera (D to F), or AcMNPV polyhedrin (G to I). (A) Electron micrograph of KRKK-granulin crystals without virions in the nucleus. (B) Association of a fibrillar structure (FS) with electron-dense spacers and a KRKK-granulin crystal. (C) Light micrograph of hemocytes infected with the KRKK-granulin recombinant AcMNPV, adjacent to fat body in a fourth-instar larva of T. ni. Note the large intranuclear KRKK-granulin crystals. (D to F) Electron micrographs of tetrahedral crystals produced by the granulin-polyhedrin chimera. (D) Intranuclear tetrahedral crystals with associated fibrillar structures (FS), electron-dense spacers, and masses of unoccluded virions near the nuclear membrane (arrows). (E and F) Corner of a tetrahedral crystal illustrating dense structure (E), and virions discernible in the central area of a crystal (F). (G to I) Light and electron micrographs illustrating characteristic intranuclear polyhedron formation (G and H) and virion occlusion (H and I) by the AcMNPV recombinant virus that produces AcMNPV polyhedrin. The bars equal 1 μm in panels A and B, 3 μm in panel C, 300 nm in panels D to F, 10 μm in panel G, 1 μm in panel I, and 300 nm in panel H.
Expression of hybrid granulin-polyhedrin gene.
Cells infected with the recombinant AcMNPV producing the granulin-polyhedrin chimera produced one or a few large tetrahedral inclusions per cell, most of which occurred in the nucleus (Fig. 3C and D). Like the granulin inclusions, these were unevenly spaced in the nucleus.
Viewed by electron microscopy, the tetrahedral crystals appeared dense and unfractured and contained AcMNPV virions, although at a much lower concentration than wild-type polyhedra (Fig. 4D to F). As in the AcMNPV recombinants that produced granulin, unoccluded virions accumulated at the nuclear membrane of Ac/FB/AT-infected cells. Fibrillar p10 networks and electron-dense spacers were also associated with the tetrahedral occlusions.
Expression of granulin and KRKK-granulin by AcMNPV in T. ni larvae.
Light microscopy of plastic sections showed that granulin inclusions in both Ac/FB/TGO infection and Ac/FB/TGO/K infection were mostly nuclear, smaller, and fewer in number than the granulin inclusions produced in cell culture infections. The Ac/FB/TGO/K virus produced larger inclusions than Ac/FB/TGO. Only a few inclusions were present per fat body cell, and typically only one inclusion was present per hemocyte (Fig. 4C).
Electron microscopy of larvae infected with Ac/FB/TGO/K showed essentially similar results to those described above for Tn5 cells. Fat body tissue contained inclusions that were still cuboidal but had edges more rounded than the inclusions produced in tissue culture cells (not shown). These inclusions contained no virions, associated with p10, and were aligned with electron-dense spacers as described above. In some cases, nucleocapsids could be seen aligned end-on with granulin inclusions. Ac/FB/TGO was not examined by electron microscopy because, by light microscopy, visible inclusions were rare.
Expression of AcMNPV polyhedrin by recombinant AcMNPV.
In contrast to the results obtained with the AcMNPV-expressed granulin constructs, the Ac/FB/APO control construct, which expressed the AcMNPV polyhedrin gene under control of the AcMNPV polyhedrin promoter, produced what appeared to be typical AcMNPV polyhedra (Fig. 4G to I). These polyhedra were of normal size, formed in the zone around the virogenic stroma, and occluded many virions in patterns characteristic of wild-type AcMNPV polyhedra.
The results of the present study demonstrate that TnGV granulin cannot substitute efficiently for polyhedrin in the AcMNPV occlusion process. Granulin without the KRKK nuclear localization signal occluded small numbers of virions in some cells, but no occluded virions were observed in KRKK-granulin inclusions. Moreover, both types of granulins formed large cuboidal crystals, resembling neither characteristic AcMNPV polyhedra nor TnGV granules. A granulin-polyhedrin hybrid was able to partially rescue occlusion, suggesting that the C-terminal region of polyhedrin is involved in occlusion but is not sufficient for normal occlusion. Occlusions produced by the hybrid protein resembled neither polyhedra nor granulin crystals. These results provide evidence that initiation and continuation of virion occlusion require specific domain-domain interactions between the occlusion body molecule and the virion envelope, probably proteins on the envelope surface, as suggested previously (4, 6–8, 16, 17), and that these interactions failed to occur because the homologous domains of granulin and AcMNPV virions were poorly compatible. In the absence of normal interactions between the AcMNPV virion and granulin, large granulin crystals formed by default in the nucleus and cytoplasm of the infected cells.
Cuboidal or other aberrant crystals which occlude virions poorly if at all have been reported for several mutant AcMNPV polyhedrins (3–6). The mutations in some of these polyhedrins had potentially disruptive effects on the folding of the polyhedrin protein and therefore most likely accounted for the lack of characteristic virion occlusion and formation of typical AcMNPV polyhedra. In the present study, however, at least the wild-type granulin, when produced by AcMNPV, should have yielded a granulin molecule with a normally folded structure capable of normal granulin-granulin interactions. If so, the failure of the wild-type granulin to occlude most AcMNPV virions further supports the hypothesis that poor occlusion was due to a lack of compatible interactions between granulin and the AcMNPV virion. Nevertheless, the occurrence of virions in some of the granulin crystals and the exclusion of other structural viral components, such as fibrillar structures, nucleocapsids, or electron-dense spacers, suggest that some weak but specific interaction occurred between granulin and the AcMNPV virion that was enhanced by partial replacement of granulin in the granulin-polyhedrin chimera.
The low level of virion occlusion obtained when wild-type granulin was substituted for polyhedrin in the AcMNPV resembled the AcMNPV FP mutant phenotype, caused by a deletion of the 25-kDa protein gene (11). FP mutants produce a small number of polyhedra, which contain few or no virions, and it has been suggested that the FP phenotype is caused by slower accumulation of polyhedrin, so that a critical concentration of polyhedrin required for occlusion is not reached (21). To eliminate this as a cause of the low level of virion occlusion obtained with granulin, we constructed the KRKK-granulin, which clearly resulted in a higher concentration of granulin in the nucleus (Fig. 3C and D). That we observed no virions in the crystals formed by KRKK-granulin again suggests a lack of compatible interaction between granulin and the AcMNPV virion.
Though granulin failed to interact compatibly with AcMNPV virions, it did interact with another AcMNPV protein involved in polyhedron formation. The p10 protein is a component of fibrillar networks in the nucleus and cytoplasm of infected cells (22, 24, 26, 29, 30) that is not required for production of infectious polyhedra (26). Fibrillar networks were associated with granulin crystals and with hybrid crystals in our recombinant AcMNPV strains, indicating that AcMNPV p10 is able to interact with TnGV granulin. This result is similar to the observation that Spodoptera exigua MNPV (SeMNPV) p10 formed fibrillar networks in infected cells when expressed by AcMNPV (25). It is less likely that the polyhedron envelope protein (PEP) interacted compatibly with granulin, KRKK-granulin, or the hybrid granulin-polyhedrin. PEP occurs in electron-dense spacers associated with the p10-containing fibrillar structures found in the nucleus of baculovirus-infected cells as well as in the polyhedrin envelope (13, 23–25, 28, 30). Electron-dense spacers did align along the occlusion surfaces in Tn5 cells infected with the recombinant viruses (Fig. 3F and 4A, B, and D). However, in some cells in advanced stages of disease, the granulin or mutant polyhedra were clumped together, forming a single large irregular crystal, suggesting that the crystals had no delimiting envelope. Thus, it appears that p10 can interact with granulin to bring it into close proximity with AcMNPV PEP, as occurs normally during occlusion formation, but that interactions between AcMNPV PEP and granulin may not be sufficient for envelope formation. The cuboidal shape of the granulin occlusions may also reflect an inability to be “sealed” with a polyhedron envelope, as Orgyia pseudotsugata MNPV (OpMNPV) with a deleted PEP gene produced polyhedra with a cuboidal shape distinct from the more beveled wild-type OpMNPV polyhedra (15).
Individual NPV species produce polyhedra of a characteristic shape, providing some evidence that occlusion body shape is determined in part by the primary amino acid sequence of the occlusion protein (4, 6, 8, 10, 27). The formation of large cuboidal crystals by wild-type granulin reported here, however, makes it clear that occlusion body shape is determined by more than the primary amino acid sequence alone. Nevertheless, the formation of cuboidal crystals by granulin and of tetrahedral crystals by the granulin-polyhedrin chimera provides experimental evidence that the amino acid sequence of the occlusion protein affects occlusion body shape.
Previous studies on the molecular basis of baculovirus virion occlusion and occlusion body formation have focused on polyhedrin mutants or polyhedrin fusion proteins with unusual phenotypes (3–8, 20, 21). Except for the production of the closely related SfMNPV polyhedrin in AcMNPV (14), heterologous expression of occlusion proteins has not been used to study the occlusion process. Our results indicate that substituting full and partial regions of occlusion body proteins among distantly related viruses will be useful for defining the molecular determinants of virion occlusion and occlusion body formation and shape.
Acknowledgments
We thank Dennis Bideshi for his constructive review of the research and the manuscript.
This research was supported in part by USDA Competitive Grant 95-37312-1634 to B.A.F.
REFERENCES
- 1.Akiyoshi D, Chakerian R, Rohrmann G F, Nesson M H, Beaudreau G S. Cloning and sequencing of the granulin gene from the Trichoplusia ni granulosis virus. Virology. 1985;141:328–332. doi: 10.1016/0042-6822(85)90267-3. [DOI] [PubMed] [Google Scholar]
- 2.Arnott H J, Smith K M. An ultrastructural study of the development of a granulosis virus in the cells of the moth Plodia interpunctella (Hbn.) J Ultrastruct Res. 1968;21:251–268. doi: 10.1016/s0022-5320(67)80095-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown M, Faulkner P, Cochran M A, Chung K L. Characterization of two morphology mutants of Autographa californica nuclear polyhedrosis virus with large cuboidal inclusion bodies. J Gen Virol. 1980;50:309–316. [Google Scholar]
- 4.Carstens E, Krebs A, Gallerneault C. Identification of an amino acid essential to the normal assembly of Autographa californica nuclear polyhedrosis virus polyhedra. J Virol. 1986;58:684–688. doi: 10.1128/jvi.58.2.684-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carstens E B, Lin-Bai Y, Faulkner P. A point mutation in the polyhedrin gene of a baculovirus, Autographa californica MNPV, prevents crystallization of occlusion bodies. J Gen Virol. 1987;68:901–905. doi: 10.1099/0022-1317-68-3-901. [DOI] [PubMed] [Google Scholar]
- 6.Carstens E, Williams G, Faulkner P, Partington S. Analysis of polyhedra morphology mutants of Autographa californica nuclear polyhedrosis virus: molecular and ultrastructural features. J Gen Virol. 1992;73:1471–1479. doi: 10.1099/0022-1317-73-6-1471. [DOI] [PubMed] [Google Scholar]
- 7.Duncan R, Faulkner P. Bromodeoxyuridine-induced mutants of Autographa californica nuclear polyhedrosis virus defective in occlusion body formation. J Gen Virol. 1982;62:369–373. doi: 10.1099/0022-1317-62-2-369. [DOI] [PubMed] [Google Scholar]
- 8.Duncan R, Chung K L, Faulkner P. Analysis of a mutant of Autographa californica nuclear polyhedrosis virus with a defect in the morphogenesis of the occlusion body macromolecular lattice. J Gen Virol. 1983;64:1531–1542. doi: 10.1099/0022-1317-64-7-1531. [DOI] [PubMed] [Google Scholar]
- 9.Federici B A. Mosquito baculovirus: sequence of morphogenesis and ultrastructure of the virion. Virology. 1980;100:1–9. doi: 10.1016/0042-6822(80)90546-2. [DOI] [PubMed] [Google Scholar]
- 10.Federici B A. Ultrastructure of baculoviruses. In: Granados R R, Federici B A, editors. The biology of baculoviruses. I. Boca Raton, Fla: CRC Press, Inc.; 1986. pp. 61–88. [Google Scholar]
- 11.Fraser M J. FP mutation of nuclear polyhedrosis viruses: a novel system for the study of transposon-mediated mutagenesis. In: Maramorosch K, editor. Biotechnology in invertebrate pathology and cell culture. New York, N.Y: Academic Press, Inc.; 1987. pp. 265–293. [Google Scholar]
- 12.Gardiner G R, Stockdale H. Two tissue culture media for production of lepidopteran cells and nuclear polyhedrosis viruses. J Invertebr Pathol. 1975;25:363–370. [Google Scholar]
- 13.Gombart A F, Pearson M N, Rohrmann G F, Beaudreau G S. A baculovirus polyhedral envelope-associated protein: genetic location, nucleotide sequence, and immunocytochemical characterization. Virology. 1989;169:182–193. doi: 10.1016/0042-6822(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez M A, Smith G E, Summers M D. Insertion of the SfMNPV polyhedrin gene into an AcMNPV polyhedrin deletion mutant during viral infection. Virology. 1989;170:160–175. doi: 10.1016/0042-6822(89)90363-2. [DOI] [PubMed] [Google Scholar]
- 15.Gross C H, Russell R L Q, Rohrmann G F. Orgyia pseudotsugata baculovirus p10 and polyhedron envelope protein genes: analysis of their relative expression levels and role in polyhedron structure. J Gen Virol. 1994;75:1115–1123. doi: 10.1099/0022-1317-75-5-1115. [DOI] [PubMed] [Google Scholar]
- 16.Harrap K. The structure of nuclear polyhedrosis viruses. III. Virus assembly. Virology. 1972;50:133–139. doi: 10.1016/0042-6822(72)90353-4. [DOI] [PubMed] [Google Scholar]
- 17.Harrap K A, Robertson J S. A possible infection pathway in the development of a nuclear polyhedrosis virus. J Gen Virol. 1968;3:221–225. [Google Scholar]
- 18.Hooft van Iddekinge B J L, Smith G E, Summers M D. Nucleotide sequence of the polyhedrin gene of Autographa californica nuclear polyhedrosis virus. Virology. 1983;131:561–565. doi: 10.1016/0042-6822(83)90522-6. [DOI] [PubMed] [Google Scholar]
- 19.Horton R M, Ho S N, Pullen J K, Hunt H D, Cai Z, Pease L R. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis D L, Bohlmeyer D A, Garcia A J. Requirements for nuclear localization and supramolecular assembly of a baculovirus polyhedrin protein. Virology. 1991;185:795–810. doi: 10.1016/0042-6822(91)90551-l. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis D L, Bohlmeyer D A, Garcia A J. Enhancement of polyhedrin nuclear localization during baculovirus infection. J Virol. 1992;66:6903–6911. doi: 10.1128/jvi.66.12.6903-6911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Wilk F, van Lent J W M, Vlak J M. Immunogold detection of polyhedrin, p10 and virion antigens in Autographa californica nuclear polyhedrosis virus-infected Spodoptera frugiperda cells. J Gen Virol. 1987;68:2615–2623. [Google Scholar]
- 23.van Lent J W M, Groenen J T M, Klinge-Roode E C, Rohrmann G F, Zuidema D, Vlak J M. Localization of the 34 kDa polyhedron envelope protein in Spodoptera frugiperda cells infected with Autographa californica nuclear polyhedrosis virus. Arch Virol. 1990;111:103–114. doi: 10.1007/BF01310508. [DOI] [PubMed] [Google Scholar]
- 24.van Oers M M, Flipsen J T M, Reusken C B E M, Sliwinsky E L, Goldbach R W, Vlak J M. Functional domains of the p10 protein of Autographa californica nuclear polyhedrosis virus. J Gen Virol. 1993;74:563–574. doi: 10.1099/0022-1317-74-4-563. [DOI] [PubMed] [Google Scholar]
- 25.van Oers M M, Flipsen J T M, Reusken C B E M, Vlak J M. Specificity of baculovirus p10 functions. Virology. 1994;200:513–523. doi: 10.1006/viro.1994.1214. [DOI] [PubMed] [Google Scholar]
- 26.Vlak J, Klinkenberg F, Zaal K, Usmany M, Klinge-Roode E, Geervliet J, Roosien J, van Lent J. Functional studies on the p10 gene of Autographa californica nuclear polyhedrosis virus using a recombinant expressing a p10-beta-galactosidase fusion gene. J Gen Virol. 1988;69:765–776. doi: 10.1099/0022-1317-69-4-765. [DOI] [PubMed] [Google Scholar]
- 27.Vlak J M, Rohrmann G F. The nature of polyhedrin. In: Maramorosch K, Sherman K E, editors. Viral insecticides for biological control. New York, N.Y: Academic Press, Inc.; 1985. pp. 489–542. [Google Scholar]
- 28.Whitt M A, Manning J S. A phosphorylated 34-kDa protein and a subpopulation of polyhedrin are thiol linked to the carbohydrate layer surrounding a baculovirus occlusion body. Virology. 1988;163:33–42. doi: 10.1016/0042-6822(88)90231-0. [DOI] [PubMed] [Google Scholar]
- 29.Williams G V, Rohel D Z, Kuzio J, Faulkner P. A cytopathological investigation of Autographa californica nuclear polyhedrosis virus p10 gene function using insertion/deletion mutants. J Gen Virol. 1989;70:187–202. doi: 10.1099/0022-1317-70-1-187. [DOI] [PubMed] [Google Scholar]
- 30.Zuidema D, Klinge-Roode E C, van Lent J W M, Vlak J M. Construction and analysis of an Autographa californica nuclear polyhedrosis virus mutant lacking the polyhedral envelope. Virology. 1989;173:98–108. doi: 10.1016/0042-6822(89)90225-0. [DOI] [PubMed] [Google Scholar]