Abstract
The efficiency of adenovirus-mediated gene transfer is now well established. However, the cellular and the humoral immune responses triggered by vector injection lead to the rapid elimination of the transduced cells and preclude any efficient readministration. The present investigation focuses on the role of tumor necrosis factor alpha (TNF-α), a proinflammatory cytokine, and the related cytokine lymphotoxin α (LTα), in mounting an immune reaction against recombinant adenovirus vectors. After gene transfer in the liver, mice genetically deficient for both cytokines (TNF-α/LTα−/−), in comparison with normal mice, presented a weak acute-phase inflammatory reaction, a reduction in cellular infiltrates in the liver, and a severely impaired T-cell proliferative response to both Adenoviral and transgene product antigens. Moreover, we observed a strong reduction in the humoral response to the vector and the transgene product, with a drastic reduction of anti-adenovirus immunoglobulin A and G antibody isotypes. In addition, the reduction in antibody response observed in TNF-α/LTα−/− and TNF-α/LTα+/− mice versus TNF-α/LTα+/+ mice links antibody levels to TNF-α/LTα gene dosage. Due to the absence of neutralizing antibodies, the TNF-α/LTα knockout mice successfully express a second gene transduced by a second vector injection. The discovery of the pivotal role played by TNF-α in controlling the antibody response against adenovirus will allow more efficient adenovirus-based strategies for gene therapy to be proposed.
Adenovirus is a powerful vector for gene transfer to many tissues. Subsequent to infection, however, a strong two-phase immune response develops, impairing transgene expression: a polymorphonuclear leukocyte infiltration occurs within the first few days postinfection (p.i.) (24, 31, 60), followed by a specific immunoclearance of the infected cells. The immune effectors that come into play have been characterized in liver- and lung-directed gene transfer models. First, major histocompatibility complex (MHC) class I-restricted cytotoxic T lymphocytes (CTL) directed toward viral antigens and the transgene product target the transduced cells (11, 20, 34, 57, 61). Presentation of exogenous viral antigens by MHC class II molecules has also been implicated to induce CD4+ T cells of the Th1 subset that strengthen the cytotoxic response, as well as CD4+ T cells of the Th2 subset involved in mounting an efficient humoral response (62). The B-cell response to an adenoviral infection consists essentially of immunoglobulin G (IgG) serum antibodies, but IgA antibodies also appear within the lungs following airway administration (62). Since some of these antibodies are neutralizing, efficient adenovirus readministration is prevented (9, 14, 19). Finally, serum antibodies have been implicated in reducing the levels of the transgene product in cases for which the transgene encodes a secreted protein (31, 57).
Different strategies are being developed to counteract both arms of the host response to adenovirus infection. The first approach relies on modifying the vector backbone to limit its ability to induce a strong cellular response. E1-deleted vectors with a temperature-sensitive mutation introduced in the E2A gene were first shown to enhance transgene persistence by decreasing the cellular response (16). Vectors defective for both E1 and E4 have also been shown to lead to long-term survival of transduced hepatocytes in C57BL/6 mice immunotolerant for the transgene product (11). Similar conclusions were reached by others who showed that systemic administration of an E1/E4-defective adenovirus correlated with fewer CTLs and a prolonged transgene expression (20, 59). Adenovirus vectors with larger deletions are now being engineered that may decrease further the cellular arm of the immune response to the vector (29, 36). Although deletions of viral genes represent a potent approach for inhibiting the cellular response to the vector, it does not address the issues that stem from the humoral response directed against the capsid components.
A different means to control the host response aims at interfering directly with the many steps of this process, including inflammation. For example, a recombinant adenovirus encoding the interleukin-1 (IL-1) receptor antagonist was tested but failed to block virus-induced inflammation (40). In another study, tolerance induction following intrathymic or oral administration of adenoviral antigens was shown to be effective in abrogating the recognition phase due to the deletion or anergy of the cognate lymphocytes, translating into long-term gene delivery and efficient readministration (10, 28, 58). Administration of immunosuppressive drugs such as cyclophosphamide or cyclosporine has also been used to block the cellular and humoral arms of the immune response (50). Blocking of cell adhesion and costimulation molecules has also been tested. For example, neutralization of CD40 ligand by antibody administration has been reported to block CTL response and production of virus-specific neutralizing antibodies (63). IL-12 administration aimed at increasing the Th1/Th2 ratio has been shown to inhibit production of IgA-neutralizing antibodies and to allow successful readministration in the lung (64). By contrast, inclusion of an IL-10-like cytokine of viral origin capable of decreasing the Th1/Th2 ratio has been reported to inhibit the cellular component of the response (47). Finally, overexpression of adenovirus E3 gp19K protein has been shown to downregulate both the levels of MHC class I molecules at the cell surface and CTL induction (6, 34). Although effective, most of these strategies, however, create a profound immunosuppression. It is therefore important to assess the role of other effectors in the host response against adenoviral vectors.
Tumor necrosis factor alpha (TNF-α) and lymphotoxin α (LTα) are known to be important players during the development of the immune response. TNF-α, which is mainly produced by activated macrophages and T cells, exists as a homotrimeric secreted molecule that binds either to TNF receptor 1 (TNFRI or p55) or TNF receptor 2 (TNFRII or p75). TNF-α also exists as a transmembrane protein that binds to these same receptors for signal transduction (25). LTα is another related homotrimeric secreted molecule, expressed by B and T lymphocytes, which also binds to TNFRI and TNFRII. LTα also associates with lymphotoxin β (LTβ) to form different heterotrimeric complexes: the LTα2β1 trimer binds to both TNF-α receptor types, the LTα1β2 trimer binds exclusively to a different receptor (LTβ receptor) (5, 8). Binding to TNFRI triggers various biological responses, such as cytolysis and inflammation. For example, TNF-α-induced activation of phospholipase A2 is most likely involved in cytolysis and leads to the liberation of arachidonic acid and the synthesis of prostaglandins and leukotrienes, which are very potent mediators of inflammation (32).
The importance of TNF-α as a major actor in the immune response against adenovirus is supported by several independent observations. For example, TNF-α has been detected at a very early stage following adenovirus administration to the lungs (24). The pivotal role of TNF-α in controlling adenovirus infection is further emphasized by the existence of a set of adenovirus-encoded genes from the E3B region that counteract its function: the 14.7-kDa protein and the 10.4-kDa–14.5-kDa heterodimer act by different mechanisms to inhibit TNF-α-induced cytolysis and phospholipase A2 activation (30). In particular, the 10.4-kDa–14.5-kDa complex blocks the TNF-α-induced translocation of cytosolic phospholipase A2 to the plasma membrane, impairing the release of inflammation mediators (13). This complex also impairs FasL/Fas cytotoxicity by the release or degradation of cell surface Fas (49, 56). The E3 14.7-kDa protein interferes with TNF-α-induced apoptosis through its interaction with the caspase FLICE (7). The biological significance of the E3B region in controlling the immune response is further supported by assessing the in vivo behavior of specific mutant adenoviruses. For example, administration of mutants, lacking all or parts of the E3B region, into the lungs of both permissive (cotton rats) and nonpermissive (mice) animals has been correlated with an increased alveolar infiltration, compared with wild-type infection, an increase that was most likely made possible because the recruitment of inflammatory cells by TNF-α byproducts was no longer inhibited (23, 51). To investigate the contribution of TNF-α and its related LTα in the immune response against adenovirus, we have monitored the immune parameters affected by the systemic administration of vectors in mice genetically deficient for both cytokines (1).
MATERIALS AND METHODS
Mice.
Generation of TNF-α/LTα−/− mice by homologous recombination and the TNF-α/LTα+/− and TNF-α/LTα+/+ mice have been described (1). The mice were maintained on a mixed 129Sv × C57BL/6 genetic background in the animal facilities of the Institut Gustave Roussy (IGR) under specified pathogen-free conditions.
Adenovirus vectors.
AdRSVβgal and Adα1hAT encoding a nuclear β-galactosidase and the human α1-antitrypsin (αAT), respectively, were as described previously (22, 54). Ad-dl324 was used as an antigen in the antibody assay (55). A recombinant adenovirus AdCO1 encoding no transgene was used in the proliferation assay (26). The viral stocks were prepared on 293 cells and purified twice on CsCl gradients. Desalting was performed by using Pharmacia G50 columns (Orsay, France), and viruses were frozen in phosphate-buffered saline (PBS)–10% glycerol at −80°C. Titers were calculated as PFU on 911 cells (18).
Animals.
A volume of 100 μl containing 5 × 109 PFU of recombinant adenovirus diluted in PBS was injected in the retroorbital plexus of 2-month-old mice. Blood samples were collected before the virus injection and at different intervals thereafter. The sera were prepared and analyzed for serum amyloid P component (SAP), αAT, and antibodies. When AdRSVβgal was injected, animals were sacrificed at different days, and livers were removed for histological analysis and transgene expression assay.
Measurement of SAP.
The quantity of SAP present in mouse sera was quantified by enzyme-linked immunosorbent assay (ELISA). A 96-well microtiter plate (Nunc Maxisorp, Roskilde, Denmark) was coated with 50 μl of sheep antimurine SAP antibody per well (Calbiochem, La Jolla, Calif.) diluted 1:1,000 in 50 mM NaHCO3; after overnight incubation at 4°C, the wells were washed five times with TBSMT (Tris-buffered saline [TBS], 5% nonfat dry milk [Regilait; Nestlé, Saint-Martin-Belle-Roche, France], 0.02% Tween 20 [Sigma, Saint Quentin Fallovier, France]). Wells were blocked with TBSMT for 2 h at room temperature on a rocking platform shaker. After being washed as above, wells were incubated in TBSMT with serial dilutions of mice sera or standard murine SAP (Calbiochem). After a 2-h incubation with shaking and washing as above, wells were incubated with 50 μl of rabbit anti-murine SAP antibody (Calbiochem) diluted 1:2,000 in TBSMT. After incubation and washing as described above, wells were incubated with 50 μl of anti-rabbit antibody conjugated with alkaline phosphatase (Promega, Madison, Wis.) diluted 1:5,000 in TBSMT. After incubation and a washing as described above, SAP was detected by a 20-min incubation with developing solution (alkaline phosphatase substrate kit; Bio-Rad Laboratories, Richmond, Calif.). The A405 was determined by using a microplate reader (Bio-Rad).
Human αAT assay.
The expression of αAT was monitored by a sandwich ELISA as described earlier (42). Briefly, 96-well microtiter plates (Nunc) were coated with a rabbit anti-human αAT antibody (Cappel; Organon Teknika, Fresnes, France), blocked with 5% milk in TBS-Tween; dilutions of sera were then added. The linked αAT was revealed by using a rabbit anti-human αAT Ab (Boehringer Mannheim, Meylan, France) and an alkaline phosphatase-conjugated goat anti-rabbit Ab (Promega).
Determination of adenovirus-specific antibodies.
The presence of adenovirus-specific antibodies in the sera was determined by ELISA. Microplates with 96 wells (Nunc) were coated with 100 ng of inactivated adenovirus dl324 (55) and blocked with 5% milk in TBS-Tween; serial dilutions of the sera were then added. Bound antibody was detected with peroxidase-conjugated anti-mouse isotypes IgG+IgM (Jackson Immunoresearch Laboratories, West Grove, Pa.), IgG2a, IgG1, IgA, or IgM (Southern Biotechnology Associates, Birmingham, Ala.) goat antibodies. The peroxidase was revealed by incubation with the substrate o-phenylenediamine dihydrochloride (Sigma) for 30 min. The reaction was stopped by the addition of 3 N HCl, and a spectrophotometric reading was obtained at 490 nm.
Anti-adenoviral neutralizing antibody assay.
293 cells cultured in complete minimal essential medium (GIBCO-BRL) in 96-well plates (50,000 cells/well) were incubated with recombinant adenovirus (multiplicity of infection = 10) and serum dilution. Typically, a suspension of AdRSVβgal (5 × 105 PFU in 100 μl) was mixed with serial dilutions of serum samples decomplemented for 30 min at 56°C. This mixture was incubated with the 293 cells for 1 h at 37°C. Then, 100 μl of complete medium was added, and the cells were cultured for 19 h. Fluorometric methodology, which allows a more accurate estimation of transduction, was used. Briefly, cells were washed and incubated with 100 μl of lysis buffer (6 mM Na2HPO4, 10 mM KCl, 0.1 mM MgSO4, 50 mM 2-mercaptoethanol, and 0.5% Triton X-100) containing 0.1 mg of βgal substrate (4-methyl-umbelliferyl-β-d-galactoside; Sigma) for 1 h at 37°C. After excitation at 360 nm, the resulting fluorescence was measured at 460 nm with a Cytofluor 2300 (Millipore Corp., Bedford, Mass.). For each serum dilution, the percentage of transduction was calculated as follows: (experimental value − background value [without virus])/(positive control [100% transduction] − background value) × 100. The titer of neutralizing antibody is defined as the highest dilution which gives a percentage of transduction lower than 50%.
Determination of β-galactosidase-specific antibodies.
Plates (96-well; Nunc) were coated with 100 ng of β-galactosidase (Sigma) and treated as described above. Serial dilutions of the sera in 5% milk in TBS-Tween were added, and bound antibody was detected with peroxidase-conjugated anti-mouse isotypes IgG+IgM (Jackson Immunoresearch Laboratories). The peroxidase activity was measured as described above.
Histochemical analysis and histopathology.
After sacrifice of the animals, livers were removed at different days p.i., immediately embedded in OCT compound (Tissue-Tek; Miles Laboratories Inc., Naperville, Ill.), and snap-frozen in liquid nitrogen. For β-galactosidase activity, cryosections (10 μm) were processed by postfixing in 0.37% formaldehyde–0.2% glutaraldehyde in PBS, stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactosidase), and counterstained with hematoxylin and eosin. For inflammation analysis, liver sections were counterstained with hematoxylin and eosin and then classified as described into four groups according to the level of cellular infiltrate as follows: group 1, absent or inconspicuous infiltration; group 2, slight infiltration; group 3, moderate infiltration; and group 4, severe infiltration (11).
Immunostaining.
Acetone-fixed frozen sections of liver (5 μm) were incubated with either a rat anti-CD4 antibody at a dilution of 1:3,000 or a rat anti-CD8 antibody at a dilution of 1:1,000 (PharMingen, Inc., San Diego, Calif.). For detection of macrophages, anti-Mac1 antibody was used at a dilution of 1:600 (PharMingen). After being washed, an alkaline phosphatase-conjugated rabbit anti-rat antibody (DAKO, Trappes, France) was added at a dilution of 1:50. In order to amplify the signal, an APAAP complex specific for rat (rat antibodies to calf intestinal alkaline phosphatase plus calf intestinal alkaline phosphatase) was added at a dilution of 1:25 (DAKO). Endogenous phosphatases were inhibited with levamisole (DAKO). Alkaline phosphatase activity was revealed with Fast Red substrate (DAKO), and the liver sections were counterstained with hematoxylin. As a negative control, the primary antibody was omitted in each immunostaining.
Lymphoproliferation assay.
Mouse splenocytes were obtained at different times after AdRSVβgal administration. Quadruplicate cultures of lymphocytes (2.5 × 105 cells) were incubated in 96-well plates (Nunc) in 200 μl of RPMI supplemented with 0.6% mouse serum and 50 μM 2-mercaptoethanol with β-galactosidase (0.6 μg/ml; Sigma), AdRSVβgal (10 PFU/cell), or AdCO1 (10 PFU/cell, heat inactivated or not) or without antigen. Proliferation was determined on day 5 in culture after an 18-h pulse with [3H]thymidine (specific activity, 5 Ci/mmol; NEN, Le Blanc-Mesnil, France) with 1 μCi/well. Results were expressed in counts per minute (Δcpm) and calculated as Δcpm = mean cpm with antigen − mean cpm without antigen.
Cytokine release assay.
Spleen cells (5 × 106) from AdRSVβgal-infected animals were cultured in 24-well plates (Nunc) in 2 ml of RPMI supplemented with 0.6% mouse serum and 50 μM 2-mercaptoethanol. For detection of proinflammatory cytokines, splenocytes were prepared at day 1 p.i. and cultured in the absence of any antigen, and cell-free supernatants were examined at 24 h in duplicate for the presence of IL-6 and TNF-α using commercial kits (R&D Systems, Abingdon, England). For analysis of the T-helper cytokine profile, spleen cells were removed at different times and cultured either with no antigen or with heat-inactivated AdCO1 (10 PFU/cell) or recombinant β-galactosidase (0.6 μg/ml). After 48 h, cell-free supernatants were collected and tested in duplicate for the presence of IL-2, gamma interferon (IFN-γ), and IL-4 by using commercial ELISA kits (R&D Systems).
RESULTS
A reduced inflammatory response was unaccompanied by a sustained expression of transgene in TNF-α/LTα−/− mice following recombinant adenovirus administration.
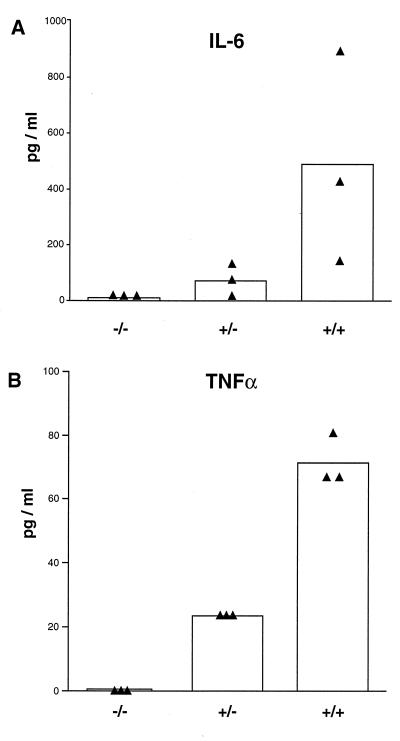
In order to analyze the role played by TNF-α and LTα in the immune response directed against E1-deleted adenovirus, we administered 5 × 109 PFU of AdRSVβgal to TNF-α/LTα+/+, TNF-α/LTα+/−, and TNF-α/LTα−/− mice. We first monitored the level of SAP, an acute-phase protein produced at a very early step after the inflammatory stimulus with a pic at around 24 to 36 h (53). In each experimental group, an increase in the level of SAP is observed at day 1 p.i. after adenovirus administration and is maintained until day 2 p.i. (Table 1). However, the quantity of SAP remains less in TNF-α/LTα−/− than in TNF-α/LTα+/− and TNF-α/LTα+/+ mice (80.75 μg/ml versus 114.35 and 116.15, respectively, at day 1). These data lead to a difference in SAP levels between day 1 before the virus injection and day 2 p.i. that is lower in TNF-α/LTα−/− mice than in the control TNF-α/LTα+/− or TNF-α/LTα+/+ mice (4.4 μg/ml versus 57 and 40.7 μg/ml, respectively). We also analyzed two other parameters of the inflammatory response: namely, the production of two proinflammatory cytokines TNF-α and IL-6. Because these cytokines were undetectable in mice sera, supernatants of splenocytes of animals infected with adenovirus for 1 day were tested for the presence of both cytokines. A strong reduction in IL-6 levels was observed in TNF-α/LTα−/− mice (10.4 ± 7.4 pg/ml) versus TNF-α/LTα+/− (71.9 ± 44.7 pg/ml) and TNF-α/LTα+/+ (489 ± 310.2 pg/ml) mice (Fig. 1A). Interestingly, TNF-α/LTα+/− mice produce less TNF-α than TNF-α/LTα+/+ mice (23.4 ± 0 pg/ml versus 71.5 ± 6.4 pg/ml [Fig. 1B]).
TABLE 1.
Measurement of SAP levels after administration of recombinant adenovirus
| Mouse group | SAP level (μg/ml)a on day:
|
||
|---|---|---|---|
| −1 (preinfection) | 1 p.i. | 2 p.i. | |
| TNF-α/LTα+/+ | 55.95 ± 25.6 | 116.15 ± 27.5* | 96.65 ± 25.7** |
| TNF-α/LTα+/− | 61.95 ± 27.87 | 114.35 ± 33.68 | 118.9 ± 42.95* |
| TNF-α/LTα−/− | 54.45 ± 19.2 | 80.75 ± 35.1 | 58.8 ± 17.9 |
The values represent the means ± the standard deviations for six mice. ∗ and ∗∗ represent statistically significant differences (P < 0.05 and P < 0.01, respectively) compared to TNF-α/LTα−/− animals (Student t test).
FIG. 1.
Correlation between TNF-α/LTα gene dosage and production of proinflammatory cytokines following adenovirus infection. The production of IL-6 (A) and TNF-α (B) in the supernatant of splenocytes of TNF-α/LTα−/−, TNF-α/LTα+/−, and TNF-α/LTα+/+ mice infected for 1 day was monitored by ELISA. The bar graph depicts the mean of data (n = 3) from splenocyte cultures for each mouse genotype; each triangle represents individual mouse data.
X-Gal staining performed on liver sections at days 4, 8, and 16 showed that in the TNF-α/LTα−/− mice, β-galactosidase expression did not last any longer than it did in the controls; few hepatocytes were still expressing β-galactosidase at day 16 (data not shown).
Transgene-expressing cells are eliminated by the cellular immune response.
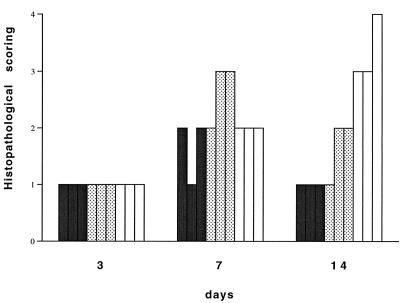
Animals injected with AdRSVβgal were analyzed for their liver cellular infiltrates (see Materials and Methods). Figure 2 depicts the increased histopathological scoring observed for all three mouse groups between days 3 and 14. There is no significant difference in the quantity of cellular infiltrates between the different groups at days 3 and 7, but the infiltrate at day 14 is clearly less important in the TNF-α/LTα−/− mice than in the TNF-α/LTα+/+ mice. Indeed, the cellular infiltrate in TNF-α/LTα−/− mice never exhibits foci of inflammatory cells (grades 3 and 4 of the histopathological scoring) in liver parenchyme as was observed in TNF-α/LTα+/+ mice.
FIG. 2.
Cellular infiltrate in liver of TNF-α/LTα-deficient mice. The relative cellular infiltrate in liver sections expressed as a histopathological score (see Materials and Methods) was evaluated at days 3, 7, and 14 p.i. Bars represent individual scores (n = 3) of TNF-α/LTα−/− (solid bars), TNF-α/LTα+/− (stippled bars), or TNF-α/LTα+/+ (open bars) mice.
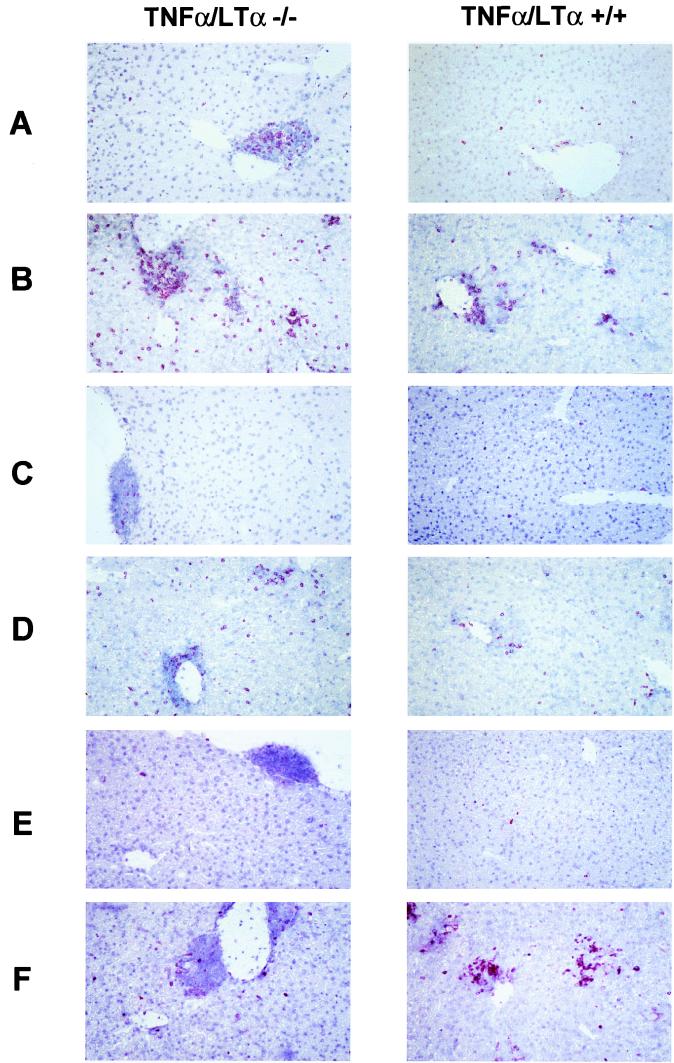
We used immunostaining to characterize the immune cells involved in the cellular infiltrate and responsible for the elimination of transgene-expressing cells. Figure 3A and C show, respectively, CD4+ or CD8+ cells present at basal levels in the liver of noninfected TNF-α/LTα−/− and TNF-α/LTα+/+ mice. While TNF-α/LTα+/+ mice present a nearly complete absence of CD4+ and CD8+ cells, TNF-α/LTα−/− mice show clusters of cells in the periportal and perivascular regions containing CD4+ and CD8+ cells. A similar accumulation of lymphocytes was previously described for LTα−/− mice and was attributed to a homing of lymphocytes to liver because of a general defect in the organogenesis of secondary lymphoid tissues (3). At day 7 of the adenoviral infection, the levels of CD4 and CD8 cells are augmented in both TNF-α/LTα−/− and TNF-α/LTα+/+ mice (Fig. 3B and D). The presence of CD8+ T cells in the liver parenchyme may explain the complete elimination of β-galactosidase-expressing cells at day 16 p.i. in both groups of mice.
FIG. 3.
Characterization of cellular infiltrate in the liver of adenovirus-infected mice. Immunostaining of cells positive for CD4 (A and B), CD8 (C and D), and Mac1 (E and F) was performed on liver sections of either noninfected (A, C, and E) or infected (B, D, and F) TNF-α/LTα−/− (left) and TNF-α/LTα+/+ (right) mice. Magnification, ×200.
Figure 3E shows the absence of Mac1-positive cells in noninfected TNF-α/LTα+/+ mice and the presence of few Mac1-positive cells among the lymphocytes clustered in the periportal and perivascular regions of TNF-α/LTα−/− mice. Interestingly, at day 7 p.i., foci of Mac1-positive cells are present in the liver parenchyme of TNF-α/LTα+/+ mice, while TNF-α/LTα−/− mice exhibit an increase in Mac1 staining only in the periportal and perivascular regions (Fig. 3F).
Impaired antibody response in TNF-α/LTα−/− mice.
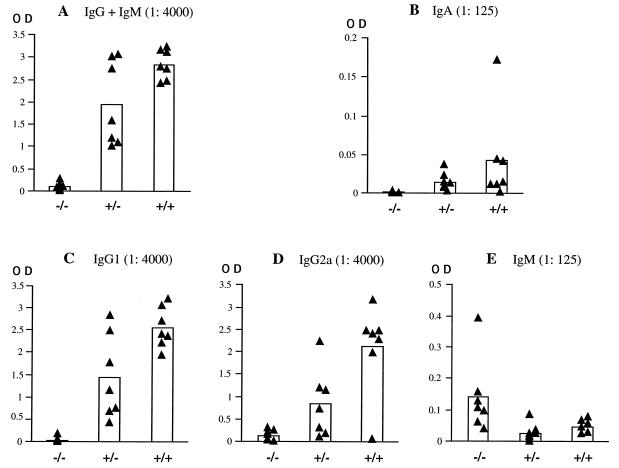
We investigated the humoral response against adenovirus in all three groups of mice. The antibody level against the adenoviral capsid was analyzed in the sera at day 29 after intravenous injection of 5 × 109 PFU of Adα1hAT. We observed a marked decrease in the level of total IgM and IgG antibodies in the sera of TNF-α/LTα−/− mice compared with the other mice (Fig. 4A). A similar defect was noticed for antibodies of the IgA isotype (Fig. 4B). Quantification of IgG subisotypes IgG1 and IgG2a indicates that the levels of both of these are very much reduced (Fig. 4C and D, respectively); it should be pointed out, however, that while some TNF-α/LTα+/− and TNF-α/LTα+/+ mice failed to produce significant levels of IgG2a, all of them produced IgG1 antibodies emphasizing the importance of this isotype in response to adenovirus systemic administration. On the contrary, levels of the IgM antibodies were significantly higher in the TNF-α/LTα−/− mice (Fig. 4E).
FIG. 4.
Impaired humoral response against adenovirus in TNF-α/LTα-deficient mice. Sera of TNF-α/LTα+/+, TNF-α/LTα+/−, and TNF-α/LTα−/− mice were analyzed for adenovirus-specific antibodies of different isotypes: A, IgG+IgM antibodies; B, IgA antibodies; C, IgG1 antibodies; D, IgG2a antibodies; and E, IgM antibodies. The values expressed as OD490 represent relative quantities of antibody at serum dilutions of 1:4,000 (IgG+IgM, IgG1, and IgG2a) and 1:125 (IgM and IgA). The black triangles represent antibody levels at day 29 p.i. in the serum of individual mice, and the columns represent the mean of each group.
To determine whether among the antibodies barely detectable in the TNF-α/LTα−/− mice sufficient neutralizing antibodies are present to neutralize a second adenovirus administration, we specifically evaluated the level of neutralizing antibodies in the same sera at day 29. Table 2 shows that while the titers of neutralizing antibodies in the TNF-α/LTα+/+ and TNF-α/LTα+/− mice range from 20 to 160 and from 40 to 160, respectively, the titers in the sera of the TNF-α/LTα−/− mice remain below 20 (the lowest dilution tested for technical reasons).
TABLE 2.
Measurement of neutralizing antibody titer following administration of recombinant adenovirus
| Mouse | Antibody titera for group:
|
||
|---|---|---|---|
| TNF-α/LTα−/− | TNF-α/LTα+/− | TNF-α/LTα+/+ | |
| 1 | <20 | 160 | 80 |
| 2 | <20 | 40 | 160 |
| 3 | <20 | 20 | 40 |
| 4 | <20 | 40 | 80 |
| 5 | <20 | 40 | 80 |
| 6 | <20 | 80 | 160 |
| 7 | <20 | 80 | 160 |
| 8 | <20 | 80 | |
The values represent antibody titers of individual mice in the sera at day 29 p.i. of 5 × 109 PFU of Adα1hAT.
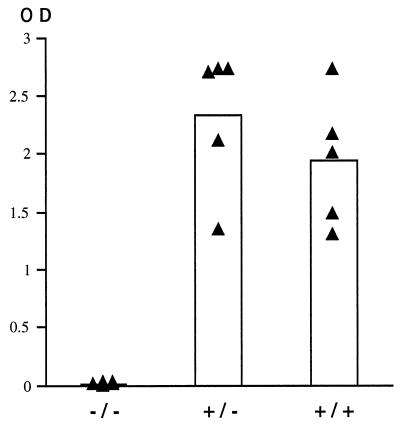
We also determined whether the TNF-α/LTα−/− mice are able to mount an immune response against the product of the transgene. Because β-galactosidase is known to be immunogenic in a B6 background (10), mice were injected with 5 × 109 PFU of AdRSVβgal. As illustrated in Fig. 5, sera (1:4,000 dilution) from all of the TNF-α/LTα+/− and TNF-α/LTα+/+ mice revealed a strong antibody response (IgG+IgM) against β-galactosidase (optical densities [OD] of 2.33 ± 0.549 and 1.94 ± 0.510, respectively). In sharp contrast, TNF-α/LTα−/− mice evidenced a dramatically impaired response (OD = 0.007 ± 0.007).
FIG. 5.
Impaired antibody response against the product of the transgene in TNF-α/LTα-deficient mice. TNF-α/LTα+/+, TNF-α/LTα+/−, and TNF-α/LTα−/− mice were injected with AdRSVβgal (5 × 109 PFU). The values expressed as OD490 represent relative quantities of antibody at a serum dilution of 1:4,000 (IgG+IgM). The black triangles represent antibody levels at day 15 p.i. in the serum of individual mice, and the columns represent the mean of each group.
The absence of neutralizing antibodies allows successful virus readministration.
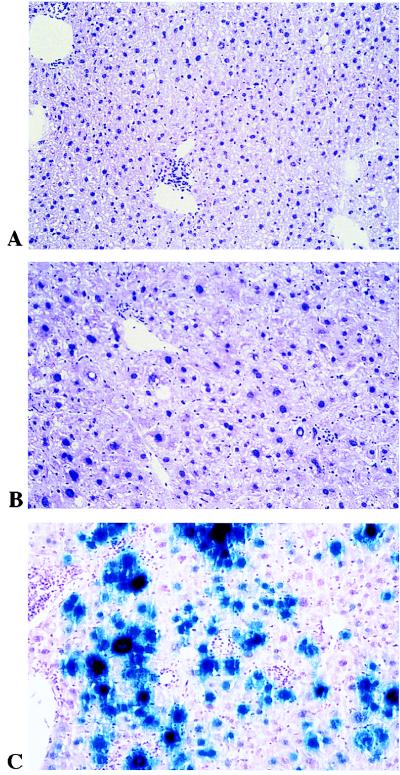
Because of the absence of neutralizing antibodies in the TNF-α/LTα−/− mice, we analyzed the efficiency of a second administration. Mice were first injected with Adα1hAT (5 × 109 PFU), followed by AdRSVβgal (8.8 × 109 PFU) 5 months later. As expected, measurement of αAT levels in the serum of TNF-α/LTα+/+, TNF-α/LTα+/−, and TNF-α/LTα−/− mice revealed a long-term expression (data not shown). Indeed, αAT was previously reported as nonimmunogenic in the C57BL/6 background (4), and this observation was valid for our animals established on a mixed C57BL/6 × 129Sv background. Indeed, Figure 6 shows the level of β-galactosidase expression in the liver at day 3 after this second administration. TNF-α/LTα+/+ and TNF-α/LTα+/− mice failed to express β-galactosidase after readministration (Fig. 6A and B). In sharp contrast, a remarkable expression of β-galactosidase was detected in TNF-α/LTα−/− mice (Fig. 6C). This expression in TNF-α/LTα−/− mice is maintained at day 8 but is no longer detectable by day 15 (data not shown). The cellular infiltrate of mononuclear cells which develops just next to the β-galactosidase-positive hepatocytes in TNF-α/LTα−/− mice explains the loss of transgene expression. As was described for the first injection (Table 2), we observed that the titer of neutralizing antibodies at day 15 after the second injection in the TNF-α/LTα−/− mice still remains less than 20 (Table 3). In comparison, the antibody titer in TNF-α/LTα+/+ and TNF-α/LTα+/− mice rises and ranges from 1,280 to 10,240 and from 2,560 to 5,120, respectively.
FIG. 6.
Efficient readministration of recombinant adenovirus in TNF-α/LTα-deficient mice. Mice previously injected with Adα1hAT were secondarily injected with AdRSVβgal (8.8 × 109 PFU) and sacrificed at day 3 following the second adenovirus administration. β-Galactosidase expression in liver sections after X-Gal staining is shown for TNF-α/LTα+/+ (A), TNF-α/LTα+/− (B), and TNF-α/LTα−/− (C) mice. Magnification, ×200.
TABLE 3.
Neutralizing antibody titers following repeated injection of recombinant adenovirus
| Expt group | Mouse | Antibody titera at
|
|
|---|---|---|---|
| First injection | Second injection | ||
| TNF-α/LTα−/− | 1 | <20 | <20 |
| 2 | <20 | <20 | |
| 3 | <20 | <20 | |
| TNF-α/LTα+/− | 1 | 160 | 1,280 |
| 2 | 80 | 5,120 | |
| 3 | 80 | 10,240 | |
| TNF-α/LTα+/+ | 1 | 160 | 2,560 |
| 2 | 80 | 5,120 | |
| 3 | 160 | 5,120 | |
The values represent neutralizing antibody titers after the first injection of 5 × 109 PFU of Adα1hAT (sera at day 29) and the second injection of 8.8 × 109 PFU of AdRSVβgal (sera at day 15).
To determine more accurately the efficiency of readministration, we then sought to determine whether mice already primed with AdRSVβgal exhibit the same level of αAT expression after delivery of Adα1hAT as do naive mice. A similar level of αAT expression at day 3 p.i. was detected in both groups of mice (157.6 ± 73.7 and 202 ± 2 ng/ml for sensitized and naive mice, respectively), which demonstrates that sensitized TNF-α/LTα−/− mice are as efficient as naive mice in their αAT expression.
The time course of antibody response correlates with the level of TNF-α and LTα.
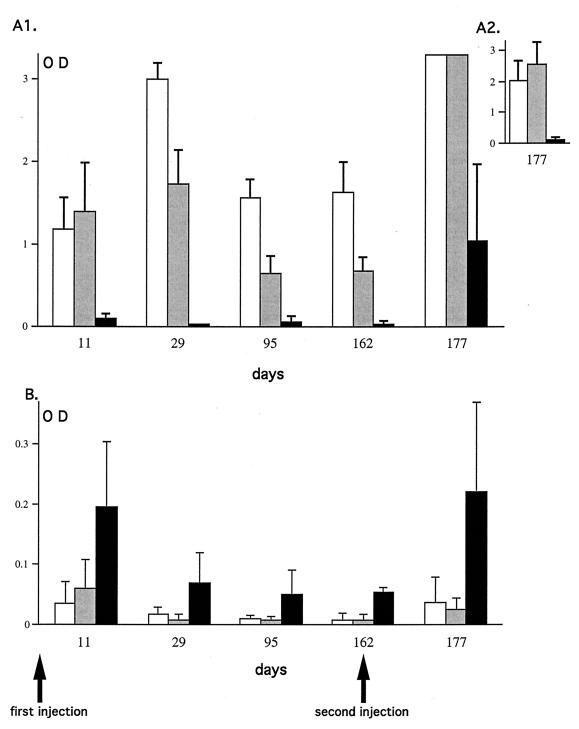
We analyzed the antibody response in the sera of TNF-α/LTα+/+, TNF-α/LTα+/−, and TNF-α/LTα−/− mice at different time points from the first administration of adenovirus (5 × 109 PFU of Adα1hAT) till after the second injection (8.8 × 109 PFU of AdRSVβgal) 5 months later. At all times tested, the IgG+IgM antibody response in TNF-α/LTα−/− mice was very low, whereas the antibody response in TNF-α/LTα+/− and TNF-α/LTα+/+ mice rose to a peak at day 29 and then decreased and stabilized between days 95 and 162 (Fig. 7A1). Even after the second injection the antibody response was still lower in TNF-α/LTα−/− mice compared to the controls (day 177, Fig. 7A1). This is most obvious in Fig. 7A2, where a practically undetectable level of anti-adenovirus antibody in the sera of TNF-α/LTα−/− mice at a dilution of 1:32,000 can be seen compared to the controls. The antibody response of the IgM isotype was higher in TNF-α/LTα−/−, mice with a maximum level evident by day 11. It decreased and remained stable until it peaked again 15 days after adenovirus readministration (Fig. 7B). This higher level of IgM antibody observed in the TNF-α/LTα−/− mice compared to TNF-α/LTα+/− and TNF-α/LTα+/+ mice is consistent with a block in the isotype switch of the humoral response. Interestingly, Fig. 7 shows that at day 11, the IgG+IgM antibody response was slightly higher in TNF-α/LTα+/− mice than in TNF-α/LTα+/+ mice (Fig. 7A1), which correlates well with the difference in the IgM levels observed the same day (Fig. 7B). At the other time points analyzed (days 29, 95, and 162 p.i.), the IgG+IgM antibody response against adenovirus in TNF-α/LTα+/− mice was lower than in TNF-α/LTα+/+ mice. Experiments carried out on larger experimental groups demonstrated that these differences between TNF-α/LTα+/+ and TNF-α/LTα+/− mice are statistically significant (P < 0.05) at all of the times tested, suggesting a link between antibody response against adenovirus and TNF-α/LTα gene dosage (data not shown).
FIG. 7.
Time course of antibody response in sera of mice deficient or not for TNF-α/LTα. Sera of TNF-α/LTα+/+ (white bars), TNF-α/LTα+/− (gray bars), and TNF-α/LTα−/− (black bars) mice were obtained at different days (11, 29, 95, and 162) after the first adenovirus administration (5 × 109 PFU of Adα1hAT) and at 15 days (day 177) after the second adenovirus administration (8.8 × 109 PFU of AdRSVβgal). The sera were tested for the presence of IgG+IgM antibodies (upper panel) or IgM (lower panel). The values correspond to the mean (n = 3) of relative amounts (OD490) of antibodies in sera at dilutions of 1:4,000 (A1), 1:32,000 (A2), and 1:500 (B). Accurate estimation of antibody levels at day 177 was made on sera at a dilution of 1:32,000 in order to be within the linear range of the ELISA (A2). The standard deviations are shown.
Impaired proliferative response in TNF-α/LTα−/− mice.
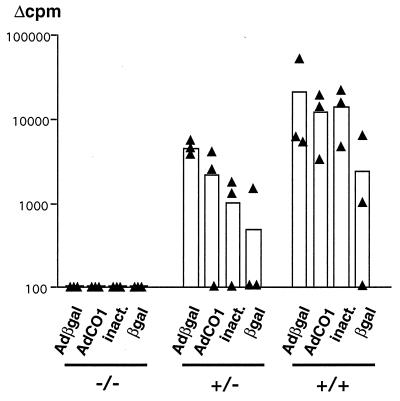
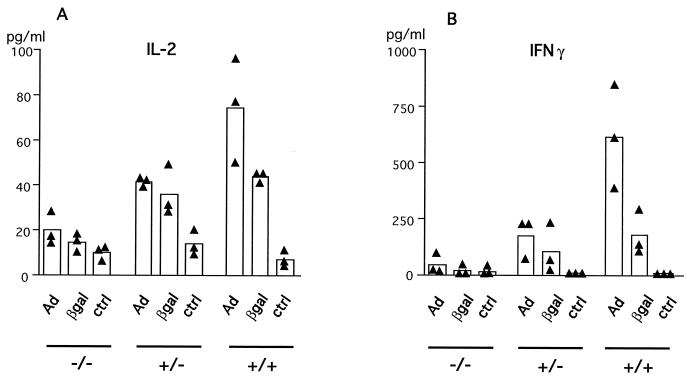
We analyzed the proliferative capacity of splenocytes of AdRSVβgal-infected mice at different times p.i. Data presented in Fig. 8 depicts the proliferative capacity of lymphocytes from mice of the three genotypes at day 14 p.i. with 5 × 109 PFU of AdRSVβgal. TNF-α/LTα−/− mice were profoundly impaired in their proliferative response against both viral and β-galactosidase antigens, while TNF-α/LTα+/− mice present an intermediate profile compared to TNF-α/LTα+/+ mice. Similar results were consistently obtained with splenocytes at different times p.i. (data not shown). It should be noticed that the important difference in the proliferative capacity between mice within each group may reflect the fact that the mice used in this study are not inbred but were maintained on a mixed 129Sv × C57BL/6 background. Nevertheless, an intermediate pattern of proliferation was usually observed in TNF-α/LTα+/− (Fig. 8 and data not shown), suggesting a link with TNF-α/LTα gene dosage. Because the T-helper response to recombinant adenovirus by intravenous delivery is known to implicate the Th1 subset in particular (61), we measured the level of IL-2 and IFN-γ produced by splenocytes at day 22 p.i. after in vitro restimulation with antigen. Figure 9 indicates that viral proteins (heat-inactivated adenovirus) and β-galactosidase both induce the production of high levels of IL-2 (Fig. 9A) and IFN-γ (Fig. 9B) by splenocytes of TNF-α/LTα+/+ mice (Fig. 9B) compared with unstimulated splenocytes. Upon restimulation, the levels of both cytokines were lower in TNF-α/LTα+/− mice and even lower in TNF-α/LTα−/− mice. These results are in agreement with the defect in proliferation observed for TNF-α/LTα+/− and TNF-α/LTα−/− mice (Fig. 8). In all three groups of mice, we detected an inconspicuous level of IL-4 (data not shown), an indicator of Th2 T cells; this is most likely because of the paucity of this population, as described by others in another model of liver gene transfer (61).
FIG. 8.
Deficient lymphoproliferative response in TNF-α/LTα-deficient mice. Spleen cells from mice at day 14 p.i. with 5 × 109 PFU of AdRSVβgal were stimulated in vitro with AdRSVβgal (10 PFU/cell [Adβgal]), an adenovirus with no transgene (10 PFU/cell) that was either viable (AdCO1) or heat-inactivated (inact.), or β-galactosidase (βgal). The data presented on a logarithmic scale are expressed in counts per minute as indicated in Materials and Methods. The triangles represent the data (mean of quadruplicates) from the spleen of one animal, and the bars represent the mean (n = 3) of each experimental group.
FIG. 9.
Defects in Th1 cytokine production in TNF-α/LTα mice following adenovirus administration. Spleen cells from AdRSVβgal-infected mice at day 22 p.i. were cultured in the presence of AdCO1 (Ad) or β-galactosidase (βgal) or in medium alone (ctrl). Supernatants were collected after 2 days and were tested in duplicate for the presence of IL-2 (A) or IFN-γ (B). Black triangles represent data from individual mice, and the bars represent the mean (n = 3) of each group.
DISCUSSION
The absence of TNF-α and LTα in mice results in an impaired immune response to recombinant adenovirus; this response is characterized by an attenuated acute-phase inflammation and the presence of a minor cellular infiltrate in the liver. These mice show a lack of proliferative response and an impaired humoral response against both the virus capsid and the transgene product. We report here for the first time that the absence of antiadenovirus neutralizing antibodies in this context allows an efficient secondary adenovirus-mediated gene transduction. An interesting observation lies in the correlation between TNF-α/LTα gene dosage and the intensity of antibody response. A detailed analysis of antibody isotypes demonstrates the lack of serum antibodies not only of the IgG but also of the IgA isotype following adenovirus administration in the absence of both TNF-α and LTα. That an increase in the IgM/IgG ratio is apparent in our study is consistent with the absence of isotype switching in TNF-α/LTα−/− mice, and this correlates with an abnormal splenic microarchitecture (33, 48). As shown in Fig. 6, these antibodies were, however, inefficient in preventing virus readministration. In another study, a disabled antibody response has been evidenced in mice after administration of an E1-deleted recombinant adenovirus expressing the E3 region from the cytomegalovirus promoter (27). In contrast to our study, a general impairment of virus-specific antibodies was documented, including IgM (27). Different immunosuppression mechanisms are therefore involved upon adenovirus injection in TNF-α/LTα-deficient mice versus wild-type mice injected with such an E3-overexpressing virus.
We observed a weak acute-phase inflammation process in TNF-α/LTα−/− and TNF-α/LTα+/− mice compared to wild-type animals, with the most obvious differences at day 2 p.i. One explanation for the weak acute-phase inflammatory process we observed may be due to the fact that TNF-α/LTα−/− and TNF-α/LTα+/− express no, or only a low level of, TNF-α, a strong inducer of mouse acute-phase proteins (43). Because IL-6 is a promoter of acute-phase proteins (53), the low level of SAP observed in TNF-α/LTα−/− and TNF-α/LTα+/− splenocytes after adenovirus infection compared to TNF-α/LTα+/+ mice may also be explained by the limited ability of these mice to produce IL-6. Finally, it should be emphasized that the low levels of IL-6 in TNF-α/LTα−/− mice compared to TNF-α/LTα+/− and to TNF-α/LTα+/+ mice are in agreement with the role of TNF-α as a positive regulator of IL-6 levels (52).
Binding of TNF-α on TNFRI is essential for upregulation of adhesion molecules such as E-selectin and VCAM-1 and for the production of chemoattractant cytokines that are required for leukocyte organ infiltration (17, 44, 45). We thus assessed the extent of liver infiltration in our TNF-α/LTα−/− model and found a significant reduction compared to TNF-α/LTα+/+ mice at day 16 p.i. but not at earlier time points. In contrast, an impaired intrahepatic recruitment of leukocytes has been reported as early as day 7 p.i. in TNF-α/LTα−/− mice (15). This difference most likely results from the absence of secondary lymphoid organs in our knockout mice due to the lack of LTβ receptor signaling in the absence of LTα. We thus believe that leukocyte recruitment from the periportal and perivascular regions of the liver may be quicker in the absence of LTα, translating at early time points p.i. into a pattern of inflammation in TNF-α/LTα−/− mice that is similar to that in TNF-α/LTα+/+ mice. Indeed, immunostaining performed on liver sections demonstrated an increase of CD8+ and CD4+ cells in both groups of mice as early as day 7 p.i. These cells most likely illustrate an ongoing immune process by CTL and T-helper cells that might explain the absence of prolonged expression of transgene-expressing cells in TNF-α/LTα−/− mice. However, compared with TNF-α/LTα+/+ mice, TNF-α/LTα−/− mice are impaired in their ability to recruit Mac1-positive cells since there is no evidence of clusters in the liver parenchyme.
After intravenous adenovirus administration in TNF-α/LTα−/− mice, we observed a deficient ability of T-helper lymphocytes to proliferate in response to either viral or transgene product antigen after in vitro restimulation. This deficiency is associated with a lack of production of Th1 cytokines. Such a defect in Th1 response was reported for TNF-α/LTα−/− mice during infection with Candida albicans (41). The absence of a T-helper proliferative response may lead to the absence of cooperation with B cells for the production of antibodies directed against a T-dependent antigen such as the adenovirus. The absence of a T-helper response may also impair the cooperation with cytotoxic T cells and may explain why TNF-α/LTα−/− mice exhibit an attenuated CTL response directed against vaccinia virus or lymphocytic choriomeningitis virus (17). Because such a reduction of the cytotoxic or proliferative response was not observed in TNF-α−/− mice (15, 39), this defect most likely stems from the interruption of LTβ receptor signaling and is possibly correlated with the aberrant splenic microarchitecture.
Independent studies in different knockout models have helped to clarify the respective roles of LTα and TNF-α in the humoral immune response to various antigens. For example, mice lacking LTα exhibit a defect in secondary lymphoid organ development and an unusual architecture of the spleen that translate into an impaired humoral immune response (3). LTα−/− mice also present a defective isotype switching that has been assigned to the breakdown of signaling through the LTβ receptor, since normal switching occurs in both TNFRI−/− or TNFRII−/− mice (33). Both TNF-α−/− and TNFRI−/− mice exhibit a complete lack of splenic germinal centers, primary B-cell follicles, and follicular dendritic cells, but they remain capable of mounting an IgM and IgG antibody response. These mice, however, fail to maintain a sustained IgG antibody response, apparently because follicular dendritic cells do not deliver a proper stimulation to activated B cells within the germinal centers. Because such a lack of IgG antibodies against adenovirus has been described in TNF-α knockout mice (15), signalization through TNF-α binding to TNFRI is likely to be required for a full IgG antibody response (33, 46). Recently, the use of fusion proteins encoding soluble TNFR-p55 or LTβ-R led to conclusions slightly different from those obtained with different TNF/LT knockout mice. In particular, this approach helped to discriminate between the role of TNF-α and LTα in development versus their physiological role in adults. Thus, in adult mice the inhibition of TNFR-p55 did not affect the splenic architecture or the humoral response against sheep erythrocytes, while inhibition of LTα/β signaling led to profound defects such as the absence of B-cell follicles in the spleen, the absence of germinal center formation, and impaired production of immunoglobulin to sheep erythrocytes (38). It remains to be clarified whether the apparent dominant influence of the LTα/β system relative to TNF in the humoral response in adults is true for adenoviral antigen. Indeed, while adenovirus encodes three proteins which antagonize the TNFR-p55 pathway (13, 30), no LTα/β pathway antagonist activity has yet been assigned to E3 region products; this discovery would support a crucial role of LTα/β in the antiadenovirus immune response.
The role of TNF-α in antibody response is further supported by the in vitro observation that soluble and membrane-bound TNF-α can rescue the Burkitt lymphoma B-cell line Ramos from B-cell receptor-induced cell death (35). Furthermore, cell-associated TNF-α on activated CD4+ T cells has been shown to deliver a costimulatory signal to human B cells (2, 12, 37). Taken together, these data clearly implicate TNF-α as a major player in B-cell activation and antibody response. Our comparison of the antibody response in TNF-α/LTα+/+ and TNF-α/LTα+/− mice correlates the magnitude of the antibody response with the TNF-α/LTα gene dosage. Although we could not detect TNF-α in the serum of all virus-infected groups, levels of this cytokine in serum have been reported to be 20- to 100-fold lower in samples from heterozygous TNF-α/LTα+/− mice than in their wild-type counterparts when a very potent TNF-α activator (e.g., lipopolysaccharide) was used (1). More interestingly, we demonstrated that the level of TNF-α produced by activated splenocytes is significantly lower in TNF-α/LTα+/− mice than in TNF-α/LTα+/+ mice. The overall data link antibody levels to TNF-α concentrations. This correlation is further supported by an enhanced antibody response against T-cell-dependent antigen following administration of recombinant TNF-α to mice (21).
We have found no effect of TNF-α and LTα on the duration of transgene expression (αAT or β-galactosidase) in our murine knockout model. In contrast, TNF-α deficiency has been recently reported to markedly attenuate the clearance of circulating chloramphenicol acetyltransferase enzyme encoded by a recombinant adenovirus (AdCAT) (15). This apparent contradiction may result from the use of different promoters (the adenovirus major late promoter for Adα1hAT, the LTR of Rous sarcoma virus [RSV] for AdRSVβgal, and the immediate-early promoter of cytomegalovirus for AdCAT), as the weaker inflammatory setting in our knockout model might have impaired the activity of the RSV promoter. Indeed, an extinction of RSV-driven β-galactosidase expression has been shown for an E1E4-defective adenovirus that correlated with a weaker inflammation in mice (11). The evaluation of transgene expression driven by a house-keeping gene promoter in our knockout model may help to answer this issue.
In summary, TNF-α plays roles at different levels of the immune response. First, as a proinflammatory cytokine it can trigger early events of inflammation. Second, as an effector of cytolysis of TNF receptor-bearing cells, it can eliminate the transduced cells while recruiting leukocytes and macrophages following the liberation of inflammation mediators. Third, TNF-α can provide a survival signal to B lymphocytes. Finally, membrane-bound TNF-α on T-helper cells can provide additional costimulatory signals to activated B cells. Strategies aimed at blocking this cytokine may thus prove useful for gene therapy applications requiring multiple adenovirus administrations.
ACKNOWLEDGMENTS
We are most grateful to S. Esselin for assistance. We are profoundly indebted to L. Franqueville for viral stock preparations. We also thank N. DiFalco and D. Grandjon, as well as all of the staff of the animal facilities of the IGR. We thank all of the staff of the Service Multimedia of the IGR for photographic work. We express our gratitude to Gahéry-Ségard for helpful advice in measurement of β-galactosidase activity. Finally, we thank P. Bobé, M. Robert-Le Meur, A. Maron, and K. Sollerbrant for helpful discussions.
This work was supported in part by the CNRS, the Association Française de Lutte contre la Mucoviscidose (AFLM), and the Association Française pour la Myopathie (AFM). K.B. was supported by the AFLM and Rhône-Poulenc Gencell.
REFERENCES
- 1.Amiot F, Boussadia O, Cases S, Fitting C, Lebastard M, Cavaillon J-M, Milon G, Dautry F. Mice heterozygous for a deletion of the tumor necrosis factor-alpha and lymphotoxin-alpha genes: biological importance of a nonlinear response of tumor necrosis factor-alpha to gene dosage. Eur J Immunol. 1997;27:1035–1042. doi: 10.1002/eji.1830270434. [DOI] [PubMed] [Google Scholar]
- 2.Aversa G, Punnonen J, de Vries J E. The 26-kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J Exp Med. 1993;177:1575–1585. doi: 10.1084/jem.177.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks T A, Rouse B T, Kerley M K, Blair P J, Godfrey V L, Kuklin N A, Bouley D M, Thomas J, Kanangat S, Mucenski M L. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 4.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain-related variations in adenoviral mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 5.Browning J L, Ngam-Ek A, Lawton P, DeMarinis J, Tizard R, How E P, Hession C, O’Brine-Greco B, Foley S F, Ware C F. Lymphotoxin-beta, a novel member of the TNF family forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 6.Bruder J T, Jie T, McVey D L, Kovesdi I. Expression of gp19k increases the persistence of transgene expression from an adenovirus vector in the mouse lung. J Virol. 1997;71:7623–7628. doi: 10.1128/jvi.71.10.7623-7628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Tian J, Kovesdi I, Bruder J T. Interaction of the adenovirus 14.7-kDa protein with FLICE inhibits Fas Ligand-induced apoptosis. J Biol Chem. 1998;273:5815–5820. doi: 10.1074/jbc.273.10.5815. [DOI] [PubMed] [Google Scholar]
- 8.Crowe P D, VanArsdale T L, Walter B N, Ware C F, Hession C, Ehrenfels B, Browning J L, Din W S, Goodwin R G, Smith C A. A lymphotoxin-beta specific receptor. Science. 1994;264:707–710. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- 9.Dai Y, Schwarz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Cellular and humoral responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Matteo R P, Chu G, Ahn M, Chang E, Barker C F, Markmann J F. Long-lasting adenovirus transgene expression in mice through neonatal intrathymic tolerance induction without the use of immunosuppression. J Virol. 1997;71:5330–5335. doi: 10.1128/jvi.71.7.5330-5335.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedieu J-F, Vigne E, Torrent C, Jullien C, Mahfouz I, Caillaud J-M, Aubailly N, Orsini C, Guillaume J-M, Opolon P, Delaère P, Perricaudet M, Yeh P. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. J Virol. 1997;71:4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Prete G, De Carli M, D’Elios M M, Fleckenstein I M, Fickenscher H, Fleckenstein B, Almerigogna F, Romagnani S. Polyclonal B cell activation induced by herpesvirus saimiri-transformed human CD4+ T cell clones. J Immunol. 1994;152:4872–4879. [PubMed] [Google Scholar]
- 13.Dimitrov T, Krajcsi P, Hermiston T W, Tollefson A E, Hannink M, Wold W S M. Adenovirus E3-10.4K/14.5K protein complex inhibits tumor necrosis factor-induced translocation of cytosolic phospholipase A2 to membranes. J Virol. 1997;71:2830–2837. doi: 10.1128/jvi.71.4.2830-2837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong J-Y, Wang D, Van Ginkel F W, Pascual D W, Frizzell R A. Systematic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum Gene Ther. 1996;7:319–331. doi: 10.1089/hum.1996.7.3-319. [DOI] [PubMed] [Google Scholar]
- 15.Elkon K B, Liu C-C, Gall J G, Trevejo J, Marino M W, Abrahamsen K A, Song X, Zhou J-L, Old L J, Crystal R G, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelhardt J F, Ye X, Doranz B, Wilson J M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eugster H-P, Müller M, Karrer U, Car B D, Schnyder M, Eng V M, Woerly G, Le Hir M, di Padova F, Aguet M, Zinkernagel R, Bluethmann H, Ryffel B. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double deficient-mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 18.Fallaux F J, Kranenburg O, Cramer S J, Houweling A, van Ormondt H, Hoeben R C, van der Eb A J. Characterization of 911: a new helper cell line for the titration and propagation of early-region-1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 19.Gahéry-Ségard H, Juillard V, Gaston J, Lengagne R, Pavirani A, Boulanger P, Guillet J-G. Humoral immune response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 1997;27:653–659. doi: 10.1002/eji.1830270312. [DOI] [PubMed] [Google Scholar]
- 20.Gao G-P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiara P, Boraschi D, Nencioni L, Ghezzi P, Tagliabue A. Enhancement of in vivo immune response by tumor necrosis factor. J Immunol. 1987;139:3676–3679. [PubMed] [Google Scholar]
- 22.Gilardi P, Courtney M, Pavirani A, Perricaudet M. Expression of human alpha-1 antitrypsin using a recombinant adenovirus vector. FEBS Lett. 1990;267:60–62. doi: 10.1016/0014-5793(90)80287-s. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg H S, Lundholm-Beauchamp U, Horswood R L, Pernis B, Wold W S M, Chanock R M, Prince G A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci USA. 1989;86:3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginsberg H S, Moldawer L L, Sehgal P, Redington M, Kilian P L, Chanock R M, Prince G A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grell M, Douni E, Wajant H, Löhden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 26.Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yeh P, Lu H. Angiostatin gene transfer: inhibition of tumor growth in vivo by blockage of endothelial cell proliferation associated with a mitosis arrest. Proc Natl Acad Sci USA. 1998;95:6367–6372. doi: 10.1073/pnas.95.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilan Y, Droguett G, Roy Chowdhury N, Li Y, Sengupta K, Thummala N R, Davidson A, Roy Chowdhury J, Horwitz M S. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilan Y, Prakash R, Davidson A, Jona V, Droguett G, Horwitz S M, Chowdhury N R, Chowdhury J R. Oral tolerization to adenoviral antigens permits long-term gene expression using recombinant adenoviral vectors. J Clin Invest. 1997;99:1098–1106. doi: 10.1172/JCI119238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochanek S, Clemens P R, Mitani K, Chen H H, Chan S, Caskey C T. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krajcsi P, Tollefson A E, Anderson C W, Wold W S M. The adenovirus E3 14.5-kDa protein, which is required for down-regulation of the epidermal growth factor receptor and prevention of tumor necrosis factor cytolysis, is an integral membrane protein oriented with its C terminus in the cytoplasm. J Virol. 1992;66:1665–1673. doi: 10.1128/jvi.66.3.1665-1673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuzmin A I, Finegold M J, Eisensmith R C. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Ther. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 32.Laster S M, Wold W S M, Gooding L R. Adenovirus proteins that regulate susceptibility to TNF also regulate the activity of PLA2. Semin Virol. 1994;5:431–442. [Google Scholar]
- 33.Le Hir M, Bluethmann H, Kosco-Villebois M H, Muller M, di Padova F, Moore M, Ryffel B, Eugster H-P. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee M G, Abina M A, Haddada H, Perricaudet M. The constitutive expression of the immunomodulatory gp19k protein in E1-, E3-adenoviral vectors strongly reduces the host cytotoxic T cell response against the vector. Gene Ther. 1995;2:1–7. [PubMed] [Google Scholar]
- 35.Lens S M A, Tesselaar K, Den Drijver B F A, Van Oers M H J, Van Lier R A W. A dual role for both CD40-ligand and TNF alpha in controlling human B cell death. J Immunol. 1996;156:507–514. [PubMed] [Google Scholar]
- 36.Lieber A, He C-Y, Kirillova I, Kay M A. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macchia D, Almerigogna F, Parronchi P, Ravina A, Maggi E, Romagnani S. Membrane tumor necrosis factor alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature. 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 38.Mackay F, Majeau G R, Lawton P, Hochman P S, Browning J L. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur J Immunol. 1997;27:2033–2042. doi: 10.1002/eji.1830270830. [DOI] [PubMed] [Google Scholar]
- 39.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCoy R D, Davidson B L, Roessler B J, Huffnagle G B, Janich S L, Laing T J, Simon R H. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum Gene Ther. 1995;6:1553–1560. doi: 10.1089/hum.1995.6.12-1553. [DOI] [PubMed] [Google Scholar]
- 41.Mencacci A, Cenci E, Del Sero G, Fe d’Ostiani C, Mosci P, Montagnoli C, Bacci A, Bistoni F, Quesniaux V F, Ryffel B, Romani L. Defective co-stimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-alpha double-deficient mice infected with Candida albicans. Int Immunol. 1998;10:37–48. doi: 10.1093/intimm/10.1.37. [DOI] [PubMed] [Google Scholar]
- 42.Michalski J P, McCombs C C, Sheth S, McCarthy M, deShazo R. A modified double antibody sandwich enzyme-linked immunosorbent assay for measurement of alpha-1-antitrypsin in biologic fluids. J Immunol Methods. 1985;83:101–112. doi: 10.1016/0022-1759(85)90063-8. [DOI] [PubMed] [Google Scholar]
- 43.Mortensen R F, Shapiro J, Lin B-F, Douches S, Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988;140:2260–2266. [PubMed] [Google Scholar]
- 44.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak T W, Holzmann B, Krönke M. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- 45.Ohmori Y, Wyner L, Narumi S, Armstrong D, Stoler M, Hamilton T A. Tumor necrosis factor-α induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am J Pathol. 1993;142:861–870. [PMC free article] [PubMed] [Google Scholar]
- 46.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF-alpha-deficient mice: a critical requirement for TNF-alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. Proc Natl Acad Sci USA. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin L, Ding Y, Pahud D R, Robson N D, Shaked A, Bromberg J S. Adenovirus-mediated gene transfer of viral interleukin-10 inhibits the immune response to both alloantigen and adenoviral antigen. Hum Gene Ther. 1997;8:1365–1374. doi: 10.1089/hum.1997.8.11-1365. [DOI] [PubMed] [Google Scholar]
- 48.Ryffel B, Di Padova F, Schreier M H, Le Hir M, Eugster H-P, Quesniaux V F J. Lack of type 2 T cell-independent B cell responses and defect in isotype switching in TNF-lymphotoxin alpha-deficient mice. J Immunol. 1997;158:2126–2133. [PubMed] [Google Scholar]
- 49.Shisler J, Yang C, Walter B, Ware C F, Gooding L R. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol. 1997;71:8299–8306. doi: 10.1128/jvi.71.11.8299-8306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith T A G, White B D, Gardner J M, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 51.Sparer T E, Tripp R A, Dillehay D L, Hermiston T W, Wold W S, Gooding L R. The role of human adenovirus early region 3 proteins (gp19k, 10.4K, 14.5K, and 14.7K) in a murine pneumonia model. J Virol. 1996;70:2431–2439. doi: 10.1128/jvi.70.4.2431-2439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starnes H F, Pearce M K, Tewari A, Yim J H, Zou J-C, Abrams J S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-α challenge in mice. J Immunol. 1990;145:4185–4191. [PubMed] [Google Scholar]
- 53.Steel D M, Whitehead A S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 54.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thimmappaya B, Weinberger C, Schneider R J, Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982;31:543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- 56.Tollefson A E, Hermiston T W, Lichtenstein D L, Colle C F, Tripp R A, Dimitrov T, Toth K, Wells C E, Doherty P C, Wold W S M. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature. 1998;392:726–729. doi: 10.1038/33712. [DOI] [PubMed] [Google Scholar]
- 57.Tripathy S K, Black H B, Goldwasser E, Leiden J. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 58.Walter J, You Q, Hagstrom J N, Sands M, High K A. Successful expression of human factor IX following repeat administration of an adenoviral vector in mice. Proc Natl Acad Sci USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Greenburg G, Bunch D, Farson D, Finer M H. Persistent transgene expression in mouse liver following in vivo gene transfer with a delta E1/delta E4 adenovirus vector. Gene Ther. 1997;4:393–400. doi: 10.1038/sj.gt.3300404. [DOI] [PubMed] [Google Scholar]
- 60.Wolff G, Worgall S, van Rooijen N, Song W-R, Harvey B-G, Crystal R G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Ertl H C, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]