Abstract
Background
Robust data are lacking regarding the optimal route, duration, and antibiotic choice for gram-negative bloodstream infection from a complicated urinary tract infection source (GN-BSI/cUTI).
Methods
In this multicenter observational cohort study, we simulated a 4-arm registry trial using a causal inference method to compare effectiveness of the following regimens for GN-BSI/cUTI: complete course of an intravenous β-lactam (IVBL) or oral stepdown therapy within 7 days using fluoroquinolones (FQs), trimethoprim-sulfamethoxazole (TMP-SMX), or high-bioavailability β-lactams (HBBLs). Adults treated between January 2016 and December 2022 for Escherichia coli or Klebsiella species GN-BSI/cUTI were included. Propensity weighting was used to balance characteristics between groups. The 60-day recurrence was compared using a multinomial Cox proportional hazards model with probability of treatment weighting.
Results
Of 2571 patients screened, 759 (30%) were included. Characteristics were similar between groups. Compared with IVBLs, we did not observe a difference in effectiveness for FQs (adjusted hazard ratio, 1.09 [95% confidence interval, .49–2.43]) or TMP-SMX (1.44 [.54–3.87]), and the effectiveness of TMP-SMX/FQ appeared to be optimal at durations of >10 days. HBBLs were associated with nearly 4-fold higher risk of recurrence (adjusted hazard ratio, 3.83 [95% confidence interval, 1.76–8.33]), which was not mitigated by longer treatment durations. Most HBBLs (67%) were not optimally dosed for bacteremia. Results were robust to multiple sensitivity analyses.
Conclusions
These real-world data suggest that oral stepdown therapy with FQs or TMP-SMX have similar effectiveness as IVBLs. HBBLs were associated with higher recurrence rates, but dosing was suboptimal. Further data are needed to define optimal dosing and duration to mitigate treatment failures.
Keywords: antimicrobial stewardship, β-lactams, complicated urinary tract infection, gram-negative bacteremia, real-world evidence
Oral antibiotic stepdown therapy is a safe, convenient, less costly alternative to outpatient intravenous antibiotics for gram-negative bloodstream infections (GN-BSIs) [1–5]. However, data are lacking regarding the optimal oral agent, dose, and duration for complicated urinary tract infection (cUTI), which suffers from variable definitions in published literature and (when defined as structural or functional urologic abnormalities) often comprises a minority of included patients in GN-BSI studies [6–15]. Randomized trials have found a duration of 7 days to be sufficient for afebrile cUTI treated with a fluoroquinolone (FQ) or trimethoprim-sulfamethoxazole (TMP-SMX), whereas >7 days may be needed for febrile cUTI (depending on the chosen definition of treatment success) [16, 17]. Recent observational studies suggest that 7–10 days may be appropriate for GN-BSI from a cUTI source (GN-BSI/cUTI) [18] and that oral β-lactams (BLs) might be associated with slightly higher recurrence rates of questionable clinical significance [19].
Many of these studies were limited by small sample size, variable definitions of treatment failure, and underrepresentation or underdosing of oral BLs. Further data are needed to optimize oral stepdown therapy for GN-BSI/cUTI. Herein, we present real-world data from a large, observational, multicenter cohort study using a target trial emulation with a causal inference method to compare effectiveness between IVBLs and oral stepdown with BLs, FQs, or TMP-SMX for GN-BSI/cUTI.
METHODS
Patients and Data Collection
The study took place in the Intermountain Health integrated network of 23 hospitals and emergency departments (EDs), 38 urgent cares, and 300 primary care clinics in Utah and Idaho, serving >1.5 million patients each year. Using the enterprise data warehouse, we identified a screening population of unique patients ≥18 years of age with matching positive blood and urine cultures for Escherichia coli, Klebsiella pneumoniae, or Klebsiella oxytoca obtained during an ED or hospital encounter between January 2016 and December 2022.
Data related to hospital course, demographics, laboratory values, and microbiology were extracted electronically from the enterprise data warehouse, whereas data on comorbid conditions, imaging, severity of illness, antibiotic treatment, recurrent infection, readmissions, and deaths were abstracted manually from the electronic medical record (EMR) by trained record reviewers using a standardized data collection tool (see Appendix in the Supplementary Materials). Data were collected through 90 days after hospital discharge. The study met all STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) requirements for observational studies [20] and ISPOR criteria for comparative effectiveness [21], and it was approved by the Intermountain Institutional Review Board; it was granted a waiver of patient consent due to its design and less-than-minimal risk to subjects.
Inclusion and Exclusion Criteria
Male and female patients with cUTI, defined by the presence of structural or functional urologic abnormalities, were included in the initial screening cohort. We excluded patients who had concomitant infections during the index encounter (besides GN-BSI/cUTI), polymicrobial cultures, hospital-onset bacteremia, or a nonurinary source of bacteremia (eg, prostatitis, epididymo-orchitis, or renal abscess). Furthermore, we excluded patients who were pregnant, died in the hospital, were discharged with hospice, transferred to a facility outside our healthcare system, or were lost to follow-up after discharge (defined as no further notes or follow-up visits in the record). Patients were also excluded if they had a prolonged hospitalization (>14 days), did not receive an effective intravenous antibiotic within 24 hours of the index blood culture, received >7 days of intravenous antibiotics before discharge or oral stepdown, received multiple oral antibiotics, or received an oral antibiotic to which the blood or urine isolate was not susceptible or if EMR data were incomplete regarding antibiotic treatment.
Microbiology Procedures
Blood culture susceptibilities were performed on either BD Phoenix (BD Diagnostic Systems) or MicroScan Walkaway (Beckman Coulter) panels depending on the processing facility, whereas all urine cultures were processed on MicroScan Walkaway panels. Granular minimum inhibitory concentration (MIC) data were lacking for many isolates reported only as “susceptible” in our EMR, and we often had to infer susceptibility for oral antibiotics (eg, cephalexin and amoxicillin) based on surrogate intravenous antibiotics reported on the panel (eg, cefazolin and ampicillin, respectively). Because of this limitation, we preplanned an analysis limited to isolates confirmed to be susceptible by current Clinical and Laboratory Standards Institute (CLSI) breakpoints (see Sensitivity Analyses section).
Enrollment Window and Index Day 0
Index day 0 for the simulated trial was defined as the index blood culture date. The enrollment window was defined as index day 1 through day 7 to reflect real-world variation in the time to culture positivity and clinical stability (ie, afebrile and hemodynamically stable). Patients were permitted to transition from intravenous to oral antibiotics at any time during the enrollment window per the treating physician's discretion, provided that they were transitioned to oral stepdown therapy or discharged on IVBL therapy by index day 7.
Comparator Groups
Patients were classified into 1 of 4 comparator groups based on the definitive treatment they received: intravenous BL (IVBL), oral FQ (levofloxacin and ciprofloxacin), oral TMP-SMX, or high-bioavailability oral BLs (HBBLs; amoxicillin, amoxicillin-clavulanate, and cephalexin). For sensitivity analyses, we also evaluated a fifth group receiving low-bioavailability oral BLs (LBBLs; cefdinir and cefuroxime). IVBLs were used as the reference group in all comparisons. Oral antibiotic dosing and duration was per prescriber choice in this real-world study. However, we prespecified a descriptive analysis of patients who received bacteremia dosing per Delphi expert consensus recommendations [10] (with appropriate adjustments for renal impairment; all taken orally): ciprofloxacin (750 mg every 12 hours), levofloxacin (750 mg every 24 hours), TMP-SMX (5 mg/kg every 12 hours; eg, approximately 2 double-strength tablets every 12 hours for a 70-kg patient), amoxicillin (1000 mg every 8 hours), amoxicillin/clavulanic acid (875–1000 mg every 8 hours), and cephalexin (1000 mg every 6 hours).
Outcomes and Primary Analysis
The primary outcome was recurrence-free days through index day 60. Recurrence was defined as positive blood or urine culture for the same organism (regardless of susceptibility results). If only the urine culture was positive (ie, no evidence of recurrent bacteremia), manual review of the EMR had to indicate that a symptomatic urinary tract infection (UTI) was diagnosed and treated with antibiotics in order to count as a recurrence. Otherwise, positive urine cultures without documented symptoms and treatment were classified as asymptomatic bacteriuria and were not counted toward the primary outcome. Secondary outcomes included all-cause mortality, Clostridioides difficile infection, and UTI-related readmission rates. All ED or hospital readmissions were manually reviewed to determine whether they were UTI related, defined as either an adverse event to the antibiotic or ongoing, worsening, or recurrent UTI as the reason for readmission. Adjudication of all patients with a primary or secondary outcome was performed by the same infectious diseases pharmacist (J. J. V.) to ensure consistency.
Statistical Analysis
To address exchangeability, we used a 4-comparator multinomial regression model to estimate propensity weights for choosing one antibiotic group over the others (R; twang package) [22–25]. The propensity model was fitted using covariates identified from relevant GN-BSI literature [2, 18] and causal diagrams: age, sex, chronic kidney disease, Charlson comorbidity index, Pitt bacteremia score, allergy or resistance to FQs/TMP-SMX/BLs, and small hospital size (<200 beds; due to observed variation in prescribing patterns between large and small hospitals in our health system). We selected an optimally balanced model for the primary analysis based on the absolute standardized mean differences, minimum P values, and effective sample sizes (Supplementary Table 1). Furthermore, we assessed covariate overlap via propensity score box plots, which, together with the balance table, supported causal estimation (Supplementary Figure 1). We then compared recurrence risk between groups using a propensity score–weighted multinomial Cox proportional hazards model. The model was censored at the time of death, administration of antibiotics during a subsequent ED/hospital admission (for indications other than GN-BSI/UTI), or last known follow-up within 90 days (rather than prespecifying separate per-protocol and intention-to-treat analyses). Duration of intravenous antibiotic treatment, in days, was included as a time-dependent covariate in the model because the enrollment window allowed up to 7 days of intravenous antibiotic treatment before oral switch.
Regarding antibiotic treatment duration, in a slight divergence from a strict trial emulation method (whereby duration subgroups would ideally be stratified at enrollment), we chose to optimize power and recognize uncertainty in the existing evidence [11, 18, 26] by including total antibiotic duration as a continuous, time-dependent variable in the primary analysis. We then conducted prespecified analyses to explore associations between recurrence and total duration. To determine an optimal cutoff point for dichotomizing duration, we used a receiver operating characteristic curve to plot 60-day recurrence versus antibiotic duration and calculated the Youden index [27] that optimized sensitivity and specificity for all antibiotics. We then refit the primary Cox models, using the duration cutoff point as an interaction term, to estimate the effect of each oral antibiotic on recurrence stratified by short versus long duration. Results were displayed by creating cumulative incidence plots.
Sensitivity Analyses
Several sensitivity analyses were planned a priori. First, we evaluated the primary analysis for recurrence at index days 30 and 90. Second, we expanded the BL group in the primary analysis to include all BLs (LBBLs and HBBLs together). Third, we limited the BL group in the primary analysis to only those patients receiving HBBLs whose blood and urine isolates were confirmed to be susceptible at current CLSI breakpoints (cefazolin ≤MIC 2 mg/L if they received cephalexin, ampicillin MIC ≤8 mg/L if they received amoxicillin, or amoxicillin-clavulanate MIC ≤8/4 mg/L if they received amoxicillin-clavulanate) [28]. Fourth, we conducted a variation of the primary analysis with restricted inclusion criteria allowing 1–4 days (instead of 1–7 days) of intravenous antibiotics before oral switch. Finally, we fitted a new 3-group Cox model to compare LBBLs, HBBLs, and IVBLs.
RESULTS
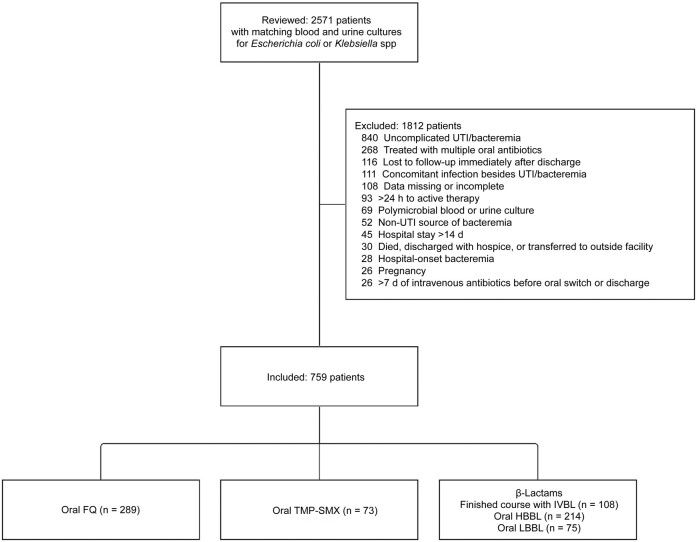
Of 2571 patients reviewed, 759 (30%) met inclusion criteria (Figure 1). There were 289 patients in the FQ group, 73 in the TMP-SMX group, 214 in the HBBL group, and 108 in the IVBL group. Most patients (713 [94%]) were treated in the hospital, and 190 (25%) required intensive care (Table 1). Patients were predominantly female (470 patients [62%]) and >65 years of age (461 [61%]). Baseline characteristics were similar across the oral stepdown groups, except that patients receiving TMP-SMX had the highest incidence of urinary retention, kidney stones, and hydronephrosis (26%, 53%, and 55%, respectively). Patients receiving definitive IVBLs had more comorbid conditions, urologic abnormalities, and extended-spectrum β-lactamase–producing isolates than the oral stepdown groups. Most patients (702 [92%]) achieved clinical stability within the first 3 days, and nearly all (751 [99%]) were initially treated with an IVBL before oral stepdown or discharge on definitive IVBL therapy. The median time to oral switch (interquartile range) was 3 (3–4) days, and the median total duration of antibiotic treatment was 14 (11–15) days. Oral antibiotic prescribing patterns varied by care venue: ED patients were most likely to receive an HBBL (22 of 46 [48%]), hospitalized patients were most likely to receive an FQ (278 of 713 [39%]), and patients transitioned to TMP-SMX more frequently received care at smaller hospitals (38 of 73 [52%]). More patients treated with FQs received consensus-recommended dosing compared with those treated with TMP-SMX (69% vs 4%; P < .001) or HBBLs (69% vs 43%; P < .001) (Table 1).
Figure 1.
Patient inclusion/exclusion. Abbreviations: FQ, fluoroquinolone; HBBL, high-bioavailability β-lactam; IVBL, intravenous β-lactam; LBBL, low-bioavailability β-lactam; TMP-SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
Table 1.
Demographics, Comorbid Conditions, and Antibiotic Treatment
| Baseline Characteristics | Patients, No. (%)a | ||||
|---|---|---|---|---|---|
| FQ (n = 289) | TMP-SMX (n = 73) | HBBL (n = 214) | IVBL (n = 108) | LBBLb (n = 75) | |
| Age, median (IQR), y | 67 (55–76) | 68 (56–78) | 73 (61–81) | 72 (61–79) | 71 (60–77) |
| Female sex | 188 (65) | 37 (51) | 135 (63) | 72 (67) | 38 (51) |
| Diabetes | 138 (48) | 27 (37) | 104 (49) | 54 (50) | 31 (41) |
| Chronic kidney disease (stage II or higher) | 80 (28) | 19 (26) | 80 (37) | 48 (44) | 23 (31) |
| Charlson comorbidity index, median (IQR) | 6 (3–9) | 4 (2–8) | 6 (4–9) | 7 (5–10) | 5 (3–8) |
| Allergy to FQ, TMP-SMX, or BL | 18 (6) | 2 (3) | 4 (2) | 13 (12) | 12 (16) |
| History of kidney stones | 107 (37) | 35 (48) | 51 (24) | 31 (29) | 26 (35) |
| Immunocompromisedc | 47 (16) | 5 (7) | 47 (22) | 41 (38) | 13 (17) |
| Urinary retention or neurogenic bladder | 55 (19) | 19 (26) | 39 (18) | 25 (23) | 10 (13) |
| Benign prostatic hypertrophy | 51 (18) | 18 (25) | 35 (16) | 16 (15) | 16 (21) |
| Baseline urinary catheterization | 28 (10) | 14 (19) | 27 (13) | 21 (19) | 7 (9) |
| Chronic urinary incontinence | 30 (10) | 4 (5) | 33 (15) | 12 (11) | 8 (11) |
| Urologic stricture, stenosis, or obstruction | 35 (12) | 7 (10) | 20 (9) | 13 (12) | 8 (11) |
| Cancer or mass of bladder, prostate, or kidney | 22 (8) | 7 (10) | 27 (13) | 7 (6) | 5 (7) |
| >2 Positive urine cultures in past year | 13 (4) | 6 (8) | 10 (5) | 11 (10) | 4 (5) |
| Urologic procedure in previous 2 wk | 12 (4) | 3 (4) | 8 (4) | 8 (7) | 3 (4) |
| Baseline stent or nephrostomy tube | 10 (3) | 3 (4) | 9 (4) | 7 (6) | 1 (1) |
| Cystocele or urologic fistula | 6 (2) | 2 (3) | 7 (3) | 3 (3) | 2 (3) |
| Other urologic abnormalitiesd | 7 (2) | 0 (0) | 4 (2) | 2 (2) | 2 (3) |
| Index bacteremia characteristics | |||||
| Active kidney/ureteral stone | 130 (45) | 39 (53) | 69 (32) | 30 (28) | 27 (36) |
| Hydronephrosis or hydroureter | 106 (37) | 40 (55) | 63 (29) | 35 (32) | 26 (35) |
| Ureteral stent or nephrostomy tube placed | 75 (26) | 26 (36) | 41 (19) | 25 (23) | 23 (31) |
| Received care at small hospital (<200 beds) | 105 (36) | 38 (52) | 88 (41) | 36 (33) | 34 (45) |
| Admitted to hospital | 278 (96) | 69 (95) | 192 (90) | 105 (97) | 69 (92) |
| Discharged from emergency department | 11 (4) | 4 (5) | 22 (10) | 3 (3) | 6 (8) |
| Length of stay, median (IQR), h | 69 (48–87) | 71 (51–91) | 72 (49–96) | 91 (56–108) | 64 (36–87) |
| Pitt bacteremia score, median (IQR) | 2 (1–3) | 3 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Admitted to intensive care unit | 50 (17) | 20 (27) | 58 (27) | 40 (37) | 22 (29) |
| Received vasopressors | 17 (6) | 15 (21) | 31 (14) | 19 (18) | 15 (20) |
| Achieved clinical stability within 3 d | 269 (93) | 63 (86) | 203 (95) | 100 (93) | 67 (89) |
| Microbiology | |||||
| Escherichia coli | 234 (81) | 61 (84) | 175 (82) | 88 (81) | 64 (85) |
| Klebsiella species | 55 (19) | 12 (16) | 39 (18) | 20 (19) | 11 (15) |
| FQ resistant | 0 (0) | 13 (18) | 31 (14) | 50 (46) | 14 (19) |
| TMP-SMX resistant | 52 (18) | 0 (0) | 47 (22) | 43 (40) | 20 (27) |
| Cefazolin resistant | 33 (11) | 10 (14) | 3 (1) | 40 (37) | 7 (9) |
| ESBL-producing isolate | 4 (1) | 1 (1) | 0 (0) | 23 (21) | 0 (0) |
| Antibiotic treatment | |||||
| Empiric IVBL | 283 (98) | 73 (100) | 212 (99) | 108 (100) | 75 (100) |
| Empiric intravenous FQ | 6 (2) | 0 (0) | 2 (1) | 0 (0) | 0 (0) |
| Time to active intravenous therapy, median (IQR), h | 1.1 (0.6–1.8) | 0.7 (0.3–1.8) | 1.2 (0.5–1.8) | 1.6 (0.7–2.2) | 1.2 (0.4–1.9) |
| Duration of active inpatient intravenous therapy, median (IQR), d | 3 (3–4) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 3 (2–4) |
| Duration of active oral therapy, median (IQR), d | 10 (7–12) | 10 (7–11) | 10 (7–11) | NA | 10 (7–11) |
| Total duration, median (IQR), d | 13 (10–15) | 13 (11–15) | 13 (11–15) | 15 (13–16) | 13 (11–15) |
| Received recommended oral dosinge | 199 (69) | 3 (4) | 93 (43) | NA | NAf |
Abbreviations: BL, β-lactam; ESBL, extended-spectrum β-lactamase, FQ, fluoroquinolone, HBBL, high-bioavailability BL; IQR, interquartile range; IVBL, intravenous BL; LBBL, low-bioavailability BL; NA, not applicable; TMP-SMX, trimethoprim-sulfamethoxazole.
aData represent no. (%) of patients unless otherwise specified.
bThe LBBL group was included in sensitivity analyses but not in the primary analysis.
cImmunocompromised was defined as follows: human immunodeficiency virus/AIDS with a CD4 cell count <200/µL, neutropenia with an absolute neutrophil count <500/µL, or receiving any of the following medications at the time of admission: antirejection medications following transplant, chemotherapy, tumor necrosis factor α inhibitors, disease-modifying antirheumatic drugs, or maintenance steroids with equivalent prednisone dose ≥20 mg.
dOther urologic abnormalities included urostomy (n = 9), neobladder (n = 2), bladder sling (n = 1), polycystic kidney disease (n = 1), solitary kidney (n = 1), and chronic interstitial cystitis (n = 1).
eRecommended oral antibiotic doses were based on consensus guidance from Heil et al [10].
fLBBLs are not routinely recommended for GN-BSI due to pharmacokinetic concerns and lack of clinical data.
Crude 60-day recurrence occurred in 111 patients (14.6%) and was lowest (overall and when considering bacteremia and UTI separately) in those receiving FQ or TMP-SMX stepdown (Table 2). Recurrence was primarily driven by recurrent UTIs (12.0% vs 2.6% for recurrent bacteremia + UTI). Unadjusted all-cause 90-day mortality and C difficile infection rates were low overall (1.7%, and 1.2%, respectively) and did not differ significantly between groups.
Table 2.
Unadjusted Primary and Secondary Outcomes
| Outcome | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| FQ (n = 289) | TMP-SMX (n = 73) | HBBL (n = 214) | IVBL (n = 108) | LBBL (n = 75) | |
| Primary outcomes | |||||
| Recurrence within 60 d | 21 (7.3) | 7 (9.6) | 46 (21.5) | 22 (20.4) | 15 (20.0) |
| Recurrent bacteremia + UTI | 3 (1.0) | 1 (1.4) | 9 (4.2) | 5 (4.6) | 2 (2.7) |
| Recurrent UTI only | 18 (6.2) | 6 (8.2) | 37 (17.3) | 17 (15.7) | 13 (17.3) |
| Secondary outcomes | |||||
| Recurrence within 30 d | 12 (4.2) | 4 (5.5) | 31 (14.5) | 15 (13.9) | 10 (13.3) |
| Recurrence within 90 d | 26 (9.0) | 11 (15.1) | 56 (26.2) | 32 (29.6) | 17 (22.7) |
| UTI-related readmission within 90 d | 28 (9.7) | 11 (15.1) | 46 (21.5) | 35 (32.4) | 13 (17.3) |
| Clostridioides difficile infection within 90 d | 0 (0.0) | 1 (1.4) | 3 (1.4) | 4 (3.7) | 1 (1.3) |
| All-cause 90-d mortality rate | 3 (1.0) | 1 (1.4) | 5 (2.3) | 2 (1.9) | 2 (2.7) |
Abbreviations: FQ, fluoroquinolone; HBBL, high-bioavailability β-lactam; IVBL, intravenous β-lactam; LBBL, low-bioavailability β-lactam; TMP-SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
In the primary analysis, compared with a full course of IVBL, we did not observe a difference in recurrence with oral stepdown to FQs (adjusted hazard ratio [aHR], 1.09 [95% confidence interval (CI), .49–2.43]) or TMP-SMX (aHR 1.44 [.54–3.87]). However, the recurrence risk was nearly 4-fold higher with HBBLs (aHR, 3.83 [95% CI, 1.76–8.33]; P < .001) (Figure 2). Receiver operating characteristic curve analysis identified 10 days as the best cutoff point in our data set for differentiating the effect of total antibiotic duration on 60-day recurrence, although there were not large differences in discrimination using other cutoff points (Supplementary Figure 2). In the modified primary analysis model, each antibiotic group was stratified by short (≤10 days) versus longer (>10 days) total duration. Compared with “longer-duration IVBL” as the referent group, we did not observe significant differences in recurrence risk for short-duration IVBL (aHR, 1.17 [95% CI, .29–4.74]), longer duration with FQ stepdown (0.99 [.38–2.58]), or longer duration with TMP-SMX stepdown (1.00 [.29–3.49]). However, longer duration with HBBL stepdown (aHR, 4.35 [1.78–10.66]), short duration with HBBL stepdown (3.68 [1.36–9.91]), and short duration with TMP-SMX stepdown (5.28 [1.36–20.46]) were all associated with significantly higher recurrence risk (Figure 3). Short duration with FQ stepdown was associated with an aHR of 1.82 (.64–5.23), which was not significantly different, but this analysis was limited by small sample size.
Figure 2.
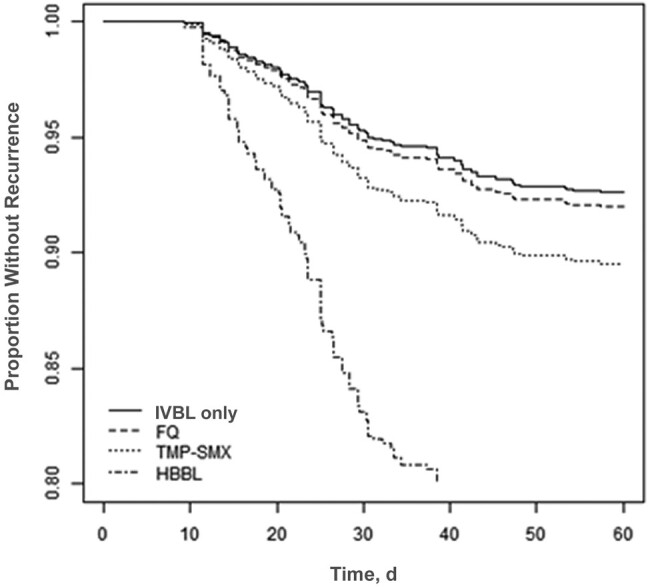
Recurrence-free days for gram-negative bloodstream infection from a complicated urinary tract infection source, comparing intravenous β-lactam (IVBL) versus oral stepdown therapies. Data represent cumulative incidence curves generated from the propensity-weighted Cox proportional hazards models. Abbreviations: FQ, fluoroquinolone; HBBL, high-bioavailability β-lactam; TMP-SMX, trimethoprim-sulfamethoxazole (all taken orally).
Figure 3.
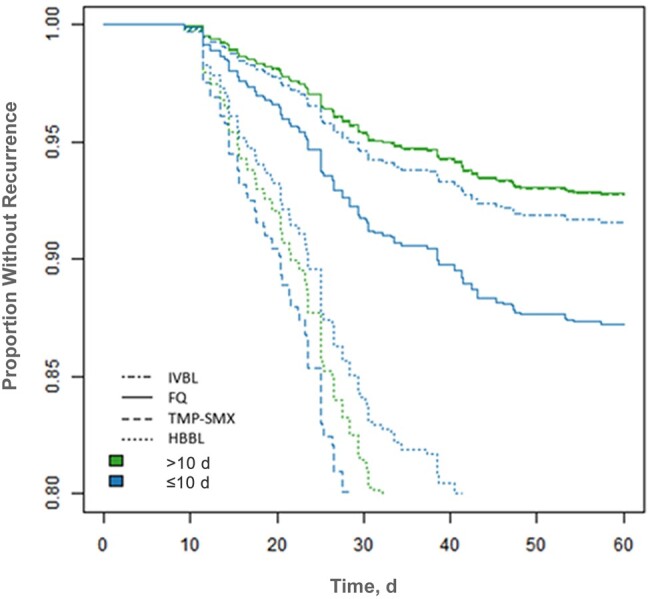
Recurrence-free days through day 60 for gram-negative bloodstream infection from a complicated urinary tract infection source, based on treatment regimen and total duration. Data represent cumulative incidence curves generated from the propensity-weighted Cox proportional hazards models. Abbreviations: FQ, fluoroquinolone, HBBL, high-bioavailability β-lactam; IVBL, intravenous β-lactam; TMP-SMX, trimethoprim-sulfamethoxazole (all taken orally).
Results were robust to all sensitivity analyses (Supplementary Figures 3–7). We did not detect a difference in recurrence risk between HBBLs and LBBLs, both of which were associated with significantly higher recurrence than IVBL (Supplementary Figure 8). Given concerns with low HBBL dosing in our study, we conducted a limited post hoc subgroup analysis of dosing and 60-day recurrence stratified by renal function and recurrence stratified by dosing (using unadjusted data). This suggested that preserved renal function (Supplementary Table 2) and suboptimal dosing (Supplementary Table 3) might both contribute to HBBL recurrence, although this analysis was limited by small sample size.
DISCUSSION
Our real-world data provide several important insights on treatment of GN-BSI/cUTI. First, we observed no difference in effectiveness between a full IVBL course and oral stepdown to FQs or TMP-SMX. This finding was most notable for the TMP-SMX group, which had more patients with urologic abnormalities and fewer patients receiving recommended dosing [10] than the FQ group. Together with other published data [6, 9, 12, 18, 19], our findings suggest that oral stepdown with FQ or TMP-SMX should be preferred for GN-BSI/cUTI once clinical stability is achieved on intravenous therapy.
Second, FQ and TMP-SMX stepdown appeared to be optimized with total durations longer than 10 days. While 2 randomized trials [7, 11] have found comparable outcomes with shorter (ie, 7-day) durations for GN-BSI, it is important to note that both trials excluded patients with lack of source control (often a concern for patients with cUTI), and one specifically excluded patients with moderate to severe hydronephrosis [11] (which was prevalent in our data set). It is possible that 7-day courses for GN-BSI from a UTI source are most effective when the patient either lacks structural or functional urologic abnormalities or has source control. Of note, we observed no significant difference in recurrence risk for short versus long duration with FQ stepdown, but the recurrence risk for short-course TMP-SMX stepdown was strikingly higher. These findings, particularly for TMP-SMX, raise the question of how best to optimize dosing and duration for effectiveness while limiting toxicity. Further study is needed to clarify whether interventions such as higher dosing combined with shorter duration could optimize outcomes for TMP-SMX stepdown therapy.
Finally, oral stepdown with HBBLs was associated with the highest risk of recurrence. While some studies have not observed higher recurrences with HBBLs [8, 13, 14], this finding is consistent with a previous meta-analysis and multicenter VA cohort study [9, 19]. HBBL recurrences were not mitigated by longer treatment duration in our data set, but this analysis was limited by small sample size and suboptimal dosing. It is possible that optimally dosing HBBL or prolonging treatment durations (>10 days) might close the outcomes gap in patients with GN-BSI/cUTI, but further study is needed.
Our study adds robust, real-world evidence to a growing knowledge base and had many advantages, including large sampling of only patients with cUTI, granular data from manual record review, use of a causal inference method, capturing recurrent symptomatic UTI in addition to bacteremia, and prolonged 90-day surveillance in an integrated health system. However, we acknowledge some important limitations, including unmeasured confounders that may have influenced prescribing choices and incomplete data that may have contributed to recurrence risk (eg, patient compliance, postdischarge change in antibiotics, or bacterial virulence). We were unable to control for provider-level or facility-level variability. The timing of postdischarge urologic procedures for source control and restoration of urinary flow (eg, lithotripsy with ureteral stent removal) might have influenced recurrence in any of the treatment groups; however, we could not accurately capture this information because many procedures took place outside our healthcare system. Readmissions and recurrences outside our system were not captured, which may have led to underreporting of outcomes. We could not distinguish between recurrence and new infection during retrospective review; however, sensitivity analyses at days 30, 60, and 90 yielded similar results.
Our restrictive inclusion criteria limited the study patients to only 30% of the screened population, which limits external validity, and our conclusions may apply only to patients who achieve clinical stability within 3 days on intravenous therapy. Furthermore, we acknowledge that “cUTI” represents a heterogeneous group of patients, and our findings might apply differently to various subgroups. Finally, our ability to evaluate the effectiveness of HBBLs was limited by current susceptibility testing practices, including lack of granular MICs, use of surrogate intravenous antibiotics to infer susceptibility of oral agents, and lack of systemic susceptibility breakpoints for oral BLs. All of these limitations should be considered when designing future studies.
In conclusion, our data suggest that oral FQs and TMP-SMX are similar in effectiveness to IVBL therapy for GN-BSI/cUTI and may be considered for oral therapy transitions when the isolate is susceptible. TMP-SMX effectiveness might be optimized with total durations longer than 10 days, although further study is needed. Conversely, HBBL stepdown therapy was associated with higher recurrence rates regardless of treatment duration. Further studies are needed to determine whether optimized dosing and/or extended treatment duration can mitigate the higher risk of recurrence observed with HBBLs.
Supplementary Material
Acknowledgments
We thank the Intermountain Research and Medical Foundation for funding this project; telehealth, infectious diseases, and pharmacy leadership for their support in conducting this work; and all those who assisted with data collection.
Financial support. This work was supported by the Intermountain Research and Medical Foundation (grant 846).
Contributor Information
John J Veillette, Infectious Diseases Telehealth Service, Intermountain Health, Murray, Utah, USA; Department of Pharmacy, Intermountain Medical Center, Murray, Utah, USA.
Stephanie S May, Infectious Diseases Telehealth Service, Intermountain Health, Murray, Utah, USA; Department of Pharmacy, Intermountain Medical Center, Murray, Utah, USA.
Sameer Alzaidi, Pharmacy Services, Intermountain Health, Taylorsville, Utah, USA.
Jared Olson, Department of Pharmacy, Primary Children's Hospital, Salt Lake City, Utah, USA; Division of Pediatrics, University of Utah, Salt Lake City, Utah, USA.
Allison M Butler, Statistical Data Center, Intermountain Health, Murray, Utah, USA.
C Dustin Waters, Department of Pharmacy, McKay-Dee Hospital, Ogden, Utah, USA.
Katarina Jackson, Department of Pharmacy, Intermountain Medical Center, Murray, Utah, USA.
Mary A Hutton, Department of Pharmacy, Utah Valley Hospital, Provo, Utah, USA.
Brandon J Webb, Division of Clinical Epidemiology and Infectious Diseases, Intermountain Medical Center, Murray, Utah, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Tamma PD, Cosgrove SE. Which trial do we need? Early oral antibiotic therapy for the treatment of gram-negative bloodstream infections. Clin Microbiol Infect 2023; 29:670–2. [DOI] [PubMed] [Google Scholar]
- 2. Tamma PD, Conley AT, Cosgrove SE, et al. Antibacterial resistance leadership group: association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med 2019; 179:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hospenthal DR, Waters CD, Beekmann SE, Polgreen PM. Practice patterns of infectious diseases physicians in transitioning from intravenous to oral therapy in patients with bacteremia. Open Forum Infect Dis 2020; 7:ofz386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rieger KL, Bosso JA, MacVane SH, et al. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017; 37:1479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omrani AS, Abujarir SH, Ben Abid F, et al. Switch to oral antibiotics in gram-negative bacteraemia: a randomized, open-label, clinical trial. Clin Microbiol Infect 2024; 30:492–498. [DOI] [PubMed] [Google Scholar]
- 6. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 2000; 283:1583–90. [DOI] [PubMed] [Google Scholar]
- 7. von Dach E, Albrich WC, Brunel A-S, et al. Effect of C-reactive protein–guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA 2020; 323:2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mack T, Hiles JJ, Wrin J, Desai A. Use of fluoroquinolones or sulfamethoxazole-trimethoprim compared to β-lactams for oral step-down therapy in hospitalized patients with uncomplicated Enterobacterales bacteremia. Ann Pharmacother 2023; 57:251–8. [DOI] [PubMed] [Google Scholar]
- 9. Punjabi C, Tien V, Meng L, Deresinski S, Holubar M. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs. ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis 2019; 6:ofz364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heil EL, Bork JT, Abbo LM, et al. Optimizing the management of uncomplicated gram-negative bloodstream infections: consensus guidance using a modified Delphi process. Open Forum Infect Dis 2021; 8:ofab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019; 69:1091–8. [DOI] [PubMed] [Google Scholar]
- 12. Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet 2012; 380:484–90. [DOI] [PubMed] [Google Scholar]
- 13. McAlister MJ, Rose DT, Hudson FP, Padilla-Tolentino E, Jaso TC. Oral β-lactams vs fluoroquinolones and trimethoprim/sulfamethoxazole for step-down therapy for Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae bacteremia. Am J Health Syst Pharm 2023; 80(suppl 1):S33–41. [DOI] [PubMed] [Google Scholar]
- 14. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus β-lactams. Int J Antimicrob Agents 2018; 51:687–92. [DOI] [PubMed] [Google Scholar]
- 15. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents 2016; 48:498–503. [DOI] [PubMed] [Google Scholar]
- 16. Drekonja DM, Trautner B, Amundson C, Kuskowski M, Johnson JR. Effect of 7 vs 14 days of antibiotic therapy on resolution of symptoms among afebrile men with urinary tract infection: a randomized clinical trial. JAMA 2021; 326:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lafaurie M, Chevret S, Fontaine JP, et al. Antimicrobial for 7 or 14 days for febrile urinary tract infection in men: a multicenter noninferiority double-blind, placebo-controlled, randomized clinical trial. Clin Infect Dis 2023; 76:2154–62. [DOI] [PubMed] [Google Scholar]
- 18. McAteer J, Lee JH, Cosgrove SE, et al. Defining the optimal duration of therapy for hospitalized patients with complicated urinary tract infections and associated bacteremia. Clin Infect Dis 2023; 76:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutton JD, Stevens VW, Chang NN, Khader K, Timbrook TT, Spivak ES. Oral β-lactam antibiotics vs fluoroquinolones or trimethoprim-sulfamethoxazole for definitive treatment of Enterobacterales bacteremia from a urine source. JAMA Netw Open 2020; 3:e2020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147:573–7. [DOI] [PubMed] [Google Scholar]
- 21. Massarweh NN, Haukoos JS, Ghaferi AA. ISPOR reporting guidelines for comparative effectiveness research. JAMA Surg 2021; 156:673–4. [DOI] [PubMed] [Google Scholar]
- 22. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 23. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 2013; 32:3388–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridgeway G, McCaffrey D, Morral A, et al. Toolkit for weighting and analysis of nonequivalent groups: a guide to the twang package. R Foundation. 2008. Available at: https://cran.r-project.org/web/packages/twang/vignettes/twang.pdf. Accessed 20 November 2023. [Google Scholar]
- 25. Burgette L, Griffin B, McCaffrey D. Propensity scores for multiple treatments: a tutorial for the mnps function in the twang package. R Foundation. 2021. Available at: https://cran.r-project.org/web/packages/twang/vignettes/mnps.pdf. Accessed 20 November 2023. [Google Scholar]
- 26. Molina J, Montero-Mateos E, Praena-Segovia J, et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: a randomized, controlled trial. Clin Microbiol Infect 2022; 28:550–7. [DOI] [PubMed] [Google Scholar]
- 27. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–5. [DOI] [PubMed] [Google Scholar]
- 28. Humphries RM, Abbott AN, Hindler JA. Understanding and addressing CLSI breakpoint revisions: a primer for clinical laboratories. J Clin Microbiol 2019; 57:e00203-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.