Abstract
Introduction:
The coronavirus disease 2019 (COVID-19) pandemic may have influenced the prevalence and seasonality of acute respiratory viral infections. The aim of the study was to investigate the prevalence of all viruses causing acute viral respiratory infections before and after social distancing measures were lifted
Methods:
Cross-sectional study where outpatients and inpatients at Kyunghee University Hospital were examined. From January 2021 to December 2022, respiratory samples were analyzed using multiplex reverse transcriptase real-time polymerase chain reaction
Results:
Of 3953 samples obtained, 412 (10.42%) were positive for acute respiratory viral infection, and 502 viruses were detected. The number of viral infections increased from 184 in 2021 to 318 in 2022. Human metapneumovirus was detected from August to November 2022. Human bocavirus (HBoV) was frequently detected from April to June 2021; however, in 2022, HBoV was frequently detected from July to October. Human parainfluenza virus 3 was rarely detected after its initial frequent detection from October to December 2021 but was continuously observed after frequent detection in September 2022. Co-infection occurred in 78 (18.9%) cases. The most common combination of simultaneous infections was human rhinovirus–HBoV (n = 30, 38.5%)
Conclusions:
During the COVID-19 pandemic, the incidence of acute respiratory viral infection decreased significantly but increased in 2022 when measures were lifted. The prevalence and seasonality of respiratory viral infections have changed since the pandemic. Our findings contribute to the prediction of an effective response to changes in the prevalence of respiratory viruses.
Keywords: Acute viral infection, coronavirus disease 2019, influenza, prevalence rate, respiratory, seasonality, virus
INTRODUCTION
Acute respiratory viral infections are classified as Grade 4 infectious diseases. Understanding such infections, including their incidence, trends (epidemic signs and early detection), and high-risk groups, is important. For instance, many respiratory viral infections are caused by adenovirus, bocavirus, human coronavirus, influenza virus, metapneumovirus, parainfluenza virus, respiratory syncytial virus (RSV), and rhinovirus.[1] Most of these viruses cause upper respiratory infection in adults without comorbidities, and the prognoses of these individuals improve with short-term medication. However, hospitalization is often required for lower respiratory tract infections, particularly in children, and results in the death of several children annually.[2,3] According to the Korea Centers for Disease Control and Prevention’s (KCDCP) annual sample monitoring report,[4] the number of positive cases of infection is increasing annually owing to the increase in the older population.[5] Moreover, each viral infection epidemic is affected by various seasons.[4]
Since coronavirus disease 2019 (COVID-19) was first reported in 2019, it has spread rapidly, and in March 2020, after the infectious disease became prevalent worldwide, the World Health Organization declared COVID-19 as a pandemic.[6] Various measures have been implemented by each country,[7] including the Republic of Korea,[8] to curb its spread. These measures, such as limiting face-to-face activity, private gatherings, and business hours, have caused variations in the prevalence of respiratory viral infections.[8,9,10,11,12,13,14]
Previous studies have only investigated the prevalence of select respiratory viruses; therefore, we aimed to investigate the prevalence of all viruses that cause acute viral respiratory infections. We examined the prevalence of these Grade 4 infectious diseases both before and after social distancing measures were lifted. We also analyzed the prevalence and seasonality of each virus based on the results of respiratory virus (RV) multiplex reverse transcription (RT) real-time polymerase chain reaction (PCR) from a single tertiary hospital.
METHODS
Target and data collection
In this cross-sectional study, we analyzed respiratory specimen results obtained from outpatients and inpatients at Kyunghee University Hospital to assess the prevalence and seasonality of each virus and compare the findings with prepandemic data from January 2021 to December 2022. After obtaining 3953 samples (1775 in 2021 and 2178 in 2022) from the nasal cavity and pharynx, culture tests were conducted in liquid transport media, and 412 positive results (10.4%) were obtained, compared, and analyzed. We did not consider whether patients had duplicate samples.
Virus detection
Samples were analyzed for the presence of 14 respiratory viruses (the human adenovirus [HAdV], RSV-A, RSV-B, influenza A virus [IAV], influenza B virus [IBV], human parainfluenza virus 1 [HPIV-1], human parainfluenza virus 2 [HPIV-2], human parainfluenza virus 3 [HPIV-3], human rhinovirus [HRV], human metapneumovirus [HMPV], human coronavirus 229E [HCoV-229E], human coronavirus NL63 [HCoV-NL63], human coronavirus OC43 [HCoV-OC43], and human bocavirus [HBoV]).
Samples
Nasopharyngeal swabs from the pharynx and nasal cavity were collected in the ASAN Transport Medium (ASAN Pharm, Seoul, the Republic of Korea). All specimens were stored in a 4°C refrigerator until they were processed and examined within 24 h.
Methods of analyses
Nucleic acid was extracted from samples using the AdvanSure E3 System (Advanced Nucleic Acid R Kit, Advanced Nucleic Acid R Tube; LG Chemistry). All tests were conducted immediately after the extraction of the nucleic acid. Samples were analyzed using the multiplex/RT-PCR method with the AdvanSure RV-plus real-time RT-PCR kit (LG Chemistry, Seoul, Republic of Korea), with four replicates for each sample, according to the manufacturer’s instructions.
Validity
The cycle threshold (Ct) value of the control substance was considered valid only when the results were within the range indicated in the manufacturer’s instructions. All tests contained one positive and one negative control solution, and the amplification curves of all tests were recorded.
Interpretation
A Ct value of <27, induced in each channel from the wavelength of the fluorescent material of each sample, was set as a positive standard and interpreted according to the table provided by the manufacturer. In addition, when signals of two or more types of viruses were detected, the results were analyzed by interpreting them as indicating a simultaneous coinfection.
We analyzed participant characteristics based on data from patient records and investigated the association of sex, age, and time of infection with the virus test results. The results were then also compared and analyzed by period (monthly), sex, and age.
Ethics
This study was reviewed and approved by the Clinical Trials Ethics Committee of Dankook University. This study retrospectively analyzed existing test results without including any personal patient information. As patient information was not included, the need for informed consent from the included patients was waived.
RESULTS
Prevalence by sex
Of the 3953 samples requested, 412 (10.42%) were positive for viral infection, and 502 viruses were detected. Among all patients tested, 2404 (60.81%) were men and 1549 (39.19%) were women, and the total numbers of positive viral infections were 288 (7.29%) among men and 214 (5.41%) among women. The predominant virus among men and women was HPIV-2 (male/female ratio: 3.00), followed by IAV (male/female ratio: 2.50), HCoV-OC43 (male/female ratio: 2.00), and RSV-B (male/female ratio: 1.54). For HCoV-229E, the male/female ratio could not be obtained because no positive results were detected in the samples collected from women [Table 1].
Table 1.
Prevalence of viruses by sex
| Virus | Total number of positive patients, n (%) | Ratio of positive cases to total number of samples (%) | Males, n (%) | Females, n (%) | Male/female ratio |
|---|---|---|---|---|---|
| HAdV | 31 (6.18) | 0.78 | 15 (5.20) | 16 (7.48) | 0.94 |
| RSV-A | 40 (7.97) | 1.01 | 23 (7.99) | 17 (7.94) | 1.35 |
| RSV-B | 33 (6.57) | 0.83 | 20 (6.94) | 13 (6.07) | 1.54 |
| IAV | 7 (1.39) | 0.18 | 5 (1.74) | 2 (0.93) | 2.50 |
| IBV | 0 | 0 | 0 | 0 | 0.00 |
| HPIV-1 | 10 (1.99) | 0.25 | 5 (1.74) | 5 (2.34) | 1.00 |
| HPIV-2 | 4 (0.8) | 0.10 | 3 (1.04) | 1 (0.47) | 3.00 |
| HPIV-3 | 61 (12.15) | 1.54 | 34 (11.80) | 27 (12.62) | 1.26 |
| HRV | 148 (29.48) | 3.74 | 89 (30.90) | 59 (27.57) | 1.51 |
| HMPV | 29 (5.78) | 0.73 | 14 (4.86) | 15 (7.01) | 0.93 |
| HCoV-229E | 4 (0.8) | 0.10 | 4 (1.39) | 0 | N/A |
| HCoV-NL63 | 8 (1.59) | 0.20 | 3 (1.04) | 5 (2.34) | 0.60 |
| HCoV-OC43 | 33 (6.57) | 0.83 | 22 (7.64) | 11 (5.14) | 2.00 |
| HBoV | 94 (18.73) | 2.38 | 51 (17.71) | 43 (20.10) | 1.19 |
| Total | 502 (100) | 412 (10.42) | 288 (100) | 214 (100) | 1.35 |
Total number of positive cases=Counts for each virus/sum of positive cases × 100. Ratio of positive cases to total number of samples=Number of cases positive for each virus/total number of samples × 100. Males, females=Number of males or females positive for each virus/number of total positive males or females × 100. HAdV: Human adenovirus, HBoV: Human bocavirus, HCoV-229E: Human coronavirus 229E, HCoV-NL63: Human coronavirus NL63, HCoV-OC43: Human coronavirus OC43, HMPV: Human metapneumovirus, HPIV-1: Human parainfluenza virus 1, HPIV-2: Human parainfluenza virus 2, HPIV-3: Human parainfluenza virus 3, HRV: Human rhinovirus, IAV: Influenza A virus, IBV: Influenza B virus, N/A: Results could not be calculated because the number for one sex is zero, RSV-A: Respiratory syncytial virus A, RSV-B: Respiratory syncytial virus B
Annual occurrence of each virus
There were 184 viral infections in 2021 and 318 in 2022, which were detected among 412 patients [Figure 1]. Among the 412 virus-positive patients in 2021 and 2022, the number of patients who tested positive for viruses other than HBoV, HPIV-3, and HRV was significantly higher in 2022 than in 2021. HCoV-NL63, HCoV-OC43, IAV, HMPV, and HPIV-2 were not detected in 2021 but were detected in 2022. RSV-A (n = 38), HCoV-OC43 (n = 33), HMPV (n = 29), RSV-B (n = 15), HAdV (n = 9), HPIV-1 (n = 8), HCoV-NL63 (n = 8), and IAV (n = 7) were more frequently detected in 2022 than in 2021 (figures in parentheses indicate the increase in the number of cases in 2022 when compared with the numbers in 2021).
Figure 1.
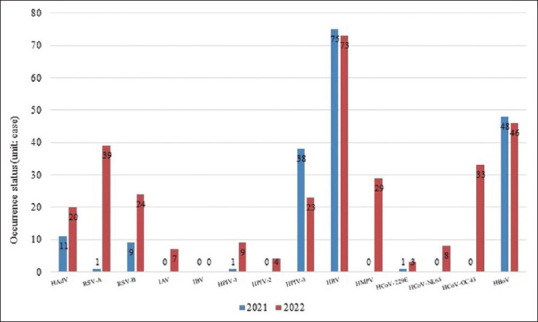
Annual occurrence of each virus. Graph comparing the number of positive cases, in which the 14 types of viruses were detected in 2021 and 2022 based on the respiratory virus multiplex real-time polymerase chain reaction conducted at the Kyunghee Medical Center. HAdV: Human adenovirus, RSV-A: Respiratory syncytial virus-A, RSV-B: Respiratory syncytial virus-B, IAV: Influenza A virus, IBV: Influenza B virus, HPIV-1: Human parainfluenza virus 1, HPIV-2: Human parainfluenza virus 2, HPIV-3: Human parainfluenza virus 3, HRV: Human rhinovirus, HMPV: Human metapneumovirus, HCoV-229E: Human coronavirus 229E, HCoV-NL63: Human coronavirus NL63, HCoV-OC43: Human coronavirus OC43, HBoV: Human bocavirus
Virus detection patterns by month in 2021 and 2022
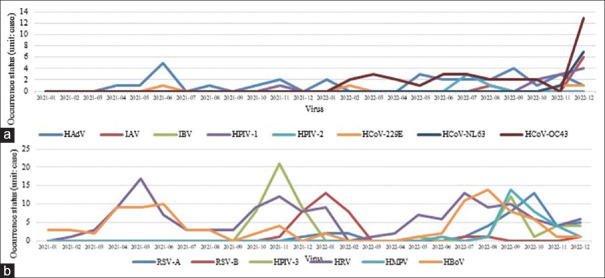
HAdV was detected consistently throughout these years [Figure 2a], and HBoV was frequently detected in the spring (April–June) of 2021. However, in 2022, HBoV was frequently detected in summer to fall (July–October) [Figure 2b]. HRV was frequently detected in the spring and winter (April–June and October–January, respectively) of 2021, although it was frequently detected from the late spring to summer (May–September) of 2022.
Figure 2.
Monthly number of cases of each virus detected from 2021 to 2022. (a) The graphs show the positive numbers of human adenovirus, influenza A virus, influenza B virus, human parainfluenza virus-1, human parainfluenza virus-2, human coronavirus 229E, human coronavirus NL63, and human coronavirus OC43 detected using multiplex reverse transcription real-time polymerase chain reaction. The tests were conducted at the Kyunghee Medical Center from 2021 to 2022, (b) The graphs show the positive numbers of respiratory syncytial virus-A, respiratory syncytial virus-B, human metapneumovirus-3, human rhinovirus, human metapneumovirus, and human bocavirus detected using multiplex reverse transcription real-time polymerase chain reaction. The tests were conducted at the Kyunghee Medical Center from 2021 to 2022. HAdV: Human adenovirus, IAV: Influenza A virus, IBV: Influenza B virus, HPIV-1: Human parainfluenza virus 1, HPIV-2: Human parainfluenza virus 2, HCoV-229E: Human coronavirus 229E, HCoV-NL63: Human coronavirus NL63, HCoV-OC43: Human coronavirus OC43, RSV-A: Respiratory syncytial virus A, RSV-B: Respiratory syncytial virus B, HPIV-3: Human parainfluenza virus 3, HRV: Human rhinovirus, HMPV: Human metapneumovirus, HBoV: Human bocavirus
HPIV-1 was rarely detected before October 2022 and consistently detected thereafter. HPIV-2 was detected only in July and August 2022. HPIV-3 was rarely detected after its frequent detection from the fall to the winter of 2021 (October–December) and consistently detected after being frequently detected in September 2022.
RSV-A was rarely detected in 2021 but was detected in the winter between December 2021 and February 2022 and consistently detected in the summer (July) of 2022. RSV-B was rarely detected earlier but was frequently detected from late 2021 to early 2022 (November 2021 to March 2022). IAV was rarely detected earlier but was detected at the end of 2022, whereas IBV was not detected in either 2021 or 2022. HCoV-229E was rarely detected at any time. HCoV-NL63 and HCoV-OC43 were rarely detected and only in late 2022. HMPV was detected from the summer to fall of 2022 (August–November).
Coinfection
Coinfection was observed in 78 (18.9%) cases, of which 68 (87.2%) were dual infection, 8 (10.3%) were triple infection, and 2 (2.6%) were quadruple infection cases [Table 2]. While most coinfection cases involved HRV (n = 50, 33.8%), HBoV exhibited the highest rate of coinfection (n = 46, 48.9%). The most common combination of viruses in cases of coinfection was HRV–HBoV (n = 30, 38.5%), followed by HPIV-3–HRV (n = 9, 11.5%) and HAdV–HRV (n = 8, 10.3%).
Table 2.
Occurrence of coinfections according to the number of viral infections
| Dual, n (%) | Triple, n (%) | Quadruple, n (%) |
|---|---|---|
| 68 (87.2) cases | 8 (10.3) cases | 2 (2.6) cases |
DISCUSSION
Prevalence by sex
The test results from the Kyunghee Medical Center demonstrated clear differences in terms of the male/female ratio for HPIV-3 and IAV infections. However, it remains unclear whether this result was significant because these viruses were detected in very few samples. In addition, most viruses were detected more frequently in men than in women; the positive rates relative to the total number of samples were 7.29% for men and 5.41% for women, which were not significantly different. Most studies have also shown that sex differences in prevalence were not significant.[3,14,15,16,17,18] In this limited-sample, retrospective study, sex difference was also not significant.
Prevalence of respiratory viral infections from 2021 to 2022
According to RV statistics,[19] which include the sample surveillance of infectious diseases on the Korea Disease Control and Prevention Agency Infected Disease home page, 16,555 cases were reported in 2021 and 30,605 cases in 2022. The 2021/2022 ratio was 0.54, similar to the result observed at the Kyunghee Medical Center in this study. The prevalence of respiratory viral infections following the COVID-19 pandemic has shown a significant decrease worldwide.[11,13] According to the 2021–2022 Influenza and Respiratory Viruses Laboratory Surveillance Report in the Public Health Weekly Report, the Republic of Korea, the annual outbreaks of respiratory viral infection remained low during the COVID-19 pandemic and increased during the second half of 2021 until the first half of 2022.[12] Before the COVID-19 outbreak (2018–2019), more than 80,000 cases of respiratory viral infection were reported annually, but these gradually increased after a sharp decline in 2020,[13] which is presumed to be due to an increase in face-to-face activities.[12] This also suggests that the number of respiratory viral infection cases may increase further in 2023 during the process of lifting the requirement of mandatory wearing of masks and recovery of daily life.
Detection patterns of influenza in 2021–2022
At the Kyunghee Medical Center, IAV was not detected in 2021 but was detected in 2022. This result was similar to that of the 2021–2022 Influenza and Respiratory Viruses Laboratory Surveillance Report in the Public Health Weekly Report, the Republic of Korea[12] (period during the second half of 2020 to the first half of 2021, not detected; period during the second half of 2021 to the first half of 2022, n = 38 cases of IAV [H3N2] detected). In addition, IBV was not observed at Kyunghee Medical Center and in the KCDCP statistics.[12,20] A comparison of this result with the findings reported in studies from the United States revealed that the prevalence of influenza decreased sharply in April 2020, showing a significantly lower prevalence than that before the pandemic in 2021, which then increased in 2022.[10,11,21,22] Moreover, influenza type A viruses, but not type B viruses, were mostly detected internationally, with results similar to Korean trends.[10,11,21,22] Currently, influenza type B viruses are rarely detected, and only Type A viruses are detected. However, type B viruses are detected in Asia and other countries.[11] As overseas travel and face-to-face activities increase, prevention and vaccination are considered important for both viruses, and continuous attention and observation are required to prevent a so-called “twindemic.”
Detection patterns of acute respiratory viral infection in 2021–2022
The RV test results from the Kyunghee Medical Center did not consider virus subtypes for comparison with local data. Therefore, these results were compared with the statistics on the KCDCP’s infectious diseases website [Table 3].[19]
Table 3.
Annual comparison of most frequently detected viruses between Kyunghee Medical Center and Korean Centers for Disease Control and Prevention
| Year | Institution | 1st (%) | 2nd (%) | 3rd (%) |
|---|---|---|---|---|
| 2021 | Kyunghee Medical Center | HRV (40.8) | HBoV (26.1) | HPIV (21.2) |
| KCDCP | HRV (41.9) | HPIV (26.7) | HBoV (19.4) | |
| 2022 | Kyunghee Medical Center | HRV (23.0) | RSV (19.8) | HBoV (14.5) |
| KCDCP | HRV (28.2) | RSV (27.5) | HPIV (12.3) |
None of the differences were statistically significant. HBoV: Human bocavirus, HPIV: Human parainfluenza virus, HRV: Human rhinovirus, KCDCP: Korean Centers for Disease Control and Prevention, RSV: Respiratory syncytial virus
The most commonly detected virus was HRV, which was detected at a similar incidence at both institutions. However, in 2021, HBoV was more prevalent than HPIV at the Kyunghee Medical Center, whereas HPIV was more prevalent than HBoV according to the KCDCP statistics. In 2022, the second-most common virus was RSV, which was detected at a similar incidence at both institutions. However, HBoV, at the Kyunghee Medical Center, and HPIV, in the KCDCP statistics, had the second highest positivity rate. On comparing these results with those of the previous 3 years (2018–2020), HRV was the most commonly observed virus annually, and RSV, HPIV, and HCoV were mainly detected before the pandemic.[14,19] Furthermore, HBoV was increasingly detected in 2021 and 2022. However, studies from the United States and Canada have reported that HCoV, HRV, and RSV had the highest prevalence before and during the pandemic,[9,11,23] with similar trends observed in the Republic of Korea. In contrast to the trends observed in the Republic of Korea, where the outbreak status of HBoV was identified as a fourth-class epidemic, it is difficult to compare the positivity rates in other countries, as these rely mainly on study results.[4] Notably, prior studies conducted in Europe have shown that the prevalence rates can vary from 2% to 40% by the country and period.[24,25] As HBoV prevalence has clearly increased in the Republic of Korea, it is important to establish relevant preventive methods and treatments.
Monthly detection trends for each virus
Most of the viruses targeted in this study showed trends similar to those reported in prior studies from the Republic of Korea and the United States.[2,3,14,15,23] However, HMPV is seasonally detected from the late winter to spring in the Republic of Korea,[3,14] and in 2022, it had the highest positivity rate in the fall (September–November). HBoV is not seasonally detected, and in prior studies, several HBoV infections have been detected in the spring,[14] and in 2022, HBoV had the highest positivity rate from the summer to the fall (July–October). Regarding HPIV, serotypes 1 and 2 are mainly prevalent in the fall and winter, and HPIV is frequently detected in the spring and summer, with most studies showing similar trends.[2,3,14,15,23] However, based on the results from the Kyunghee Medical Center in this study, HPIV-1 and HPIV-2 followed the same trends as those observed in previous reports, whereas HPIV-3 infection occurred in the fall and winter of 2021 and in the summer and fall of 2022, in contrast to previous findings. This discovery suggests that changes occurred in virus detection trends during the pandemic. However, as this study did not include the entire pandemic period, and the pandemic is yet to be completely resolved, we will continue analyzing new variations in the trends and collect findings that will facilitate effective responses moving forward.
Coinfections
According to data from the Republic of Korea and England, the coinfection rates of respiratory viruses range from 10% to 40%,[15,26,27,28] and the results vary by age, region, and period. The results of these previous studies showed trends similar to those observed in the Republic of Korea. However, among the types of coinfection, dual infection was the most common at 87.2%, with trends similar to those reported in prior studies.[15,26,28] According to previous studies, HRV, HAdV, and RSV mainly occur as coinfections in the Republic of Korea.[15,26,27] Internationally, RSV, HRV, and HAdV[28,29] are often reported to cause coinfections. In addition, HAdV–HRV,[15,26,27] which is generally the most common coinfection in the Republic of Korea, accounted for a low rate of 10.3% of the total simultaneous infections. HRV–HBoV was the most frequently observed coinfection in the present study (38.5%) and could lead to an increase in the prevalence of HBoV and coinfections. In addition, considering the epidemiological characteristics of HBoV, it is frequently involved in coinfection.[24,25] According to international studies on HBoV, this virus epidemiologically presents more coinfections than single infections.[24,25] In the Republic of Korea, the respiratory management guidelines of the KCDCP[4] have reported HBoV coinfection rates of up to 61.7%. While some previous studies[17,27,29,30] have suggested that coinfection does not significantly affect disease severity, others have indicated that coinfection does increase disease severity.[16] As only a few studies have been conducted on simultaneous infections, further research is necessary for an in-depth understanding of coinfections. In particular, studies on coinfection and countermeasures are required to mitigate the increasing prevalence of HBoV, which causes coinfections at a high rate.
This study has several limitations. First, the findings of this study cannot be used to determine the prevalence rate across the country as they only reflect the test results of a single institution located in Seoul. Second, this was a retrospective study that investigated a limited number of samples. Third, we believe that the results obtained for some viruses did not reach statistical significance because of their low representation in the sample. Finally, this study did not include all periods during the COVID-19 outbreak in 2019 and did not assess detailed patient information, such as disease name, clinical symptoms, treatment, and patient age. To complement this study, additional information from various institutions should be analyzed to reflect the differences over the pandemic period as well as regional prevalence. As the pandemic is not yet completely resolved, it is necessary to study future prevalence trends through continuous monitoring.
CONCLUSIONS
This study included patients across all age groups and identified the epidemic period of each virus by classifying virus subtypes. In addition, this study confirmed that the prevalence of viruses varied in the pre- and during-pandemic periods. Therefore, the findings of this study could contribute to the prediction of effective responses to changes in the prevalence of respiratory viruses.
Research quality and ethics statement
This study was approved by Dankook University Institutional Review Board (IRB number: 2023-03-004). The authors followed applicable EQUATOR Network guidelines during the conduct of this research project.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Korea Disease Control and Prevention Agency. Status of Disease Monitoring and Investigation Systems. Cheongju-si: Korea Disease Control and Prevention Agency; [[Last accessed on 2023 Feb 10]]. Available from: https://www.kdca.go.kr/contents.es?mid=a20510010100 . [Google Scholar]
- 2.Sohn YJ, Choi YY, Yun KW, Choi EH, Lee HJ. Epidemiology and clinical characteristics of parainfluenza virus type 4 in Korean children: A single center study, 2015-2017. Korean Pediatr Infect Vaccine. 2018;25:156–64. [Google Scholar]
- 3.Lee HJ, Park JH, Kim M, Kim JH, Baek HS. Clinical efficacy of respiratory virus detection by using the FilmArray method in children admitted with respiratory infection. Korean Allergy Asthma Respir Dis. 2021;9:12–20. [Google Scholar]
- 4.Park HK. Guidelines for Managing Respiratory Infectious Diseases in 2021. 1st ed. Cheongju-si: Korea Disease Control and Prevention Agency; 2022. pp. 121–89. [Google Scholar]
- 5.Korea Disease Control and Prevention Agency. Acute Respiratory Viral Infection. Cheongju-si: Korea Disease Control and Prevention Agency; [[Last accessed on 2021 Jan 13]]. Available from: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do . [Google Scholar]
- 6.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CM, Kim HL, Kim SH, Oh JY, Kim IH. Major National Trends Related To COVID-19 Response and Transition to the Quarantine System. Policy Report. Cheongju-si: Korea Disease Control and Prevention Agency. 2021. [[Last accessed on 2021 Nov 04]]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=717440&act=view# .
- 8.Kim M. COVID-19 Changes in Response to Infectious Diseases Over the Last Three Years. Sejong-si: Ministry of Health and Welfare; 2023. [[Last accessed on 2021 Jan 13]]. Available from: https://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&CONT_SEQ=374685&page=1 . [Google Scholar]
- 9.Groves HE, Piché-Renaud PP, Peci A, Farrar DS, Buckrell S, Bancej C, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg Health Am. 2021;1:100015. doi: 10.1016/j.lana.2021.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic –United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013–9. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2023;21:195–210. doi: 10.1038/s41579-022-00807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee NJ, Woo SH, Lee J, Rhee JE, Kim EJ. 2021-2022 influenza and respiratory viruses laboratory surveillance report in the republic of Korea. Public Health Wkly Rep. 2023;16:53–65. [Google Scholar]
- 13.Sullivan SG, Carlson S, Cheng AC, Chilver MB, Dwyer DE, Irwin M, et al. Where has all the influenza gone?The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25:2001847. doi: 10.2807/1560-7917.ES.2020.25.47.2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JM, Jung HD, Cheong HM, Lee A, Lee NJ, Chu H, et al. Nation-wide surveillance of human acute respiratory virus infections between 2013 and 2015 in Korea. J Med Virol. 2018;90:1177–83. doi: 10.1002/jmv.25069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Kim JM, Choi SK, Cho YJ, Kim SJ, Kim JR. Prevalence of respiratory viral infection using multiplex reverse transcriptase-polymerase chain reaction. Korean J Fam Pract. 2021;11:210–6. [Google Scholar]
- 16.Sun H, Sun J, Ji W, Hao C, Yan Y, Chen Z, et al. Impact of RSV coinfection on human bocavirus in children with acute respiratory infections. J Trop Pediatr. 2019;65:342–51. doi: 10.1093/tropej/fmy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JS, Chu SY, Shin YY, Ryu IK, Tang CL, Choi J, et al. Comparison of clinical severity between single- and coinfections of respiratory syncytial virus and influenza virus with common respiratory viruses. Korean Allergy Asthma Respir Dis. 2019;7:86–91. [Google Scholar]
- 18.Caini S, de Mora D, Olmedo M, Portugal D, Becerra MA, Mejía M, et al. The epidemiology and severity of respiratory viral infections in a tropical country: Ecuador, 2009-2016. J Infect Public Health. 2019;12:357–63. doi: 10.1016/j.jiph.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korea Disease Control and Prevention Agency. Infectious Disease Homepage. Cheongju-si: Korea Disease Control and Prevention; [[Last accessed on 2023 Feb 10]]. Available from: https://www.kdca.go.kr/npt/biz/npp/iss/ariStatisticsMain.do . [Google Scholar]
- 20.Korea Disease Control and Prevention Agency. Infectious Disease Homepage. Cheongju-si: Korea Disease Control and Prevention Agency; [[Last accessed on 2023 Feb 10]]. Available from: https://www.kdca.go.kr/npt/biz/npp/iss/influenzaStatisticsMain.do . [Google Scholar]
- 21.National Center for Immunization and Respiratory Diseases. 2021-2022 Flu Season Summary. Centers for Disease Control and Prevention. 2023. [[Last accessed on 2023 Jan 12]]. Available from: https://www.cdc.gov/flu/season/faq-flu-season-2021-2022.htm .
- 22.Merced-Morales A, Daly P, Abd Elal AI, Ajayi N, Annan E, Budd A, et al. Influenza activity and composition of the 2022-23 influenza vaccine –United States, 2021-22 Season. MMWR Morb Mortal Wkly Rep. 2022;71:913–9. doi: 10.15585/mmwr.mm7129a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanti M, Birger R, Ud-Dean M, Filip I, Morita H, Comito D, et al. Longitudinal active sampling for respiratory viral infections across age groups. Influenza Other Respir Viruses. 2019;13:226–32. doi: 10.1111/irv.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polo D, Lema A, Gándara E, Romalde JL. Prevalence of human bocavirus infections in Europe. A systematic review and meta-analysis. Transbound Emerg Dis. 2022;69:2451–61. doi: 10.1111/tbed.14233. [DOI] [PubMed] [Google Scholar]
- 25.Falahi S, Sayyadi H, Abdoli A, Kenarkoohi A, Mohammadi S. The prevalence of human bocavirus in<2-year-old children with acute bronchiolitis. New Microbes New Infect. 2020;37:100736. doi: 10.1016/j.nmni.2020.100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JK, Jeon JS, Kim JW, Rheem I. Epidemiology of respiratory viral infection using multiplex rt-PCR in Cheonan, Korea (2006-2010) J Microbiol Biotechnol. 2013;23:267–73. doi: 10.4014/jmb.1212.12050. [DOI] [PubMed] [Google Scholar]
- 27.Kwon Y, Cho WJ, Kim HM, Lee J. Single or dual infection with respiratory syncytial virus and human rhinovirus: Epidemiology and clinical characteristics in hospitalized children in a rural area of South Korea. Korean Pediatr Infect Vaccine. 2019;26:99–111. [Google Scholar]
- 28.Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol Infect. 2015;143:37–47. doi: 10.1017/S0950268814000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meskill SD, O’Bryant SC. Respiratory virus co-infection in acute respiratory infections in children. Curr Infect Dis Rep. 2020;22:3. doi: 10.1007/s11908-020-0711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cebey-López M, Herberg J, Pardo-Seco J, Gómez-Carballa A, Martinón-Torres N, Salas A, et al. Does viral co-infection influence the severity of acute respiratory infection in children? PLoS One. 2016;11:e0152481. doi: 10.1371/journal.pone.0152481. [DOI] [PMC free article] [PubMed] [Google Scholar]