Abstract
Background:
In January 2021, vericiguat, a soluble guanylate cyclase (sGC) stimulator, was approved by the US FDA to reduce the risk of cardiovascular death and heart failure (HF) hospitalization among patients with a recent worsening HF event based on the VICTORIA trial.
Objective:
To leverage a contemporary US registry of patients hospitalized for heart failure (HF) to characterize patients who may be candidates for vericiguat based on FDA label and the VICTORIA trial eligibility criteria.
Methods:
We studied patients hospitalized for HF with ejection fraction (EF) <45%) across 525 sites in the Get With The Guidelines (GWTG)-HF registry between January 2014 – December 2020. We applied approximate FDA label criteria (excluding eGFR<15 mL/min/1.73 m2, dialysis, or patients with heart transplantation or durable mechanical circulatory support) and eligibility criteria for the VICTORIA trial to the GWTG-HF cohort.
Results:
Among 241,057 patients with EF<45% in the GWTG-HF registry, 221,730 (92%) could be candidates for vericiguat under the FDA label and 92,249 (38%) would have been eligible for VICTORIA. The most frequent reasons for ineligibility for the FDA label were eGFR<15 mL/min/1.73 m2 (5.7%) and dialysis (1.6%). Although there were more women and Black patients among patients in GWTG-HF, most clinical characteristics were qualitatively similar with patients enrolled in the VICTORIA trial. Among Medicare beneficiaries in GWTG-HF eligible for vericiguat by either FDA label or VICTORIA criteria, 12-month post-discharge rates of mortality (36–37%), HF hospitalization (33–35%), all-cause hospitalization (64–66%), and mean healthcare expenditure (US$25,106-$25,428) were high.
Conclusions:
Data from a large, contemporary US registry of patients actively hospitalized for HF with EF<45% suggest that approximately 4 in 10 patients meet the criteria of the VICTORIA trial and more than 9 of 10 patients are potential candidates for vericiguat based on the FDA label. Contemporary Medicare beneficiaries hospitalized for HFrEF and eligible for vericiguat face high rates of post-discharge mortality and readmission, and accrue substantial healthcare costs.
Keywords: vericiguat, heart failure, hospitalization, trial
CONDENSED ABSTRACT
Among 241,057 US patients hospitalized for HF with EF<45% in the Get With The Guidelines-Heart Failure registry, 92% would be candidates for vericiguat under the FDA label. Clinical characteristics, post-discharge outcomes, and healthcare expenditure were generally similar among the full registry HFrEF population, FDA label candidates, and patients enrolled in the VICTORIA trial, suggesting strong applicability of vericiguat to US clinical practice. Contemporary Medicare beneficiaries hospitalized for HFrEF and eligible for vericiguat face high rates of post-discharge mortality and readmission, and accrue substantial healthcare costs.
INTRODUCTION
Despite recent therapeutic advancements in heart failure with reduced ejection fraction (HFrEF), many patients continue to experience worsening HF events and face exceptionally high risk of subsequent death and worsening health status.(1–3) The VerICiguaT Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) demonstrated that vericiguat, a soluble guanylate cyclase (sGC) stimulator, reduced the risk of mortality and HF hospitalizations in patients with HFrEF who had recently been hospitalized or had received intravenous diuretics within 3 months of randomization on the background of GDMT.(4) The US Food and Drug administration (FDA) subsequently approved vericiguat in January 2021 for use in adults with symptomatic chronic HF and an ejection fraction less than 45% following a hospitalization for HF or the need for outpatient intravenous diuretics. Current American Heart Association/American College of Cardiology guidelines now give vericiguat a Class 2b (Level of Evidence: B-R) recommendation for use in selected high-risk patients already on GDMT who experience a recent worsening HF event.(5)
However, despite regulatory approval and inclusion within practice guidelines, limited data are available characterizing the applicability of the VICTORIA trial and vericiguat to US clinical practice. Data are especially limited among the high-risk subset of patients actively hospitalized for HFrEF, a population where approximately 1 in 4 patients die or are readmitted within 30 days of discharge.(6,7) Patients actively hospitalized for HFrEF were included within VICTORIA (11% of trial population), and studies across multiple GDMTs have consistently shown in-hospital initiation of medical therapies to be associated with substantial improvements in post-discharge medication use and patient outcomes.(4,8,9) Thus, understanding the representativeness of a VICTORIA trial eligibility criteria to patients actively hospitalized for HFrEF in US clinical practice, as well as the proportion eligible for vericiguat by the FDA label, could inform the potential impact of vericiguat in a care setting where implementation may be most effective. In this context, the objectives of the current study were to leverage a contemporary registry of US patients hospitalized for HFrEF to understand the proportion of patients who may be candidates for vericiguat, and to characterize the clinical profile, outcomes, and healthcare expenditure of eligible patients.
METHODS
Data Source:
These analyses used the Get With the Guidelines-Heart Failure (GWTG-HF) registry, which is an ongoing, observational, hospital-based, quality improvement registry formed by the American Heart Association in 2005.(10) The registry includes patients hospitalized for HF, and those who developed significant HF symptoms during hospitalization such that HF was the primary diagnosis. IQVIA (Parsippany, New Jersey) serves as the data collection and coordinating center for the American Heart Association’s GWTG programs, and the Duke Clinical Research Institute (Durham, NC) serves as the data analytic center. Baseline characteristics and subsequent data are collected via case report forms and includes demographics, medical history, laboratory, and biochemical data. The GWTG-HF protocol was approved by the institutional review boards at each participating center. In order to assess post-discharge outcomes and healthcare costs, participants 65 years of age and older with fee-for-service Medicare coverage were linked to Medicare data using a validated technique.(11)
Patient Population:
We identified adults age 18 years or older hospitalized for HF between January 1, 2014, and December 30, 2020, across 687 sites participating in the GWTG-HF registry. Only patients with non-missing information on left ventricular ejection fraction, eGFR, and systolic blood pressure were included. Patients who had ejection fraction (EF) <45%, left against medical advice, who transferred to an acute care facility, discharged to hospice, or had unknown discharge disposition were also excluded. The remaining group included patients hospitalized for HF with EF <45% and available data.
Study Cohorts:
Three study groups were identified within GWTG-HF: hospitalized HF with EF<45%, FDA label patients, and VICTORIA trial eligible patients. Criteria to define the FDA label for vericiguat included patients with eGFR ≥15 mL/min/1.73 m2, and excluded those with a history of dialysis, left ventricular assist device, or heart transplant. To define the VICTORIA trial eligible group, the strict trial inclusion and exclusion criteria from the VICTORIA trial were mapped to the GWTG-HF population with EF<45% in pre-specified fashion.(4) These specific criteria applied to GWTG-HF were: inclusions - systolic blood pressure ≥100 mm Hg, eGFR ≥15 mL/min/1.73m2; elevated natriuretic peptide concentration (N-terminal pro-B-type natriuretic peptide ≥1000 pg/mL if in sinus rhythm or ≥1600 pg/mL if atrial fibrillation, or B-type natriuretic peptide ≥300 pg/mL if in sinus rhythm or ≥500 pg/mL if atrial fibrillation); exclusions – discharge prescription of nitrates, left ventricular assist device, heart transplant or listed for heart transplant, dialysis therapy, no history of HF prior to hospitalization, intravenous inotrope therapy during index hospitalization, coronary revascularization or cardiac surgery during index hospitalization, non-compliance to HF medications, or limited life expectancy (as reason for not considering implantable cardioverter-defibrillator therapy). As all patients in GWTG-HF have an index HF hospitalization by definition, all patients met VICTORIA criteria for having a recent worsening HF event.
Outcomes:
In-hospital outcomes included in-hospital mortality, length of index hospital stay, and discharge disposition. Among patients ≥65 years old linked to Medicare fee-for-service claims and discharged alive, the following clinical outcomes were assessed at 30 days and 12 months post-discharge: all-cause mortality, all-cause readmission, HF readmission, and composite all-cause mortality or HF readmission. To assess associated healthcare expenditure, average Medicare Part A per-patient costs were calculated during index hospitalization, 30 days post-discharge, and 12 months post-discharge.
Statistical Analysis:
The proportion of hospitalized HF with EF<45% in GWTG-HF who met criteria for the 1) VICTORIA eligible group, and 2) the FDA label group were assessed. Patient characteristics were described and compared for all 3 GWTG-HF groups: VICTORIA eligible patients, FDA label patients, and hospitalized HF with EF<45%. Counts and percentages were presented for categorical/binary variables. Mean ±standard deviation or median (25th, 75th) were presented for continuous variables. Published data on patient characteristic and medication use from the VICTORIA trial were also juxtaposed for reference.(4,12)
Among GWTG-HF patients ≥65 years linked to CMS who were discharged alive, event rates for post-discharge clinical outcomes were separately assessed among patients in the overall hospitalized HF with EF<45% group, FDA label patients, and VICTORIA trial eligible patients. For all-cause readmission and HF readmission outcomes, the cumulative incidence function accounted for the competing risk of death. Additionally, cumulative incidence curves were generated to visualize each time-to-event endpoint for each patient population. To assess healthcare costs, total unadjusted payments made by Medicare (Part A) at 12 months were calculated as the claim payment amount plus the product of claim pass thru per diem amount and claim utilization day count. All costs were standardized to 2019 US dollar amounts (i.e., the last year of available cost data) using Market Basket Update and Productivity Adjustment published on the CMS website. For post-discharge costs, the cumulative costs were plotted in each patient group; increment of costs by time (number of days post discharge) was calculated as the sum of costs occurred on that day divided by the number of patients at risk at that time point. Cumulative costs at 12 months were averaged over the number of patients at risk, accounting for the competing risk of death and differential length of time observed. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Selection
Between January 1, 2014, and December 30, 2020, the GWTG-HF registry included 992,850 adults hospitalized for HF. Of this sample, 487,520 (49.1%) were excluded for EF ≥45% or missing, 157,109 (15.8%) were excluded due to missing data on eGFR or systolic blood pressure, and 37,164 (3.7%) were excluded due to having left against medical advice, discharged to another acute care facility, hospice care, or unknown discharge disposition (Figure 1). The remaining 241,057 (24.3%) patients comprised the group of hospitalized HF with EF<45% in GWTG-HF and served as 1 of the aforementioned 3 GWTG-HF groups for comparison.
Figure 1. Identifying the Proportion of Patients Hospitalized for Heart Failure with Ejection Fraction <45% that are Candidates for Initiation of Vericiguat in the Get With The Guidelines-Heart Failure Registry.
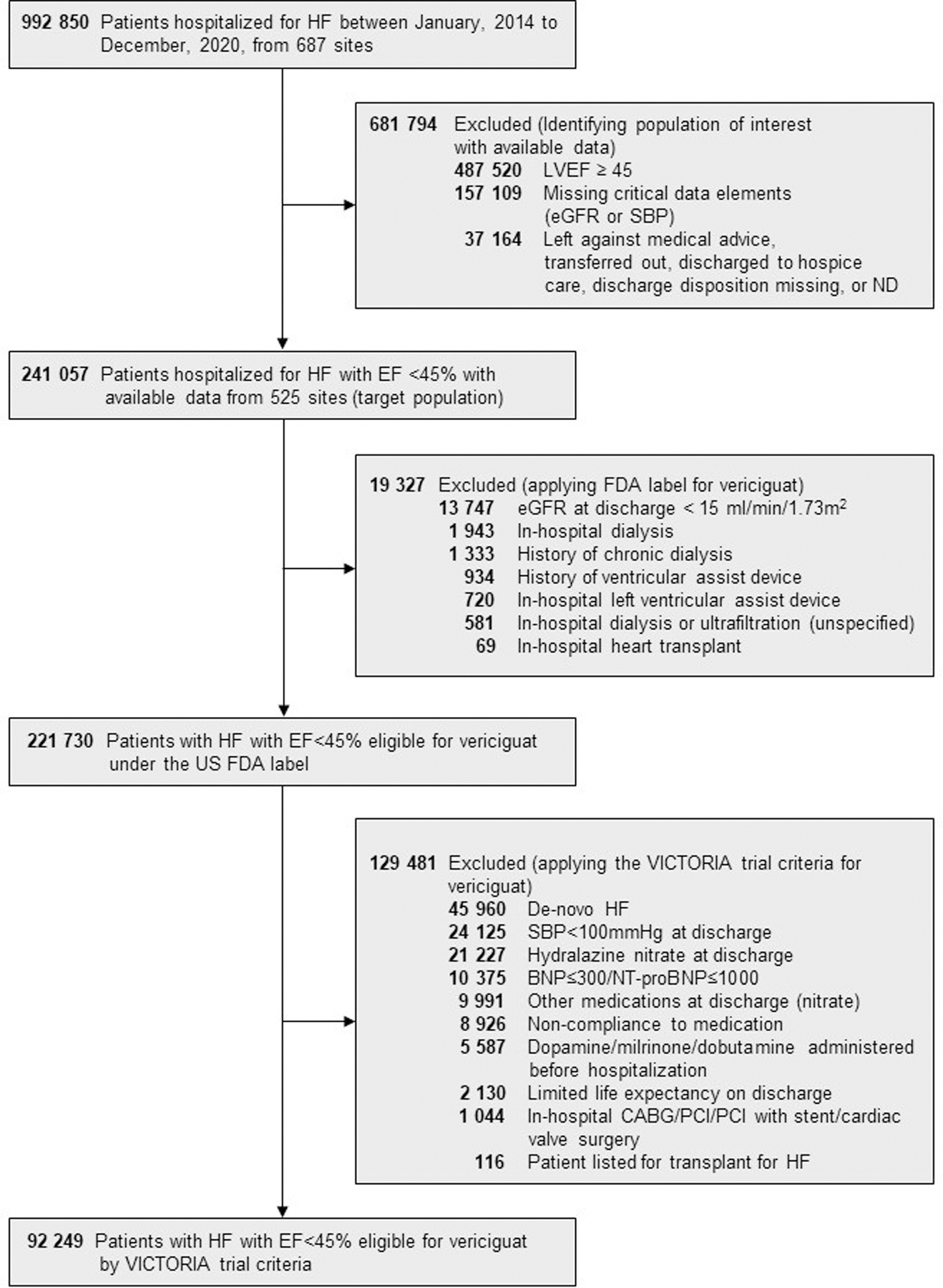
BNP=B-type natriuretic peptide; CABG=coronary artery bypass grafting; eGFR=estimated glomerular filtration rate; FDA=Food and Drug Administration; HF=heart failure; LVEF=left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCI=percutaneous coronary intervention; SBP=systolic blood pressure
Candidates for Vericiguat Initiation Based on FDA label and VICTORIA Trial Criteria
The frequency and percentage of patients hospitalized for HF with EF<45% within GWTG-HF who met the criteria for the FDA label group and VICTORIA eligible group were 221,730 (92.0%) and 92,249 (38.3%), respectively. The most common reasons for patients being excluded from the FDA label cohort was eGFR <15 mL/min/1.73 m2 (n=13,747; 5.7%), in-hospital dialysis (n=1,943; 0.8%), and history of chronic dialysis (n=1,333; 0.6%). The most common reasons patients were excluded from the VICTORIA trial group were no history of HF prior to admission (i.e., de novo HF) (n=45,960; 19.1%), patients being on nitrates (31,218; 12.9%), systolic blood pressure <100 mmHg (24,125; 10.0%), non-compliance to HF medications (8,926; 3.7%), and intravenous inotropes during index hospitalization (5,587; 2.3%).
Among patients with HF and EF<45% receiving background “triple therapy” with ACEI/ARB/ARNI, beta-blocker, and MRA (n=55,096; 22.9%), 54,392 (98.7%) were projected to meet criteria for the FDA label. This cohort of 54,392 patients receiving triple therapy and eligible for vericiguat comprised 22.6% of the full population hospitalized for HF with EF<45%.
Clinical Profiles of Participants in GWTG-HF and the VICTORIA Trial:
Patient characteristics by VICTORIA eligible patients, FDA Label patients, and the hospitalized HF with EF<45% population in GWTG-HF are shown in in Table 1, alongside characteristics of participants actually enrolled in the global VICTORIA trial (Central Illustration). Among GWTG-HF participants, demographic and clinical characteristics were generally similar among patients in the hospitalized HF with EF<45% group, FDA label candidates, and VICTORIA trial eligible patients, with few exceptions. Compared with the hospitalized HF with EF<45% population in GWTG-HF, GWTG-HF patients meeting FDA label criteria had higher eGFR (median [25th, 75th] eGFR 57 [39–77] versus 54 [35–75] mL/min/1.732). In comparisons between the population within GWTG-HF with EF<45% and VICTORIA trial eligible patients within GWTG-HF, patients meeting strict trial criteria were older (median [25th, 75th] age, 72 [61–82] years vs 69 [58–80] years), more likely to be White (65.2% vs 60.5%), and more likely to have history of atrial fibrillation (43.1% vs 36.8%). By comparison, patients recruited into the actual VICTORIA randomized clinical trial were younger, less likely to be Black, and were more likely to have an ICD (Table 1).
Table 1.
Patient Characteristics in the VICTORIA Trial and the GWTG-HF Registry
| Variable | VICTORIA Trial Population (RCT) | VICTORIA Eligible Patients (GWTG-HF) | FDA Label Patients (GWTG-HF) | Hospitalized HF with EF<45% (GWTG-HF) | Standardized Differences | ||
|---|---|---|---|---|---|---|---|
|
N=5,050 |
[A] N=92,249 |
[B] N=221,730 |
[C] N=241,057 |
[A] vs [B] | [A] vs [C] | [B] vs [C] | |
| Demographics | |||||||
| Age, median (25th, 75th) | 69 (60–76) | 72 (61 – 82) | 70 (58 – 80) | 69 (58 – 80) | 15.3 | 16.8 | 1.4 |
| Female | 2.7 | 2.4 | 0.4 | ||||
| Female | 1208 (23.9) | 33,459 (36.3) | 77,513 (35.0) | 84,710 (35.1) | |||
| Race/Ethnicity | 8.6 | 10.1 | 1.9 | ||||
| White | 3239 (64.1) | 60,152 (65.2) | 135,906 (61.3) | 145,757 (60.5) | |||
| Black | 249 (4.9) | 20,758 (22.5) | 57,402 (25.9) | 63,338 (26.3) | |||
| Hispanic | N/R | 7,287 (7.9) | 18,044 (8.1) | 20,175 (8.4) | |||
| Asian | 1,132 (22.4) | 1,392 (1.5) | 3,555 (1.6) | 4,177 (1.7) | |||
| Other | 430 (8.5) | 2,660 (2.9) | 6,823 (3.1) | 7,610 (3.2) | |||
| Insurance Status | 11.8 | 11.1 | 1.2 | ||||
| Medicare - Private/HMO/Other | N/R | 3,506 (4.1) | 12,179 (6.0) | 12,658 (5.7) | |||
| Medicare | N/R | 43,919 (51.9) | 95,581 (47.1) | 104,185 (47.3) | |||
| Medicaid | N/R | 14,537 (17.2) | 38,602 (19.0) | 42,356 (19.2) | |||
| Private/HMO/Other | N/R | 22,609 (26.7) | 56,761 (27.9) | 61,279 (27.8) | |||
| Medical History | |||||||
| Ejection fraction, % | 30 (23–35) | 28 (20 – 35) | 27 (20 – 35) | 27 (20 – 35) | 7.2 | 5.4 | 1.7 |
| AFib/Flutter medical history | 2269 (45.0) | 39,743 (43.1) | 80,693 (37.3) | 86,708 (36.8) | 11.9 | 13.0 | 1.0 |
| AFib/Flutter at presentation or during hospitalization | NR | 30,063 (32.6) | 64,765 (29.2) | 69,305 (28.8) | 7.3 | 8.3 | 1.0 |
| COPD or Asthma | 863 (17.1) | 31,974 (34.7) | 70,872 (32.8) | 76,766 (32.6) | 4.1 | 4.5 | 0.4 |
| Depression | N/R | 13,029 (14.1) | 30,502 (14.1) | 33,492 (14.2) | 0.1 | 0.2 | 0.3 |
| Prior MI | N/R | 25,016 (27.1) | 56,135 (25.9) | 61,671 (26.2) | 2.7 | 2.2 | 0.5 |
| Peripheral Vascular Disease | 630 (12.5) | 11,135 (12.1) | 24,264 (11.2) | 27,590 (11.7) | 2.7 | 1.2 | 1.6 |
| Hyperlipidemia | N/R | 54,101 (58.7) | 120,894 (55.9) | 132,226 (56.1) | 5.7 | 5.3 | 0.5 |
| Hypertension | 3,993 (79.1) | 77,903 (84.5) | 180,707 (83.5) | 197,509 (83.8) | 2.8 | 2.0 | 0.8 |
| Diabetes | 2,377 (47.1) | 42,386 (46.0) | 97,645 (44.6) | 109,143 (45.8) | 2.9 | 0.4 | 2.4 |
| Chronic kidney disease | N/R | 19,285 (20.9) | 43,315 (20.0) | 56,936 (24.2) | 2.3 | 7.7 | 10.0 |
| Dialysis, Chronic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8,580 (3.6) | 0.0 | 27.5 | 27.5 |
| Prior PCI | N/R | 21,034 (22.8) | 47,406 (21.9) | 52,057 (22.1) | 2.2 | 1.8 | 0.4 |
| Prior CABG | N/R | 21,382 (23.2) | 44,934 (20.8) | 49,286 (20.9) | 5.9 | 5.5 | 0.4 |
| CRT | 739 (14.7) | 10,428 (13.6) | 21,683 (11.6) | 23,511 (11.6) | 6.0 | 6.0 | 0.0 |
| Ischemic Etiology | N/R | 57,974 (62.9) | 125,103 (57.8) | 137,013 (58.1) | 10.4 | 9.8 | 0.7 |
| Smoking | N/R | 17,813 (19.3) | 49,802 (22.5) | 53,113 (22.0) | 7.8 | 6.7 | 1.0 |
| Discharge Vital Signs and Laboratory Values | |||||||
| Heart rate, bpm | 72 (64–81) | 77 (68 – 88) | 78 (69 – 88) | 78 (69 – 88) | 5.1 | 5.1 | 0.0 |
| Systolic blood pressure, mmHg | 119 (109–131) | 119 (109 – 132) | 117 (105 – 131) | 117 (105 – 132) | 18.3 | 16.1 | 1.9 |
| BMI, kg/m2 | 26.8 (23.7–30.9) | 27.8 (23.7 – 33.2) | 27.9 (23.7 – 33.5) | 27.8 (23.7 – 33.4) | 2.3 | 1.3 | 1.0 |
| eGFR, mL/min/1.73 | 58 (41–77) | 55 (39 – 75) | 57 (39 – 77) | 54 (35 – 75) | 4.0 | 8.3 | 11.9 |
| Potassium, mEq/L | 4.5 (4.2–4.8) | 4.0 (3.7 – 4.4) | 4.0 (3.7 – 4.4) | 4.1 (3.8 – 4.4) | 2.5 | 6.5 | 4.1 |
| BNP, pg/mL | 747.9 (452.4–1,340) | 1,248 (725 – 2,261) | 1,112 (562 – 2,118) | 1,160 (581 – 2,253) | 18.3 | 12.9 | 4.8 |
| NT-proBNP, pg/mL | 3,377 (1,992–6,380) | 6,738 (3,359 – 13,800) | 6,193 (2,888 – 13,062) | 6,663 (3,033 – 14,758) | 10.5 | 2.7 | 7.3 |
| Hospital Characteristics | |||||||
| Number of Beds | N/R | 381 (250 – 549) | 394 (259 – 581) | 394 (260 – 581) | 7.6 | 8.3 | 0.7 |
| Geographic Region | 5.4 | 5.6 | 0.3 | ||||
| West | N/R | 15,573 (16.9) | 40,766 (18.4) | 44,564 (18.5) | |||
| South | N/R | 30,762 (33.3) | 75,187 (33.9) | 81,631 (33.9) | |||
| Midwest | N/R | 18,838 (20.4) | 45,299 (20.4) | 49,188 (20.4) | |||
| Northeast | N/R | 27,070 (29.3) | 60,470 (27.3) | 65,664 (27.2) | |||
| Rural Location | N/R | 2,997 (3.2) | 6,276 (2.8) | 6,671 (2.8) | 2.4 | 2.8 | 0.4 |
| Teaching Status | N/R | 73,808 (81.0) | 179,596 (81.8) | 195,581 (82.0) | 2.2 | 2.6 | 0.4 |
| Heart Transplant Hospital | N/R | 5,506 (8.1) | 17,100 (10.5) | 19,711 (11.1) | 8.4 | 10.3 | 2.0 |
Data represent median (25th – 75th) or n (%).
Data reflect the proportion of patients within each group that were enrolled from various types of hospitals.
Abbreviations: EF, ejection fraction; BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HMO, health maintenance organization; ICD, implantable cardioverter-defibrillator; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Central Illustration. Applicability of Vericiguat to US Patients Hospitalized for HF with EF<45%.
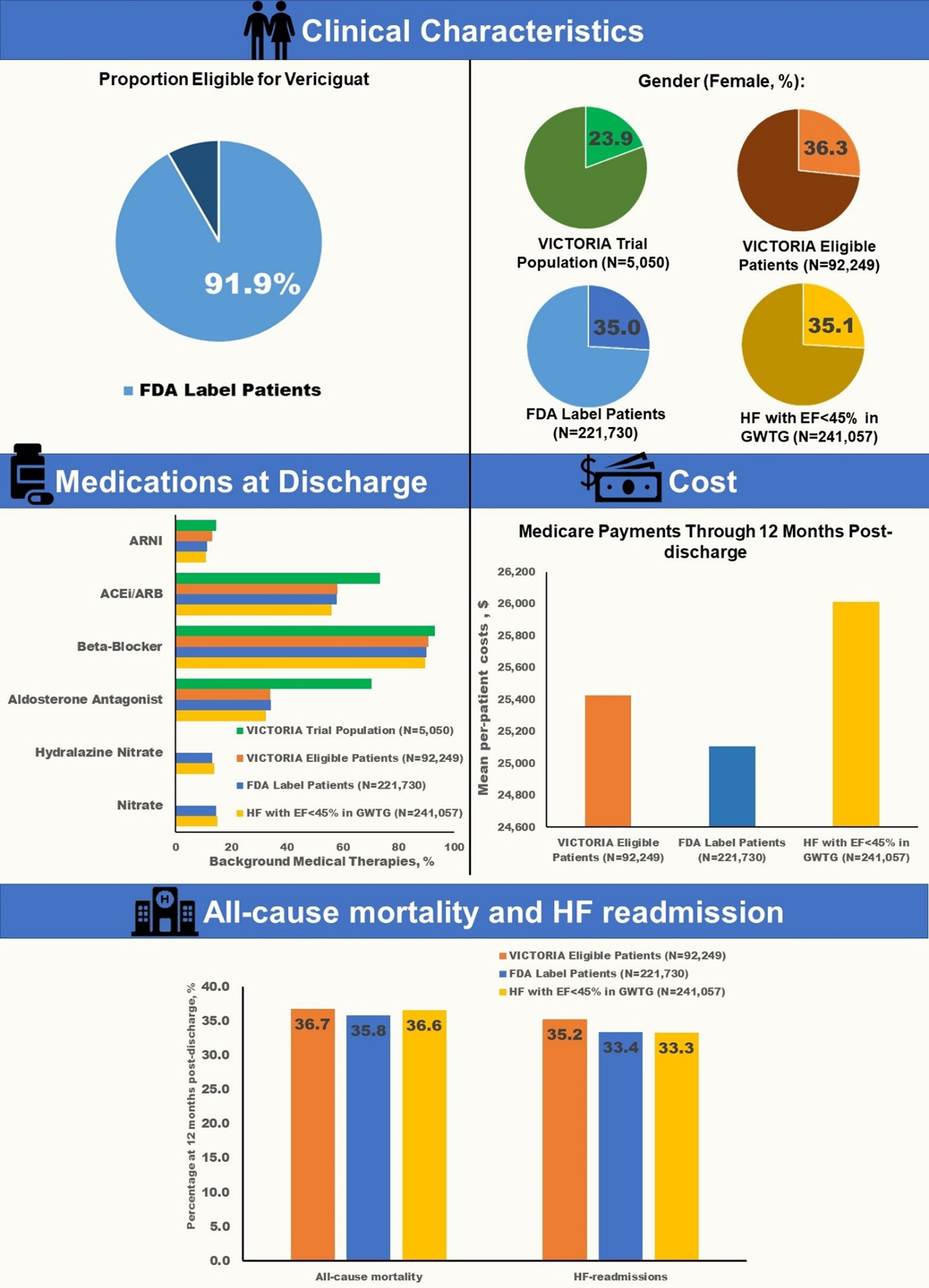
Clinical characteristics, clinical outcomes, and clinical outcomes among VICTORIA eligible patients, FDA label patients, the hospitalized HF with EF <45% population in GWTG-HF, as well as patients enrolled in the VICTORIA randomized trial. ARNI=Angiotensin receptor neprilysin inhibitor; ARB= Angiotensin receptor blocker; ACEI= Angiotensin converting enzyme inhibitor.
Medications prescribed to VICTORIA eligible patients, FDA label patients, and patients with hospitalized HF with EF<45% within GWTG-HF, as well as VICTORIA trial population, are shown in Figure 2. Approximately, one third of patients were on mineralocorticoid antagonist at discharge in each of the GWTG-HF groups (i.e., VICTORIA eligible patients, FDA label patients, and hospitalized HF with EF<45%), compared with 70% in the population enrolled in the VICTORIA randomized trial. Prescription rates of ARNI at discharge were less than 15% in all groups.
Figure 2. Background Medical Therapy in the VICTORIA Trial and GWTG-HF Registry.
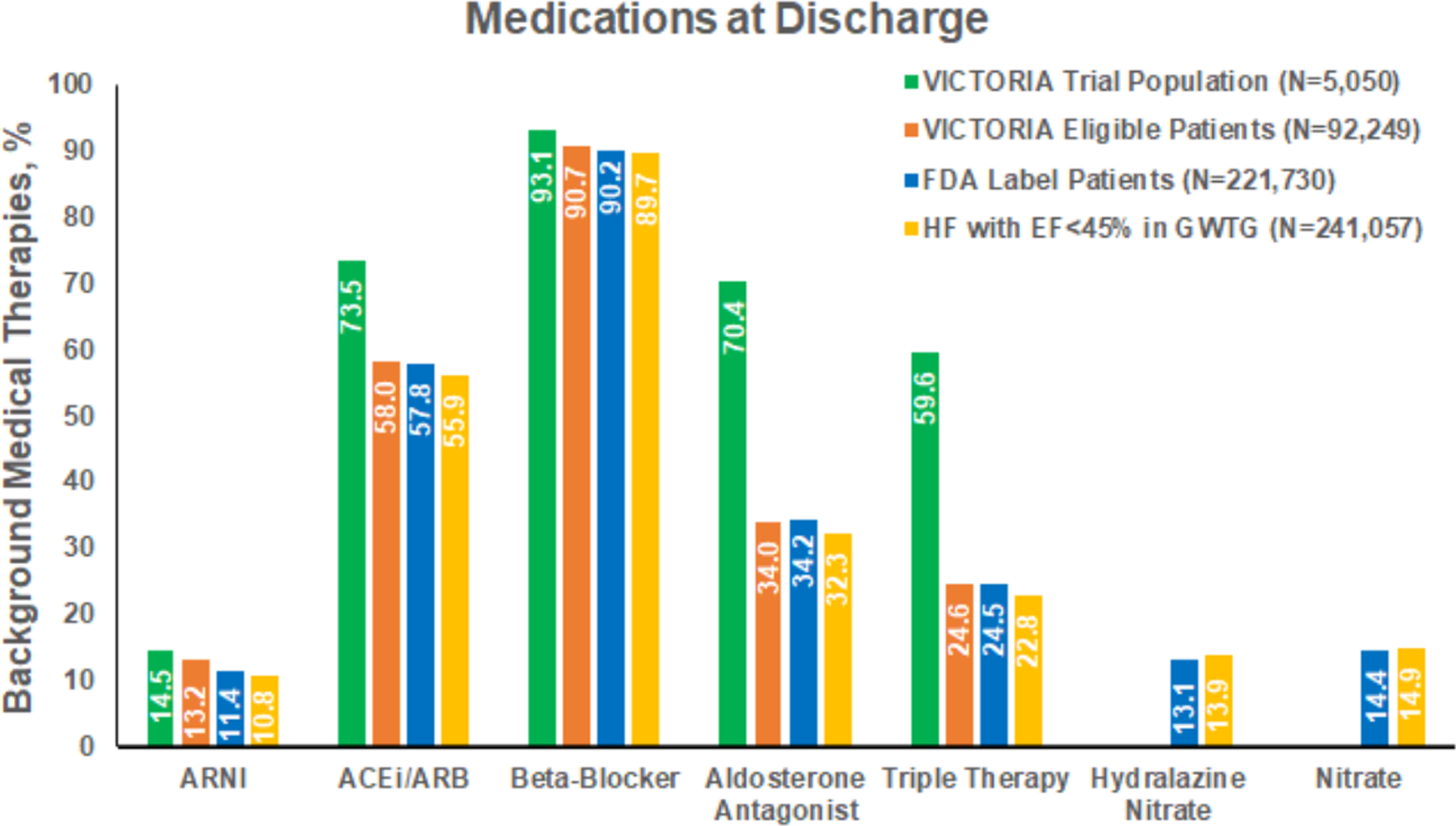
Shows the medications by VICTORIA eligible patients, FDA label patients, hospitalized HF with EF <45% population, and VICTORIA randomized trial population. Percentages represent proportion of patients prescribed these medications at discharge among all groups except VICTORA trial population where the percentages represent the proportion of patients who were on these medications at baseline.
In-hospital Outcomes:
Among the 3 GWTG-HF groups, data on in-hospital mortality, discharge disposition and length of stay are shown in Table 2. In-hospital mortality rates (2.8% vs 2.5% vs 3.0%) were similar across VICTORIA eligible patients, FDA label patients, and hospitalized HF with EF<45% in GWTG-HF, respectively. Median (interquartile range) length of stay was 4 (2–6) days in each group.
Table 2.
Clinical Outcomes of Patients Eligible for Vericiguat in GWTG-HF
| Outcomes | VICTORIA Eligible Patients (GWTG-HF)* |
FDA Label Patients (GWTG-HF)* |
Hospitalized HF with EF<45% (GWTG-HF)* |
|---|---|---|---|
| In-Hospital Outcomes * | |||
| Discharge Disposition | |||
| Home | 75,494 (81.8) | 184,630 (83.3) | 198,893 (82.5) |
| Other Health Care Facility | 14,139 (15.3) | 31,629 (14.3) | 35,025 (14.5) |
| Expired | 2,616 (2.8) | 5,471 (2.5) | 7,139 (3.0) |
| In-hospital Death | 2,616 (2.8) | 5,471 (2.5) | 7,139 (3.0) |
| Length of Stay, days | |||
| Median (25th, 75th) | 4 (2 – 6) | 4 (2 – 6) | 4 (2 – 6) |
| Mean (SD) | 4.5 (4.0) | 4.9 (4.5) | 5.0 (4.9) |
| 30-day Endpoints * | |||
| All-cause mortality | 890 (5.7) | 2,192 (6.2) | 2,448 (6.5) |
| All-cause readmission | 3,402 (22.0) | 7,674 (21.6) | 8,352 (22.1) |
| HF readmission | 1,407 (9.1) | 3,219 (9.1) | 3,466 (9.2) |
| Mortality or HF readmission | 2,113 (13.6) | 4,995 (14.0) | 5,464 (14.4) |
| 12-month Endpoints * | |||
| All-cause mortality | 5,703 (36.73) | 12,751 (35.80) | 13,872 (36.55) |
| All-cause readmission | 10,114 (66.16) | 22,497 (64.23) | 24,236 (64.90) |
| HF readmission | 5,374 (35.23) | 11,660 (33.37) | 12,406 (33.29) |
| Mortality or HF readmission | 8,586 (55.30) | 19,019 (53.40) | 20,518 (54.06) |
For in-hospital outcomes, the denominators used for the three groups were 92,249, 221,730 and 241,057 respectively. For 30-day and 12-month outcome analyses, the demoniators for the three groups were 15,527, 35,614, and 37,952. #FDA= Food and Drug Administration; GWTG= Get With The Guidelines; HFrEF= Heart Failure with Reduced Ejection Fraction; SD= Standard Deviation; HF= Heart Failure.
Post-discharge Outcomes:
Among patients age ≥65 years linked to Medicare, 12-month mortality (36.7% vs 35.8% vs 36.6%), HF hospitalization (35.2% vs 33.3% vs 33.2%), and all-cause hospitalization (66.2% vs 64.2% vs 64.9%) were similar across VICTORIA eligible patients, FDA label patients, and the hospitalized HF with EF<45% population, respectively. Similar consistency was observed for 30-day endpoints (Table 2). Figure 3 shows the cumulative incidence plots for the 3 cohorts for all-cause mortality, all-cause readmission, and HF readmission.
Figure 3. Post-discharge Outcomes of Heart Failure Patients Eligible for Vericiguat.
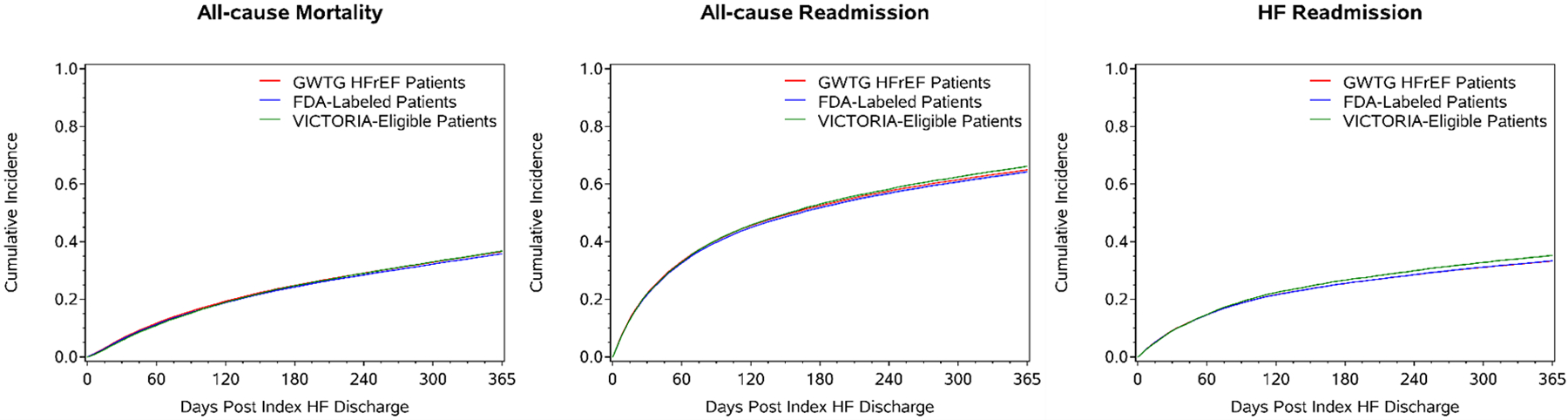
Shows the cumulative incidence plots of all-cause mortality, all cause readmission and heart failure admission for the 3 cohorts: hospitalized HF with EF<45% population, FDA label and VICTORIA eligible patients. The 3 cohorts are represented by red, blue and green colors respectively.
Medicare Expenditures:
Among GWTG-HF patients ≥65 years linked to CMS, cost during index admission, cost at 30 days’ post discharge and cost at 12-months post index discharge for the hospitalized HF with EF<45% population, FDA label patients, and VICTORIA eligible patients are shown in Table 3 and Figure 4. There were modest differences in mean per-patient cost during index admission across groups (US$14,236 vs $13,677 vs $12,969), with the hospitalized HF with EF<45% group having highest costs and VICTORIA eligible patients having lowest costs. During the post-discharge period, mean cost though 30 days (US$5,066 vs $4,928 vs $4,827), and 12 months (US$26,013 vs $25,106 vs $25,428) were qualitatively similar across all 3 groups.
Table 3.
Medicare Part A Payments in 2019 US Dollars for Patients Eligible for Vericiguat
| All discharged alive patients | All readmitted patients | |||||
|---|---|---|---|---|---|---|
| VICTORIA Eligible Patients (GWTG-HF) |
FDA Label Patients (GWTG-HF) |
Hospitalized HF with EF<45% (GWTG-HF) |
VICTORIA Eligible Patients (GWTG-HF) |
FDA Label Patients (GWTG-HF) |
Hospitalized HF with EF<45% (GWTG-HF) |
|
| (N=15,527) | (N=35,614) | (N=37,952) | (N=10,114) | (N=22,497) | (N=24,236) | |
| Cost during index admission | ||||||
| Mean | 12,969 | 13,677 | 14,236 | 12,974 | 13,661 | 14,298 |
| Std Dev | 12,133 | 13,751 | 16,165 | 11,885 | 13,154 | 16,159 |
| Median | 9,012 | 9,355 | 9,524 | 9,091 | 9,434 | 9,642 |
| 10th Pctl | 4,658 | 4,740 | 4,801 | 4,734 | 4,802 | 4,883 |
| Q1 | 6,290 | 6,471 | 6,637 | 6,393 | 6,588 | 6,803 |
| Q3 | 14,924 | 15,743 | 16,111 | 14,918 | 15,836 | 16,242 |
| 90th Pctl | 26,443 | 27,481 | 28,114 | 26,333 | 27,193 | 27,875 |
| 99th Pctl | 58,425 | 66,660 | 71,396 | 57,090 | 64,796 | 70,113 |
| Cost at 30d post index discharge | ||||||
| Mean | 4,827 | 4,928 | 5,066 | 7,241 | 7,647 | 7,779 |
| Std Dev | 17,088 | 18,453 | 18,412 | 20,454 | 22,620 | 22,439 |
| Median | 0 | 0 | 0 | 0 | 0 | 0 |
| 10th Pctl | 0 | 0 | 0 | 0 | 0 | 0 |
| Q1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q3 | 0 | 0 | 0 | 9,304 | 9,466 | 9,696 |
| 90th Pctl | 15,243 | 15,139 | 15,607 | 21,409 | 22,168 | 22,459 |
| 99th Pctl | 57,696 | 59,753 | 60,956 | 69,694 | 73,055 | 73,765 |
| Cost at 1y post index discharge | ||||||
| Mean | 25,428 | 25,106 | 26,013 | 38,675 | 39,379 | 40,377 |
| Std Dev | 41,626 | 43,229 | 44,167 | 46,173 | 48,828 | 49,636 |
| Median | 11,036 | 10,278 | 10,746 | 24,514 | 24,480 | 25,164 |
| 10th Pctl | 0 | 0 | 0 | 6,790 | 6,793 | 6,934 |
| Q1 | 0 | 0 | 0 | 11,254 | 11,246 | 11,521 |
| Q3 | 34,172 | 33,183 | 34,365 | 49,356 | 49,671 | 51,034 |
| 90th Pctl | 68,104 | 67,487 | 69,537 | 86,368 | 85,841 | 88,478 |
| 99th Pctl | 184,530 | 198,008 | 202,931 | 218,404 | 241,490 | 247,191 |
PCTL=Percentile; STD Dev= Standard Deviation; D= Days; Y=Year; FDA= Food and Drug Administration; GWTG= Get With The Guidelines; HFrEF= Heart Failure with Reduced Ejection Fraction
Figure 4. Healthcare Expenditure of Heart Failure Patients Eligible for Vericiguat.
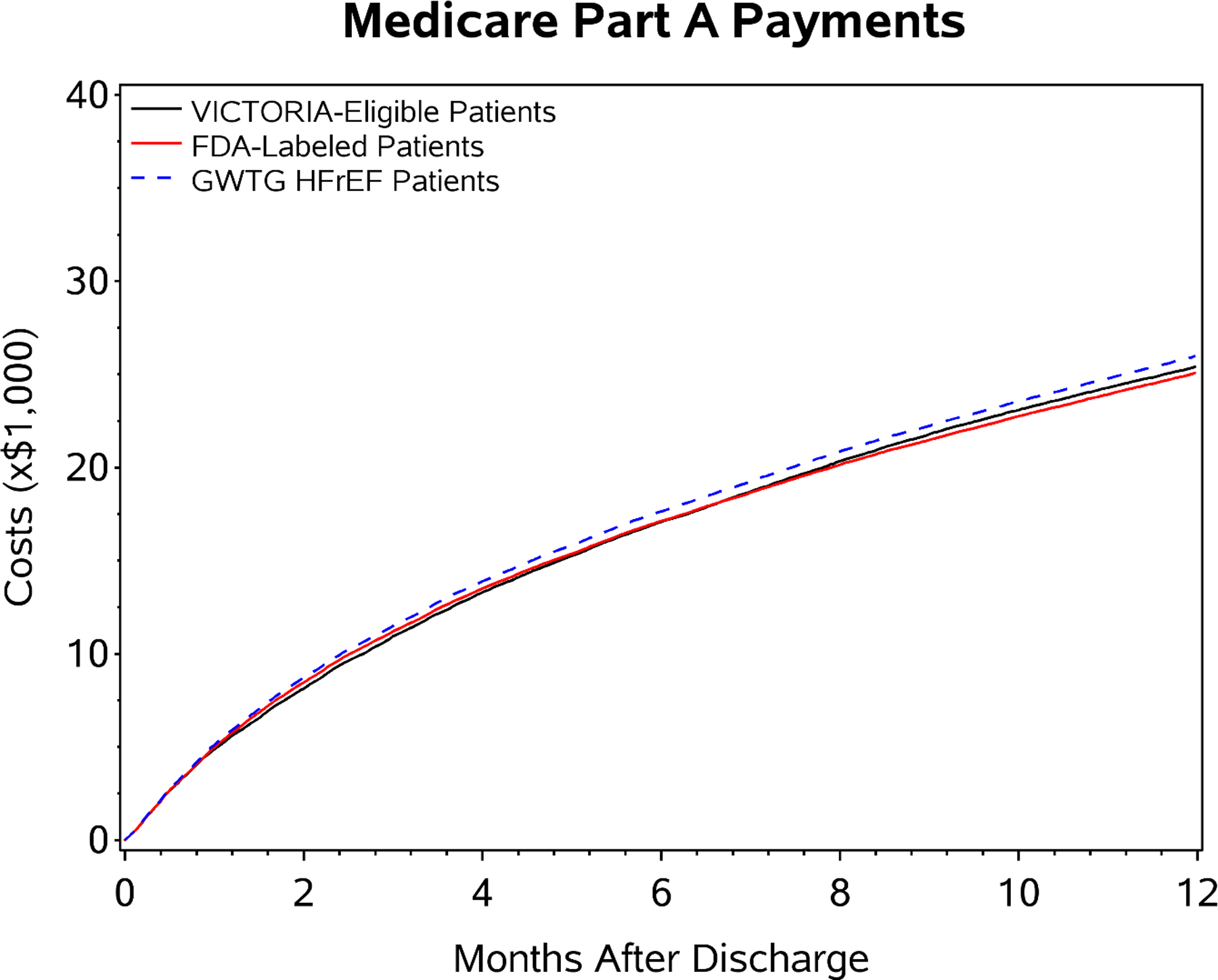
Graph depicts cumulative costs in each patient group divided by number of patients at risk at that timepoint accounting for competing risk of death. Mean per-patient cost over 12-months post-discharge are shown for the hospitalized HF with EF<45% population (blue), FDA label patients (red), and VICTORIA eligible patients (black), respectively.
DISCUSSION
In a contemporary registry cohort of patients hospitalized for HFrEF in the United States, more than 9 of 10 patients may be candidates for vericiguat based on the FDA label, and approximately 4 of 10 were estimated to meet eligibility criteria for the VICTORIA trial. The most common reason for not meeting FDA label criteria was eGFR <15 mL/min, whereas most common reasons for patients being ineligibile for the VICTORIA trial were no history of HF prior to admission, concurrent nitrate therapy, and systolic blood pressure <100 mmHg. Patients meeting and not meeting VICTORIA trial eligibility criteria had generally similar baseline characteristics, supporting generalizability of the FDA label and the VICTORIA trial to US clinical practice. Among patients age ≥65 years linked to Medicare, 12-month mortality, HF hospitalization, and all-cause hospitalization were also similar across VICTORIA eligible patients, FDA label patients, and the hospitalized HF with EF<45% population in GWTG-HF. Of note, 12-month mortality and HF hospitalizations were well above 30% in all groups, indicating that contemporary Medicare beneficiaries hospitalized for HFrEF and eligible for vericiguat face high post-discharge risk of adverse clinical outcomes.
Multiple prior studies have evaluated the representativeness of clinical trial populations in real-world clinical practice. For instance, approximately 7 of every 10 individuals hospitalized for HFrEF may meet criteria for in-hospital initiation of sacubitril/valsartan, while 8 out of every 10 individuals would be candidates for dapagliflozin use in HFrEF based on the FDA label.(13,14) A recent analysis of outpatients with HFrEF from the PINNACLE registry showed that 1 in 4 HFrEF patients met the VICTORIA trial inclusion criterion of worsening HF event, and that characteristics of this subpopulation were similar to those enrolled in VICTORIA.(15) Our results also support broad applicability of vericiguat to US patients hospitalized for HF, as more than 90% of patients hospitalized for HF with EF<45% in GWTG-HF were candidates for vericiguat based on the FDA label. These data also once again highlight differences between clinically actionable criteria within regulatory labels for medical therapies versus the strict inclusion and exclusion within randomized clinical trials. By comparison with prior analyses of trial eligibility criteria within the GWTG-HF population, the 38% of patients meeting strict selection criteria for the VICTORIA trial was comparable to the proportion meeting DAPA-HF trial criteria (44%), and higher than the proportion meeting criteria for the PIONEER-HF trial (21%).(13,14)
The VICTORIA trial was unique from many other HFrEF trials in that it included higher-risk patients who had experienced a recent worsening HF event and allowed enrollment with severe kidney disease with eGFR as low as 15 mL/min/1.73 m2.(4) In the context of these selection criteria, it is notable that across most key patient characteristics, the profile of patients enrolled in the VICTORIA trial was consistent with participants in the GWTG-HF registry. For example, median EF, systolic blood pressure, eGFR and comorbidities such as diabetes, hypertension and peripheral vascular disease were similar among the groups. Nonetheless, some differences did exist. Specifically, patients enrolled in the VICTORIA trial were younger, less likely to be Black, and more likely to have an ICD and MRA prescription compared with the GWTG-HF cohort.
Among all 3 groups of GWTG-HF participants, we observed consistently high rates of post-discharge mortality and hospitalization, as well as healthcare expenditure. However, aside from consistency across groups, the absolute magnitude of the event rates and costs supports the substantial unmet need in improving care for these patients. Specifically, whether by VICTORIA trial or FDA label criteria, more than 35% of patients who would have been eligible for vericiguat died within 12 months of discharge. More than 1 out of every 2 patients experienced death or HF readmission during that same timeframe. These event rates were considerably higher than those observed in the VICTORIA trial itself, where annualized rates of death or HF hospitalization were 40.1 events per 100 patient-years.(4) This difference in event rate suggests that despite application of trial eligibility criteria and some relative similarities in clinical characteristics between the VICTORIA trial and GWTG-HF populations (e.g., blood pressure, diabetes, atrial fibrillation), patients hospitalized for HF with EF<45% in routine clinical practice who are candidates for vericiguat have particularly poor prognosis.
Current data from US clinical practice highlight the need for both rapid sequence in-hospital initiation of quadruple medical therapy for HFrEF (i.e., ARNI, beta-blocker, MRA, SGLT2i) as tolerated, as well as consideration of in-hospital initiation of novel therapies such as vericiguat to further reduce clinical risk.(8,16) In our current analysis, we observed that only a third of patients were prescribed MRA, and less than 15% of the patients were on ARNI at discharge. Considering the magnitude and early onset of benefit with each of the mortality-reducing medications for HFrEF, maximizing use of these therapies among eligible patients by time of hospital discharge will be paramount to improving post-discharge outcomes.(8,9) However, it is also important to emphasize that some patient subsets face more difficult challenges with tolerability or eligibility for components of quadruple medical therapy (e.g., stage IV-V chronic kidney disease, low blood pressure). Among these high-risk subsets, additional therapeutic options proven to be both efficacious and well tolerated are needed. Moreover, it is important to acknowledge the residual risk of death and hospitalization that persists, even in the setting of complete provision of quadruple medical therapy.(17) In this context, vericiguat has been shown to reduce the incidence of death from cardiovascular causes or hospitalization for HF by an absolute event-rate reduction of 4.2 events per 100 patient-years with a number needed to treat of 24 patients, incremental to background use of GDMT.(18) If these benefits of vericiguat in VICTORIA were fully realized in the current GWTG-HF Medicare population, the higher absolute event rates in clinical practice suggest that the number needed to treat to prevent one cardiovascular death or hospitalization for HF might be even lower. Furthermore, nearly 9 out of 10 individuals in the VICTORIA trial were adherent to the 10 mg dose of vericiguat.(4) These trial data, in combination with broad generalizability to US clinical practice and high absolute event rates seen here, suggest that early adoption of vericiguat could have a sizeable impact on improving outcomes among patients hospitalized for HFrEF, in addition to focused efforts to maximize use and dosing of background quadruple medical therapy as tolerated.
Limitations
Limitations of this study should be considered. First, while previous studies have suggested that patients enrolled in GWTG-HF have similar demographics and clinical characteristics to real world clinical practice cohorts, hospital participation in GWTG-HF is voluntarily and the registry may not entirely represent all patients receiving care across all hospitals in the United States.(19) Second, despite comprehensive review of data elements and definitions in GWTG-HF and the VICTORIA trial selection criteria, and pre-specified inclusion of only those variables with reasonable alignment between datasets, we cannot exclude the potential influence of differing variable definitions across datasets. Likewise, some exclusion criteria in the VICTORIA trial (e.g., concurrent use of phosphodiesterase type 5 inhibitors) are not captured in GWTG-HF and could not be incorporated within current estimates of US patients eligible for the VICTORIA trial. Third, in our primary analyses of determining eligibility, a requirement that patients were already receiving each foundational component of GDMT was not applied. In contrast with the VICTORIA trial, background use of core GDMTs was substantially lower in GWTG-HF. The current analysis does not inform appropriate sequencing of vericiguat with other GDMTs, and should not suggest that vericiguat initiation be prioritized above pillars of quadruple medical therapy (all of which have Class I, level of evidence A guideline recommendations). Fourth, current data regarding proportion of patients eligible for vericiguat should be interpreted in the context of estimates reflecting data collected near time of discharge from a HF hospitalization. It is possible that vericiguat eligibility status could change during the early post-discharge period, and criteria from the VICTORIA trial support eligibility extending for up to 6 months following a HF hospitalization. Lastly, the clinical outcomes and expenditure analyses were limited to Medicare beneficiaries age 65 years or older, and the extent to which these results are generalizable to younger patients is unclear.
Conclusions
In conclusion, these data from a large, contemporary US registry suggest that more than 9 of 10 patients actively hospitalized for HF with EF<45% may be candidates for vericiguat based on the FDA label, and approximately 4 of 10 patients based on criteria for the VICTORIA trial. Although there were more women and Black patients among patients in GWTG-HF, most clinical characteristics were qualitatively similar with patients enrolled in the VICTORIA trial, further supporting generalizability of the FDA label and the VICTORIA trial to US clinical practice. Patients hospitalized for HFrEF and eligible for vericiguat face high absolute rates of post-discharge mortality, readmission, and healthcare expenditure, supporting the urgent need to improve in-hospital implementation of quadruple medical therapy for HFrEF as tolerated, as well as added consideration for in-hospital initiation of vericiguat.
PERSPECTIVES.
Competency in Patient Care:
Data from a large, contemporary US registry suggest that more than 9 out of 10 patients hospitalized for HF with EF<45% may be candidates for vericiguat based on the FDA label.
Competency in Medical Knowledge:
Within GWTG-HF, clinical characteristics, post-discharge outcomes, and healthcare expenditure were generally similar among the hospitalized HF with EF<45% population, FDA label candidates, and VICTORIA trial eligible patients. Clinical characteristics of GWTG-HF participants were also qualitatively similar to patients enrolled in the global VICTORIA trial, suggesting strong generalizability of vericiguat to US clinical practice.
Translational Outlook:
Patients hospitalized for HF with EF<45% and eligible for vericiguat face high absolute rates of post-discharge mortality, readmission, and healthcare expenditure. Data from the VICTORIA trial, in combination with broad applicability to US clinical practice and high absolute event rates seen in the current study, suggest that adoption of vericiguat could have a sizeable impact on improving outcomes among patients hospitalized for HFrEF, in addition to focused efforts to maximize use and dosing of background quadruple medical therapy as tolerated.
FUNDING SOURCES
This study was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The Get With The Guidelines–Heart Failure (GWTG-HF) program is provided by the American Heart Association and sponsored, in part, by Novartis, Boehringer Ingelheim and Eli Lilly Diabetes Alliance, Novo Nordisk, Sanofi, AstraZeneca, and Bayer.
ABBREVIATIONS
- EF
ejection fraction
- FDA
Food and Drug Administration
- GWTG-HF
Get With The Guidelines Heart Failure
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- sGC
soluble guanylate cyclase
- VICTORIA
VerICiguaT Global Study in Subjects with Heart Failure with Reduced Ejection Fraction
Footnotes
DISCLOSURES
Drs. Lautsch and Hilkert, are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Dr. Allen reports grant support from NIH and PCORI, and consulting fees from ACI Clinical, American Heart Association, Boston Scientific, Cytokinetics, Novartis, and UpToDate. Dr. Fonarow reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Janssen, Medtronic, Merck Sharp & Dohme LLC (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), and Novartis. Dr. Albert reports grant support from Novartis and AstraZeneca and consulting fees from Boston Scientific, Boehringer Ingelheim/Lilly, Cytokinetics, and Merck Sharp & Dohme LLC (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA). Dr. Butler has served as a consultant to Abbott, Adrenomed, Arena Pharma, Array, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Cardior, CVRx, Eli Lilly, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Sequana Medical, V-Wave Limited, and Vifor. Dr. Greene has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association (#929502), National Heart Lung and Blood Institute, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck Sharp & Dohme LLC (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim/ Lilly, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, and Sanofi; serves as a consultant for Amgen, Bayer, Boehringer Ingelheim/ Lilly, Bristol Myers Squibb, CSL Vifor, Corteria Therapeutics, Merck Sharp & Dohme LLC (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), PharmaIN, Roche Diagnostics, Sanofi, Tricog Health, and Urovant Pharmaceuticals; and has received speaker fees from Boehringer Ingelheim and Cytokinetics. All other authors report no disclosures.
REFERENCES
- 1.Greene SJ, Mentz RJ, Felker GM. Outpatient Worsening Heart Failure as a Target for Therapy: A Review. JAMA Cardiol 2018;3:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler J, Yang M, Manzi MA et al. Clinical Course of Patients With Worsening Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2019;73:935–944. [DOI] [PubMed] [Google Scholar]
- 3.Okumura N, Jhund PS, Gong J et al. Importance of Clinical Worsening of Heart Failure Treated in the Outpatient Setting: Evidence From the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Circulation 2016;133:2254–62. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong PW, Pieske B, Anstrom KJ et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263–e421. [DOI] [PubMed] [Google Scholar]
- 6.Greene SJ, Triana TS, Ionescu-Ittu R et al. Patients Hospitalized for De Novo Versus Worsening Chronic Heart Failure in the United States. J Am Coll Cardiol 2021;77:1023–1025. [DOI] [PubMed] [Google Scholar]
- 7.Shah KS, Xu H, Matsouaka RA et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 8.Greene SJ, Butler J, Fonarow GC. In-hospital initiation of quadruple medical therapy for heart failure: making the post-discharge vulnerable phase far less vulnerable. Eur J Heart Fail 2022;24:227–229. [DOI] [PubMed] [Google Scholar]
- 9.Rao VN, Murray E, Butler J et al. In-Hospital Initiation of Sodium-Glucose Cotransporter-2 Inhibitors for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2021;78:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smaha LA, American Heart A. The American Heart Association Get With The Guidelines program. Am Heart J 2004;148:S46–8. [DOI] [PubMed] [Google Scholar]
- 11.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pieske B, Patel MJ, Westerhout CM et al. Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail 2019;21:1596–1604. [DOI] [PubMed] [Google Scholar]
- 13.Fudim M, Sayeed S, Xu H et al. Representativeness of the PIONEER-HF Clinical Trial Population in Patients Hospitalized With Heart Failure and Reduced Ejection Fraction. Circ Heart Fail 2020;13:e006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaduganathan M, Greene SJ, Zhang S et al. Applicability of US Food and Drug Administration Labeling for Dapagliflozin to Patients With Heart Failure With Reduced Ejection Fraction in US Clinical Practice: The Get With the Guidelines-Heart Failure (GWTG-HF) Registry. JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler J, Djatche LM, Lautsch D, Yang L, Patel MJ, Mentz RJ. Representativeness of the VICTORIA Trial Population in Clinical Practice: Analysis of the PINNACLE Registry. J Card Fail 2021;27:1374–1381. [DOI] [PubMed] [Google Scholar]
- 16.Greene SJ, Ezekowitz JA, Anstrom KJ et al. Medical Therapy During Hospitalization for Heart Failure With Reduced Ejection Fraction: The VICTORIA Registry. J Card Fail 2022;28:1063–1077. [DOI] [PubMed] [Google Scholar]
- 17.Greene SJ, Fonarow GC, Butler J. Risk Profiles in Heart Failure: Baseline, Residual, Worsening, and Advanced Heart Failure Risk. Circ Heart Fail 2020;13:e007132. [DOI] [PubMed] [Google Scholar]
- 18.Butler J, Anstrom KJ, Armstrong PW. Comparing the Benefit of Novel Therapies Across Clinical Trials: Insights From the VICTORIA Trial. Circulation 2020;142:717–719. [DOI] [PubMed] [Google Scholar]
- 19.Curtis LH, Greiner MA, Hammill BG et al. Representativeness of a National Heart Failure Quality-of-Care Registry Comparison of OPTIMIZE-HF and Non-OPTIMIZE-HF Medicare Patients. Circ-Cardiovasc Qual 2009;2:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]


