Abstract
Long non-coding RNAs (lncRNAs) outnumber protein-coding transcripts, but their functions remain largely unknown. In this Review, we discuss the emerging roles of lncRNAs in the control of gene transcription. Some of the best characterized lncRNAs have essential transcription cis-regulatory functions that cannot be easily accomplished by DNA-interacting transcription factors, such as XIST, which controls X-chromosome inactivation, or imprinted lncRNAs that direct allele-specific repression. A growing number of lncRNA transcription units, including CHASERR, PVT1 and HASTER (also known as HNF1A-AS1) act as transcription-stabilizing elements that fine-tune the activity of dosage-sensitive genes that encode transcription factors. Genetic experiments have shown that defects in such transcription stabilizers often cause severe phenotypes. Other lncRNAs, such as lincRNA-p21 (also known as Trp53cor1) and Maenli (Gm29348) contribute to local activation of gene transcription, whereas distinct lncRNAs influence gene transcription in trans. We discuss findings of lncRNAs that elicit a function through either activation of their transcription, transcript elongation and processing or the lncRNA molecule itself. We also discuss emerging evidence of lncRNA involvement in human diseases, and their potential as therapeutic targets.
Introduction
In the mid-1980s, transcripts that lacked obvious open reading frames were identified in the Drosophila melanogaster bithorax locus1. At the time, the authors of the study hypothesized that these transcripts might be by-products of enhancer activity, that their transcription could regulate adjacent genes, that they might regulate splicing or translation in trans, or that they might encode atypical polypeptides. Others speculated that bithorax non-coding RNAs promote or inhibit the activity of nearby enhancers2. Decades later, these conjectures have been proven to be remarkably insightful, as careful analyses of individual long non-coding RNAs (lncRNAs) have largely confirmed each of these models.
lncRNAs are defined as transcripts that are longer than 500 nucleotides and do not encode proteins3. In practice, this is a catch-all definition that encompasses different types of transcripts that do not have an obvious protein-coding potential, and some lncRNAs defined in this fashion have turned out to encode micropeptides4,5. The number of annotated lncRNAs has been growing steadily, and currently includes more than 20,000 lncRNAs6, most of which have no known function. A relatively small number of lncRNAs, however, has been linked to a wide range of biological processes through disparate molecular mechanisms3.
Many of the best characterized lncRNAs have been implicated in the regulation of gene transcription7,8, often through defined molecular interactions. These studies have raised several crucial questions. Importantly, are there lncRNA types that execute distinct regulatory functions? Considering our current models of gene transcription, which are largely shaped by our understanding of how regulatory protein complexes are recruited to cis-acting DNA elements, what is the purpose of lncRNAs that regulate gene transcription? Which functions do lncRNAs carry out that cannot be easily enacted by DNA-interacting proteins, and what are the underlying mechanisms?
In this Review, we discuss the accumulating evidence that points to major modes through which lncRNAs participate in the regulation of gene transcription. For the purpose of this Review, we have considered lncRNA transcription units regardless of whether their regulatory function is conferred by the lncRNA molecules, the process of lncRNA transcription or the lncRNA promoter. Given that experimental perturbations of lncRNAs can theoretically disrupt other established transcription-regulating components, we specifically focused on lncRNA functions that cannot be easily ascribed to conventional enhancers, promoters or regulatory proteins. We emphasize functions and mechanisms that have been shown to operate at more than one lncRNA locus, in particular those that have been supported through orthogonal experimental tools. We discuss lncRNAs that act in cis or in trans to promote gene transcription, and lncRNA loci that act as cis-regulatory elements to stabilize the transcription of genes encoding transcription factors (TFs), or as allele-specific repressors. Finally, we discuss how our understanding of lncRNA function is helping unravel the impact of non-coding genome variation in human diseases, and the potential of lncRNAs as therapeutic targets.
Transcription activation in cis by lncRNAs
Genes with complex expression patterns are controlled by enhancers and other DNA elements located in their surrounding genomic regions, which often extend to hundreds of thousands of base pairs. These regulatory domains adopt 3D configurations known as topologically associating domains (TADs), which enable enhancers to gain spatial proximity to the genes they regulate, while restricting their interactions with genes from nearby TADs. This spatial framework enables regulatory elements to act in cis, that is, to control genes located on the same DNA molecule. Multiple lncRNAs have been shown to function in such cis-regulatory domains, sometimes through poorly understood mechanisms.
Many lncRNAs spatially interact with neighbouring mRNA-expressing genes, and the expression of these lncRNA–coding gene pairs correlates across tissues and individuals9–11. Genetic experiments have revealed that some of these lncRNAs have a cis-activating function12–20. Although active enhancers also produce non-coding transcripts called enhancer RNAs (eRNAs)21,22, cis-activating lncRNAs are often distringuished from eRNAs because the promoters of cis-activating lncRNAs are flanked by nucleosomes with histone modifications typical of promoters, such as high levels of histone H3 trimethylated at lysine 4 (H3K4me3), instead of conventional enhancer modifications such as H3 methylated at lysine 4 (H3K4me1)23. Furthermore, lncRNAs are preferentially spliced and are stable, as opposed to eRNAs, which are generally shorter than 500 nucleotides, unspliced and rapidly degraded21,22. Despite these differences, the distinction between putative cis-activating lncRNAs and eRNAs can be blurry, as stable multi-exonic transcripts are also formed at regions carrying classic active-enhancer chromatin signatures.
The null hypothesis for candidate cis-activating lncRNAs is that they are passively produced from active DNA enhancer regions, and that the lncRNA molecules are thus merely ‘transcription noise’. An example is mouse Lockd, a spliced lineage-specific lncRNA which, as its name indicates, is expressed 5 kb downstream of Cdkn1b, the gene that encodes the cell cycle regulator p27 (ref. 24). Whereas deletion of the entire Lockd locus in mouse erythroblasts results in reduced transcription of Cdkn1b, premature termination of Lockd does not affect Cdkn1b levels25. This finding was taken to indicate that Cdkn1b is positively regulated by a cis DNA element, but that the transcription of Lockd or the transcribed Lockd itself is dispensable for Cdkn1b expression26.
Studies at other cis-activating lncRNA loci, however, support the notion that lncRNAs are not simply passive by-products of transcription. In this section, we discuss different mechanisms through which lncRNA transcripts or the process of lncRNA transcription can contribute to cis-activating functions12–19.
lncRNAs that function as scaffolds
Some lncRNAs, exemplified by the lncRNA HOTTIP, have been proposed to form a local concentration gradient that provides a scaffold for locus-specific recruitment of regulatory complexes, which in turn regulate the transcription of neighbouring genes27–29 (Fig. 1a). A related proposed mechanism is the RNA-mediated feedback model30. In this model, low levels of nascent RNA initially enhance the formation of transcriptional condensates that promote transcription, whereas transcript elongation elevates local RNA concentration, which dissolves the condensates and reduces transcription (Fig. 1b). The key effector in this process is the charge balance of electrostatic interactions provided by RNA molecules, which is proportional to RNA length and local abundance, whereas the importance of the RNA sequence itself is minor. Notably, the cyclic nature of this process suggests that the RNA concentration is the basis of the burst kinetics of promoters’ activity, which cannot be explained by classic promoter–enhancer interaction and function31.
Fig. 1 |. Mechanisms of transcription activation in cis by long non-coding RNAs.
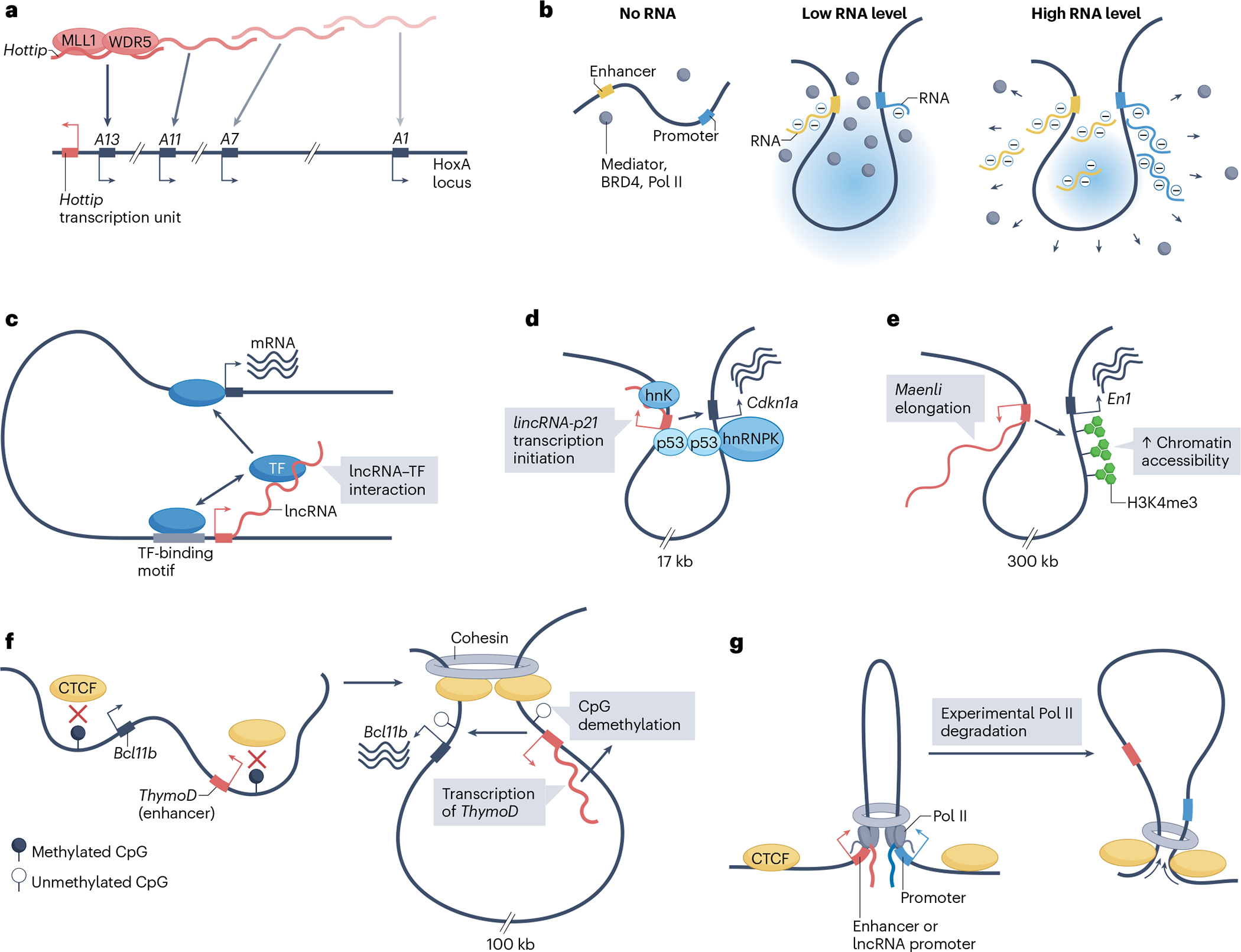
a, The long non-coding RNA (lncRNA) Hottip is expressed from the HoxA locus and serves as a scaffold for the local recruitment of the histone methyltransferase complex comprising MLL1 (also known as KMT2A) and WDR5 to HoxA gene (A1–A13) sites of transcription. Consistent with local activity, RNAi-mediated depletion of Hottip preferentially affects Hottip proximal, compared with distal, HoxA genes (fading colour gradient). b, Local RNA abundance provides feedback on transcription initiation. Left: the Mediator complex, the histone-acetylation reader bromodomain-containing protein 4 (BRD4) and RNA polymerase II (Pol II) are present in low abundance at transcriptionally inactive promoter and enhancer elements. Middle: upon transcription initiation, nascent RNAs produced from promoter and enhancer regions nucleate the formation of a condensate, which increases the local concentration of transcription regulators, thereby causing a burst in transcription. Right: as transcription proceeds, the increase in local RNA abundance beyond a certain threshold generates electrostatic repulsive forces that disperse the transcriptional condensates, thereby ending the transcription burst. c, Many transcription factors (TF) have RNA-binding domains, which potentially interact with nascent transcripts, including of lncRNAs. These lncRNA–TF interactions could contribute to the targeting or the strength of association of the TFs to their genomic target sites by taking advantage of their pre-existing 3D proximity. d, lincRNA-p21 and its cis-activated target, the neighbouring gene Cdkn1a (encoding p21), are in 3D proximity, and are co-regulated by the TF p53. Transcription initiation of lincRNA-p21 is sufficient to enhance the expression of Cdkn1a by creating a scaffold for the recruitment of the Cdkn1a transcriptional co-activator heterogeneous nuclear ribonucleoprotein K (hnRNPK). e, Transcription elongation of the lncRNA Maenli increases local histone H3 trimethylated at lysine 4 (H3K4me3), which is a mark of transcriptionally active chromatin, and promotes the expression of the neighbouring gene En1. f, Transcription of a lncRNA in the Bcl11b locus named thymocyte differentiation factor (ThymoD) promotes the demethylation of CTCF-binding sites and, therefore, CTCF recruitment and chromatin reorganization. This process brings the ThymoD-associated enhancer region in proximity with their target, the promoter of Bcl11b, resulting in transcription activation. g, Pol II recruitment to enhancers and promoters blocks chromatin-loop extrusion and stabilizes the loops at a configuration that brings active enhancers in proximity of active promoters. Experimental degradation of Pol II leads to the formation of larger loops, extrusion of which is now limited by CTCF.
A broad range of TFs were recently shown to harbour RNA-binding domains32. The resulting RNA–TF interactions, which also have limited RNA sequence specificity, were shown to enhance the binding of TFs to chromatin and to promote their ability to activate transcription of episomal reporters32. Although the effects and biological contexts in vivo of these types of RNA–TF interactions remain to be elucidated, this model suggests a plausible function for some cis-activating lncRNAs (Fig. 1c). The notion that lncRNAs modulate TFs is supported by earlier findings on individual lncRNAs, such as a report that the human lncRNA A-ROD recruits TFs upon its release from chromatin to promote the expression of its neighbouring protein-coding gene DKK1 (ref. 33).
Cis-activation by transcription initiation of lncRNAs
Mouse lincRNA-p21 (also known as Trp53cor1) is a spliced and polyadenylated lncRNA transcribed 17 kb upstream of Cdkn1a (encoding the cell cycle inhibitor p21). lincRNA-p21 illustrates the functional importance of transcription initiation34. In this locus, the promoters of lincRNA-p21 and Cdkn1a engage in constitutive 3D chromatin interactions, and both harbour p53 response elements that confer responsiveness to p53 during cellular stress15 (Fig. 1d). Deletion of the lincRNA-p21 p53 response element, the lincRNA-p21 promoter or the entire locus in mice led to reduced p21 levels and function, indicating the presence of a Cdkn1a cis-activating element in lincRNA-p21 (refs. 13,15,35). Indeed, interference with lincRNA-p21 transcription initiation significantly reduced Cdkn1a expression, indicating that the earliest steps of production of nascent lincRNA-p21 are required for Cdkn1a cis-activation15,36 (Fig. 1d). By contrast, premature transcription termination, abrogation of splicing or ribozyme-mediated degradation of lincRNA-p21 had no effect on Cdkn1a, indicating that the mature transcript is dispensable for p21 upregulation15. A similar mechanism was observed for the activation of the developmental gene Eomesodermin (Eomes) (a TF) by the lncRNA Meteor (mesendoderm transcriptional enhancer organizing region)16 and for the stimulation of the inflammation regulator Ptgs2 by lincRNA-Cox2 (ref. 18). Collectively, these examples indicate that lncRNA transcription initiation and minimal elongation are cis-activating processes of a class of lncRNAs.
It remains to be determined how transcription activation of some lncRNA promoters evokes them to function as enhancers, although several mechanisms have been proposed, including the possibility that nascent lncRNA transcripts tethered to the locus by RNA polymerase II (Pol II) may enhance the recruitment of additional factors. The recruitment includes factors that can contribute to the formation of transcription condensates (Fig. 1b), TFs (Fig. 1c) or transcriptional cofactors, as exemplified by the recruitment of the Cdkn1a activator heterogeneous nuclear ribonucleoprotein K (hnRNPK) by lincRNA-p21 (refs. 13,34) (Fig. 1d).
Cis-activation by lncRNA-transcript elongation and processing
For some lncRNAs, transcript elongation or processing, rather than initiation, is functionally important. Maenli (also known as Gm29348), a multi-exonic mouse lncRNA transcribed 300 kb upstream of the homeobox TF En1, illustrates a transcription-based activation mechanism with important implications for limb development17. A series of genetically engineered mouse strains revealed that Maenli elongation is required to establish a permissive chromatin environment for En1 expression in cis in limbs17 (Fig. 1e). Elongation and splicing of the lncRNA Blustr were also proposed to regulate the neighbouring Polycomb-group gene Sfmbt2 (ref. 37). Blustr length, splicing and rate of transcription — but not specific sequence elements in the mature Blustr transcript — contribute to its cis-activating function37.
Evidence for the role of splicing in promoting cis-activation of transcription comes also from genome-scale observations that the production of multi-exonic non-coding transcripts from strong enhancers is evolutionarily constrained38. Accordingly, splicing motifs in lncRNAs, rather than exonic sequences or expression levels of lncRNAs, are under strong purifying selection39,40. Importantly, individuals who carry nucleotide variants at splice sites of enhancer lncRNAs exhibit changes in local chromatin epigenetic signatures and altered expression of target mRNAs41.
Cis-activation through 3D genome reconfiguration
lncRNA transcription has been shown to influence 3D chromatin architecture and enhancer–promoter interactions at several loci, including the lncRNAs PLUT in human or thymocyte differentiation factor (ThymoD) in mouse, as well as the protocadherin-α gene cluster lncRNAs12,19,42. The transcription of ThymoD lncRNA from an enhancer of the Bcl11b gene was linked to the demethylation of CpG dinucleotides at CTCF-binding sequences in the locus, which increases CTCF occupancy19 (Fig. 1f). The resulting changes in chromatin topology reposition the enhancer region in greater proximity to its target, the Bcl11b gene promoter19 (Fig. 1f). A related mechanism was described for the stochastic selection of alternative protocadherin-α promoters42. Each protocadherin-α promoter overlaps with an antisense lncRNA, and transcription activation of individual antisense lncRNAs causes demethylation of CTCF-binding sites and increased CTCF binding, which enables the formation of long-range chromatin loops with a distal cluster of enhancers42.
In addition to these studies of individual loci, recent work suggests that Pol II recruitment to lncRNAs could have a widespread influence on 3D genome organization. Transcript elongation of lncRNAs was shown to dissociate long-range chromatin contacts (loops) by displacing CTCF and cohesin, without necessarily modifying DNA methylation at the CTCF-binding sites43. By contrast, recent genome-scale studies have illustrated how Pol II recruitment and productive elongation from enhancers and promoters can block the extrusion of chromatin loops, thereby increasing contacts between enhancers and promoters44,45 (Fig. 1g). Although the impact of transcription on 3D genome conformation remains unsettled, these findings raise the possibility that the cis-activation function of some transcribed lncRNAs is mediated by the effects of transcription initiation or processing on spatial genome organization.
eRNA contribution to enhancer function
Early studies proposed that eRNAs mediate essential enhancer functions, such as the recruitment of Pol II and loading of the Mediator complex to protein-coding target genes, or the regulation of CTCF-mediated chromatin remodelling to enable enhancer–promoter interactions46–49. However, these functions were primarily determined using RNAi and antisense oligonucleotides to deplete eRNAs, which in addition to RNA inhibition have been shown to induce epigenetic changes in the chromatin of target loci50–52.
More recent work, based on varied perturbation tools, has supported the notion that eRNAs could elicit similar functions to those described for cis-activating lncRNAs. For example, eRNAs, similar to other lncRNAs, have been reported to contribute to the formation of local condensates, which lead to enhanced gene expression through increased formation and stabilization of transcription co-activator complexes53,54. Furthermore, the suggested regulatory impact of RNA–TF interactions theoretically applies to both eRNAs and lncRNAs32. One study has specifically focused on eRNAs containing Alu repetitive sequences and found that they can promote enhancer–promoter pairing by forming RNA duplexes with promoter-associated RNAs55.
The spatial effects of Pol II recruitment could be relevant to the cis-activating function of enhancers and cis-activating lncRNA loci, by blocking loop extrusion and promoting proximity to target genes44,45 (Fig. 1g). Pol II recruitment to enhancers has also been proposed to increase enhancer–promoter contacts by tethering both elements at transcriptionally active hubs or condensates56. These findings suggest that enhancer transcription is important for normal enhancer–promoter interactions.
Although the analysis of cis-activating transcription units has largely focused on lncRNA promoters and eRNAs, active promoters of protein-coding genes can also have long-range enhancer activity57. The conditions in which transcription regulation functions are enacted by eRNA, lncRNA or mRNA promoters are poorly understood.
lncRNAs as local rheostats of transcription-factor genes
In contrast to lncRNAs that contribute to the activation of transcription by enhancers, other lncRNAs carry out specialized rheostat-like functions in cis.
Many lncRNA genes are located in the vicinity of TF genes9,58,59, and in numerous studies the knockout of a lncRNA has led to moderately increased expression of a TF gene in the same TAD (Table 1). Further analyses of some of these individual lncRNAs indicate that they are fundamentally different from transcription silencers, which repress their target genes60,61, or from enhancers that confer cell type-specific gene activation. Instead, these lncRNAs regulate already-active genes and tune their transcription to ensure appropriate gene product concentrations (Fig. 2a). We refer to such lncRNAs as gene expression stabilizers, in analogy to voltage stabilizers, which are devices used to protect electronic equipment from excessively high voltage levels.
Table 1.
Long non-coding RNAs whose loss results in increased expression of a neighbouring transcription-factor gene
| lncRNA | TF gene | Effect in cis or in trans | lncRNA transcription required | Modulation of enhancer– promoter contacts | TF–lncRNA feedback | Response signalling | Mouse phenotype of lncRNA loss | Dosage-sensitive phenotype of the human TF gene | Refs.a |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Halr1 |
Hoxa1
Hoxa2 |
Cis | Yes | Yes | Negative feedback | Retinoic acid | - | Haploinsufficiency: ear defects | 62–64 |
| HASTER | HNF1A | Cis | No | Yes | Negative feedback | Unknown | Diabetes | Haploinsufficiency: diabetes | 70 |
| CHASERR | CHD2 | Cis | Yes | Yes | Negative feedback | Unknown | Lethal | Haploinsufficiency: neurodevelopmental | 71 |
| Hand2os1 | Hand2 | Unknown | Unknown | Unknown | Unknown | Unknown | Cardiac abnormalities | Haploinsufficiency: cardiac | 65,66 |
| Hdnr | Hand2 | Unknown | Yes | Unknown | Possibleb | Unknown | Cardiac abnormalities | Haploinsufficiency: cardiac | 67 |
| Flicr | Foxp3 | Cis | Yes | Unknown | Unknown | IL-2 | Reduced type 1 diabetes | No (X-linked autoimmune disease) | 69 |
| PVT1 | MYC | Cis | Yes | Yes | Possibleb | Stress, p53 | Loss of function: tumour suppression | Gain of function: oncogenic | 14,74 |
| METEOR | EOMES | Unknown | No | Yes | Unknown | Unknown | - | - | 16 |
| Evf2 | Dlx6 | Cis | No | Unknown | Unknown | Unknown | Neurodevelopmental | Haploinsufficiency: split-hand and foot malformation | 72 |
| Playrr | Pitx2 | Unknown | Yes | Unknown | Negative cross-regulation | Unknown | Cardiac arrhythmias | Haploinsufficiency: Rieger syndrome | 75 |
| NXTAR | AR | Unknown | Yes | Unknown | Negative cross-regulation | Androgen | - | Gain of function : oncogenic | 78 |
| FENDRR | FOXF1 | Possibly trans | Yes | Unknown | Negative feedback | Unknown | Vascular malformations (AVCD-MPV) | Haploinsufficiency: vascular malformations (AVCD-MPV) | 68 |
AVCD-MPV, alveolar capillary dysplasia and misalignment of pulmonary veins.;
References of long non-coding RNA (lncRNA) mutation studies showing increased expression of a proximal transcription regulator gene.
Evidence for regulation of a lncRNA gene by the transcription factor (TF), but no direct evidence for a feedback mechanism.
Fig. 2 |. Long non-coding RNAs as cis-acting transcription stabilizers.
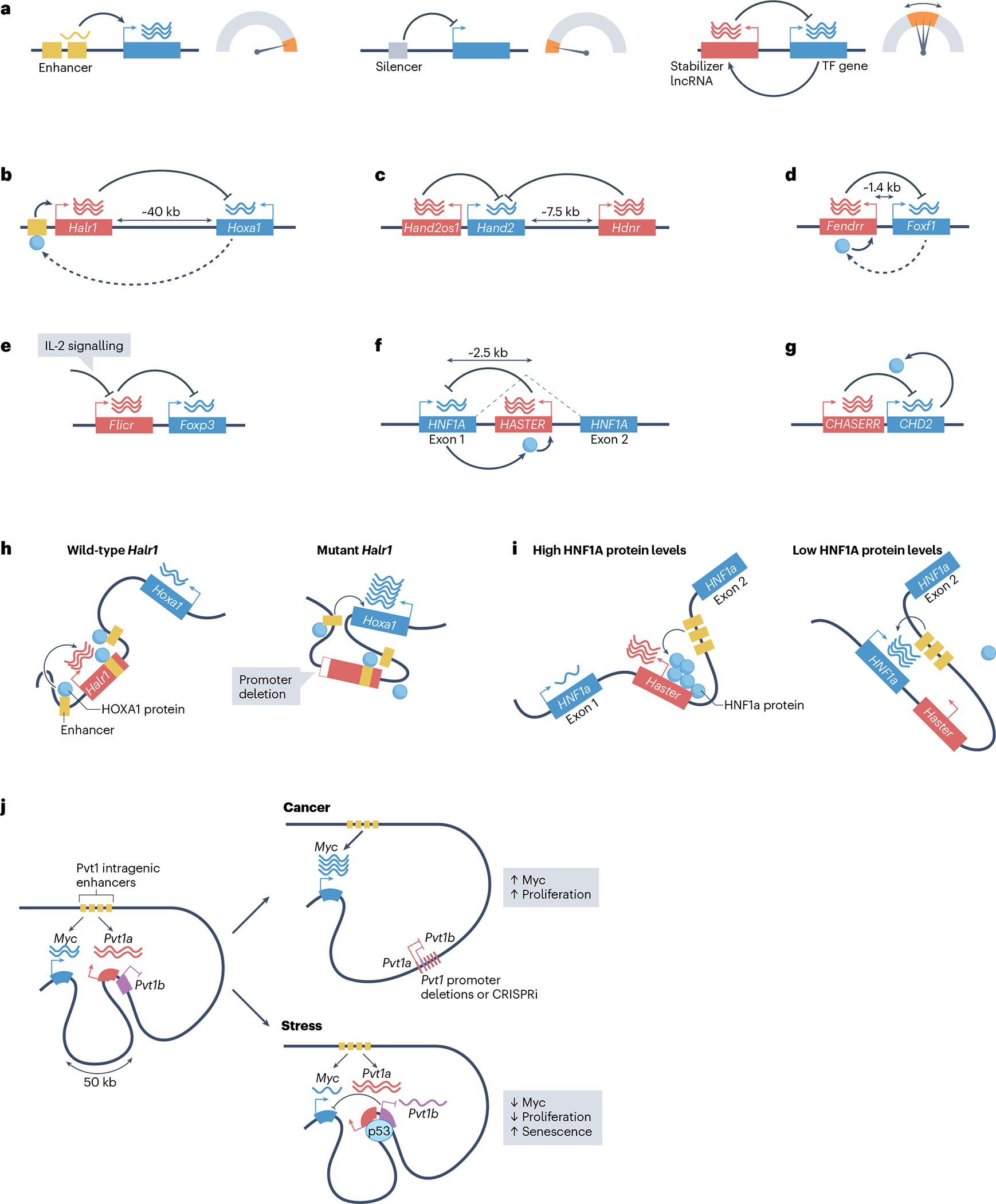
a, Whereas enhancers promote cell type-specific gene activation and silencers prevent the expression of their target genes, transcription-stabilizing long non-coding RNAs (lncRNAs) act in cis to tune the transcription level of dosage-sensitive transcription-factor (TF) genes. b–g, Examples of lncRNAs (in red) that act as transcription stabilizers of adjacent genes, all of which encode transcription regulators (in blue). The lncRNAs are Halr1 (b), Hand2os1 (c), FENDRR (d), Flicr (e), HASTER (f) and CHASERR (g) — loss of function of all of these lncRNAs caused increased expression of the adjacent gene. HNF1 homeobox A (HNF1A) and chromodomain helicase DNA-binding protein 2 (CHD2) (blue circles) enhance the inhibitory effects of the adjacent lncRNAs, and therefore provide negative feedback. Homeobox A (HOXA1) and forkhead box F1 (FOXF1) proteins are positive regulators of the lncRNAs Halr1 and FENDRR, respectively, suggesting they could also form a negative feedback loop. Some transcription-stabilizing lncRNAs modulate their target genes in a signal-responsive manner; for example, interleukin-2 (IL-2) acts on Flicr (e) to reduce high Foxp3 expression levels in regulatory T cells. Two lncRNAs, Hand2os1 and Hdnr (c), restrict Hand2 expression. h,i, The promoters of transcription-stabilizing lncRNAs modulate interactions between their target TFs genes and local enhancers. h, Left: in pluripotent cells, Halr1 binds and sequesters proximal enhancers of Hoxa1, which dampens retinoic acid-induced expression of Hoxa1. Right: deletion of the Halr1 promoter increases enhancer–Hoxa1 interactions. HOXA1 (blue circles) binds to local enhancers and activates Halr1, which restrains Hoxa1 expression. Left and right in h depict retinoic acid-induced cells. i, The Haster promoter limits interactions between the Hnf1a promoter and intragenic enhancers. This effect is accentuated at high concentrations of HNF1A protein, thereby providing negative feedback on Hnf1a transcription. j, The active Pvt1 lncRNA promoter acts as a boundary element that associates with enhancers located within the Pvt1 gene body and limits access of the Myc promoter to these enhancers. Experimental inhibition of the transcription activity of the Pvt1 promoter through targeted promoter deletions or CRISPR inactivation (CRISPRi) leads to increased Myc promoter–enhancer engagement, high Myc transcription and increased cellular proliferation. The Pvt1 locus also harbours a p53-dependent isoform, Pvt1b, which downregulates Myc transcription during stress, decreases cell proliferation and increases cell senescence without apparent changes in Myc–enhancer contacts.
One of the earliest examples of such a stabilizer is the mouse lncRNA Halr1 (also known as Haunt or linc-HOXA1), which is located ~40 kb from the homeobox TF gene Hoxa1. Three studies showed that Halr1 depletion or mutations in its promoter led to increased expression of Hoxa1 and other genes of the Hoxa cluster in pluripotent stem cells exposed to retinoic acid62–64 (Fig. 2b), indicating that Halr1 guards against inappropriately elevated Hoxa gene expression.
Similarly, two mouse lncRNA transcription units curtail the expression of Hand2, which encodes a cardiac basic helix–loop–helix TF (Fig. 2c). One is the lncRNA Hand2os1 (also known as Upperhand, lncHand2 or HAND2-AS1), which is transcribed divergently of Hand2 (refs. 65,66). Deletion of two Hand2os1 distal exons or of a much broader Hand2os1 sequence in mice causes increased cardiac expression of Hand2 mRNA, which leads to augmented expression of HAND2-dependent genes in specific cardiac cell subpopulations66. The other lncRNA, Hdnr (also known as Handsdown), is located downstream of Hand2. Insertion of a polyadenylation signal to prematurely terminate Hdnr transcription also increases Hand2 levels, whereas its CRISPR-based induction reduces Hand2 expression67.
The lncRNA FENDRR and the TF gene FOXF1 share an ~1.4 kb promoter region in the mouse and human genomes (Fig. 2d). The replacement of mouse Fendrr exon 1 with a polyadenylation cassette that interrupts Fendrr transcription resulted in increased Foxf1 mRNA levels in the caudal end of embryonic day 8.5 and 9.5 mouse embryos, and ectopic expression of Foxf1 mRNA in the heart of embryonic day 12.5 embryos68. Mutations in several other lncRNA genes similarly elicit moderately increased expression of adjacent transcription regulator genes, including Flicr69, HASTER (also known as HNF1A-AS1)70, CHASERR71, Evf2 (also known as Dlx6os1)72, Hotair73, Pvt114,74 and Playrr75 (Table 1 and Fig. 2e–g).
Fine-tuning the expression of transcription-factor genes through feedback
The dampening effect of lncRNAs on adjacent TF genes could theoretically represent a steady-state tonic inhibition, but some lncRNAs have been shown to act as robust feedback circuits that maintain stable concentrations of regulatory proteins within a narrow concentration range. An example of such a rheostat-like mechanism is the human lncRNA named HASTER. HASTER transcription starts in the first intron of HNF1A, which encodes a homeodomain TF, and proceeds in antisense orientation to HNF1A (ref. 70) (Fig. 2f). High HNF1A protein concentrations result in the direct activation of the HASTER promoter, and this leads to inhibition of HNF1A transcription70. Consequently, deletion of the HASTER promoter in mice or human stem cell-derived hepatocytes boosted HNF1A expression70. This indicates that HNF1A and HASTER form a classic negative feedback loop that prevents HNF1A overexpression (Fig. 2f).
This type of feedback likely exists at other loci. The lncRNA Halr1, which limits retinoic acid-induced transcription of Hoxa1, is positively regulated by HOXA1 (ref. 63) (Fig. 2b). Depletion studies have shown that FOXF1 is a positive regulator of the lncRNA FENDRR76, which in turn inhibits FOXF1 (ref. 68) (Fig. 2d), and that HAND2 binds at two sites in the Hdnr locus, which could conceivably modulate the inhibitory function of Hdnr77.
Other types of feedback appear to operate in some lncRNA–TF gene pairs. A recent study reported in a preprint points to a negative cross-regulatory feedback loop formed by the mouse lncRNA Playrr and its adjacent homeodomain TF gene Pitx275 (Table 1). These two genes are expressed in mutually exclusive heart domains, and loss of a Playrr splice site leads to increased and inappropriate Pitx2 expression patterns, causing cardiac arrythmia75. The human lncRNA NXTAR also forms negative cross-regulatory feedback with the AR gene, which in turn inhibits NXTAR78.
These examples raise the question of why TF genes require feedback. Negative feedback systems dampen fluctuations and can provide a cell type-specific range for TF concentrations. This is important because TF concentrations are crucial determinants of genomic-site binding choices79. Lineage-selective TFs are often expressed at different levels across cell types, and typically bind to different cell type-specific genomic sites80,81. Tight regulation of TF concentrations may be particularly relevant for pioneer TFs, which bind DNA sequences in inaccessible chromatin. HNF1A, in particular, has typical pioneer factor properties, such as the ability to bind nucleosomal DNA82, and a capacity to activate silent genes in fibroblasts83. Accordingly, livers of Haster mutant mice, in which HNF1A concentrations are abnormally high, exhibit widespread genomic binding of HNF1A and chromatin opening at HNF1A recognition sequences that are normally inaccessible in hepatocytes, leading to aberrant, ectopic gene transcription70. Feedback from lncRNAs could therefore tune TF concentrations to ensure the specificity of cell type-specific gene programmes.
Studies of mouse and human CHASERR lncRNA have exemplified another remarkable feedback system that controls a chromatin remodeller instead of a DNA-binding TF71. The lncRNA CHASERR is transcribed upstream on the same strand of the CHD2 gene (Fig. 2b). Heterozygous deletions of mouse Chaserr promoter or gene body, or lncRNA depletion, increased Chd2 expression, indicating that Chaserr inhibits Chd2 (ref. 71). The authors postulated that Chaserr transcription interferes with transcription of the downstream Chd2 gene. They also found that CHD2 forms an autoregulatory feedback loop by binding to Chaserr transcripts — and to the Chaserr gene — which promotes Chaserr interference of Chd2 (ref. 71) (Fig. 2g). Failure of this feedback in Chaserr mutants causes increased CHD2 expression, which in turn decreases the expression of many other genes that are located downstream of CHD2-bound transcription units.
Mechanisms of modulation of transcription-factor genes
Genetic experiments have begun to shed light on how stabilizer lncRNAs tune the expression of TF genes. Although lncRNA–TF gene pairs vary greatly in their relative orientations or genomic distance (Fig. 2b–g), perturbation studies have revealed commonalities in their mode of action.
Several studies have demonstrated that the mechanism by which transcription stabilizer lncRNAs modulate the expression of adjacent TF genes occurs in cis. The demonstration that a lncRNA exerts its effects in cis rules out the possibility that the local regulatory activity is carried out by RNA-encoded polypeptides, as well as other indirect mechanisms. cis effects have been demonstrated using heterozygous mutant models that can unequivocally ascertain whether only the chromosome that carries the mutant allele exhibits altered expression of the TF gene. In practice, this analysis can be carried out using either heterozygous lncRNA mutations bred on hybrid mouse strain backgrounds, thereby allowing to distinguish between the two chromosomes, or with compound heterozygotes in which the lncRNA and TF mutations are on separate chromosomes70,71. Other studies have pointed to a cis effect by showing that ectopic lncRNA expression does not rescue the lncRNA-null mice69. The case of Fendrr differs from that of other stabilizer lncRNAs in that ectopic expression was shown to partially rescue the mouse Fendrr mutant phenotype68, which suggests that at least some effects of Fendrr occur in trans. This possibility was supported by another study, which deleted a sequence in Fendrr forming a putative DNA–DNA–RNA triplex with various potential target sequences, and found that it partially phenocopies other Fendrr mutants84. However, these experiments have not fully addressed whether the rheostat-like function of Fendrr on the Foxf1 gene, with which it shares a promoter region and a closely related developmental phenotype, also occurs through this type of trans mechanism68.
Cis-regulatory effects can be mediated through RNA-dependent or DNA-dependent mechanisms. A functional requirement of lncRNA transcription for expression stabilization has been demonstrated using transcription termination alleles or CRISPR-based transactivation for some, but not all, stabilizer lncRNAs66,67,69,71. Likewise, genetic perturbations or RNA degradation experiments have shown that the RNA itself is functionally important for the inhibitory effects of Chaserr71, Flicr69 and Hdnr67 on adjacent genes. By contrast, blocking transcription of HASTER using nuclease-deficient Cas9 as a roadblock or by inserting a polyadenylation site, as well as activation of HASTER transcription through a modified CRISPR–Cas9 system did not have an effect on HNF1A mRNA expression70. Moreover, overexpression of HNF1A separation-of-function mutants that lacked the ability to transactivate genes, and therefore did not activate HASTER transcription, still resulted in feedback inhibition of the endogenous HNF1A gene, an effect that required an intact HASTER promoter70. Thus, HNF1A interactions with the HASTER promoter, but not HASTER transcript elongation or transcripts, appear to be important for the HASTER–HNF1A transcription feedback.
Chromatin conformation capture studies have shown that the promoters of several transcription stabilizer lncRNAs, including HASTER70, PVT174, Meteor16, Halr163,64 and Chaserr71, limit interactions between enhancers and their target genes, which consequently dampens gene transcription (Fig. 2h–j). In the case of HASTER, increased HNF1A concentrations led to enhanced binding to the HASTER promoter, which further limited interactions between HNF1A intronic enhancers and the HNF1A promoter (Fig. 2i). These independent studies suggest that enhancer competition may be a prevalent mode through which lncRNAs control the expression of TF genes (Fig. 2h–j). In summary, to maintain homeostatic expression levels of TFs, cis-acting lncRNAs deploy transcription-dependent and RNA-dependent mechanisms, but also compete with enhancers of their target genes, and several lncRNAs appear to simultaneously use more than one of these molecular mechanisms14,71,74.
Dual positive and negative regulatory functions
Numerous lncRNA loci intertwine positive and negative cis-regulatory functions14,16,64,70,74. For example, a comprehensive genetic dissection of Meteor showed that its transcription is required to activate Eomes in pluripotent cells, whereas the Meteor promoter limits Eomes expression during neuronal differentiation16. Likewise, a deletion of the Haster promoter in mice led to increased HNF1A expression in all hepatocytes, whereas this same deletion caused variegated HNF1A expression in pancreatic β-cells, with some cells showing marked HNF1A overexpression and others complete HNF1A silencing70. The Haster promoter, therefore, has a cell type-specific cis-activating function in addition to its negative feedback.
Some of the observed dual phenotypes might occur because stabilizer lncRNAs are often embedded in enhancer clusters. Different genetic alterations in a locus with a complex interspersion of positive and negative regulatory elements can easily lead to opposite phenotypes. For example, an allele that produces a premature termination of Hand2os1 transcription causes a severe loss of cardiac Hand2 expression65, whereas Hand2os1 deletions cause Hand2 upregulation65,66. Likewise, small deletions in Halr1 and RNA perturbations have resulted in increased expression of HoxA genes, whereas other deletions in the Halr1 locus have uncovered HoxA-activating sequences62–64.
Signal-induced modulation of transcription-factor genes by lncRNAs
For some cis-regulatory lncRNAs, modulation of the neighbouring gene can be triggered by cellular and environmental perturbations. In this manner, lncRNAs can endow signal responsiveness to a single gene, rather than act on a wide gene expression programme. For example, in humans and mice, PVT1 is a collection of alternatively spliced lncRNAs initiated ~50 kb downstream of the TF oncogene MYC, which accumulate locally and downregulate MYC transcription14,74,85. A study in human breast cancer cell lines showed that the PVT1 promoter limits long-range interactions between the MYC promoter and PVT1 intragenic enhancers, and therefore reduces MYC expression74 (Fig. 2j). Human and mouse PVT1 are part of an additional inhibitory mechanism that involves the expression of a stress-dependent, p53-inducible transcript isoform termed Pvt1b, which is initiated at a downstream transcription start site and whose production inhibits transcription of Myc without insulating Myc from its enhancers14 (Fig. 2j). The stress-induced Pvt1b isoform reverses transcription activation by p53 into a local inhibitory signal, thereby limiting Myc levels and reducing cell proliferation. Interestingly, Pvt1b production leads to both proliferation arrest by Myc downregulation within hours of stress, and to long-term Myc repression, which is associated with activation of cell senescence, suggesting it has a role in epigenetic reprogramming of the Myc locus14,86.
Flicr is another signal-responsive transcription stabilizer that dampens the expression of Foxp3, which encodes a forkhead TF that controls regulatory T cells69 (Fig. 2e). The disruption of mouse Flicr promoters, a mutation of a Flicr splice donor site or targeted degradation of Flicr all led to increased expression of Foxp3 and its target genes, and to a relative depletion of regulatory T cell subpopulations that express low Foxp3 levels69. This process can be modulated by interleukin-2 (IL-2), which inhibits Flicr expression, thus promoting Foxp3 expression and regulatory T cell expansion69.
Whereas Pvt1 and Flicr tune the transcription level of active genes, other signal-responsive lncRNAs elicit transcription switches. The mouse and human lncRNA MORRBID, for example, rapidly downregulates the neighbouring pro-apoptotic gene BCL2L11 (also known as BIM), in response to cytokines and viral infections, thus promoting the survival of myeloid and CD8+ T cells87,88. Bcl2l11 downregulation is accompanied by deposition of the gene-repressive H3K27me3-modified chromatin88. A plant lncRNA named COOLAIR, which inactivates the expression of the vernalization TF FLOWERING LOCUS C (FLC) during the autumn to winter transition89, provides fascinating insights into how a lncRNA carries out a signal-responsive binary switch (Box 1).
Box 1. A plant long non-coding RNA as a paradigm of environmental switch.
The Arabidopsis thaliana long non-coding RNA (lncRNA) COOLAIR exemplifies how a lncRNA can function as a binary switch. COOLAIR is a gene comprising a group of antisense, alternatively spliced lncRNA isoforms that overlap the gene body and promoter of the vernalization transcription factor (TF) FLC gene255–257. COOLAIR responds to environmental cues such as the first seasonal frost, and switches off FLC expression during the autumn to winter transition89. In vivo analysis of structural conformations of individual COOLAIR RNA molecules revealed striking cold-dependent enrichment of specific structural isoforms258. Interestingly, these transcript structural variants preferentially occur in a key region of complementarity between COOLAIR and the transcription start site of FLC. The structural variability might influence the ability of the lncRNA to form an R-loop at the 5′ end of FLC, mediate DNA–DNA–RNA triplex formation between COOLAIR and the FLC transcription start site or promote the recruitment of a protein complex to the FLC transcription start site259. Although the exact mechanism by which COOLAIR suppresses FLC is unclear, this finding reveals a new dimension of RNA-based cis-regulation, namely the capacity to be dynamically altered by adopting alternative structural conformations in response to environmental cues258.
Cis-regulatory lncRNAs as allele-specific repressors
Another notable gene regulatory activity that cannot be explained solely through the general framework of TFs interacting with specific DNA sequences is the selective silencing of one of two homologous chromosomal loci. Some of the best characterized lncRNAs accomplish this type of function, including the lncRNA XIST, which regulates X-chromosome inactivation (XCI), and imprinted lncRNAs that control parent-of-origin allele-specific repression.
X-chromosome inactivation
XCI in mammals ensures X-linked dosage compensation between cells of females and males by inactivating one of the two X chromosomes in female cells90–92. This process is controlled by the X-inactivation centre (Xic), a genomic region that integrates X-chromosome counting information with random selection of one of the two female X chromosomes for inactivation93–96.
The lncRNA gene XIST, which has a central role in XCI, is located in the Xic and is selectively transcribed from what will become the silenced X chromosome (Xi)90,97 (Fig. 3a). XIST transcription is regulated by neighbouring activating and repressive cis-regulatory lncRNAs, which are partitioned into two adjacent TADs98. In mice, the lncRNAs Jpx, Ftx and Xert are located in the same TAD as Xist and promote its transcription in cis99,100 (Fig. 3a). This regulation has been demonstrated also in human cell models, and by a 453-kb deletion in a human female that overlaps JPX and FTX and caused markedly skewed XIST expression from the intact chromosome101. Mechanistically, XIST transcription and accumulation depend on JPX transcription in human cells, or on the accumulation of mature Jpx RNA in mice100 (Fig. 3b). Furthermore, Jpx can also act in cis and in trans to activate Xist by binding and displacing CTCF from the Xist promoter102.
Fig. 3 |. Control of X-chromosome inactivation by long non-coding RNAs.
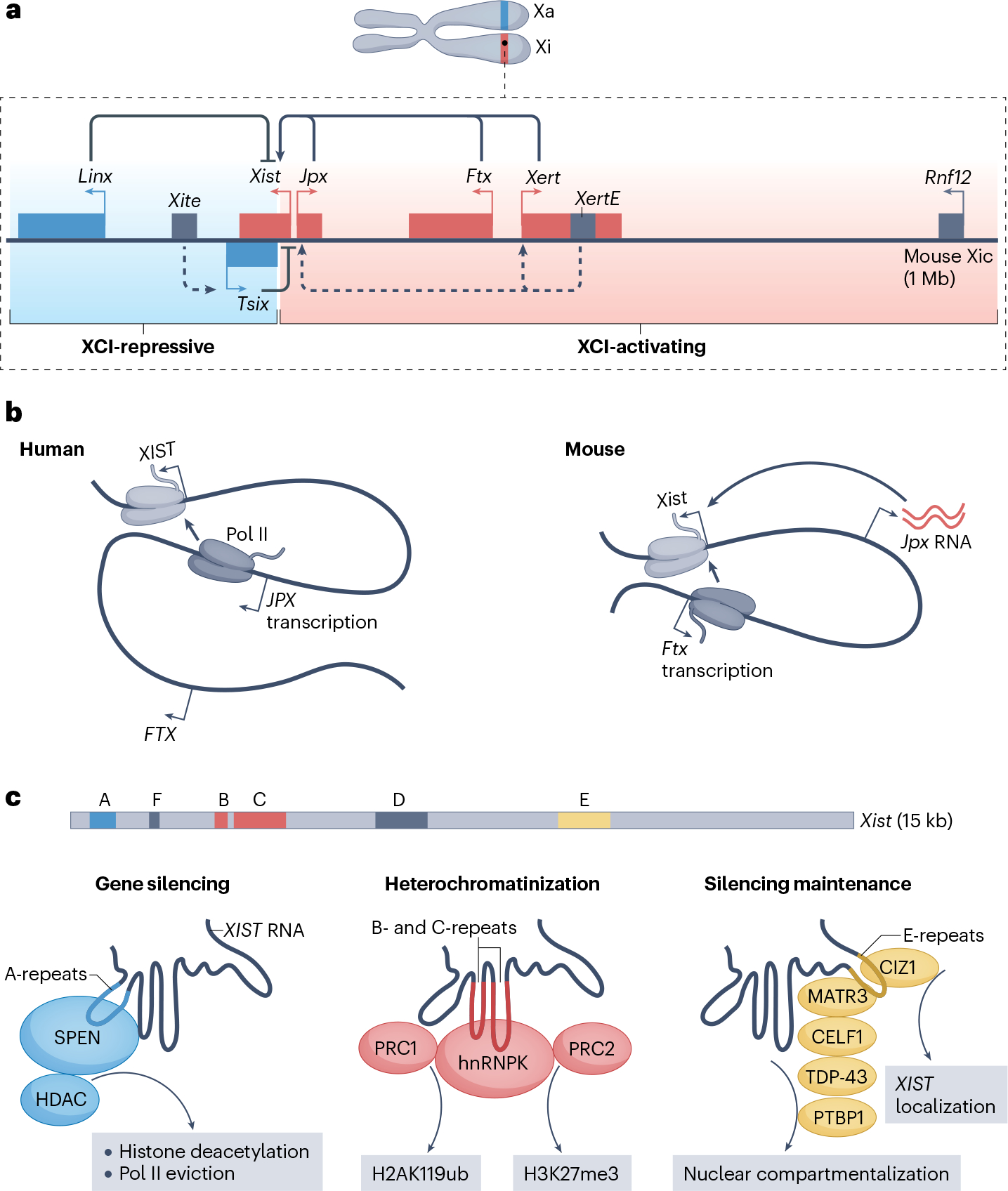
a, Transcription activation of mouse Xist. The X-inactivation centre (Xic) shows two topologically associating domains (TADs) in mouse cells. In one TAD (blue background), the long non-coding RNAs (lncRNAs) Linx and Tsix — antisense transcript of Xist — and the Tsix enhancers, termed Xite, are located. On the active X chromosome (Xa), Tsix transcription suppresses Xist transcription, whereas the Linx promoter acts across the TAD boundary to limit Xist expression in cis. In the other TAD (red background), the lncRNAs Xist, Jpx, Ftx and Xert are located. Xert enhancers, termed XertE, promote both Xert and Xist transcription on the inactive X chromosome (Xi). Following X-chromosome inactivation (XCI), Jpx and Ftx maintain Xist expression and accumulation at Xi. b, Similarities and differences between XIST regulation by JPX and FTX in human and mouse. In human, whereas FTX is not essential for XIST regulation, JPX transcription, but not the mature RNA, contributes to polymerase II (Pol II) loading and XIST transcription and accumulation. In mouse, Ftx transcription promotes Xist transcription, whereas mature Jpx transcript is responsible for Xist transcriptional activation and accumulation. c, XIST mediates transcriptional gene silencing at the X chromosome. XIST RNA highlighting its repeat regions A–F and showing the role of A-repeats in promoting the initial steps of gene silencing through SPEN-mediated and histone deacetylase (HDAC)-mediated histone deacetylation and RNA Pol II eviction; the role of B-repeats and C-repeats in heterochromatinization through recruitment of Polycomb repressive complex 1 (PRC1) and PRC2 downstream of heterogeneous nuclear ribonucleoprotein K (hnRNPK); and the role E-repeats in the CIP1-interacting zinc finger protein 1 (CIZ1)-dependent maintenance of XIST localization at Xi and in recruiting RNA-binding proteins to mediate the nuclear compartmentalization of Xi. H2AK119ub, histone H2A ubiquitylated at lysine 119; H3K27me3, histone H3 trimethylated at lysine 27; MATR3, matrin 3; PTBP1, polypyrimidine tract-binding protein 1; TDP-43, TAR DNA-binding protein 43.
By contrast, the lncRNA Tsix, which inhibits Xist transcription in cis, is located in an adjacent TAD, along with the Xite enhancer elements, which promote Tsix activation103,104 (Fig. 3a). The lncRNA Linx also maps to this TAD and acts as a distant cis-inhibitor of Xist105. This effect is exerted by the active Linx promoter, and is independent of Linx transcript elongation or effects on Tsix105. Heterozygous inactivation of Tsix, Xite or Linx prior to the onset of XCI shows that they are essential cis-regulators of Xist expression in mice104–106.
Elegant studies have shown that once the Xi is selected, Xi-specific expression and accumulation of Xist is both necessary and sufficient for chromosome-wide gene repression in cis. Early experiments established that an inducible Xist transgene can silence autosomes in cis in embryonic stem cells107,108. Molecular and genetic deletion studies have since revealed that repetitive sequences and structural elements within the Xist RNA are central to its ability to recruit regulatory proteins to Xi109–113 (Fig. 3c). The A-repeats of Xist adopt structural features that are recognized by SPEN (also known as MSX2-interacting protein), a transcription co-repressor that mediates the recruitment of chromatin modifying complexes that promote histone deacetylation, evict Pol II and contribute to the early steps of X-linked gene silencing111,114–117. B-repeats and C-repeats have been implicated in the scaffolding of hnRNPK, which mediates the recruitment of Polycomb repressive complex 1 (PRC1) and the subsequent activity of PRC2 at Xi118–122, although a recent study also describes independent binding of PRC2 to A-repeats during initiation of XCI123. The E-repeats mediate the assembly of RNA-binding proteins (RBPs) such as CIP1-interacting zinc finger protein (CIZ1), polypyrimidine tract-binding protein 1 (PTBP1), matrin 3 (MATR3), TAR DNA-binding protein 43 (TDP-43) and CELF1, which promotes chromatin compaction and maintenance of late-stage Xi in a phase-separated compartment124,125.
Ultimately, the accumulation of Xist-scaffolded protein complexes at Xi begins a succession of events that initiate, spread and maintain transcriptional gene silencing97,126 through the formation of a repressive chromatin state127–129 and reconfiguration of the chromosomal architecture112,130,131.
Parent-of-origin allelic repression
Imprinted loci provide another mechanism of lncRNA-dependent cis-regulation. There are at least 29 imprinted domains in the mouse and human genomes, harbouring more than 150 imprinted genes that are organized in clusters132. Imprinting of such loci is critically dependent on differentially methylated regions that span ~1–3 kb and acquire parent-of-origin specified epigenetic states during gametogenesis133. A seminal discovery in genomic imprinting was the identification of lncRNAs that are transcribed from the unmethylated allele in a differentially methylated region, and contribute to the repression of imprinted genes from the same locus134–140.
Although imprinted lncRNAs exhibit considerable sequence and gene-structure diversity, they also share key similarities. Imprinted lncRNAs accumulate in the chromatin at the loci from which they are expressed141, frequently exerting bidirectional, long-range silencing of multiple genes in cis142. Furthermore, transcription-based and RNA-based mechanisms have been proposed to cooperatively contribute to allele-specific transcription repression by these lncRNAs142.
Imprinted lncRNAs cause allele-specific silencing of coding genes with which they overlap143,144. Genetic experiments in mice have shown that promoter deletions or premature transcription termination of imprinted lncRNAs disrupts their silencing functions145–148. For example, prevention of Airn or Kcnq1ot1 transcription read-through into the coding genes Igf2r and Kcnq1, respectively, causes reactivation of the paternal alleles of these genes146–150. At the well-studied Airn–Igf2r locus, transcription interference has been ascribed either to promoter occlusion, where Airn transcription of Igf2r antisense prevents the recruitment of the transcription initiation machinery at the Igf2r promoter, or to a mechanism in which the Airn transcript actively removes the transcription machinery from the nascent Igf2r transcript147 (Fig. 4). A related proposed mechanism is the collision of converging elongating Pol II complexes, exemplified by transcription of the lncRNA Ube3a-ATS, which leads to premature Ube3a termination at the paternal allele151. Support for this model comes from the observation that although both maternal and paternal Ube3a promoters are actively transcribed152, either premature termination or antisense oligonucleotide-mediated degradation of paternal Ube3a-ATS prior to the overlap with the paternal Ube3a transcripts de-repress paternal Ube3a expression152,153. Despite the unequivocal evidence that antisense transcription is important for silencing by imprinted lncRNAs, it remains to be established why only some antisense transcripts evoke this effect, given that mammalian genomes harbour a myriad of convergent sense–antisense transcripts that are co-expressed in the same cells154.
Fig. 4 |. Long non-coding RNA-mediated allele-specific repression at imprinted loci.
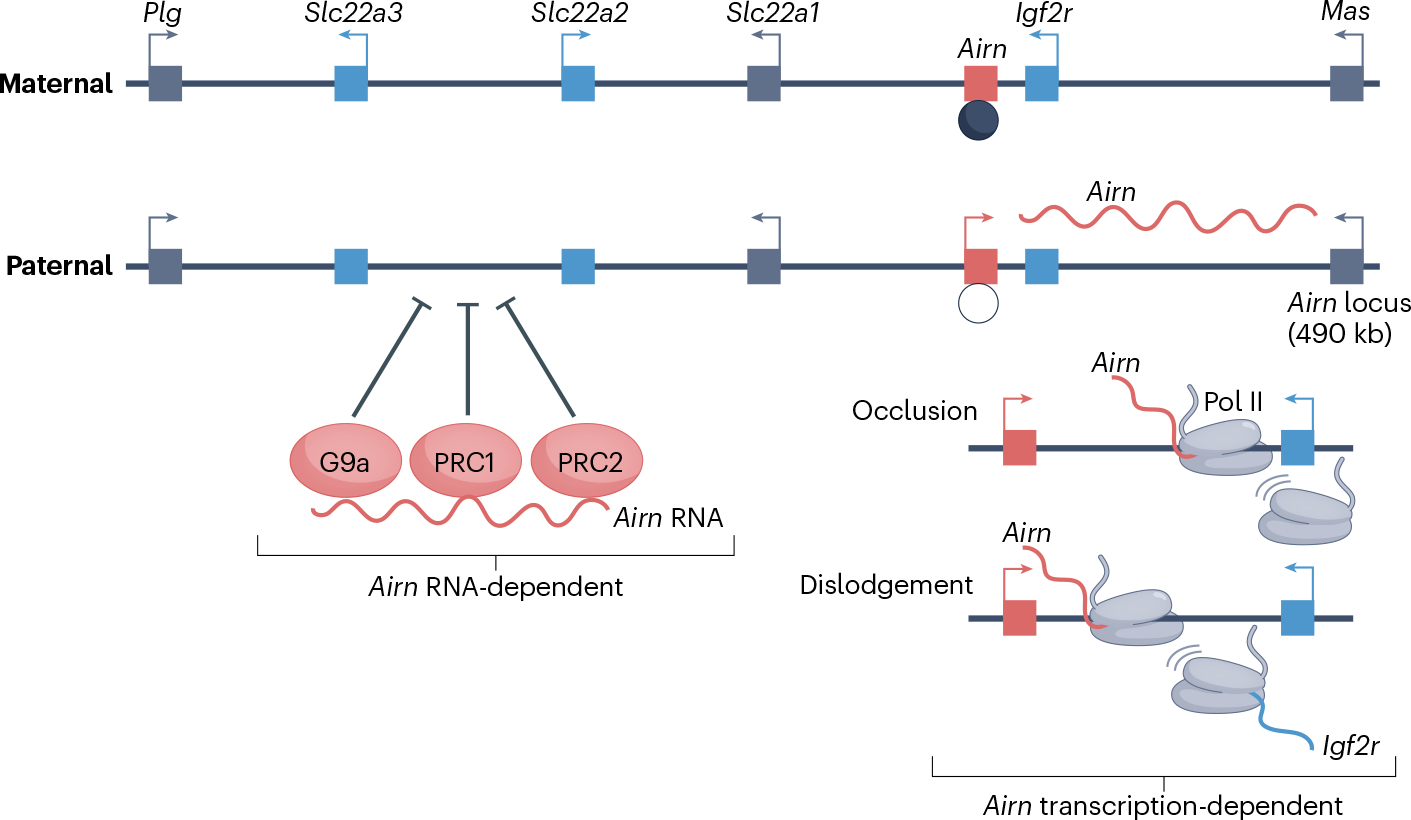
The mouse imprinted Igf2r locus, which includes the long non-coding RNA (lncRNA) Airn. Maternally expressed (blue), paternally expressed (red), and biallelic-expressed (grey) genes are shown. The circle underneath Airn denotes a differentially methylated region, with a black filling indicating methylation of the maternal allele. Bottom right: occlusion and dislodgement models for transcription-mediated repression of Igf2r by Airn. Bottom left: Airn RNA-dependent recruitment of the gene-repressing chromatin modifier complexes Polycomb repressive complex 1 (PRC1), PRC2 and G9a, which repress the Airn-distal genes Slc22a2 and Slc22a3. Airn RNA is drawn as an unstructured molecule owing to lack of structural information. Pol II, polymerase II.
Imprinted loci, however, harbour multiple genes that do not overlap with imprinted lncRNAs yet show allele-specific silencing. In these cases, lncRNAs serve as scaffolds for other regulatory complexes. Repressed alleles at several imprinted loci are heavily enriched in H3K27me3, and several studies describe cell type-specific direct interactions of the lncRNAs Airn, Kcnq1ot1 and Meg3 with PRC1, PRC2 and the H3K9 methyltransferase G9a (also known as EHMT2)139,155–162 (Fig. 4). Two recent studies have highlighted the role of Airn in PRC spreading over a 15 Mb domain in mouse trophoblast stem cells, showing a strong correlation between Airn expression, PRC occupancy at CpG islands and local changes in the chromatin architecture137,163. This finding is broadly consistent with some studies showing that RNA interactions are essential for genomic occupancy of PRC2 (ref. 164). Such mechanisms therefore explain how local RNA-dependent functions contribute to silencing of non-overlapping genes. One open question is whether the interaction of imprinted lncRNAs with protein-binding factors is mediated by specific lncRNA sequences and/or structures, as proposed for XIST.
Imprinted loci also undergo profound monoallelic changes in CTCF binding and local 3D genome organization, which can insulate genes from enhancers165. There is evidence that RNA–protein interactions are important for CTCF binding166,167, and imprinted lncRNAs have been proposed to contribute to local allele-specific 3D genome changes, although more evidence is needed to define the precise role of imprinted lncRNAs in 3D genome organization168,169.
The analysis of imprinted lncRNAs, therefore, has offered unique insights into how transcription-based and RNA-based mechanisms cooperate to enact gene repression in cis.
Transcription regulation by lncRNAs in trans
Single-molecule imaging studies have shown that some lncRNA molecules are exclusively localized at their transcribed locus, whereas other lncRNAs disperse throughout the nucleus and could thus function in trans. In early studies, global changes in gene expression observed following lncRNA inhibition implied that the lncRNAs have such a trans-regulatory function, but in several cases the changes were later attributed to secondary events or to off-target effects of the perturbation tools. The validation of trans-regulatory functions of lncRNAs requires considerations such as the physiological stoichiometry of the lncRNA and its targets29, evidence for direct engagement of the lncRNA with putative target regions and the ability to rescue loss-of-function phenotypes with exogenously expressed lncRNAs170.
The archetypal trans-acting lncRNA is HOTAIR, which is expressed from the mouse and human HOXC locus and was proposed to regulate the expression of genes in the distant HOXD locus through PRC2 targeting171. A large Hotair deletion in mice confirmed a role in transcription repression of HoxD genes and several imprinted loci, leading to developmental defects172. However, the contribution of Hotair to homeotic transformation and the specificity of its interaction with PRC2 were challenged by subsequent studies, one of which used more selective mutations to show that Hotair RNA primarily acts as a negative regulator in cis of adjacent HoxC TF genes73,173–175. Hotair highlights the need for using complementary experimental tools to understand the function of trans-regulatory lncRNAs.
Global transcription control through nuclear assemblies
An emerging concept in transcription control is the role of nuclear compartmentalization of regulatory factors, mediated by interactions between lncRNAs and RBPs with intrinsically disordered regions176. Two well-characterized examples of lncRNA-containing nuclear assemblies are nuclear speckles and paraspeckles, which compartmentalize the highly abundant lncRNAs MALAT1 and NEAT1, respectively, and have a role in the global regulation of transcription and RNA processing177,178. Recent studies have expanded the list of lncRNA-scaffolded nuclear assemblies (Fig. 5a). The intron-retaining, nuclear isoform of the lncRNA Charme specifically recruits MATR3–PTBP1 into nuclear aggregates that regulate chromatin at myogenic loci179–181. Another study directly visualized the definitive endoderm-specific lncRNA DIGIT (also known as GSC-DT) in condensates that contained the acetylated H3K18 reader, bromodomain-containing protein 3 (BRD3)182. Deletion of the retained intron of Charme or of DIGIT disrupted condensate formation and perturbed their respective downstream developmental programmes182. Analogously, the breast cancer-associated lncRNA mammary tumour-associated RNA 25 (MaTAR25) was found to interact with the complex purine-rich element-binding protein A (PURA)–PURB and was proposed to guide their association with the promoter of tensin-1, a key mediator of cell–matrix adhesion and metastatic migration183. How lncRNAs are targeted to one or many distant genomic sites remains an open question. In the context of nuclear assemblies, it is possible that locus specificity may be determined by either the lncRNA or the RBP.
Fig. 5 |. Mechanisms of transcription regulation by trans-acting long non-coding RNAs.
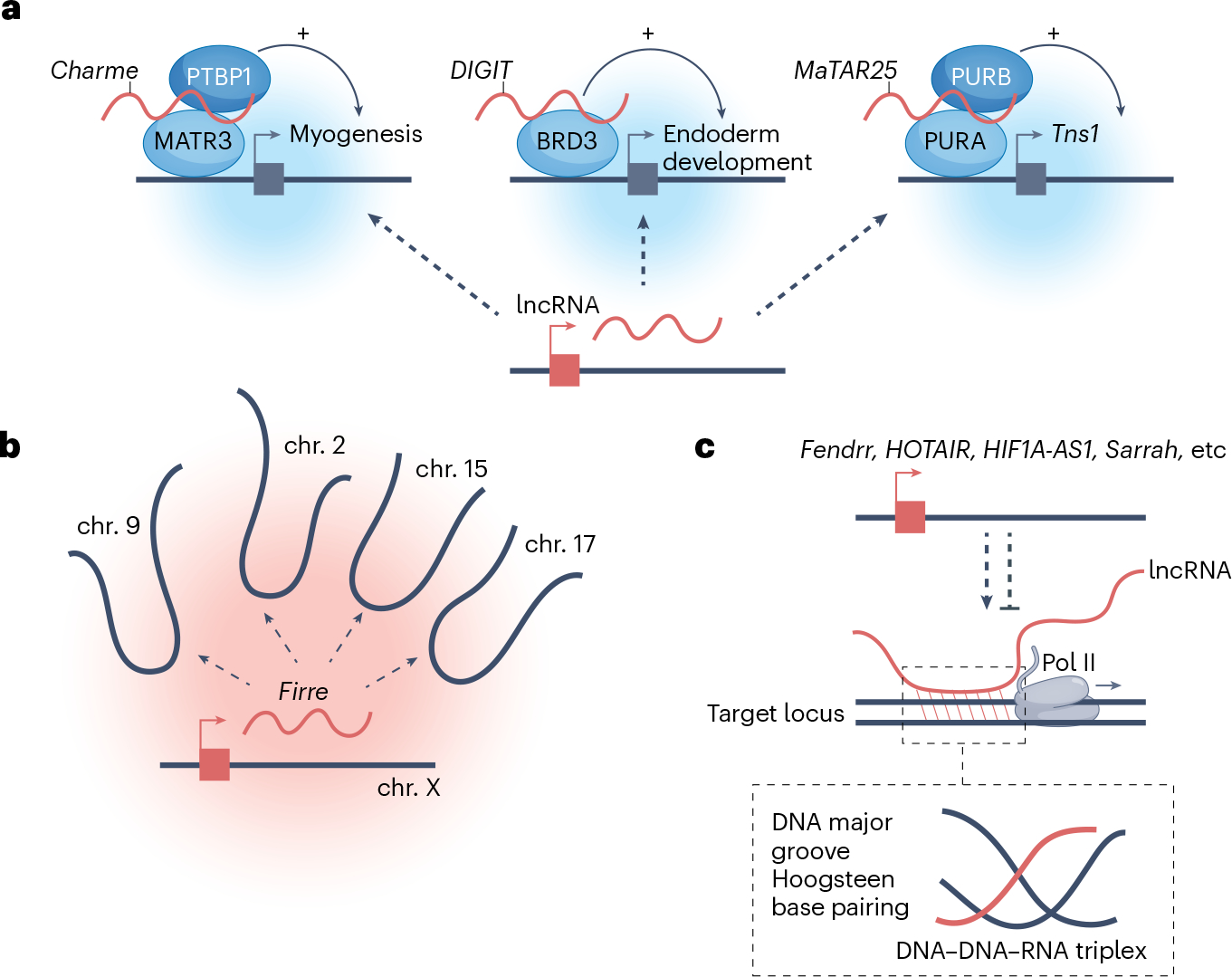
a, Association of the long non-coding RNAs (lncRNAs) Charme, DIGIT and mammary tumour-associated RNA 25 (MaTAR25) with polypyrimidine tract-binding protein 1 (PTBP1)–matrin 3 (MATR3), bromodomain-containing protein 3 (BRD3) and purine-rich element-binding protein A (PURA)–PURB, respectively, drives condensate formation (blue background) and localization at target genes. This localization promotes the activation of broad developmental or disease-associated transcription programmes. b, The lncRNA Firre promotes inter-chromosomal contacts, which facilitates the co-regulation of a genes with shared functions in energy metabolism. c, Sequence complementarity between lncRNAs and one or many genomic regions enables targeting of the lncRNA to specific loci in trans through the formation of a DNA–DNA–(lnc)RNA triplex that involves DNA major groove Hoogstein base pairing. Various lncRNAs, including Fendrr, HOTAIR, HIF1A-AS1, Sarrah and others, have been shown to engage in triplex formation and exert positive or negative effects on target-gene expression. Pol II, polymerase II.
Recent studies have also demonstrated a more general role for abundant nascent transcripts in maintaining regional chromatin compaction184. Analysis of chromatin-associated pre-mRNAs, lncRNAs and non-coding RNAs produced from repetitive regions has identified an RNA–protein scaffold that serves to counteract chromatin compaction and maintain active chromosome territories184. lncRNAs can also promote chromosomal reorganization by bringing genomic locations from different chromosomes in spatial proximity within the nucleus185. The X-linked lncRNA functional intergenic repeating RNA element (Firre) has been proposed to promote the formation of such an inter-chromosomal nuclear compartment, which contains co-regulated genes with a shared function in energy metabolism186,187 (Fig. 5b).
Engagement of targets through triplex formation
Some lncRNAs were proposed to specifically target genomic locations through the formation of hybrid DNA–DNA–RNA triplex structures. Initially, this model was put forth to explain cis-regulation by overlapping antisense lncRNAs, such as KHPS1 (refs. 188,189) and PARTICLE190. This model has been expanded to address genome-wide triplex formation based on computational identification of regions of lncRNA–DNA complementarity191–193 or experimental identification of RNAse H-resistant lncRNA–DNA heteroduplexes pulled down using an RNA–DNA-specific antibody193,194. Examples of lncRNAs proposed to engage this mechanism to repress or activate networks of genes in trans include Fendrr in mid-gestational embyos191, HOTAIR in mesenchymal stem cells192, HIF1A-AS1 in endothelial cells193 and Sarrah (also known as Oxct1as) in cardiomyocytes194 (Fig. 5c). A recent study proposed an additional role for hybrid triplexes, showing that KCNQ1OT1 forms triplexes to target gene-repressing complexes to transposable elements195. More work, however, is needed to define the extent to which DNA–DNA–RNA triplex structures are formed by lncRNAs for site-specific regulation at distant genomic sites.
Dual cis and trans regulation
Some lncRNAs have been shown to mediate regulatory activities both in cis and in trans. Notable examples include lncRNAs such as MEG3, which controls imprinting in cis but also mediates the p53 stress response196 or engages in triplex formation197; the auxin-inducible lncRNA Apolo in forming R-loops in cis and in trans as a regulator of auxin-responsive genes in plants198,199; and the cis-activating lincRNA-Cox2, which controls the expression of the neighbouring gene Ptgs2, but also modulates the expression of a wide range of immune genes through an unknown mechanism18. In particular, the trans activity of lincRNA-Cox2 was demonstrated in a mouse model by rescuing a lincRNA-Cox2 deletion with a lncRNA-expressing transgene18.
Roles of transcription-regulating lncRNAs in disease
As we begin to grasp the biological purpose of different types of regulatory lncRNAs, it becomes possible to explore their involvement in human Mendelian and polygenic diseases and oncogenesis, and their potential role as therapeutic targets.
Mendelian diseases
Identifying lncRNA gene mutations that cause Mendelian diseases poses major challenges because, unlike protein-coding sequences, there are no rules to predict the functionality of lncRNA sequence variants. Even in cases of lncRNA deletions, it is challenging to ascertain that phenotypes are not due to disruption of other functional elements, such as enhancers, located in the deleted region. Making this distinction usually requires complex genetic engineering approaches, such as combining deletions, transcription termination signals and insertion of RNA ribozymes.
Several lncRNA genes are located within genetically mapped loci that harbour Mendelian or monogenic mutations. For example, deletions encompassing the FENDRR locus lead to alveolar capillary dysplasia and misalignment of pulmonary veins (AVCD-MPV), although those deletions also disrupt elements that regulate the nearby gene FOXF1, which also harbours causal AVCD-MPV mutations76,200 (Table 1). Nonetheless, mice with disrupted Fendrr transcription recapitulate features of AVCD-MPV68,201. Likewise, variants in the lncRNA HELLPAR co-segregate with haemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, although more conclusive evidence is needed to prove the causality of distinct variants202. These examples, together with knowledge that lncRNA genes often contain or are adjacent to enhancers, highlight some of the serious challenges facing efforts to demonstrate the pathogenicity of lncRNA defects in Mendelian diseases.
The analysis of Maenli, discussed above, has illustrated how a Mendelian phenotype can be followed up with careful mouse genetic studies to specifically assess the role of a lncRNA in the disease. Two children with a limb malformation were found to harbour the same homozygous 27 kb non-coding deletion in the EN1 locus, whereas another individual with the same phenotype had a larger deletion in the same region in one allele, and an insertion in the other allele17 (Fig. 6a). They examined the syntenic mouse sequence which contains Maenli lncRNA, and either deleted the Maenli promoter or blocked its transcription by inserting a polyadenylation sequence, both of which phenocopied the human disease17. These cases suggested that the severe human developmental phenotype was caused by germ-line deletions of a cis-activating lncRNA, raising the question of how many rare or common genetic variants in lncRNAs might influence human health.
Fig. 6 |. Involvement of long non-coding RNAs in genetic diseases.
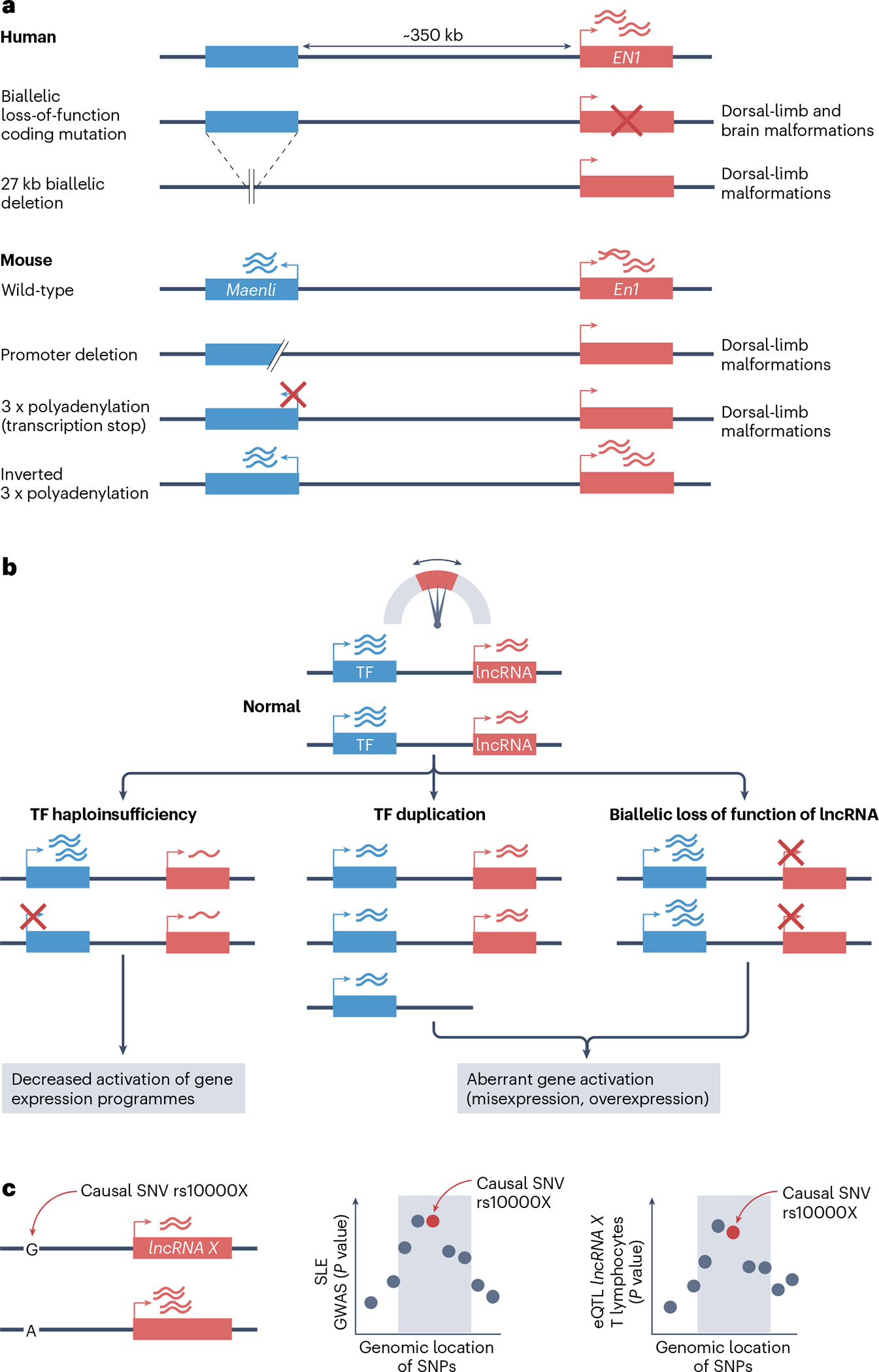
a, The human EN1 locus, which encodes a homeobox transcription factor (TF). EN1 harbours recessive coding mutations in an individual with limb and brain malformations, whereas far-upstream biallelic non-coding deletions (or a compound heterozygous deletion and insertion not shown here) cause dorsal-limb malformations. Maenli is a mouse long non-coding RNA (lncRNA) mapped to the orthologous minimal deleted region in humans. Deletion of the Maenli promoter, or insertion of polyadenylation signals of transcription termination, recapitulate limb malformations and lead to reduced En1 expression, whereas an inverted termination signal has no effect. b, Transcription stabilizers control dosage-sensitive TF genes. Small deviations in the expression levels of certain TFs can be caused by heterozygous loss-of-function mutations or duplications of TF genes, or by biallelic loss of function of the stabilizer lncRNA, causing abnormal cellular transcription with organismal phenotypes. In several examples, the defects in stabilizer lncRNAs and the dosage alterations of their target genes have the same phenotype (Table 1). c, An expression quantitative trait locus (eQTL), in which a single nucleotide variant (SNV) influences expression of a lncRNA (left). rs10000X is depicted as the identifier of a fictitious regulatory SNV that is causal for this eQTL. The two graphs on the right depict statistically significant P values of a group of adjacent SNVs for association with the presence of the autoimmune disease systemic lupus erythematosus (SLE) (top) or with a lncRNA eQTL detected in T cell lymphocytes, a cell type that is relevant to SLE (bottom). The co-localization of both sets of association P values means that the lncRNA is a plausible mediator of the disease association. Several hundred instances such as this have been identified, indicating that variation in lncRNA expression contributes to the susceptibility of common diseases. GWAS, genome-wide association studies.
Phenotypic relevance of transcription-stabilizing lncRNAs
So far, FENDRR and PVT1 are the only lncRNAs that restrain the transcription of adjacent TF genes for which there is genetic evidence of a role in human disease76,200. It is reasonable to expect that large-scale functional screens will uncover many more cis-regulatory lncRNAs, and whole genome sequencing has the potential to discover genetic defects in lncRNAs. However, not all functional genetic elements are vulnerable to disruptive mutations. For example, enhancers are thought to be relatively robust to loss of function owing to functional redundancy203–205.
Several considerations nevertheless indicate that genetic defects in stabilizer lncRNAs can result in strong phenotypes. Many of the TFs that are controlled by known stabilizer lncRNAs are very sensitive to gene dosage (Table 1). Thus, haploinsufficient germ-line mutations in HAND2, CHD2, FOXF1, HNF1A and PITX2 cause Mendelian diseases, and in some of these cases an increased dosage in mice or humans also has phenotypic consequences206–213 (Fig. 6b). Interestingly, somatic gain-of-function mutations or increased expression of MYC, the target of the lncRNA PVT1 (Table 1), constitute an established oncogenic mechanism214.
It is thus not surprising that mutations in lncRNAs that control the expression levels of these TFs frequently have phenotypes in mice (Fig. 6b). Different Hand2os1 deletions cause either abnormal heart function or heart malformations and embryonic lethality in mice66. Likewise, Hdnr disruption leads to increased Hand2 and severe cardiac malformations67,215, and deletion of Flicr decreases susceptibility for autoimmune diabetes in non-obese diabetic mice69. Pancreatic or germ-line Haster mutations cause diabetes70, and Chaserr null mutations cause embryonic lethality or severe growth retardation71 (Table 1).
Importantly, these in vivo lncRNA phenotypes are not simply associated with silencing of their target TF genes, as would be expected if there was inadvertent disruption of an enhancer, but are instead linked to increased expression of the adjacent TF genes. Interestingly, most stabilizer lncRNA mutants with organismal phenotypes exhibit only moderate changes in the expression of their target TF genes, which underscores the importance of maintaining TF concentrations within a narrow range. The strong mutant phenotypes also suggest that, unlike enhancers, cis-inhibitory lncRNAs have limited built-in redundancy. Thus, despite the challenges in annotating lncRNA mutations that are deleterious in humans, mouse genetics indicate that lncRNA defects can lead to disease.
Polygenic diseases
The most prevalent chronic human diseases, such as Alzheimer disease, coronary artery disease or type 2 diabetes, reflect the interplay of environmental factors with a large number of genetic variants. Although individual variants typically have very small effects on disease risk, the fact that they demonstrably influence human disease processes has the potential to shed light on causal mechanisms. A major fraction of susceptibility variants for common diseases identified in genome-wide association studies are non-coding, but the extent to which they act through lncRNAs is still unknown.
Numerous disease-risk variants have tight genetic co-localization with expression quantitative trait loci (eQTLs) for lncRNAs in disease-relevant cells (Fig. 6c). For example, common DNA variants that influence the expression of the lncRNA named ANRIL (also known as CDKN2B-AS1) co-localize with genetic association signals for coronary heart disease and type 2 diabetes216,217, and pancreatic islet eQTLs for LINC01512 (also known as HI-LNC77) as well as a splicing quantitative trait locus for LINC00261 co-localize with type 2 diabetes genetic association signals216. A recent systematic analysis of expression and splicing quantitative trait loci from a broad panel of tissues revealed that DNA variants influencing the expression of more than 100 lncRNAs co-localize with variants associated with 66 polygenic phenotypes. For more than 50% of these loci, the effect on the lncRNA eQTL appears to be exclusive, or stronger than effects on any protein-coding gene eQTL218. These data warrant in-depth studies to examine how specific lncRNAs can act as molecular effectors of genetic susceptibility for common diseases.
lncRNA defects in cancer
Recurrent somatic copy number variants have been identified in several lncRNA loci. Examples include PVT1 structural mutations in multiple cancer types74,219,220, amplification of FAL1 in approximately 10% of liver cancer221, amplification of SAMMSON in 10% of melanoma222 and loss of ANRIL in >50% of glioblastomas223. These regions, however, also harbour proto-oncogenes (MYC, MCL1, MITF) or the tumour suppressor CDKN2A, and are linked with enhancers, which hamper the ability to assess the pathogenic role of lncRNA defects224. Nonetheless, cancer-associated somatic structural variants such as focal deletions have been reported at the PVT1 promoter region in breast cancer and in large B cell lymphomas, as well as chromosomal rearrangements that separate PVT1 from MYC74,225. Furthermore, experimental deletions or transcription inhibition of the PVT1 promoter cause high MYC expression and increased cellular proliferation14,74
In addition to these genetic changes, abnormal expression of many lncRNAs has been linked to cancer progression224. Well-studied examples are MALAT1 overexpression, which is a strong predictor of metastasis in lung adenocarcinoma226, and increased HOTAIR expression, which correlates with progression to metastasis and poor outcomes in breast cancer227. Abnormal expression of such lncRNAs could contribute to oncogenesis regardless of their function in normal physiology. For example, a recent preprint reports that in a mouse model of lung adenocarcinoma, Malat1 overexpression is a driver of metastasis through dysregulation of gene expression and reprogramming of the tumour microenvironment228. Other studies have shown that increased HOTAIR abundance can alter the stoichiometry of PRCs, resulting in deregulation of gene expression227. Furthermore, overexpression of cis-acting lncRNAs, such as the imprinted lncRNA H19 (refs. 229–231), the gene-inhibiting lncRNAs PVT1 (refs. 85,232) and MORRBID233,234, and the gene-activating LINCRNA-P21 (ref. 235) also have oncogenic trans-regulatory activities.
X-chromosome inactivation defects
XCI is essential for development236–238 and conditional mouse deletions of Xist in the haematopoietic system cause aberrant epigenetic states and oncogenic transformation239,240. However, recent studies have revealed that XCI is not permanent in all cell lineages, as reversals can be observed in specific adult immune cell subtypes241. This reversal has been proposed to underlie the female-specific predisposition for autoimmune diseases through gene dosage increase from the X chromosome, which is known to have a high density of immunity regulating genes241–245. Another recent study, reported in a preprint, has directly implicated the immunogenicity of XIST ribonucleoprotein complexes in autoimmune disease mechanisms246.
Therapeutic modulation of lncRNAs
Regardless of whether a disease is caused by a lncRNA defect, it is sometimes possible to envision therapeutic targeting of a lncRNA to modulate a disease-relevant process, using sequence-specific RNA degradation, RNA mimetics or genome editing tools that control the transcription of lncRNAs247,248. A good example is Angelman syndrome, which is an imprinting neurodevelopmental disorder caused by deletions or mutations of the active maternal UBE3A allele. Several clinical trials are underway to activate the silenced paternal UBE3A allele with antisense oligonucleotides that block or cause degradation of the lncRNA UBE3A-ATS, following proof-of-concept studies in model systems153,248,249.
Another important therapeutic application has been the use of a lncRNA to correct abnormal gene dosage. Insertion of an inducible XIST transgene into one copy of chromosome 21 in induced pluripotent stem cells from an individual with trisomy 21 was shown to cause silencing in cis of the chromosome, and reversal of major transcriptional and other cellular defects250.
Small molecules that target and modulate the activity and stability of lncRNAs represent an additional therapeutic avenue. Compounds have been developed to target the stabilizing 3′-end triple helix structure of MALAT1, given its strong association with metastasis in solid tumours251–253. Another example is X1, a small molecule that binds XIST A-repeats and displaces PRC2 and SPEN, thereby blocking XCI254. This approach has been proposed to de-repress wild-type alleles in X-linked disorders such as Rett syndrome.
Conclusion and future perspective
Ever since the discovery of lncRNAs, efforts to understand their biological significance have met daunting challenges. Investigations started from a blank slate, with no sense of what type of molecular entities might exist under the lncRNA umbrella, or which experimental tools could be used to elucidate their function. Despite these obstacles, the past years have witnessed major breakthroughs, many of which have come from exhaustive systematic efforts to dissect the function of single lncRNAs. These studies have led to the discovery of a spectrum of lncRNA functions, including essential gene-activating functions, and specialized cis-regulatory feedback mechanisms.
These findings have also raised a long list of pressing new questions. For example, what fraction of the catalogued lncRNAs is functional, and how many lncRNAs belong to the categories identified so far? How many functional lncRNAs act through RNA-dependent mechanisms, as opposed to those that primarily involve the activation of lncRNA promoters or transcription? For lncRNAs with an ascribed function, our knowledge of the underlying mechanisms is fragmented, which surely explains several apparent paradoxes, such as the observation that the transcription of different lncRNAs can lead to either silencing or activation of their antisense genes. Likewise, transcription activation of lncRNAs has been shown to be essential for both cis-activating and inhibiting functions, but the rules that underlie these outcomes are not well understood. Another crucial gap in the field is our lack of knowledge of the major sequence determinants of lncRNA functions, and their vulnerability to disease-causing variation. Understanding the molecular underpinnings of different types of functional lncRNAs, combined with knowledge of which lncRNAs act in disease-relevant processes, holds promise for the development of new therapeutic strategies.
Acknowledgements
The authors thank T. Graff, M. Cuenca-Ardura and B. Payer for critical reading of this manuscript. This work was supported by European Research Council (789055) and Spanish Ministry of Science and Innovation (PID2021-122522OB-I00) grants to J.F., and by National Institute of Health (R01CA262286 and R37CA230580) grants to N.D.
Glossary
- CpG islands
Genomic regions of 500 nucleotides or longer with >50% CpG dinucleotide repeat content. CpG islands are associated with the transcription start sites of most housekeeping genes and as many as 40% of tissue-specific genes; they are bound by regulatory proteins
- CTCF
A zinc-finger transcription factor (TF), also known as CCCTC-binding factor, that binds specific DNA sequences and participates in the formation of chromatin loops that influence gene transcription by defining the boundaries of topologically associated domains (TADs) and bringing enhancers into proximity with promoters
- DNA–DNA–RNA triplex
A structure in which single-stranded RNA invades the major groove of double-stranded DNA and binds by forming Hoogsteen hydrogen bonds. DNA–DNA–lncRNA triplexes can be identified by pull-downs with a triplex-specific antibody
- Enhancer RNAs
(eRNAs). Non-coding RNAs that are bidirectionally transcribed from enhancer regions, and are typically ≤500 nucleotides and unstable (half-life ≤ 2 min)
- Enhancers
Genomic regions that are recognized by transcription factors (TFs) and activate and increase the transcription of genes in cis, sometimes from considerable distances. Active enhancers are flanked by nucleosomes that carry post-translational histone modifications such as histone H3 acetylated at lysine 27 (H3K27ac) and H3 methylated at lysine 4 (H3K4me1)
- Expression quantitative trait loci
(eQTL). Genetic loci in which different alleles of a DNA variant influence expression levels of coding or non-coding transcripts
- Focal deletions
Cancer-associated genomic deletions smaller than 5 Mb that affect both alleles
- Genome-wide association studies
(GWAS). Studies that survey DNA variants genome-wide to identify those showing association with a disease or trait. GWAS have been used to discover susceptibility variants for prevalent polygenic diseases. A large fraction of significant associations are found in non-coding genomic regions, indicating that they are mediated by genetic variants that influence regulatory functions
- lncRNAs that act in cis
Long non-coding RNAs (lncRNAs) that act on the same chromosome from which they are transcribed, including the regulation of a neighbouring gene, of multiple genes or of the entire chromosome
- Silencers
Genomic regions that are bound by repressive transcription factors (TFs) and decrease the transcription of genes in cis
- Splicing quantitative trait loci
Genetic loci in which different alleles influence RNA splicing patterns
- Topologically associated domains
(TADs). Genomic regions defined by having a higher frequency of long-range chromatin contacts, such as between genes and their regulatory elements, than the frequency of contacts with elements outside the region
- Transcriptional condensates
Chromatin-associated, dynamic nuclear assemblies comprising a heterogeneous mix of RNAs, transcription factors (TFs) and co-regulators that modulate transcriptional output
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Reviews Molecular Cell Biology thanks Takayuki Nojima, Igor Ulitsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Lipshitz HD, Peattie DA & Hogness DS Novel transcripts from the ultrabithorax domain of the bithorax complex. Genes. Dev. 1, 307–322 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Cumberledge S, Zaratzian A & Sakonju S Characterization of two RNAs transcribed from the cis-regulatory region of the abd-A domain within the Drosophila bithorax complex. Proc. Natl Acad. Sci. USA 87, 3259–3263 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattick JS et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 24, 430–447 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson DM et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160, 595–606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgado-Palacin L et al. The TINCR ubiquitin-like microprotein is a tumor suppressor in squamous cell carcinoma. Nat. Commun. 14, 1328 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frankish A et al. GENCODE: reference annotation for the human and mouse genomes in 2023. Nucleic Acids Res. 51, D942–D949 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil N & Ulitsky I Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 21, 102–117 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Rinn JL & Chang HY Long noncoding RNAs: molecular modalities to organismal functions. Annu. Rev. Biochem. 89, 283–308 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Moran I et al. Human β-cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 16, 435–448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabili MN et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes. Dev. 25, 1915–1927 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hon CC et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature 543, 199–204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akerman I et al. Human pancreatic β cell lncRNAs control cell-specific regulatory networks. Cell Metab. 25, 400–411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrova N et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol. Cell 54, 777–790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olivero CE et al. p53 activates the long noncoding RNA Pvt1b to inhibit myc and suppress tumorigenesis. Mol. Cell 77, 761–774.e8 (2020). This study identifies a p53-induced isoform of the lncRNA Pvt1, which acts in cis to suppress Myc transcription in response to stress and thus limits cellular proliferation.
- 15. Winkler L et al. Functional elements of the cis-regulatory lincRNA-p21. Cell Rep. 39, 110687 (2022). Systematic genetic dissection of the lincRNA-p21 locus reveals that transcription initiation of lincRNA-p21 is sufficient for stimulation of p21 expression in cis.
- 16.Gil N et al. Complex regulation of Eomes levels mediated through distinct functional features of the Meteor long non-coding RNA locus. Cell Rep. 42, 112569 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allou L et al. Non-coding deletions identify Maenli lncRNA as a limb-specific En1 regulator. Nature 592, 93–98 (2021). Genetically engineered mouse models reveal that transcript elongation of the lncRNA Maenli promotes En1 expression and supports limb development. This study demonstrates that a human developmental limb disorder is likely caused by a monogenic lncRNA defect.
- 18.Elling R et al. Genetic models reveal cis and trans immune-regulatory activities for lincRNA-Cox2. Cell Rep. 25, 1511–1524.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isoda T et al. Non-coding transcription instructs chromatin folding and compartmentalization to dictate enhancer–promoter communication and T cell fate. Cell 171, 103–119.e18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry RB, Hezroni H, Goldrich MJ & Ulitsky I Regulation of neuroregeneration by long noncoding RNAs. Mol. Cell 72, 553–567.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Santa F et al. A large fraction of extragenic RNA Pol II transcription sites overlap enhancers. PLoS Biol. 8, e1000384 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson R et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heintzman ND et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Paralkar VR et al. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood 123, 1927–1937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paralkar VR et al. Unlinking an lncRNA from its associated cis element. Mol. Cell 62, 104–110 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espinosa JM Revisiting lncRNAs: how do you know yours is not an eRNA? Mol. Cell 62, 1–2 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Wang KC et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradeepa MM et al. Psip1/p52 regulates posterior Hoxa genes through activation of lncRNA Hottip. PLoS Genet. 13, e1006677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unfried JP & Ulitsky I Substoichiometric action of long noncoding RNAs. Nat. Cell Biol. 24, 608–615 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Henninger JE et al. RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225.e24 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharp PA, Chakraborty AK, Henninger JE & Young RA RNA in formation and regulation of transcriptional condensates. RNA 28, 52–57 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oksuz O et al. Transcription factors interact with RNA to regulate genes. Mol. Cell 83, 2449–2463.e13 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ntini E et al. Long ncRNA A-ROD activates its target gene DKK1 at its release from chromatin. Nat. Commun. 9, 1636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huarte M et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groff AF et al. In vivo characterization of Linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 16, 2178–2186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuhata R et al. LincRNA-p21 exon 1 expression correlates with Cdkn1a expression in vivo. Genes. Cell 27, 14–24 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Engreitz JM et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gil N & Ulitsky I Production of spliced long noncoding RNAs specifies regions with increased enhancer activity. Cell Syst. 7, 537–547.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haerty W & Ponting CP Unexpected selection to retain high GC content and splicing enhancers within exons of multiexonic lncRNA loci. RNA 21, 333–346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuler A, Ghanbarian AT & Hurst LD Purifying selection on splice-related motifs, not expression level nor RNA folding, explains nearly all constraint on human lincRNAs. Mol. Biol. Evol. 31, 3164–3183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan JY & Marques AC The activity of human enhancers is modulated by the splicing of their associated lncRNAs. PLoS Comput. Biol. 18, e1009722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canzio D et al. Antisense lncRNA transcription mediates DNA demethylation to drive stochastic protocadherin α promoter choice. Cell 177, 639–653.e15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinz S et al. Transcription elongation can affect genome 3D structure. Cell 174, 1522–1536.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Ubelmesser N, Barbieri M & Papantonis A Enhancer–promoter contact formation requires RNAPII and antagonizes loop extrusion. Nat. Genet. 55, 832–840 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Banigan EJ et al. Transcription shapes 3D chromatin organization by interacting with loop extrusion. Proc. Natl Acad. Sci. USA 120, e2210480120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai F et al. Activating RNAs associate with mediator to enhance chromatin architecture and transcription. Nature 494, 497–501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498, 516–520 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melo CA et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol. Cell 49, 524–535 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Orom UA et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 143, 46–58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalantari R, Chiang CM & Corey DR Regulation of mammalian transcription and splicing by nuclear RNAi. Nucleic Acids Res. 44, 524–537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khvorova A Modulation of DNA transcription: the future of ASO therapeutics? Cell 185, 2011–2013 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Marasco LE et al. Counteracting chromatin effects of a splicing-correcting antisense oligonucleotide improves its therapeutic efficacy in spinal muscular atrophy. Cell 185, 2057–2070.e15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JH et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell 81, 3368–3385.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahnamoun H et al. RNAs interact with BRD4 to promote enhanced chromatin engagement and transcription activation. Nat. Struct. Mol. Biol. 25, 687–697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang L et al. Complementary Alu sequences mediate enhancer–promoter selectivity. Nature 619, 868–875 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Barshad G et al. RNA polymerase II dynamics shape enhancer–promoter interactions. Nat. Genet. 55, 1370–1380 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dao LTM et al. Genome-wide characterization of mammalian promoters with distal enhancer functions. Nat. Genet. 49, 1073–1081 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Guttman M et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulitsky I, Shkumatava A, Jan CH, Sive H & Bartel DP Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 147, 1537–1550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu N et al. Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 173, 430–442.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang B, van Weerd JH, Hamoen FL & Snyder MP Identification of non-coding silencer elements and their regulation of gene expression. Nat. Rev. Mol. Cell Biol. 24, 383–395 (2023). [DOI] [PubMed] [Google Scholar]
- 62.Maamar H, Cabili MN, Rinn J & Raj A linc-HOXA1 is a noncoding RNA that represses Hoxa1 transcription in cis. Genes. Dev. 27, 1260–1271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su G et al. Enhancer architecture-dependent multilayered transcriptional regulation orchestrates RA signaling-induced early lineage differentiation of ESCs. Nucleic Acids Res. 49, 11575–11595 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin Y et al. Opposing roles for the lncRNA haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. Cell Stem Cell 16, 504–516 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Anderson KM et al. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han X et al. The lncRNA Hand2os1/Uph locus orchestrates heart development through regulation of precise expression of Hand2. Development 146, dev176198 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Ritter N et al. The lncRNA locus handsdown regulates cardiac gene programs and is essential for early mouse development. Dev. Cell 50, 644–657.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Grote P et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 24, 206–214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zemmour D, Pratama A, Loughhead SM, Mathis D & Benoist C Flicr, a long noncoding RNA, modulates Foxp3 expression and autoimmunity. Proc. Natl Acad. Sci. USA 114, E3472–E3480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beucher A et al. The HASTER lncRNA promoter is a cis-acting transcriptional stabilizer of HNF1A. Nat. Cell Biol. 24, 1528–1540 (2022). This study shows that the promoter of the lncRNA HASTER ensures that levels of the transcripion factor HNF1A are maintained within a narrow homeostatic range. Haster deficiency causes abnormal HNF1A genomic occupancy and diabetes in mice.
- 71.Rom A et al. Regulation of CHD2 expression by the Chaserr long noncoding RNA gene is essential for viability. Nat. Commun. 10, 5092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bond AM et al. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat. Neurosci. 12, 1020–1027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amandio AR, Necsulea A, Joye E, Mascrez B & Duboule D Hotair is dispensible for mouse development. PLoS Genet. 12, e1006232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho SW et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell 173, 1398–1412.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen FL et al. The long noncoding RNA Playrr regulates Pitx2 dosage and protects against cardiac arrhythmias. Preprint at bioRxiv 10.1101/2022.09.20.508562 (2022). [DOI] [Google Scholar]
- 76.Szafranski P, Gambin T, Karolak JA, Popek E & Stankiewicz P Lung-specific distant enhancer cis regulates expression of FOXF1 and lncRNA FENDRR. Hum. Mutat. 42, 694–698 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali T & Grote P Beyond the RNA-dependent function of lncRNA genes. eLife 9, e60583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghildiyal R et al. Loss of long noncoding RNA NXTAR in prostate cancer augments androgen receptor expression and enzalutamide resistance. Cancer Res. 82, 155–168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kribelbauer JF, Rastogi C, Bussemaker HJ & Mann RS Low-affinity binding sites and the transcription factor specificity paradox in eukaryotes. Annu. Rev. Cell Dev. Biol. 35, 357–379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Golson ML & Kaestner KH Fox transcription factors: from development to disease. Development 143, 4558–4570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Servitja JM et al. Hnf1α (MODY3) controls tissue-specific transcriptional programs and exerts opposed effects on cell growth in pancreatic islets and liver. Mol. Cell Biol. 29, 2945–2959 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez Garcia M et al. Structural features of transcription factors associating with nucleosome binding. Mol. Cell 75, 921–932.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang P et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Ali T et al. Fendrr synergizes with Wnt signalling to regulate fibrosis related genes during lung development via its RNA:dsDNA triplex element. Nucleic Acids Res. 51, 6227–6237 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colombo T, Farina L, Macino G & Paci P PVT1: a rising star among oncogenic long noncoding RNAs. Biomed. Res. Int. 2015, 304208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tesfaye E et al. The p53 transcriptional response across tumor types reveals core and senescence-specific signatures modulated by long noncoding RNAs. Proc. Natl Acad. Sci. USA 118, e2025539118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotzin JJ et al. The long noncoding RNA morrbid regulates CD8 T cells in response to viral infection. Proc. Natl Acad. Sci. USA 116, 11916–11925 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kotzin JJ et al. The long non-coding RNA morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y et al. Natural temperature fluctuations promote COOLAIR regulation of FLC. Genes. Dev. 35, 888–898 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jegu T, Aeby E & Lee JT The X chromosome in space. Nat. Rev. Genet. 18, 377–389 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Deng X, Berletch JB, Nguyen DK & Disteche CM X chromosome regulation: diverse patterns in development, tissues and disease. Nat. Rev. Genet. 15, 367–378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brockdorff N, Bowness JS & Wei G Progress toward understanding chromosome silencing by Xist RNA. Genes. Dev. 34, 733–744 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Augui S, Nora EP & Heard E Regulation of X-chromosome inactivation by the X-inactivation centre. Nat. Rev. Genet. 12, 429–442 (2011). [DOI] [PubMed] [Google Scholar]
- 94.Galupa R & Heard E X-chromosome inactivation: a crossroads between chromosome architecture and gene regulation. Annu. Rev. Genet. 52, 535–566 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Furlan G & Galupa R Mechanisms of choice in X-chromosome inactivation. Cells 11, 535 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mutzel V & Schulz EG Dosage sensing, threshold responses, and epigenetic memory: a systems biology perspective on random X-chromosome inactivation. Bioessays 42, e1900163 (2020). [DOI] [PubMed] [Google Scholar]
- 97.Jacobson EC, Pandya-Jones A & Plath K A lifelong duty: how Xist maintains the inactive X chromosome. Curr. Opin. Genet. Dev. 75, 101927 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Bemmel JG et al. The bipartite TAD organization of the X-inactivation center ensures opposing developmental regulation of Tsix and Xist. Nat. Genet. 51, 1024–1034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gjaltema RAF et al. Distal and proximal cis-regulatory elements sense X chromosome dosage and developmental state at the Xist locus. Mol. Cell 82, 190–208.e17 (2022). [DOI] [PubMed] [Google Scholar]
- 100. Rosspopoff O et al. Species-specific regulation of XIST by the JPX/FTX orthologs. Nucleic Acids Res. 51, 2177–2194 (2023). Functional similarities and differences between the human and mouse lncRNAs orthologues JPX and Jpx and FTX and Ftx highlight the complementary roles of lncRNA transcription and the mature lncRNAs in XCI.
- 101.Quesada-Espinosa JF et al. First female with Allan–Herndon–Dudley syndrome and partial deletion of X-inactivation center. Neurogenetics 22, 343–346 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Sun S et al. Jpx RNA activates Xist by evicting CTCF. Cell 153, 1537–1551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loda A, Collombet S & Heard E Gene regulation in time and space during X-chromosome inactivation. Nat. Rev. Mol. Cell Biol. 23, 231–249 (2022). [DOI] [PubMed] [Google Scholar]
- 104.Ogawa Y & Lee JT Xite, X-inactivation intergenic transcription elements that regulate the probability of choice. Mol. Cell 11, 731–743 (2003). [DOI] [PubMed] [Google Scholar]
- 105.Galupa R et al. A conserved noncoding locus regulates random monoallelic xist expression across a topological boundary. Mol. Cell 77, 352–367.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee JT & Lu N Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99, 47–57 (1999). [DOI] [PubMed] [Google Scholar]
- 107.Wutz A & Jaenisch R A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol. Cell 5, 695–705 (2000). [DOI] [PubMed] [Google Scholar]
- 108.Lee JT, Strauss WM, Dausman JA & Jaenisch RA 450 kb transgene displays properties of the mammalian X-inactivation center. Cell 86, 83–94 (1996). [DOI] [PubMed] [Google Scholar]
- 109.Chu C et al. Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Markaki Y et al. Xist nucleates local protein gradients to propagate silencing across the X chromosome. Cell 184, 6212 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McHugh CA et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Minajigi A et al. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science 349, aab2276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raposo AC, Casanova M, Gendrel AV & da Rocha ST The tandem repeat modules of Xist lncRNA: a Swiss army knife for the control of X-chromosome inactivation. Biochem. Soc. Trans. 49, 2549–2560 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carter AC et al. Spen links RNA-mediated endogenous retrovirus silencing and X chromosome inactivation. eLife 9, e54508 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dossin F et al. SPEN integrates transcriptional and epigenetic control of X-inactivation. Nature 578, 455–460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jachowicz JW et al. Xist spatially amplifies SHARP/SPEN recruitment to balance chromosome-wide silencing and specificity to the X chromosome. Nat. Struct. Mol. Biol. 29, 239–249 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zylicz JJ et al. The implication of early chromatin changes in X chromosome inactivation. Cell 176, 182–197.e23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bousard A et al. The role of Xist-mediated Polycomb recruitment in the initiation of X-chromosome inactivation. EMBO Rep. 20, e48019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jansz N et al. Smchd1 targeting to the inactive X is dependent on the Xist–HnrnpK–PRC1 pathway. Cell Rep. 25, 1912–1923.e9 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Pintacuda G et al. hnRNPK recruits PCGF3/5–PRC1 to the Xist RNA B-repeat to establish polycomb-mediated chromosomal silencing. Mol. Cell 68, 955–969.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang CY, Colognori D, Sunwoo H, Wang D & Lee JT PRC1 collaborates with SMCHD1 to fold the X-chromosome and spread Xist RNA between chromosome compartments. Nat. Commun. 10, 2950 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang CY, Jegu T, Chu HP, Oh HJ & Lee JT SMCHD1 merges chromosome compartments and assists formation of super-structures on the inactive X. Cell 174, 406–421.e25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colognori D, Sunwoo H, Wang D, Wang CY & Lee JT Xist repeats A and B account for two distinct phases of X inactivation establishment. Dev. Cell 54, 21–32.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pandya-Jones A et al. A protein assembly mediates Xist localization and gene silencing. Nature 587, 145–151 (2020). Assembly of multiple RNA-binding proteins on Xist E-repeats promotes homotypic and heterotypic interactions that result in the formation of a condensate, which is essential for gene silencing. Once formed, this condensate can sustain XCI in absence of Xist.
- 125.Sunwoo H, Colognori D, Froberg JE, Jeon Y & Lee JT Repeat E anchors Xist RNA to the inactive X chromosomal compartment through CDKN1A-interacting protein (CIZ1). Proc. Natl Acad. Sci. USA 114, 10654–10659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strehle M & Guttman M Xist drives spatial compartmentalization of DNA and protein to orchestrate initiation and maintenance of X inactivation. Curr. Opin. Cell Biol. 64, 139–147 (2020). [DOI] [PubMed] [Google Scholar]
- 127.de Napoles M et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7, 663–676 (2004). [DOI] [PubMed] [Google Scholar]
- 128.Sun BK, Deaton AM & Lee JT A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol. Cell 21, 617–628 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Zhao J, Sun BK, Erwin JA, Song JJ & Lee JT Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750–756 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Engreitz JM et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, 1237973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simon MD et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature 504, 465–469 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tucci V, Isles AR, Kelsey G, Ferguson-Smith AC & Erice Imprinting G Genomic imprinting and physiological processes in mammals. Cell 176, 952–965 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Barlow DP & Bartolomei MS Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 6, a018382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guenzl PM & Barlow DP Macro lncRNAs: a new layer of cis-regulatory information in the mammalian genome. RNA Biol. 9, 731–741 (2012). [DOI] [PubMed] [Google Scholar]
- 135.Latos PA & Barlow DP Regulation of imprinted expression by macro non-coding RNAs. RNA Biol. 6, 100–106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kota SK et al. ICR noncoding RNA expression controls imprinting and DNA replication at the Dlk1–Dio3 domain. Dev. Cell 31, 19–33 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Schertzer MD et al. lncRNA-induced spread of Polycomb controlled by genome architecture, RNA abundance, and CpG island DNA. Mol. Cell 75, 523–537.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seidl CI, Stricker SH & Barlow DP The imprinted Air ncRNA is an atypical RNAPII transcript that evades splicing and escapes nuclear export. EMBO J. 25, 3565–3575 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Terranova R et al. Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Dev. Cell 15, 668–679 (2008). [DOI] [PubMed] [Google Scholar]
- 140.Tibbit CJ et al. Antisense activity across the Nesp promoter is required for Nespas-mediated silencing in the imprinted Gnas cluster. Noncoding RNA 1, 246–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quinodoz SA et al. RNA promotes the formation of spatial compartments in the nucleus. Cell 184, 5775–5790.e30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.MacDonald WA & Mann MRW Long noncoding RNA functionality in imprinted domain regulation. PLoS Genet. 16, e1008930 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pauler FM, Koerner MV & Barlow DP Silencing by imprinted noncoding RNAs: is transcription the answer? Trends Genet. 23, 284–292 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hao N, Palmer AC, Dodd IB & Shearwin KE Directing traffic on DNA — how transcription factors relieve or induce transcriptional interference. Transcription 8, 120–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andergassen D et al. The Airn lncRNA does not require any DNA elements within its locus to silence distant imprinted genes. PLoS Genet. 15, e1008268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Latos PA et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 338, 1469–1472 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Santoro F et al. Imprinted Igf2r silencing depends on continuous Airn lncRNA expression and is not restricted to a developmental window. Development 140, 1184–1195 (2013). [DOI] [PubMed] [Google Scholar]
- 148.Sleutels F, Zwart R & Barlow DP The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature 415, 810–813 (2002). [DOI] [PubMed] [Google Scholar]
- 149.Golding MC et al. Depletion of Kcnq1ot1 non-coding RNA does not affect imprinting maintenance in stem cells. Development 138, 3667–3678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mancini-Dinardo D, Steele SJ, Levorse JM, Ingram RS & Tilghman SM Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes. Dev. 20, 1268–1282 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meng L, Person RE & Beaudet AL Ube3a-ATS is an atypical RNA polymerase II transcript that represses the paternal expression of Ube3a. Hum. Mol. Genet. 21, 3001–3012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meng L et al. Truncation of Ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. PLoS Genet. 9, e1004039 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meng L et al. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature 518, 409–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Katayama S et al. Antisense transcription in the mammalian transcriptome. Science 309, 1564–1566 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Lewis A et al. Epigenetic dynamics of the Kcnq1 imprinted domain in the early embryo. Development 133, 4203–4210 (2006). [DOI] [PubMed] [Google Scholar]
- 156.Lewis A et al. Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat. Genet. 36, 1291–1295 (2004). [DOI] [PubMed] [Google Scholar]
- 157.Mohammad F, Mondal T, Guseva N, Pandey GK & Kanduri C Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 137, 2493–2499 (2010). [DOI] [PubMed] [Google Scholar]
- 158.Nagano T et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322, 1717–1720 (2008). [DOI] [PubMed] [Google Scholar]
- 159.Pandey RR et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 32, 232–246 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Redrup L et al. The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development 136, 525–530 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Umlauf D et al. Imprinting along the Kcnq1 domain on mouse chromosome 7 involves repressive histone methylation and recruitment of Polycomb group complexes. Nat. Genet. 36, 1296–1300 (2004). [DOI] [PubMed] [Google Scholar]
- 162.Wagschal A et al. G9a histone methyltransferase contributes to imprinting in the mouse placenta. Mol. Cell Biol. 28, 1104–1113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Braceros AK et al. Proximity-dependent recruitment of Polycomb repressive complexes by the lncRNA Airn. Cell Rep. 42, 112803 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Long Y et al. RNA is essential for PRC2 chromatin occupancy and function in human pluripotent stem cells. Nat. Genet. 52, 931–938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lleres D et al. CTCF modulates allele-specific sub-TAD organization and imprinted gene activity at the mouse Dlk1–Dio3 and Igf2–H19 domains. Genome Biol. 20, 272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hansen AS et al. Distinct classes of chromatin loops revealed by deletion of an RNA-binding region in CTCF. Mol. Cell 76, 395–411.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saldana-Meyer R et al. RNA interactions are essential for CTCF-mediated genome organization. Mol. Cell 76, 412–422.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kurukuti S et al. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl Acad. Sci. USA 103, 10684–10689 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Murrell A, Heeson S & Reik W Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat. Genet. 36, 889–893 (2004). [DOI] [PubMed] [Google Scholar]
- 170.Kopp F & Mendell JT Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rinn JL et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li L et al. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep. 5, 3–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davidovich C et al. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol. Cell 57, 552–558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schorderet P & Duboule D Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet. 7, e1002071 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Selleri L et al. A hox-embedded long noncoding RNA: is it all hot air? PLoS Genet. 12, e1006485 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith KP, Hall LL & Lawrence JB Nuclear hubs built on RNAs and clustered organization of the genome. Curr. Opin. Cell Biol. 64, 67–76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hutchinson JN et al. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8, 39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.West JA et al. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol. Cell 55, 791–802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ballarino M et al. Deficiency in the nuclear long noncoding RNA charme causes myogenic defects and heart remodeling in mice. EMBO J. 37, e99697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Desideri F et al. Intronic determinants coordinate charme lncRNA nuclear activity through the interaction with MATR3 and PTBP1. Cell Rep. 33, 108548 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Taliani V et al. The long noncoding RNA Charme supervises cardiomyocyte maturation by controlling cell differentiation programs in the developing heart. eLife 12, e81360 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Daneshvar K et al. lncRNA DIGIT and BRD3 protein form phase-separated condensates to regulate endoderm differentiation. Nat. Cell Biol. 22, 1211–1222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chang KC et al. MaTAR25 lncRNA regulates the Tensin1 gene to impact breast cancer progression. Nat. Commun. 11, 6438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Creamer KM, Kolpa HJ & Lawrence JB Nascent RNA scaffolds contribute to chromosome territory architecture and counter chromatin compaction. Mol. Cell 81, 3509–3525.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mele M & Rinn JL “Cat’s cradling” the 3D genome by the act of lncRNA transcription. Mol. Cell 62, 657–664 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Andergassen D et al. In vivo firre and Dxz4 deletion elucidates roles for autosomal gene regulation. eLife 8, e47214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hacisuleyman E et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat. Struct. Mol. Biol. 21, 198–206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blank-Giwojna A, Postepska-Igielska A & Grummt I lncRNA KHPS1 activates a poised enhancer by triplex-dependent recruitment of epigenomic regulators. Cell Rep. 26, 2904–2915.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 189.Postepska-Igielska A et al. lncRNA Khps1 regulates expression of the proto-oncogene SPHK1 via triplex-mediated changes in chromatin structure. Mol. Cell 60, 626–636 (2015). [DOI] [PubMed] [Google Scholar]
- 190.O’Leary VB et al. PARTICLE, a triplex-forming long ncRNA, regulates locus-specific methylation in response to low-dose irradiation. Cell Rep. 11, 474–485 (2015). [DOI] [PubMed] [Google Scholar]
- 191.Grote P & Herrmann BG The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol. 10, 1579–1585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kalwa M et al. The lncRNA HOTAIR impacts on mesenchymal stem cells via triple helix formation. Nucleic Acids Res. 44, 10631–10643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Leisegang MS et al. HIF1α-AS1 is a DNA:DNA:RNA triplex-forming lncRNA interacting with the HUSH complex. Nat. Commun. 13, 6563 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Trembinski DJ et al. Aging-regulated anti-apoptotic long non-coding RNA Sarrah augments recovery from acute myocardial infarction. Nat. Commun. 11, 2039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang X et al. KCNQ1OT1 promotes genome-wide transposon repression by guiding RNA-DNA triplexes and HP1 binding. Nat. Cell Biol. 24, 1617–1629 (2022). [DOI] [PubMed] [Google Scholar]
- 196.Uroda T et al. Conserved pseudoknots in lncRNA MEG3 are essential for stimulation of the p53 pathway. Mol. Cell 75, 982–995.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mondal T et al. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA–DNA triplex structures. Nat. Commun. 6, 7743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ariel F et al. Noncoding transcription by alternative RNA polymerases dynamically regulates an auxin-driven chromatin loop. Mol. Cell 55, 383–396 (2014). [DOI] [PubMed] [Google Scholar]
- 199.Ariel F et al. R-loop mediated trans action of the APOLO long noncoding RNA. Mol. Cell 77, 1055–1065.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 200.Szafranski P et al. Small noncoding differentially methylated copy-number variants, including lncRNA genes, cause a lethal lung developmental disorder. Genome Res. 23, 23–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sauvageau M et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife 2, e01749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van Dijk M et al. HELLP babies link a novel lincRNA to the trophoblast cell cycle. J. Clin. Invest. 122, 4003–4011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kvon EZ, Waymack R, Gad M & Wunderlich Z Enhancer redundancy in development and disease. Nat. Rev. Genet. 22, 324–336 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miguel-Escalada I et al. Pancreas agenesis mutations disrupt a lead enhancer controlling a developmental enhancer cluster. Dev. Cell 57, 1922–1936.e9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Osterwalder M et al. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554, 239–243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chenier S et al. CHD2 haploinsufficiency is associated with developmental delay, intellectual disability, epilepsy and neurobehavioural problems. J. Neurodev. Disord. 6, 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cohen ASA et al. Haploinsufficiency of the basic helix–loop–helix transcription factor HAND2 causes congenital heart defects. Am. J. Med. Genet. A 182, 1263–1267 (2020). [DOI] [PubMed] [Google Scholar]
- 208.Dirkx E et al. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nat. Cell Biol. 15, 1282–1293 (2013). [DOI] [PubMed] [Google Scholar]
- 209.Tamura M et al. Overdosage of Hand2 causes limb and heart defects in the human chromosomal disorder partial trisomy distal 4q. Hum. Mol. Genet. 22, 2471–2481 (2013). [DOI] [PubMed] [Google Scholar]
- 210.Yamagata K et al. Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384, 455–458 (1996). [DOI] [PubMed] [Google Scholar]
- 211.Luco RF et al. A conditional model reveals that induction of hepatocyte nuclear factor-1α in Hnf1α-null mutant β-cells can activate silenced genes postnatally, whereas overexpression is deleterious. Diabetes 55, 2202–2211 (2006). [DOI] [PubMed] [Google Scholar]
- 212.Gage PJ, Suh H & Camper SA Dosage requirement of Pitx2 for development of multiple organs. Development 126, 4643–4651 (1999). [DOI] [PubMed] [Google Scholar]
- 213.Tumer Z & Bach-Holm D Axenfeld–Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur. J. Hum. Genet. 17, 1527–1539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cole MD The myc oncogene: its role in transformation and differentiation. Annu. Rev. Genet. 20, 361–384 (1986). [DOI] [PubMed] [Google Scholar]
- 215.George MR et al. Minimal in vivo requirements for developmentally regulated cardiac long intergenic non-coding RNAs. Development 146, dev185314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Atla G et al. Genetic regulation of RNA splicing in human pancreatic islets. Genome Biol. 23, 196 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Holdt LM & Teupser D Long noncoding RNA ANRIL: lnc-ing genetic variation at the chromosome 9p21 locus to molecular mechanisms of atherosclerosis. Front. Cardiovasc. Med. 5, 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. de Goede OM et al. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 184, 2633–2648.e19 (2021). Identification of numerous lncRNAs as candidate mediators of genetic association signals that underly susceptibility for prevalent human diseases.
- 219.Cory S, Graham M, Webb E, Corcoran L & Adams JM Variant (6;15) translocations in murine plasmacytomas involve a chromosome 15 locus at least 72 kb from the c-myc oncogene. EMBO J. 4, 675–681 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Graham M, Adams JM & Cory S Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. Nature 314, 740–743 (1985). [DOI] [PubMed] [Google Scholar]
- 221.Hu X et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell 26, 344–357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Leucci E et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 531, 518–522 (2016). [DOI] [PubMed] [Google Scholar]
- 223.Hoadley KA et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Olivero CE & Dimitrova N Identification and characterization of functional long noncoding RNAs in cancer. FASEB J. 34, 15360–15646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hilton LK et al. The double-hit signature identifies double-hit diffuse large B-cell lymphoma with genetic events cryptic to FISH. Blood 134, 1528–1532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gutschner T, Hammerle M & Diederichs S MALAT1 — a paradigm for long noncoding RNA function in cancer. J. Mol. Med. 91, 791–801 (2013). [DOI] [PubMed] [Google Scholar]
- 227.Gupta RA et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Martinez-Terroba E et al. Overexpressed malat1 drives metastasis through inflammatory reprogramming of lung adenocarcinoma microenvironment. Preprint at bioRxiv 10.1101/2023.03.20.533534 (2023). [DOI] [Google Scholar]
- 229.Hibi K et al. Loss of H19 imprinting in esophageal cancer. Cancer Res. 56, 480–482 (1996). [PubMed] [Google Scholar]
- 230.Kondo M et al. Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene 10, 1193–1198 (1995). [PubMed] [Google Scholar]
- 231.Rainier S et al. Relaxation of imprinted genes in human cancer. Nature 362, 747–749 (1993). [DOI] [PubMed] [Google Scholar]
- 232.Tseng YY & Bagchi A The PVT1–MYC duet in cancer. Mol. Cell Oncol. 2, e974467 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cai Z et al. Targeting bim via a lncRNA morrbid regulates the survival of preleukemic and leukemic cells. Cell Rep. 31, 107816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cai Z et al. Role of lncRNA Morrbid in PTPN11(Shp2)E76K-driven juvenile myelomonocytic leukemia. Blood Adv. 4, 3246–3251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Huang Y et al. The role of lincRNA-p21 in regulating the biology of cancer cells. Hum. Cell 35, 1640–1649 (2022). [DOI] [PubMed] [Google Scholar]
- 236.Borensztein M et al. Xist-dependent imprinted X inactivation and the early developmental consequences of its failure. Nat. Struct. Mol. Biol. 24, 226–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marahrens Y, Panning B, Dausman J, Strauss W & Jaenisch R Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes. Dev. 11, 156–166 (1997). [DOI] [PubMed] [Google Scholar]
- 238.Takagi N & Abe K Detrimental effects of two active X chromosomes on early mouse development. Development 109, 189–201 (1990). [DOI] [PubMed] [Google Scholar]
- 239.Yang L, Yildirim E, Kirby JE, Press W & Lee JT Widespread organ tolerance to Xist loss and X reactivation except under chronic stress in the gut. Proc. Natl Acad. Sci. USA 117, 4262–4272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yildirim E et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 152, 727–742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Syrett CM et al. Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells. PLoS Genet. 13, e1007050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Spaziano A & Cantone I X-chromosome reactivation: a concise review. Biochem. Soc. Trans. 49, 2797–2805 (2021). [DOI] [PubMed] [Google Scholar]
- 243. Syrett CM et al. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight 4, e12671 (2019). Findings in mouse and human suggesting that XIST dysregulation and abnormal X-chromosome inactivation in T cells underlie the increased prevalence of systemic lupus erythematosus in women.
- 244.Wang J et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl Acad. Sci. USA 113, E2029–E2038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yu B et al. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 184, 1790–1803.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dou DR et al. XIST ribonucleoproteins promote female sex-biased autoimmunity. Preprint at bioRxiv 10.1101/2022.11.05.515306 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li Y et al. A noncoding RNA modulator potentiates phenylalanine metabolism in mice. Science 373, 662–673 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dindot SV et al. An ASO therapy for Angelman syndrome that targets an evolutionarily conserved region at the start of the UBE3A-AS transcript. Sci. Transl. Med. 15, eabf4077 (2023). [DOI] [PubMed] [Google Scholar]
- 249.Wolter JM et al. Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA. Nature 587, 281–284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jiang J et al. Translating dosage compensation to trisomy 21. Nature 500, 296–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Abulwerdi FA et al. Selective small-molecule targeting of a triple helix encoded by the long noncoding RNA, MALAT1. ACS Chem. Biol. 14, 223–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Donlic A, Zafferani M, Padroni G, Puri M & Hargrove AE Regulation of MALAT1 triple helix stability and in vitro degradation by diphenylfurans. Nucleic Acids Res. 48, 7653–7664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zafferani M et al. Multiassay profiling of a focused small molecule library reveals predictive bidirectional modulation of the lncRNA MALAT1 triplex stability in vitro. ACS Chem. Biol. 17, 2437–2447 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Aguilar R et al. Targeting Xist with compounds that disrupt RNA structure and X inactivation. Nature 604, 160–166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rosa S, Duncan S & Dean C Mutually exclusive sense-antisense transcription at FLC facilitates environmentally induced gene repression. Nat. Commun. 7, 13031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li P, Tao Z & Dean C Phenotypic evolution through variation in splicing of the noncoding RNA COOLAIR. Genes. Dev. 29, 696–701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu Z, Fang X, Zhu D & Dean C Autonomous pathway: flowering locus C repression through an antisense-mediated chromatin-silencing mechanism. Plant. Physiol. 182, 27–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Yang M et al. In vivo single-molecule analysis reveals COOLAIR RNA structural diversity. Nature 609, 394–399 (2022). Temperature-dependent structural alterations in the lncRNA COOLAIR underly its role as a transcription repressive switch during seasonal transition in plants.
- 259.Sun Q, Csorba T, Skourti-Stathaki K, Proudfoot NJ & Dean C R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340, 619–621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


