Abstract
Objective
The use of a proton pump inhibitor (PPI) reduces rebleeding and mortality in patients with upper gastrointestinal bleeding (UGIB). Vonoprazan is a novel oral agent with strong and sustained acid-inhibitory activity. We clarified the effect of vonoprazan compared with oral PPIs in such patients.
Methods
We analyzed the Diagnosis Procedure Combination database. The primary outcome was rebleeding, and secondary outcomes were in-hospital mortality and in-hospital mortality after rebleeding. Propensity score matching was performed to balance the comparison groups, and logistic regression analyses were used to compare the outcomes between vonoprazan and oral PPIs.
Patients
Patients on vonoprazan or oral PPIs who underwent endoscopic hemostasis for UGIB between 2014 and 2019 were included.
Results
We enrolled 78,964 patients, of whom 27,101 and 51,863 were prescribed vonoprazan and a PPI, respectively. After propensity score matching, the rebleeding rate of vonoprazan did not significantly differ from that of oral PPIs [6.4% vs. 6.1%; odds ratio (OR), 1.05; 95% confidence interval (CI), 0.98-1.13]; similarly, the in-hospital mortality rate (1.4% vs. 1.5%; OR, 0.91; 95% CI, 0.79-1.05) and in-hospital mortality after rebleeding (0.3% vs. 0.2%; OR, 1.09; 95% CI, 0.78-1.54) also did not significantly differ between the groups. The acquired findings were robust across dose-restricted analyses and several sensitivity analyses.
Conclusion
Rebleeding and in-hospital mortality risks in patients on vonoprazan were similar to those in patients on oral PPIs. Considering the higher cost of vonoprazan, oral PPIs might be an optimal oral agent as an acid-suppressive therapy in such patients.
Keywords: upper gastrointestinal bleeding, vonoprazan, proton pump inhibitor, rebleeding, in-hospital mortality
Introduction
Upper gastrointestinal bleeding (UGIB) is a common emergency disease with an incidence of 61-78 per 100,000 persons (1-3) and a mortality rate of 2-10% (3,4). According to the guidelines (5,6), patients with UGIB should undergo endoscopy with endoscopic treatment of the sites with active bleeding. However, despite the increasing availability of effective endoscopic modalities and high-quality endoscopic intervention, the rate of rebleeding has remained high at a reported 3.4-14.6% (7-9). Furthermore, rebleeding is a risk factor for mortality (10,11). Thus, the prevention of rebleeding is an important issue to be resolved in the management of patients with UGIB.
The use of proton pump inhibitors (PPIs) is a cornerstone in the management of nonvariceal UGIB (12). According to the update of the Cochrane systematic review, the use of PPIs reduces the rebleeding rate from 13.2% to 6.9% and mortality from 3.5% to 2.0% (13). Another systematic review showed that PPIs significantly decreased the rebleeding rate compared with histamine-2 receptor antagonists (H2RAs) (14). Therefore, stronger acid inhibition might further decrease rebleeding and mortality in patients with UGIB.
Vonoprazan (Takeda Pharmaceutical, Tokyo, Japan) is a new oral potassium-competitive acid blocker with strong and sustained acid-inhibitory activity (15). Compared with oral PPIs, vonoprazan has shown superiority for the eradication of Helicobacter pylori (16) and no inferiority for the healing rate of erosive esophagitis (17). Recently, we revealed that vonoprazan had a reduced effect on delayed bleeding compared with oral PPIs in gastroduodenal endoscopic submucosal dissection (ESD) (18). However, whether or not vonoprazan is more effective for preventing rebleeding than oral PPIs in patients with UGIB remains unclear.
The present study therefore investigated the effect of vonoprazan in such patients.
Materials and Methods
Study design and data source
We used the Diagnosis Procedure Combination (DPC) database to extract patient data. This database was obtained from over 1,000 acute-care hospitals throughout Japan, covering approximately 90% of all tertiary hospitals and 50% of all acute-care hospitalizations (7 million per year). The database includes the following data: patient's main characteristics; diagnoses, comorbidities present at admission and complications during hospitalization coded with the International Classification of Diseases and Related Health Problems Tenth Revision (ICD-10) (19) and text data in Japanese; procedures coded with Medical Intervention Classification master code (20) (treatment code); discharge status; medications including drugs administered daily. The use of DPC data to identify diagnoses and procedure records has been previously validated (21).
This study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine, and informed consent was waived because of the anonymity of the data.
Study population
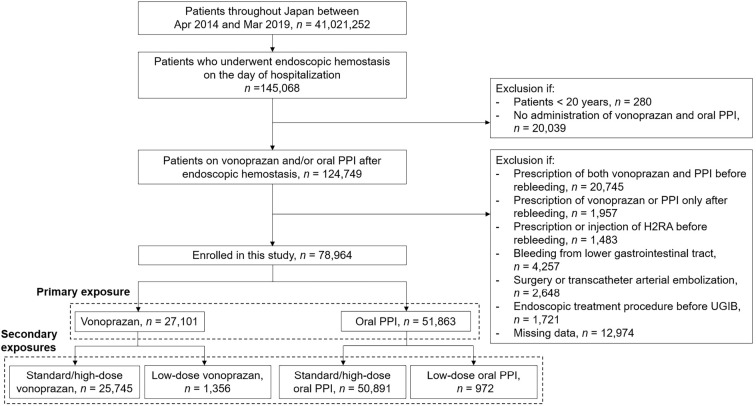
We extracted the data of adult patients (≥20 years old) who underwent upper gastrointestinal endoscopic hemostasis on the day of hospitalization and were prescribed vonoprazan or a PPI between April 2014 and March 2019. The exclusion criteria were patients 1) who were prescribed both vonoprazan and a PPI before rebleeding; 2) who were prescribed vonoprazan or a PPI only after rebleeding; 3) who were prescribed or injected with a H2RA before rebleeding; 4) who had bleeding from the lower gastrointestinal tract; 5) who underwent surgery or transcatheter arterial embolization after initial endoscopic hemostasis for UGIB because this study evaluated rebleeding after endoscopic hemostasis as a primary outcome for UGIB; 6) who underwent endoscopic treatment procedures, such as ESD, before the development of UGIB; and 7) who had missing data.
Vonoprazan and oral PPIs
The doses of vonoprazan and oral PPIs varied among patients, which might have affected the rebleeding rate. To reduce the dose-selection bias, the doses of vonoprazan and oral PPIs were categorized into standard/high and low. The standard daily dose of vonoprazan is 20 mg, which was set as the cutoff value of vonoprazan. The standard daily doses of lansoprazole, rabeprazole, esomeprazole, and omeprazole are 30, 10, 20, and 20 mg, respectively, in Japan; therefore, these were set as the cutoff values of PPIs.
Data collection and covariates
We extracted data on the age, sex, body mass index (BMI), comorbidities, concurrent medications, annual hospital volume, bleeding outcome, and discharge status. Comorbidities were assessed using the Charlson comorbidity index (22). Regarding medication, we assessed drugs associated with bleeding. Antiplatelet agents (aspirin, P2Y12 receptor antagonist, cilostazol, and other antiplatelet agents), anticoagulants (warfarin, direct oral anticoagulant, heparin, and other anticoagulants), nonsteroidal anti-inflammatory drugs, corticosteroids, and mucosal protective agents were assessed. We also assessed the data of intravenous PPIs, including the period of administration after endoscopic hemostasis. The annual hospital volume was categorized into quartiles according to the number of upper gastrointestinal treatment cases, as follows: low (<81 cases/year), intermediate (81-144 cases/year), high (145-242 cases/year), and very high (>242 cases/year).
Exposures
The primary exposure was vonoprazan or oral PPIs, regardless of the dose (Fig. 1). To reduce the dose-selection bias for the outcomes, the following groups were compared separately as the secondary exposures: 1) standard/high-dose vonoprazan or standard high-dose oral PPIs and 2) low-dose vonoprazan or standard/high-dose oral PPIs (Fig. 1).
Figure 1.
Flow diagram of the enrollment of patients. PPI: proton pump inhibitor, H2RA: histamine-2 receptor antagonist
Outcomes
Rebleeding and mortality are the two major clinical outcomes in patients with UGIB. The primary outcome was rebleeding after the initial endoscopic hemostasis. Rebleeding was defined as overt bleeding that required endoscopic hemostasis and/or transfusion at ≥2 days after the initial endoscopic hemostasis. The secondary outcome was in-hospital mortality. We also evaluated in-hospital mortality after rebleeding as another secondary outcome.
Statistical analyses
Continuous variables are expressed as the median and interquartile range, and categorical variables are expressed as the number and proportion.
We conducted 1:1 propensity score (PS) matching analyses between the comparison groups in primary and secondary exposures using the PS of each patient. The PSs were estimated by multivariate logistic regression with covariates, including the age, sex, BMI, comorbidities, current medications, annual hospital volume, and period of PPI injection. Each patient in the vonoprazan group was matched to each patient in the PPI group using a caliper width of 0.2 of the standard deviation of the logit of the PSs. The balance of the baseline characteristics between the two groups was evaluated using the standardized difference (SD); SD≤0.1 denotes a good balance of covariates (23). After each PS matching, we compared the rebleeding outcomes, in-hospital mortality outcomes, and those after rebleeding between the two groups using a logistic regression analysis. p<0.05 was considered to be statistically significant.
All statistical data were analyzed using the software programs SPSS version 25.0 for Windows (IBM, Armonk, USA) and R version 3.6.1 for Windows (The R Foundation for Statistical Computing, Vienna, Austria).
Subgroup and sensitivity analyses
We conducted subgroup and sensitivity analyses to confirm the robustness of the acquired results. First, we performed subgroup analyses based on age, sex, hemodialysis, and antithrombotic agents. We investigated whether or not the acquired odds ratios (ORs) were consistent across the subgroups by the significance of an interaction term between the two comparison groups. Second, to reveal the risk of severe rebleeding, we compared the risk of rebleeding between vonoprazan and oral PPIs when rebleeding was restricted to both endoscopic hemostasis and transfusion. Third, to remove the effect of intravenous PPIs on rebleeding to some extent, we compared the risk of rebleeding between vonoprazan and oral PPIs when patients with rebleeding within three days were excluded. Three days was set based on the median time of using intravenous PPI and that of rebleeding from the initial hemostasis. Fourth, an additional PS matching and evaluation of three clinical outcomes were performed in patients with no use of intravenous PPI before rebleeding (i.e. those who used only vonoprazan or oral PPIs as acid-suppressive therapy after the initial endoscopic hemostasis procedure for UGIB). Finally, two additional PS matchings were performed to compare the two groups when restricted to patients with gastric ulcers and those with duodenal ulcers to clarify the effect of vonoprazan on peptic ulcer bleeding.
Results
Patient characteristics
We identified a total of 288,584 patients who underwent endoscopic hemostasis on the day of hospitalization. Among them, 78,964 patients (27,101 patients in the vonoprazan group and 51,863 patients in the PPI group) were prescribed vonoprazan or a PPI after the initial endoscopic hemostasis. We conducted 1:1 PS matching and selected 27,098 pairs of vonoprazan and oral PPI users. Among them, rebleeding, in-hospital mortality, and in-hospital mortality after rebleeding were observed in 3,409, 791, and 134 patients, respectively.
The details of the baseline characteristics before and after PS matching in the primary exposure are shown in Table 1. The characteristics were well balanced after PS matching. In addition, for secondary exposures, a good balance of covariates after PS matching was demonstrated.
Table 1.
Baseline Characteristics of the Enrolled Patients.
| Before PS matching | After PS matching | ||||||
|---|---|---|---|---|---|---|---|
| Vonoprazan (n=27,101) | Oral PPI (n=51,863) | SD (%) | Vonoprazan (n=27,098) | Oral PPI (n=27,098) | SD (%) | ||
| Age (y), median (IQR) | 71 (61-81) | 71 (61-81) | 0.2 | 71 (61-81) | 71 (61-81) | 1.6 | |
| Sex, n (%) | |||||||
| Male | 18,726 (69.1) | 35,280 (68.0) | 1.9 | 18,724 (69,1) | 18,679 (69.0) | 0.2 | |
| Female | 8,375 (30.9) | 16,583 (32.0) | 1.9 | 8,374 (30.9) | 8,409 (31.0) | 0.2 | |
| BMI (kg/m2), median (IQR) | 21.9 (19.5-24.5) | 21.8 (19.5-24.3) | 3.8 | 21.9 (19.5-24.5) | 22.0 (19.6-24.5) | 0.6 | |
| CCI, median (IQR) | 0 (0-1) | 0 (0-1) | 3.0 | 0 (0-1) | 0 (0-1) | 0.0 | |
| Hemodialysis,n (%) | 638 (2.4) | 1,352 (2.6) | 1.0 | 638 (2.4) | 630 (2.3) | 0.5 | |
| Hospital volume, n (%) | |||||||
| Low (0-80) | 7,420 (27.4) | 14,551 (28.1) | 1.3 | 7,416 (27.4) | 7,253 (27.1) | 0.6 | |
| Intermediate (81-144) | 6,880 (25.4) | 13,575 (26.2) | 1.5 | 6,880 (25.4) | 7,047 (26.0) | 1.1 | |
| High (145-242) | 7,243 (26.7) | 13,283 (25.6) | 2.0 | 7,242 (26.7) | 7,107 (25.9) | 1.5 | |
| Very high (>242) | 5,558 (20.5) | 10,454 (20.2) | 0.6 | 5,557 (20.5) | 5,681 (21.0) | 1.0 | |
| Drug use, n (%) | |||||||
| Aspirin | 3,658 (13.5) | 6,129 (11.8) | 4.2 | 3,656 (13.5) | 3,638 (13.4) | 0.2 | |
| Cilostazol | 489 (1.8) | 833 (1.6) | 1.3 | 488 (1.8) | 485 (1.8) | 0.0 | |
| P2Y12RA | 1,373 (5.1) | 2,261 (4.4) | 2.7 | 1,371 (5.1) | 1,384 (5.1) | 0.0 | |
| Other antiplatelet drugs | 435 (1.6) | 683 (1.3) | 2.1 | 434 (1.6) | 404 (1.5) | 0.7 | |
| Warfarin | 1,114 (4.1) | 2,454 (4.7) | 2.4 | 1,113 (4.1) | 1,106 (4.1) | 0.0 | |
| DOAC | 1,372 (5.1) | 1,820 (3.5) | 6.6 | 1,371 (5.1) | 1,279 (4.7) | 1.5 | |
| Heparin | 1,414 (5.2) | 2,478 (4.8) | 1.5 | 1,414 (5.2) | 1,485 (5.5) | 1.1 | |
| Other anticoagulants | 16 (0.1) | 43 (0.1) | 0.0 | 16 (0.1) | 20 (0.1) | 0.0 | |
| NSAIDs | 1,454 (5.4) | 2,998 (5.8) | 1.1 | 1,454 (5.4) | 1,463 (5.4) | 0.0 | |
| Mucosal protective agents | 13,091 (48.3) | 25,168 (48.5) | 0.3 | 13,089 (48.3) | 13,162 (48.6) | 0.5 | |
| Corticosteroids | 637 (2.4) | 1,812 (3.5) | 5.2 | 637 (2.4) | 620 (2.3) | 0.5 | |
| PPI injection period (day), median (IQR) | 3 (2-4) | 3 (2-4) | 21.1 | 3 (2-4) | 3 (2-4) | 0.0 | |
PS: propensity score, PPI: proton pump inhibitor, SD: standardized difference, IQR: interquartile range, BMI: body mass index, CCI: Charlson comorbidity index, P2Y12RA: P2Y12 receptor antagonist, DOAC: direct oral anticoagulant, NSAIDs: non-steroidal anti-inflammatory drugs
Primary exposure
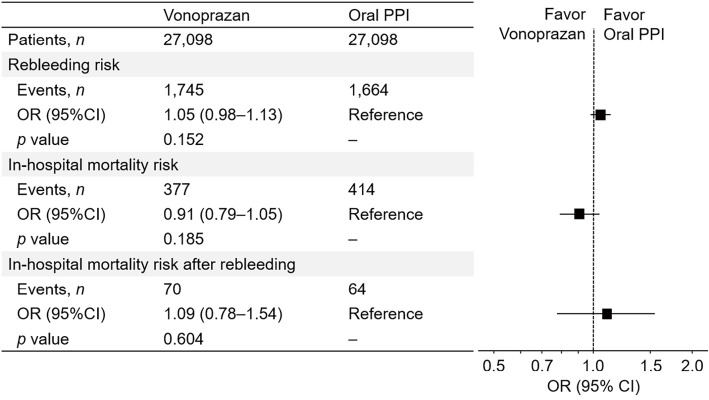
In the all-dose analysis, the rebleeding rates in the vonoprazan and oral PPI groups were 6.4% and 6.1%, respectively (Fig. 2). The OR [95% confidence interval (CI)] of vonoprazan for rebleeding, with reference to oral PPIs, was 1.05 (0.98-1.13) (Fig. 3). The in-hospital mortality rate of vonoprazan did not significantly differ from that of oral PPIs (1.4% vs. 1.5%; OR, 0.91; 95% CI, 0.79-1.05) (Fig. 2, 3). The effect of vonoprazan on in-hospital mortality after rebleeding also showed similar results (0.3% vs. 0.2%; OR, 1.09; 95% CI, 0.78-1.54) (Fig. 2, 3).
Figure 2.
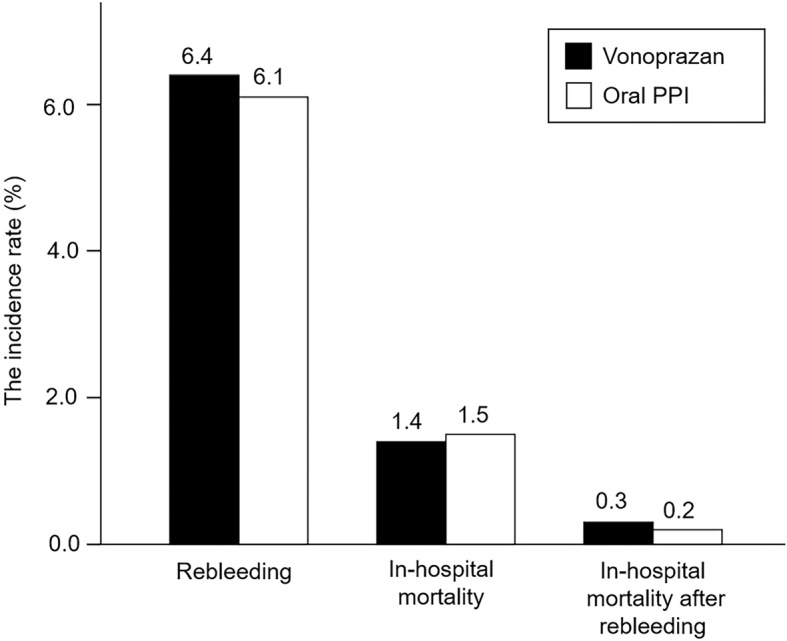
The rates of rebleeding and in-hospital mortality and in-hospital mortality after rebleeding in patients on vonoprazan and oral PPIs after PS matching. PPI: proton pump inhibitor, PS: propensity score
Figure 3.
Risk of rebleeding, in-hospital mortality, and in-hospital mortality after rebleeding for a primary exposure. PPI: proton pump inhibitor, OR: odds ratio, CI: confidence interval
Secondary exposures
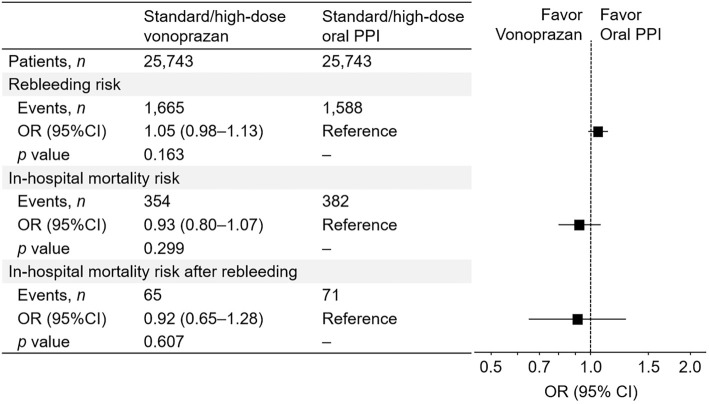
When vonoprazan and oral PPIs were restricted to the standard/high dose, the risk of vonoprazan for rebleeding (OR, 1.05; 95% CI, 0.98-1.13), in-hospital mortality (OR, 0.93; 95% CI, 0.80-1.17), and in-hospital mortality after rebleeding (OR, 0.92; 95% CI, 0.65-1.28) did not significantly differ from that of oral PPIs (Fig. 4). Similar results were acquired in the comparison of low-dose vonoprazan with standard/high-dose PPI for rebleeding (OR, 1.07; 95% CI, 0.77-1.48), in-hospital mortality (OR, 0.96; 95% CI, 0.54-1.71), and after rebleeding (OR, 1.25; 95% CI, 0.34-4.67).
Figure 4.
Risk of rebleeding and in-hospital mortality and in-hospital mortality after rebleeding for a secondary exposure (standard/high-dose vonoprazan vs. standard/high-dose oral PPI). PPI: proton pump inhibitor, OR: odds ratio, CI: confidence interval
Subgroup and sensitivity analyses
We confirmed that three clinical outcomes in vonoprazan or oral PPIs had no significant interaction across the groups stratified by age, sex, hemodialysis, and antithrombotic agent status in most subgroup analyses; however, significant interaction was observed in the subgroup analysis concerning in-hospital mortality risk in antithrombotic agent status (Table 2).
Table 2.
Subgroup Analyses for Risk of Rebleeding, In-hospital Mortality, and That after Rebleeding, according to Age, Sex, Hemodialysis, and Antithrombotic Agent Status (Vonoprazan vs. Oral PPI).
| Rebleeding risk | In-hospital mortality risk | In-hospital mortality risk after rebleeding | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| Vonoprazan vs. oral PPI | ||||||||
| Age (years) | p†=0.292 | p†=0.948 | p†=0.640 | |||||
| ≤64 | 0.98 | 0.85-1.13 | 0.97 | 0.74-1.28 | 1.25 | 0.67-2.35 | ||
| 65-74 | 1.07 | 0.94-1.21 | 0.80 | 0.62-1.04 | 1.07 | 0.58-2.00 | ||
| ≥75 | 1.08 | 0.98-1.20 | 0.95 | 0.77-1.17 | 1.02 | 0.60-1.74 | ||
| Sex | p†=0.180 | p†=0.504 | p†=0.588 | |||||
| Male | 1.09 | 0.99-1.19 | 0.88 | 0.74-1.04 | 1.02 | 0.67-1.56 | ||
| Female | 0.98 | 0.87-1.11 | 0.97 | 0.76-1.24 | 1.24 | 0.70-2.21 | ||
| Hemodialysis | p†=0.818 | p†=0.276 | p†=0.850 | |||||
| Yes | 1.09 | 0.80-1.49 | 0.64 | 0.33-1.23 | 1.24 | 0.33-4.63 | ||
| No | 1.05 | 0.98-1.13 | 0.93 | 0.33-1.09 | 1.08 | 0.76-1.54 | ||
| Antithrombotic agents | p†=0.750 | p†=0.030 | p†=0.201 | |||||
| Yes | 1.03 | 0.89-1.19 | 1.21 | 0.90-1.63 | 1.60 | 0.81-3.19 | ||
| No | 1.06 | 0.98-1.15 | 0.84 | 0.71-0.98 | 0.96 | 0.65-1.42 | ||
†p for interaction.
PPI: proton pump inhibitor, OR: odds ratio, CI: confidence interval
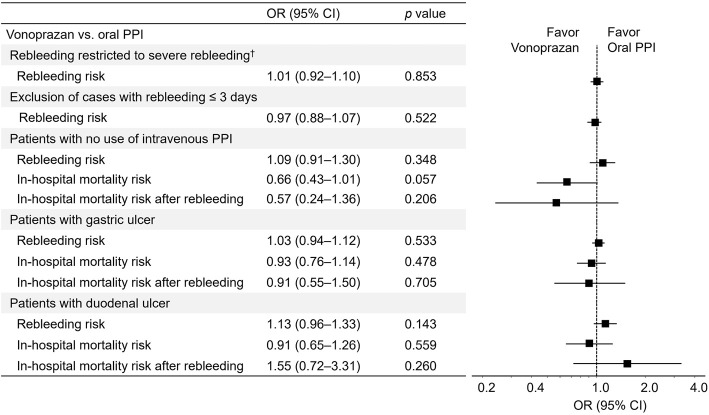
When rebleeding was limited to both endoscopic hemostasis and transfusion, the results of rebleeding (OR, 1.01; 95% CI, 0.92-1.10) were consistent. When patients with rebleeding ≤3 days were excluded, the results of rebleeding (OR, 0.97; 95% CI, 0.88-1.07) were also not significant. The baseline characteristics after three additional PS matchings in the sensitivity analyses were well balanced, and the results concerning the three clinical outcomes after these PS methods are shown in Fig. 5. In patients restricted to no use of intravenous PPIs, the risk of rebleeding, in-hospital mortality, and in-hospital mortality after rebleeding with vonoprazan did not significantly differ from that with oral PPIs. When patients were restricted to those with gastric ulcers, similar results were obtained. In addition, in patients with duodenal ulcers, no significant results were shown concerning the three clinical outcomes.
Figure 5.
Sensitivity analyses for risk of rebleeding, in-hospital mortality, and in-hospital mortality after rebleeding. †Severe rebleeding was defined as rebleeding restricted to both endoscopic hemostasis and blood transfusion. PPI: proton pump inhibitor, OR: odds ratio, CI: confidence interval
Discussion
Acid-suppressive therapy using PPIs is recommended for patients who undergo endoscopic hemostasis for UGIB in the guidelines (5,6,13) because of its effect of reducing rebleeding and mortality compared with placebo or H2RAs (13). Vonoprazan is a novel oral acid-suppressive agent with strong and sustained acid-inhibitory activity compared with PPIs (15). Our prior study revealed that vonoprazan had a lower risk for bleeding after gastroduodenal ESD than oral PPIs (18). Thus, we conducted this comparative study based on the hypothesis that vonoprazan has a lower risk of rebleeding after endoscopic hemostasis for UGIB than oral PPIs.
Contrary to our hypothesis, however, this study revealed that the rebleeding risk of vonoprazan was similar to that of oral PPIs. The risks of in-hospital mortality and in-hospital mortality after rebleeding were also similar between the two agents. When restricted to standard/high-dose vonoprazan and oral PPI, similar results were also obtained. In addition, the risk of three clinical outcomes with low-dose vonoprazan did not significantly differ from that with standard/high-dose oral PPIs. Furthermore, the study results were consistent across a range of sensitivity analyses.
The study results have important clinical implications. Based on these results, oral PPIs might have a sufficient acid-inhibitory effect for controlling rebleeding after endoscopic hemostasis for UGIB. The update of the Cochrane systematic review partially supports our results from the viewpoint of intense acid suppression (13). According to the review, no significant differences were found in the risk of rebleeding and mortality between high-dose and nonhigh-dose PPIs, with ORs of 1.25 and 1.02 for rebleeding and mortality, respectively, in high-dose PPI (13). Oral PPIs are less expensive than vonoprazan ($0.82-$1.11 vs. $1.85 in the standard daily dose in Japan). Considering the similar effect on treatment outcome and lower cost of oral PPIs, these agents might be ideal acid-suppressive oral agents for improving clinical outcomes after endoscopic hemostasis for UGIB.
Our previous study showed that vonoprazan reduced bleeding after gastroduodenal ESD compared with oral PPIs (18), which is in contrast with the similar preventive effect between the two agents noted in the present study. The reason for the discrepancy in the effect of vonoprazan for preventing bleeding between gastroduodenal ESD and UGIB is unclear. One possible explanation is a weaker effect of vonoprazan and oral PPIs than with intravenous PPIs as an initial treatment. Since intravenous vonoprazan is not commercially available, intravenous PPIs are generally used during fasting periods, and vonoprazan or oral PPI intake is started after the commencement of meals. Indeed, over 90% of the enrolled patients had been administered intravenous PPIs before the use of oral agents in both treatment arms. Thus, vonoprazan or oral PPIs following intravenous PPI might show a small effect on the clinical outcomes. In addition, we also analyzed the effect of vonoprazan in patients restricted from using intravenous PPIs, and no significant results were obtained. However, the number of patients evaluated in this analysis accounted for <10% of the enrolled patients, so caution is required for the interpretation of the findings.
Another possible explanation is the differences in the nature of artificial ulcers in gastroduodenal ESD and peptic ulcers in UGIB. Regarding the effect of PPIs on preventing bleeding compared with H2RA, previous meta-analyses in gastric ESD and UGIB showed similar positive results (14,24). However, some characteristics differ between the two types of ulcers. For instance, the risk factors for bleeding or rebleeding in patients with gastric ESD differed from those for rebleeding in patients with peptic ulcer bleeding (25-28); in particular, the effect of an antithrombotic agent on rebleeding was quite different between the two. Therefore, although the details of this issue remain unclear, the differences in the natures between the two types of ulcers might have led to different effects of vonoprazan.
We conducted PS matching using 18 covariates to balance the background characteristics of the two treatment arms. Although randomized controlled trials are the gold standard for evaluating pharmaceutical efficacy, they are frequently underpowered for identifying differences in rare events, such as rebleeding and mortality in patients with UGIB. In the present study, we initially enrolled 78,964 patients with 4,538 rebleeding and 1,284 in-hospital mortality events, and pairwise comparisons were performed after PS matching. In addition, we conducted subgroup analyses using several variables to decrease bias by characteristics related to both treatment selection and risk for rebleeding, i.e. confounding by indication. Furthermore, several sensitivity analyses showed consistent results. We believe that the large number of patients, methodology, and acquired findings in the sensitivity analyses increase the robustness of our primary findings.
However, the present study also has several limitations. First, this was an observational study; thus, it is susceptible to unmeasured confounding owing to the lack of randomization. For instance, we did not include several potential confounders, such as the location of UGIB or hemodynamic instability, as reported in previous meta-analyses (27,28). Second, we used the initially prescribed dose of the agents, and a change in the dose after the initial prescription was not considered in this study. Third, the effect of intravenous PPIs on our main results cannot be fully removed, although some sensitivity analyses confirmed the robustness of similar bleeding risk between vonoprazan and oral PPIs. Fourth, a significant interaction was shown only in the subgroup analysis of antithrombotic agents for in-hospital mortality. Why this result was obtained is unclear, but we at least feel confident that it may not be related to confounding of the more frequent use of vonoprazan in patients with antithrombotic agents than in patients without antithrombotic agents (48.6% vs. 49.6%). Fifth, we did not evaluate adverse events owing to vonoprazan or PPIs. Finally, this study has a risk of misclassification bias.
In conclusion, this large-scale population-based study first found that vonoprazan had a similar risk of rebleeding and in-hospital mortality to oral PPIs in patients who underwent endoscopic hemostasis for UGIB. Considering the higher cost of vonoprazan, PPIs might be ideal acid-suppressive oral agents in such patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 107: 1190-1195, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci 63: 1286-1293, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Abougergi MS, Travis AC, Saltzman JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc 81: 882-888, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 60: 1327-1335, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Mullady DK, Wang AY, Waschke KA. AGA clinical practice update on endoscopic therapies for non-variceal upper gastrointestinal bleeding: expert review. Gastroenterology 159: 1120-1128, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Fujishiro M, Iguchi M, Kakushima N, et al. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc 28: 363-378, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Matsuhashi T, Fukuda S, Mikami T, et al. Effects of anti-thrombotic drugs on all-cause mortality after upper gastrointestinal bleeding in Japan: a multicenter study with 2205 cases. Dig Endosc 34: 113-122, 2022. [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y, Hatta W, Koike T, et al. The use of higher dose steroids increases the risk of rebleeding after endoscopic hemostasis for peptic ulcer bleeding. Dig Dis Sci 63: 3033-3040, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Chang A, Ouejiaraphant C, Akarapatima K, Rattanaspa A, Prachayakul V. Prospective comparison of the AIMS65 score, Glasgow-Blatchford score, and Rockall score for predicting clinical outcomes in patients with variceal and nonvariceal upper gastrointestinal bleeding. Clin Endosc 54: 211-221, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuhashi T, Hatta W, Hikichi T, et al. A simple prediction score for in-hospital mortality in patients with nonvariceal upper gastrointestinal bleeding. J Gastroenterol 56: 758-768, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Chiu PW, Ng EK, Cheung FK, et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin Gastroenterol Hepatol 7: 311-316, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 340: 751-756, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the International Consensus Group. Ann Intern Med 171: 805-822, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YS, Li Q, He BS, Liu R, Li ZJ. Proton pump inhibitors therapy vs H2 receptor antagonists therapy for upper gastrointestinal bleeding after endoscopy: a meta-analysis. World J Gastroenterol 21: 6341-6351, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects - a randomised open-label cross-over study. Aliment Pharmacol Ther 42: 719-730, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65: 1439-1446, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 42: 685-695, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe H, Hatta W, Ogata Y, et al. Prevention of delayed bleeding with vonoprazan in upper gastrointestinal endoscopic treatment. J Gastroenterol 56: 640-650, 2021. [DOI] [PubMed] [Google Scholar]
- 19.Brämer GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q 41: 32-36, 1988. [PubMed] [Google Scholar]
- 20.Ministry of Health, Labour and Welfare. Various information of medical free [Internet]. [cited 2023 Mar 3]. Available from: https://shinryohoshu.mhlw.go.jp/shinryohoshu/
- 21.Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 27: 476-482, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373-383, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34: 3661-3679, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Wu Q, Liu Z, Wu K, Fan D. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion 84: 315-320, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Hatta W, Tsuji Y, Yoshio T, et al. Prediction model of bleeding after endoscopic submucosal dissection for early gastric cancer: BEST-J score. Gut 70: 476-484, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto M, Hatta W, Tsuji Y, et al. Rebleeding in patients with delayed bleeding after endoscopic submucosal dissection for early gastric cancer. Dig Endosc 33: 1120-1130, 2021. [DOI] [PubMed] [Google Scholar]
- 27.Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol 103: 2625-2632, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Iglesias P, Villoria A, Suarez D, et al. Meta-analysis: predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment Pharmacol Ther 34: 888-900, 2011. [DOI] [PubMed] [Google Scholar]