Abstract
Nontuberculous mycobacterial (NTM) infection sometimes leads to the development of pulmonary artery aneurysm (PAA), a rare but life-threatening complication. We herein report a 64-year-old woman with a history of NTM infection who presented with severe hemoptysis. Computed tomography revealed a ruptured PAA, which was treated successfully with pulmonary artery embolization. Subsequent right total pneumonectomy was performed to control infection. This case emphasizes the need to consider PAA in patients with NTM infection who present with hemoptysis. Early detection and appropriate management are critical for preventing this fatal complication.
Keywords: pulmonary artery aneurysm, nontuberculous mycobacteria
Introduction
Pulmonary artery aneurysm (PAA), a rare but life-threatening condition characterized by abnormal dilation of the pulmonary vasculature, can lead to rupture and fatal consequences (1). The diagnosis of PAA can be challenging, but imaging techniques, such as contrast-enhanced computed tomography (CT) and pulmonary angiography, are valuable for identifying the responsible vessel.
Infection is one of the most common causes of acquired PAA (2), particularly in cases of tuberculosis-affected PAA (known as Rasmussen's aneurysm), which is found in up to 5% of patients with chronic cavitary tuberculosis (3). However, PAA with nontuberculous mycobacteria (NTM) infection is a rare entity, with only a few cases reported in the literature (1,3,4).
We herein report a case of massive hemoptysis due to Rasmussen's aneurysm-like PAA caused by NTM infection that was managed successfully with pulmonary artery embolization (PAE).
Case Report
A 64-year-old woman with a history of Sjögren's syndrome had been treated with prednisolone (equivalent to 3 mg/day) for 14 years. During hospitalization due to intestinal obstruction, chest X-ray revealed an incidental finding of a cavity in the right middle lung field and infiltrating shadows in both lower lung fields. Therefore, she was referred to our department four years ago. Mycobacterium intracellulare was isolated from her sputum, and NTM infection was diagnosed. Because she had ocular disease complicated by retinal pigmentation, she was not treated with ethambutol; instead, she received rifampicin, clarithromycin, and sitafloxacin. Despite the treatment, however, the cavity continued to enlarge over the course of three years (Fig. 1A). After continuing treatment, she was transported urgently to our hospital due to massive hemoptysis.
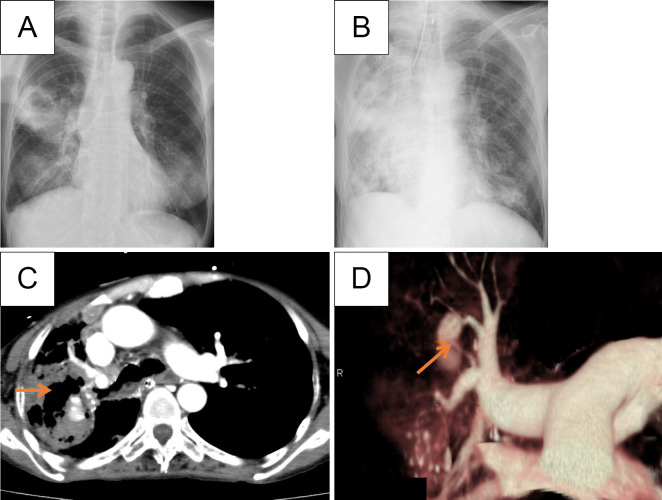
Figure 1.
A, B: Initial chest X-ray revealed a cavity in the right middle lung, which expanded during treatment of nontuberculous mycobacterial infection (A). On admission, an infiltration shadow was detected in the upper right and middle lung fields, while new ground-glass opacities and infiltration shadows were observed in both lower lung fields (B). C: Contrast-enhanced CT revealed the presence of a pulmonary arterial aneurysm within the cavity lesion. D: 3D-CT angiography illustrated the pulmonary arterial aneurysm located in A2.
At the initial visit, the following observations were made: height, 155 cm; weight, 35.4 kg; body temperature, 36.2°C; blood pressure, 131/81 mmHg; pulse, 112/min; SpO2, 95% (with a reservoir mask at 10 L/min); and respiratory rate, 32/min. Due to prominent hypoxia, the patient underwent endotracheal intubation and mechanical ventilation for respiratory management. Respiratory sounds were diminished in the right anterior chest area. Blood tests revealed an elevated inflammatory response [white blood cell count, 9,400/μL; C-reactive protein (CRP), 2.85 mg/dL] and anemia (hemoglobin, 8.3 g/dL). Chest X-ray showed enlarged infiltrative shadows in the right upper and both lower lung fields, as well as ground-glass opacities along the bronchovascular bundle in the left lung field (Fig. 1B). Three-dimensional (3D)-CT revealed a vessel branching from the right PA that was associated with the aneurysm and was suspected of being the source of the hemorrhaging (Fig. 1C, D).
Pulmonary angiography confirmed the presence of an aneurysm communicating with the ascending branch of the right PA. Contrast leakage was observed from the same site, and the PAA was identified as the source of the hemorrhaging. After embolization of the responsible vessel using a coil, the contrast leakage disappeared, and the procedure was terminated (Fig. 2A, B). Despite treatment with clarithromycin, sitafloxacin, and intravenous amikacin (500 mg/day) after PAE, the patient continued to have a positive sputum smear for acid-fast bacilli. Following PAE, the patient continued to experience persistent purulent sputum dripping, which led to unresolved inflammation in the right middle and lower lobes (Fig. 2C). As a result, daily bronchoscopic suctioning was needed. Pulmonary arteriography revealed limited blood flow in the pulmonary arteries, indicating that they were not effectively participating in alveolar gas exchange.
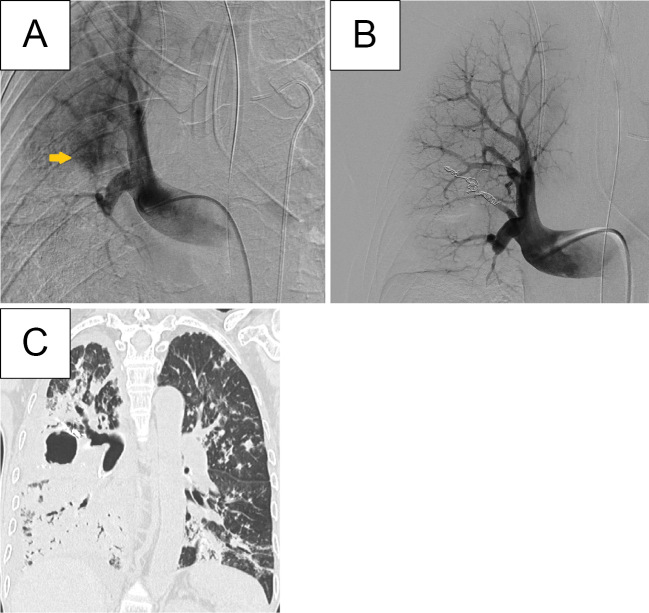
Figure 2.
A: Pulmonary artery angiography confirmed the pulmonary arterial aneurysm located in A2. B: Coil embolization of the aneurysm was completed successfully, as indicated by the absence of contrast filling in the aneurysm. C: CT after pulmonary artery embolization showed infiltrative shadows in the right middle and lower lobes, implicating persistent purulent sputum dripping.
Although resection of the right upper lobe was considered a surgical option, the presence of bronchiectasis in the middle and lower lobes, along with structural modifications of the lung, made resection of these lobes essential for infection and sputum control. Therefore, right total pneumonectomy was performed to control infection on day 28 of hospitalization. Pathological findings revealed a coil around a 45×25 mm cavity in the right upper lobe. Ziehl-Neelsen staining revealed numerous clumps of acid-fast bacilli near the area of dry necrosis. No aneurysm was identified in the region subjected to coil embolization, but many occluded artery-like vessels containing organized thrombi were observed in the vicinity (Fig. 3).
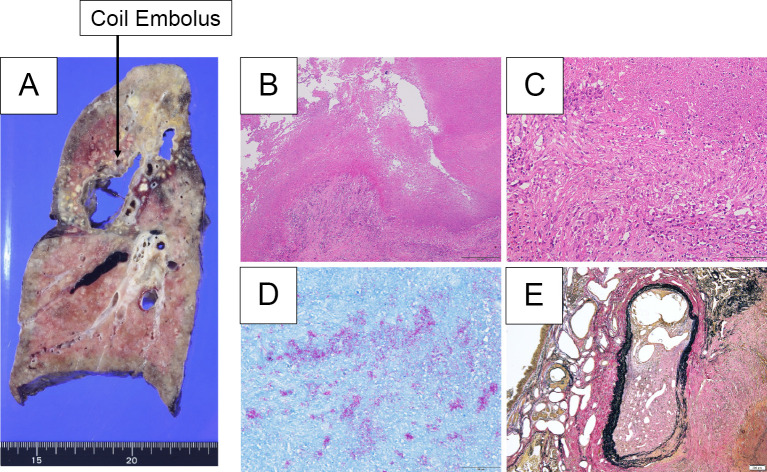
Figure 3.
A: A cavity measuring 45×25 mm was observed in the right upper lobe, with a hard surface and a brownish-to-white tone. Multiple yellowish-white nodular-to-granular lesions formed, mainly in the upper lobe. A coil embolus was confirmed adjacent to the cavity. B, C: Hematoxylin and Eosin staining revealed the formation of epithelioid cell granulomas, with caseous necrosis mainly around the cavity lesions, along with Langhans giant cells. D: Ziehl-Neelsen-staining-positive acid-fast bacilli were observed in areas of caseous necrosis. E: Elastica van Gieson stain did not reveal any aneurysms in the coil embolus; however, there were multiple artery-like vessels in the vicinity of the coil embolus that were obstructed by organized thrombus, and part of the blood vessel wall was fragile.
Three months after surgery, the patient remained free of hemoptysis. Her general condition stabilized with the use of continuous positive-pressure ventilation at night to manage persistent respiratory failure accompanied by hypercapnia. She was discharged on postoperative day 115. A postdischarge prescription was provided, including clarithromycin, sitafloxacin, and intravenous amikacin.
Discussion
PAA is classified as central or peripheral according to the site of occurrence. The central type is commonly caused by congenital heart disease or connective tissue disorders, while the peripheral type is acquired after the onset of infectious diseases, autoimmune disorders, pulmonary hypertension, or malignant tumors. Infectious PAA is typically associated with syphilis, bacterial, fungal, tuberculosis, or septic pulmonary embolism (4). Rasmussen's aneurysm refers to a PAA that develops within the cavity of tuberculosis-affected lungs (4,5). Santelli et al. reported that Rasmussen's aneurysm is found in approximately 5% of patients with chronic cavitary tuberculosis (3). In recent years, the incidence of NTM infection has markedly increased, surpassing that of tuberculosis. Two cases of Rasmussen's aneurysm caused by NTM infection have been reported (6,7). In the present case, caseous granulomas were observed predominantly in the cavity of the right upper lobe of the lung. Ziehl-Neelsen staining revealed numerous acid-fast bacilli. Contrast-enhanced CT showed a 3-cm intraluminal aneurysm, with the pulmonary artery acting as a nutrient artery. These findings are consistent with Rasmussen's aneurysm caused by tuberculosis (4,5,8).
Rupture of peripheral PAA has a high mortality rate. PAE is the first-line treatment to identify the responsible vessel and reduce PAA-associated mortality to a greater extent than surgical treatment, particularly in cases of hemodynamic instability (9,10). In contrast, surgical treatment may be the treatment of choice when bleeding has subsided but the infection persists (11,12). In the current case, the infection was controlled by total pneumonectomy. Although PAA due to NTM infection is rare, it is important to acknowledge that Rasmussen's aneurysm can occur in not only patients with tuberculosis but also those with NTM infection, particularly in cases with poor disease control.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gupta M, Agrawal A, Iakovou A, Cohen S, Shah R, Talwar A. Pulmonary artery aneurysm: a review. Pulm Circ 10: 2045894020908780, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartter T, Irwin RS, Nash G, et al. Aneurysms of the pulmonary arteries. Chest 94: 1065-1075, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Santelli ED, Katz DS, Goldschmidt AM, Thomas HA. Embolization of multiple Rasmussen aneurysms as a treatment of hemoptysis. Radiology 193: 396-398, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Kreibich M, Siepe M, Kroll J, Höhn R, Grohmann J, Beyersdorf F. Aneurysms of the pulmonary artery. Circulation 131: 310-316, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim H. Thoracic sequelae and complications of tuberculosis. Radiographics 21: 839-858, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Chiu HW, Kuo SH, Lai RS, Wu MT, Wu HF. Ambiguous presentation of Mycobacterium avium complex-associated Rasmussen aneurysm. Respirol Case Rep 5: e00219, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plantilla M, Nozu S, Nakanishi N, Ishimaru Y. A case of Rasmussen's aneurysm caused by pulmonary nontuberculous mycobacterium. Respirol Case Rep 11: e01094, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibiya K, Shigeto E, Iida K, et al. Distribution of mycobacterial antigen based on differences of histological characteristics in pulmonary Mycobacterium avium infectious diseases - consideration of the extent of surgical resection from the pathological standpoint. Pathol Res Pract 208: 53-58, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Remy-Jardin M, Remy J. Spiral CT angiography of the pulmonary circulation. Radiology 212: 615-636, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Keeling AN, Costello R, Lee MJ, et al. Rasmussen's aneurysm: a forgotten entity? Cardiovasc Intervent Radiol 31: 196-200, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Ungaro R, Saab S, Almond CH, Kumar S. Solitary peripheral pulmonary artery aneurysms. Pathogenesis and surgical treatment. J Thorac Cardiovasc Surg 71: 566-571, 1976. [PubMed] [Google Scholar]
- 12.Monchik J, Wilkins EW. Solitary aneurysm of the middle lobe artery. A case report and review of solitary peripheral pulmonary artery aneurysms. Ann Thorac Surg 17: 496-503, 1974. [DOI] [PubMed] [Google Scholar]