Abstract
Objective
The aim of this study was to evaluate the accuracy of the Raycome model M2 oscillometric upper-arm blood pressure (BP) monitor developed for ambulatory BP measurement in the general population according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Universal Standard (ISO 81060-2:2018) at rest and during dynamic exercise.
Method
Subjects were recruited to fulfill the age, gender, BP and cuff distribution criteria of the AAMI/ESH/ISO Universal Standard in the general population using the same arm sequential BP measurement method. Three cuffs of the test device were used for arm circumference 18–22 cm (small), 22–32 cm (medium) and 32–42 cm (large).
Results
For the general validation study, 106 subjects were recruited and 85 were analyzed. For validation criterion 1, the mean ± SD of the differences between the test device and reference BP readings was 0.5 ± 6.2/−0.2 ± 5.1 mmHg (systolic/diastolic). For criterion 2, the SD of the mean BP differences between the test device and reference BP per subject was 5.23/4.50 mmHg (systolic/diastolic). In the ambulatory validation study (N = 35), the mean difference was 0.4 ± 5.9/−1.1 ± 5.8 mmHg. The Raycome model M2 performed well against the standard in both the general and ambulatory validations and the Bland–Altman plots did not show any systematic variation in the error.
Conclusion
These data show that the Raycome model M2 monitor meets the requirements of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) and in the ambulatory setting, indicating its suitability for measuring BP in the general population.
Keywords: accuracy, ambulatory blood pressure monitoring, exercise, ISO protocol, validation
Introduction
The accurate measurement of blood pressure (BP) is an important prerequisite for the reliable diagnosis and efficient management of hypertension and other medical conditions. Therefore, the evaluation of the accuracy of automated devices available on the market for BP measurement in the medical environment and the community is of paramount importance [1]. Ambulatory blood pressure monitoring (ABPM) has led to improved characterization of BP patterns and hence cardiovascular risk. ABPM has clear merits, which was supported by extensive scientific evidence, and has been validated thoroughly [2]. Nonetheless, its reliance on automatic devices indicates the need for some form of control in terms of both quality and suitability for users [3]. This validation study accessed the BP measurement accuracy of the new automatic upper-arm ABPM device M2 (Shenzhen Raycome Health Technology Co, Ltd, China, which is a high-tech medical enterprise integrating production, sales and service with blood pressure management as the core of development) according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Universal Standard (ISO 81060-2:2018) in the general population [1,4,5], which was developed by researchers from the Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Methods
Test device
The Raycome model M2 automatic upper-arm ABPM device includes a monitor dock, connect cable, a standard cuff (or a small and large cuff) and a user guide. The monitor has a size of 122 × 71 × 28.8 mm and a weight of 160 g without cuff. It is designed for BP measurements on either the right or left upper arm and has a fixed tubular opening to insert the user’s arm, with an implanted single-arm cuff, which when inflated surrounds the upper arm. According to the manufacturer’s instructions, the device is suitable for arm circumference range the arm circumference of 15–42 cm. A wide light emitting diode screen presents systolic and diastolic BP, heart rate (HR) and signal. The measurement ranges are as follows: BP: 0–270 mmHg (0–36 kPa) and HR: 40–180 beats/min. The estimated accuracy is ±3 mmHg for BP and ±5% for HR. The monitor has a 4G communication interface enabling connection with a PC operating the dedicated ABPM analysis software, the software provides all of the conventional ABPM statistical and graphical data, and also reports edition and storage capabilities. One device was provided for the validation study.
Participants
Regarding to the AAMI/ESH/ISO Universal Standard, for a general population validation study of a BP monitor at least 85 subjects aged >12 years are required [1,6]. Table 1 shows the participant selection process. We recruited 106 subjects ≥18 years from patients attending the outpatient hypertension clinic (95.3%) and from hospital staff (4.7%). Of these 21 were excluded. Reasons for exclusions were arrhythmia (n = 4), references BP variability (n = 3), sound not audible (n = 4), too fragile to attend all the measurements (n = 2) and subject talking during BP measurement (n = 8). Finally, 85 subjects were analyzed (55.3% male). Thirty-five individuals from the 85 who underwent clinical validation participated in ambulatory device validation (Table 2).
Table 1.
Subjects recruited and excluded from the analysis for general validation
| Subjects | |
|---|---|
| Recruited | 106 |
| Excluded | 21 |
| Reasons for exclusion | |
| Arrhythmia | 4 |
| Reference BP variability (>12/8 mmHg for systolic/diastolic) | 3 |
| K sounds not audible | 4 |
| Talking during BP measurements | 8 |
| Too fragile | 2 |
| Analyzed | 85 |
BP, blood pressure.
Table 2.
Ambulatory validation participants’ characteristics (n = 35)
| Participants (n = 35) | ISO standard | |
|---|---|---|
| Age [range (mean ±SD)] (years) | 23–75 (52.2 ± 13.8) | NA |
| Gender (male/female) | 22/13 | NA |
| Arm circumference [range (mean ±SD)] (cm) | 21–38 (28.4 ± 5.4) | NA |
| Entry SBP R0 [range (mean ±SD)] (mmHg) | 98–198 (145.8 ± 25.6) | NA |
| Entry DBP R0 [range (mean ±SD)] (mmHg) | 64–114 (87.7 ± 14.9) | NA |
| SBP (proportion of measurements ≥160 mmHg) | 26.7 | ≥10% |
| DBP (proportion of measurements ≥100 mmHg) | 33.9 | ≥10% |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Validation team
The study was conducted in a BP measurement research laboratory by a supervisor (Y.Z.) and two trained observers (S.Y. and Z.Z.). Two observers with certificates of Good Clinical Practice (GCP) and retrained strictly in mercury BP measurement, and they were trained in the testing procedures and the operational approach of the test device with two supervisors (Y.Z. and H.Z.) additionally who also obtained the certificates of GCP before the research initiation, then one simulation test (mercury sphygmomanometer) should be conducted to confirm that the observers could normally listen the Korotkoff sound [especially the fifth phase (K5)] to determine the systolic blood pressure (SBP) and diastolic blood pressure (DBP) before the test. M.Z. and Q.Z. contributed to the participants enroll. Besides, H.M. helped with experimental data.
Reference blood pressure
Two connected (Y-tube) standard mercury sphygmomanometers (XJ11D, Shanghai Medical Instruments Co. Ltd, China), which had been calibrated before the study initiation were used for simultaneous reference auscultatory BP measurements by two observers using a dual-head teaching stethoscope (Shanghai Taihuhuamei Medical Instruments Co. Ltd, China). Four cuffs with inflatable bladder dimensions 9 × 18, 12 × 23, 14 × 28, 16 × 33 cm were used so that the length would cover 75–100% of the individual participant’s mid-arm circumference and the width 37–50% [1].
Procedure
The arm sequential method was applied according to the recommendations and practical guidance from performing and reporting validation studies [1]. Briefly, we included two entry BP measurements (reference R0 and test device T0) followed by four reference measurements (R1, R2, R3 and R4) taken alternately with three test device measurements (T1, T2 and T3). All measurements were performed on the left arm. The observers were blinded to each other’s readings and the test device results. The supervisor (Y.Z.) registered the M2 measurements and checked the observers’ measurements. In case of disagreement between the observers more than 4 mmHg, additional pairs of measurements were performed. A maximum of eight pairs of BP determinations was allowed after which the subject was excluded.
In the ambulatory validation, the validation BP measurements were performed under dynamic exercise with a HR increase of 15% above the resting HR. The measurements were performed in the seated upright position on a bicycle ergometer with the arm supported. The cuff was at the heart level by using the opposite-limb simultaneous method as per the ISO 81060-2:2018 standard. Device memory was then cleared and there was a waiting period of at least 1 min. The reference sphygmomanometer and the M2 device were swapped to the opposite arm, and BP measurements were repeated after waiting for at least another minute. The procedure of swapping devices to the opposite arm and BP measurements were repeated until six paired determinations had been performed (>1 min between device swapping and BP measurement). The allowed observer difference was ±5 mmHg.
Statistical analysis
Continuous variables are expressed as the mean ± SD, and categorical variables are expressed as frequencies or percentages. Standardized Bland–Altman scatterplots of the RBP-9801 – reference BP differences against their mean were performed. The AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) requirements were strictly followed [1]. Data were analyzed with IBM SPSS Statistics version 24 statistical software.
Study approval
The study protocol was approved by the ethics committee of the Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. All participants signed an informed consent before inclusion in the study, in accordance with the Declaration of Helsinki and WHO standards for observational studies [7]. The confidentiality of the subjects was guaranteed at all times in accordance with the provisions of current legislation on personal data protection (15/1999 of 13 December Protection of Personal Data Official Law), and the conditions were contemplated by Act 14/2007 on biomedical research.
Results
General validation study
A total of 106 individuals were recruited and 85 were analyzed. The participants’ characteristics are shown in Table 3. The Universal Standard requirements for age and gender were fulfilled [1]. The mean BP difference between the simultaneous observers’ measurements was 0.5 ± 5.5 mmHg for SBP and −0.1 ± 4.9 mmHg for DBP (range −4–4 mmHg for both SBP and DBP). Twelve BP readings with interobserver disagreement >4 mmHg were recorded and excluded in accordance with ISO standards.
Table 3.
General validation participants’ characteristics (n = 85)
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 51.3 ± 14.1 | 20–81 |
| Gender (male/female) | 47/38 | |
| Arm circumference | 30.2 ± 5.1 | 18–42 |
| Entry SBP R0 (mmHg) | 147.6 ± 27.3 | 86–210 |
| Entry DBP R0 (mmHg) | 89.5 ± 17.2 | 50–150 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
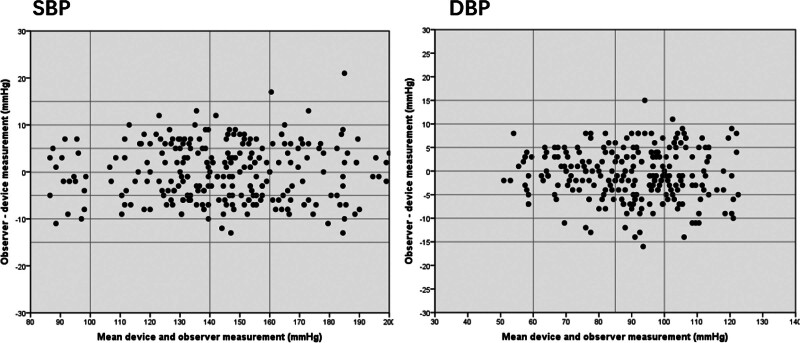
Table 4 shows the distribution of the reference BP measurements R1–R4. The Universal Standard requirements for BP distribution were fulfilled [1]. The validation analysis is shown in Table 5. Both the criteria 1 and 2 suggested ‘pass’ for SBP and DBP [1], and Bland–Altman plots are shown in Fig. 1.
Table 4.
Distribution of reference blood pressure measurements for general validation (R1–R4)
| SBP | ≤100 mmHg | ≥160 mmHg | ≥140 mmHg |
|---|---|---|---|
| 5.9% | 28.1% | 58.9% | |
| DBP | ≤60 mmHg | ≥100 mmHg | ≥85 mmHg |
| 5.8% | 31.9% | 65.2% |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 5.
General validation study results
| Pass requirement | SBP | Achieved | DBP | |
|---|---|---|---|---|
| Criterion 1 (255 BP pairs) | ||||
| Mean BP difference (mmHg) | ≤5 | 0.5 | −0.2 | |
| SD (mmHg) | ≤8 | 6.2 | 5.1 | |
| Pass | Pass | |||
| Criterion 2 (85 Subjects) | ||||
| SD (mmHg, SBP/DBP) | ≤6.92/6.95 | 5.23 | 4.50 | |
| Pass | Pass | |||
| Result | Pass |
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Fig. 1.
Bland–Altman plots of mean M2 and observer measurements for general validation study.
Ambulatory validation study
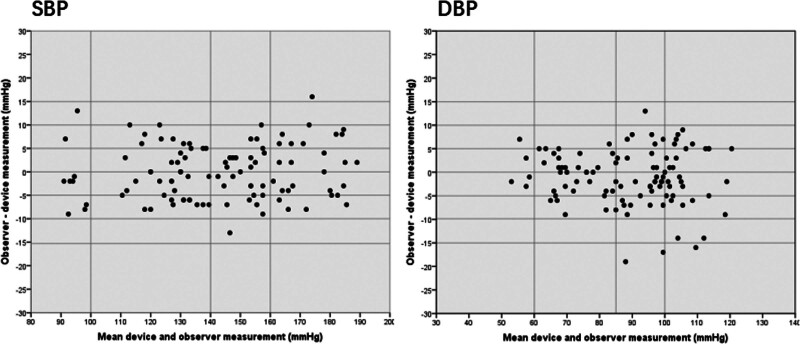
For the ambulatory investigation, 52.7% of subjects with a resting systolic BP more than 140 mmHg, the inter-arm difference of the SBP and DBP were 3.4 ± 1.5 and 2.4 ± 1.3, respectively, and the calculated mean and SD values for the difference between device and observer BP measurements were 0.4 ± 5.9 for SBP and −1.1 ± 5.8 for DBP on the basis of criterion 1 and −1.6 ± 6.39 for SBP and −3.5 ± 5.67 for DBP on the basis of criterion 2. The overall performance of the device, on the basis of both criterion 1 and criterion 2 of the standard, was very good, as shown by the calculated mean and SD values for the differences between device and observer BP values (Table 6). In addition, variation between the observer and device measurements was usually small (±10 mmHg for the majority of comparisons) and there was no systematic variation in the error based on the Bland–Altman plots (Fig. 2).
Table 6.
Ambulatory validation study results
| Pass requirement | SBP | Achieved | DBP | |
|---|---|---|---|---|
| Criterion 1 (105 BP pairs) | ||||
| Mean BP difference (mmHg) | ≤5 | 0.4 | −1.1 | |
| SD (mmHg) | ≤8 | 5.9 | 5.8 | |
| Pass | Pass | |||
| Criterion 2 (35 subjects) | ||||
| SD (mmHg, SBP/DBP) | ≤6.93/6.86 | 6.39 | 5.67 | |
| Pass | Pass | |||
| Result | Pass |
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Fig. 2.
Bland–Altman plots of mean M2 and observer measurements for ambulatory validation study.
Discussion
BP measurement is the cornerstone for hypertension clinical management, and therefore, its accuracy is of utmost importance [8]. To prevent the methodology itself from being a source of error, it is essential that the quality of the devices be rigorously evaluated as this point is central to the method’s clinical validity. This is a typical application of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) for the validation of the ambulatory BP monitor M2 in the general population and the results clearly show that the Raycome model M2 monitor provides reliable and accurate measurements. In addition, we recruited a large part of participants with BP more than 160/100 mmHg and several extremely hypertensive patients, M2 can also be measured accurately, as well as participants with BP less than 100/60 mmHg. There were no issues with the device use or the application of the validation protocol during the study. In conclusion, from the overall validation performance, the Raycome model M2 met all the quality requirements of the Universal Standard in the general population and can be recommended for the clinic.
Acknowledgements
The device was supplied by the Shenzhen Raycome Health Technology Co, Ltd., who also provided funding for the study.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, et al.; European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens 2019; 37:459–466. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien O, Pickering TG, van Montfrans GA, Di Rienzo M, Fagard R. Blood pressure monitoring. Task force i: methodological aspects. Blood Press Monit 1999; 4:279–293. [PubMed] [Google Scholar]
- 4.International Organization for Standardization. ISO 81060-2:2018. Noninvasive sphygmomanometers: Part 2: Clinical investigation of intermittent automated measurement type. https://www.iso.org/standard/73339.html. [Accessed 5 August 2020]. [Google Scholar]
- 5.Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens 2018; 36:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The 64th World Medical Congress. Declaration of Helsinki [Z]. 2013:10. [Google Scholar]
- 7.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion J-M, Mancia G, et al.; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003; 21:821–848. [DOI] [PubMed] [Google Scholar]