Abstract
BACKGROUND
Regular physical activity during childhood and adolescence is beneficial to bone development, as evidenced by the ability to increase bone density and peak bone mass by promoting bone formation.
AIM
To investigate the effects of exercise on bone formation in growing mice and to investigate the underlying mechanisms.
METHODS
20 growing mice were randomly divided into two groups: Con group (control group, n = 10) and Ex group (treadmill exercise group, n = 10). Hematoxylin-eosin staining, immunohistochemistry, and micro-CT scanning were used to assess the bone formation-related indexes of the mouse femur. Bioinformatics analysis was used to find potential miRNAs targets of long non-coding RNA H19 (lncRNA H19). RT-qPCR and Western Blot were used to confirm potential miRNA target genes of lncRNA H19 and the role of lncRNA H19 in promoting osteogenic differentiation.
RESULTS
Compared with the Con group, the expression of bone morphogenetic protein 2 was also significantly increased. The micro-CT results showed that 8 wk moderate-intensity treadmill exercise significantly increased bone mineral density, bone volume fraction, and the number of trabeculae, and decreased trabecular segregation in the femur of mice. Inhibition of lncRNA H19 significantly upregulated the expression of miR-149 and suppressed the expression of markers of osteogenic differentiation. In addition, knockdown of lncRNA H19 significantly downregulated the expression of autophagy markers, which is consistent with the results of autophagy-related protein changes detected in mouse femurs by immunofluorescence.
CONCLUSION
Appropriate treadmill exercise can effectively stimulate bone formation and promote the increase of bone density and bone volume in growing mice, thus enhancing the peak bone mass of mice. The lncRNA H19/miR-149 axis plays an important regulatory role in osteogenic differentiation.
Keywords: Exercise, Osteogenic differentiation, Bone formation, Bone mesenchymal stem cells, Autophagy
Core Tip: Adolescence is a critical period for laying the foundation for optimal peak bone mass in adulthood. Studies have shown that pre-puberty and early puberty are the periods when bones are most responsive to mechanical loading. It is essential to explore the effect of exercise on promoting bone formation in adolescence and its underlying mechanisms. In this paper, we explored the promotional effects of treadmill exercise on bone formation in growing mice and further explored the underlying mechanisms. Our results validate that moderate intensity running exercise is effective in stimulating bone formation and promoting increases in bone mineral density and bone mass, thereby enhancing peak bone mass in growing mice. Notably, the long non-coding RNA H19 (lncRNA H19)/microRNA-149 (miR-149) axis plays an important regulatory role in the osteogenic differentiation of bone mesenchymal stem cells. Overall, this paper emphasizes that exercise may promote bone formation in mice through the lncRNA H19/miR-149 axis, which may be closely related to the activation of autophagy.
INTRODUCTION
Bone tissue is a dense connective tissue that is part of the endoskeleton of vertebrates. Physiologically, bone is subjected to a dynamic mechanical environment of continuous remodeling through two coordinated and synchronized processes, including osteoblast-driven bone formation and osteoclast-driven bone resorption, which help the bone to develop an optimized morphological structure to adapt to changing loads and maintain homeostasis[1]. As a typically mechanically responsive tissue, bone can respond to stimuli of mechanical loading: physiological loading induces bone formation, whereas lack of loading or overloading can lead to bone resorption[2,3]. Mechanical loading is a major regulator of bone formation and resorption, playing a crucial role in the metabolic homeostasis of bone[4,5].
Osteoporosis is one of the most serious health problems in older women. Current strategies to prevent osteoporosis in women focus on increasing peak bone mass at skeletal maturity and reducing bone loss in midlife and late menopause[6,7]. Previous studies have demonstrated that 25%-40% of adult bone mass is gained during puberty, a critical period for laying the foundation for optimal peak bone mass in adulthood[8], which helps to reduce the chances of osteoporosis in old age[9,10]. Regular physical activity during childhood and adolescence is beneficial for musculoskeletal development, and its beneficial effects can continue into adulthood and even old age[11,12]. Therefore, exercising at an appropriate intensity during adolescence is crucial for increasing peak bone mass. Currently, however, there is a lack of clarity regarding the underlying mechanisms by which exercise promotes bone formation during puberty.
Long non-coding RNAs (lncRNAs) are by-products of RNA polymerase II transcription, a group of non-coding transcripts ≥ 200 nucleotides in length, which do not have the function of coding proteins[13,14]. Unlike microRNAs (miRNAs), which are non-coding RNAs that do not encode proteins, lncRNAs can fold into complex secondary or higher spatial structures, allowing for better target recognition[15]. Accumulating studies have shown that IncRNAs can regulate the expression of protein-coding genes epigenetically, transcriptionally, and post-transcriptionally, thereby affecting a range of biological processes, including the regulation of bone metabolism[16,17]. Studies have shown that lncRNAs exhibit the following three functions during osteogenic differentiation: (1) Regulating osteogenic differentiation by mediating epigenetic modifications; (2) Regulating osteogenic differentiation through signaling pathways; and (3) Regulating osteogenic differentiation by acting as miRNA sponges or precursor structures[18,19]. Some of the lncRNAs were found to be mechanosensitive and regulated by mechanical stress, including lncRNA H19[20-22]. LncRNA H19, located on human chromosome 11p15.5, is one of the best-known imprinted genes[23,24]. LncRNA H19 was found to be highly expressed in adult muscle tissues and upregulated during differentiation and regeneration of adult myoblasts[25]. In addition, lncRNA H19 may be involved in the regulation of atherosclerosis[26], myocardial injury[27], and osteogenic differentiation[28]. It has been shown that inhibition of lncRNA H19 promotes osteogenic differentiation of human adipogenic stem cells[29]. However, another study found that lncRNA H19 deficiency could inhibit osteogenic differentiation of bone mesenchymal stem cells (BMSCs)[30,31]. The possible reason for this is the difference in stem cell types. In addition, simple ex vivo experiments cannot adequately reflect the complex biological response processes in organisms. Therefore, the potential regulatory role of lncRNA H19 in osteogenic differentiation needs to be further elucidated.
Our previous study found that exercise protects against cartilage damage in mice by upregulating lncRNA H19[22]. Since bone is also a major effector organ of mechanical exercise, we hypothesized that exercise may promote osteogenic differentiation by modulating lncRNA H19 expression. Based on the clinical experience of exercise therapy to promote peak bone mass and the foundation of our team's previous research, this study aimed to observe the effect of moderate-intensity treadmill exercise to promote bone formation in growing-age mice and to further investigate the regulatory role of lncRNA H19 in this process, which is of great significance for bone health in the general population and for the development of new strategies for the treatment of bone-related diseases.
MATERIALS AND METHODS
Animal experiments
All procedures of this study were reviewed and approved by the Ethics Committee of Exercise Science Experiment of Beijing Sport University (Approval No. 2023026A). Twenty 3-wk-old female C57BL/6 mice were purchased from Beijing Huafukang Bio-technology Co. Ltd. and housed in an SPF-class animal laboratory environment with temperature and relative humidity at (22 ± 2) °C and 55%-75%. Normal circadian rhythms were administered. All mice are allowed to move freely within the cage. Adaptive feeding of mice for one week is required before the formal experiment begins.
After the end of adaptive feeding, twenty mice were randomly divided into two groups: Con group (control group, n = 10) and Ex group (treadmill exercise group, n = 10). Mice in the Con group were free to move around the cage, while mice in the Ex group were forced to perform treadmill exercises. The treadmill exercise program was developed based on the experience of our group's previous research[22,32,33]. The protocol during the formal treadmill exercise experiment was one hour per day, five days per week, for a total of eight weeks. It should be noted that during the formal experiment, the speed was gradually increased from 5 m/min to 15 m/min in the first five minutes at the beginning of each running exercise, followed by running at a constant speed of 15 m/min for 50 min, and the speed was gradually reduced from 15 m/min to 0 m/min in the last five minutes at the end of the running exercise. The specific treadmill running program is shown in Table 1. After eight weeks of treadmill running training, all mice were anesthetized by inhalation of isoflurane and subjected to neck transection. Before sacrifice, all mice were fed water without feeding for 24 h. No mice showed abnormal mortality in this experiment.
Table 1.
Treadmill exercise program
|
Stages
|
Time
|
Speed
|
Duration
|
| Acclimatization treadmill exercise | Day 1 | 6 m/min | 30 min |
| Day 2 | 9 m/min | 40 min | |
| Day 3 | 12 m/min | 50 min | |
| Formal treadmill exercise | Days 4 to 7 | 15 m/min | 60 min |
| Weeks 2 to 8 | 15 m/min | 60 min |
Hematoxylin-eosin staining
After PFA fixation, the mouse femur was placed in EDTA decalcification solution for 2-3 wk for decalcification. The decalcification solution was changed every other day. The standard for complete decalcification is that the needle tip of the syringe can easily penetrate the cortical bone. The samples were then dehydrated, transparent, waxed, embedded in a tissue wax block, and finally sectioned (4 μm per section). Subsequently, routine hematoxylin-eosin (HE) staining was performed according to the instructions. After staining, the sections were observed and photographed under an inverted optical microscope.
Immunohistochemical staining
Mouse femur paraffin sections were placed in a 60 ℃ oven for 30 min and then subjected to treatment with xylene, xylene, xylene, 100% ethanol, 95% ethanol, and 80% ethanol. After dewaxing, antigen repair, blocking, primary antibody incubation, secondary antibody incubation, and DAB staining were performed. The staining process was terminated after positive expression was observed under the microscope. Subsequently, the nucleus was stained using hematoxylin staining. Conventional dehydration and xylene transparency were performed. Finally, neutral resin adhesive was used for sealing. The sections were observed and photographed under an inverted optical microscope.
Immunofluorescence staining
Similar to the immunohistochemical staining steps, the mouse femur paraffin sections were sequentially subjected to dewaxing, antigen repair (microwave repair can reduce bone tissue loss), blocking, primary antibody incubation (anti-Beclin1, abmart, T55092), secondary antibody incubation, re-antigen repair, addition of primary antibody (anti-P62, abmart, T55546), secondary antibody incubation, DAPI re-staining of the cell nucleus, and blocking. Finally, images were taken under the SP8 Leica laser confocal microscope. The nucleus was stained blue (excitation wavelength 330-380 nm, emission wavelength 420 nm), Beclin1 protein was stained red (excitation wavelength 510-560 nm, emission wavelength 590 nm), and P62 protein was stained green (excitation wavelength 465-495 nm, emission wavelength 515-555 nm).
micro-CT
The mouse knee joints were carefully isolated, and the intact femur were placed in PFA fixative for 48 h for fixation before micro-CT scanning. The Skyscan1276 scanning device was used to scan the rat knee joint. The knee was placed in the Skyscan1276 ex vivo sample scanning bed. The scanning parameters were as follows: Camera pixel size (μm) = 9.01; source voltage (kV) = 69; source current (μA) = 100; image pixel size (μm) = 9.92. After scanning, NRecon and DataViewer software were used to adjust all samples to the same position in the 3D axes to facilitate subsequent more precise selection of the region of interest for reconstruction. CTan software was used for further analysis of bone microstructural parameters, including bone mineral density (BMD), bone volume fraction (BV/TV), the number of trabeculae (Tb.N), and trabecular segregation (Tb.Sp). The place where the growth plate disappeared was chosen as the starting point of the region of interest, and 100 Layers were selected upwards as the region of interest for 3D reconstruction. Finally, CTvox was used for 3D reconstruction of regions of interest. After the micro-CT scan was completed, the femur was placed in an EDTA decalcification solution for decalcification for subsequent paraffin embedding and pathological sectioning.
Extraction of primary bone marrow mesenchymal stem cells
The lower limb of the mouse was severed at the hip joint (pay attention to preserving the intact femoral trochanter). The muscles and other soft tissues of the lower limbs were carefully peeled off. Subsequently, the femur and tibia were carefully separated at the knee joint (the fibula can be detached). Sterile ophthalmic scissors were used to cut both ends of the femur and tibia to expose the bone marrow cavity. Then, a 1 mL syringe was used to draw a complete culture medium containing 10% FBS and rinse the bone marrow cavity until all the tissue in the bone marrow cavity was washed down and appeared white. Finally, a pipette was used to repeatedly blow the culture medium containing bone marrow tissue and inoculate it into a 10cm culture dish. After 4-6 d, the cells can adhere to the culture dish.
Alkaline phosphatase staining
After the osteogenic induction and differentiation of primary BMSCs, the PBS solution was washed three times, followed by PFA to fix the BMSCs for 10-15 min. PBS solution was used to wash the cells three times again. Finally, an alkaline phase (ALP) staining solution (Sigma, B5655) was used to stain the cells. BMSCs were incubated at room temperature and in a dark environment, and the staining of cells was observed in real time. After a significant blue color appeared, the staining was terminated.
Alizarin red staining
After the osteogenic induction and differentiation of primary BMSCs, the PBS solution was washed three times, followed by PFA to fix the BMSCs for 10-15 min. PBS solution was used to wash the cells three times again. Finally, an alizarin red staining solution (Servicebio, G1038) was used to stain the cells. BMSCs were incubated at room temperature, and the staining of cells was observed in real-time. After a significant red color appeared, the staining was terminated.
Bioinformatics analysis
The sequence and annotation information of lncRNA-H19 was queried through the NCBI database to understand the biological function, expression pattern, and regulatory mechanism of this gene. The sequence of lncRNA-H19 was predicted to clarify the structure of its miRNAs containing target sites. Four websites, ENCORI, mricode, DINATOOLS, and LncACTdb, were used for predictive analysis of miRNA target genes for lncRNA H19 (ENCORI: https://rnasysu.com/encori/index.php; mricode: http://www.mircode.org; DINATOOLS: http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/index; LncACTdb: http://www.bio-bigdata.net/LncACTdb/index.htm). The intersection of the four sites identified four potential target miRNAs (miR-148a, miR-185, miR-212, and miR-149).
Cell transfection experiment in vitro
Primary BMSCs were washed three times using PBS when they grew to 30%-50% fusion. Specialized media for cell transfection with low serum was used. The si-lncRNA H19 and NC with Lipofectamine® 3000 Liposome transfection reagent (Invitrogen, L3000015) were added to low-serum cell transfection-specific medium, respectively. After 8 h, a 10% FBS complete culture medium was used to replace the low serum culture medium for subsequent experiments.
RT-qPCR
The total RNA of the cells was extracted using the Trizol method to detect the expression of target genes. Briefly, cells were washed three times with PBS. Six-well plates were prepared by adding 1 mL of Trizol to each well and transferring to 1.5 mL EP tubes. Subsequently, 200 μL of chloroform was added to each well, shaken vigorously, and centrifuged at 12000 rpm, 4 °C for 15 min. An equal amount of isopropanol was added to the supernatant, mixed upside down, and then centrifuged at 12000 rpm, 4 °C for 15 min. 1 mL of 75% ethanol was added to the supernatant and centrifuged at 7500 rpm, 4 °C for 5 min. The supernatant was discarded and air-dried so that the alcohol could be completely evaporated. Add 20 μL of DPEC water to dissolve the RNA. RNA concentration was measured using a spectrophotometer. The Takara SYBRGreen kit (Takara, RR820A) was used for real-time fluorescent quantitative amplification of target genes. β-actin was used as an internal reference. The 2−ΔΔCT method was used to analyze the data. As shown in Table 2, all primers involved were designed using Primer Premier 5.0 software.
Table 2.
Primer sequences
|
Genes
|
Primer sequence (5'-3')
|
| lncRNA H19 | Forward: 5'- GCTCCACTGACCTTCTAAAC -3' Reverse: 5'- ACGATGTCTCCTTTGCTAAC -3' |
| miR-149 | Forward: 5'- TGGCTCCGTATCTTCACTCC -3' |
| β-actin | Forward: 5'-CTGTCCCTGTATGCCTCTG-3' Reverse: 5'-ATGTCACGCACGATTTCC-3' |
| ALP | Forward: 5'-CCAACTCTTTTGTGCCAGAGA-3' Reverse: 5'-GGCTACATTGGTGTTGAGCTTTT-3' |
| Runx2 | Forward: 5'-AGAGTCAGATTACAGATCCCAGG-3' Reverse: 5'-TGGCTCTTCTTACTGAGAGAGG-3' |
| BMP2 | Forward: 5'- GTGCAGATCCTAGGTTTCTCTG-3' Reverse: 5’- CAGGATCTCATTCTCTTGGATC-3’ |
| Osterix | Forward: 5'-TGGTACAAGGCAGGCATCCA-3' Reverse: 5'-GGAGCAAAGTCAGATGGGTAAGT-3' |
Western blot
RIPA lysate was used to extract total protein from BMSCs for the detection of target protein expression. Briefly, BMSCs were washed three times with PBS, and 100 μL of Ripa lysate containing 1% protein phosphatase inhibitor was added to a six-well plate. The adherent primary BMSCs were scraped off using a cell scraper and transferred to 1.5 mL EP tubes, which were shaken on ice for 20 min and then sonicated. After centrifugation at 12000 rpm for 20 min, the supernatant was taken and transferred to a 1.5 mL EP tube. Then, after measuring the protein concentration with the BCA kit to homogenize the protein concentration, the protein loading buffer was added and cooked at 100 °C for 8 min. The proteins were separated using SDS-PAGE gel electrophoresis and then transferred to PVDF membranes. Subsequently, the protein-containing PVDF membrane was subjected to blocking, primary antibody incubation, and secondary antibody incubation. Finally, the chemiluminescent solution was used to develop the protein image. Anti-ALP (Invitrogen, PA5-106391), anti-bone morphogenetic protein 2 (BMP2) (Invitrogen, PA5-85956), anti-SP7 (Invitrogen, PA5-115697), anti-Beclin1 (abmart, T55092), Anti-microtubule-associated protein 1 light chain 3 beta (LC3B) (abmart, T55992), anti-β-actin (abmart, TP70573), anti-GAPDH (abmart, MG212519S), and anti-β-tubulin (abclonal, A12289) were used as primary antibody. The interest protein grayscale values were counted using Image J software.
Data analysis
The data from each group were analyzed using SPSS 20.0 software. GraphPad Prism 8 was used to graph statistical data. Results are expressed as mean ± SD. Independent samples t-test was used to compare the differences between two groups, while one-way ANOVA and the LSD method were used to compare the differences between multiple groups. P < 0.05 indicates a significant difference.
RESULTS
Exercise promotes bone formation in growing mice
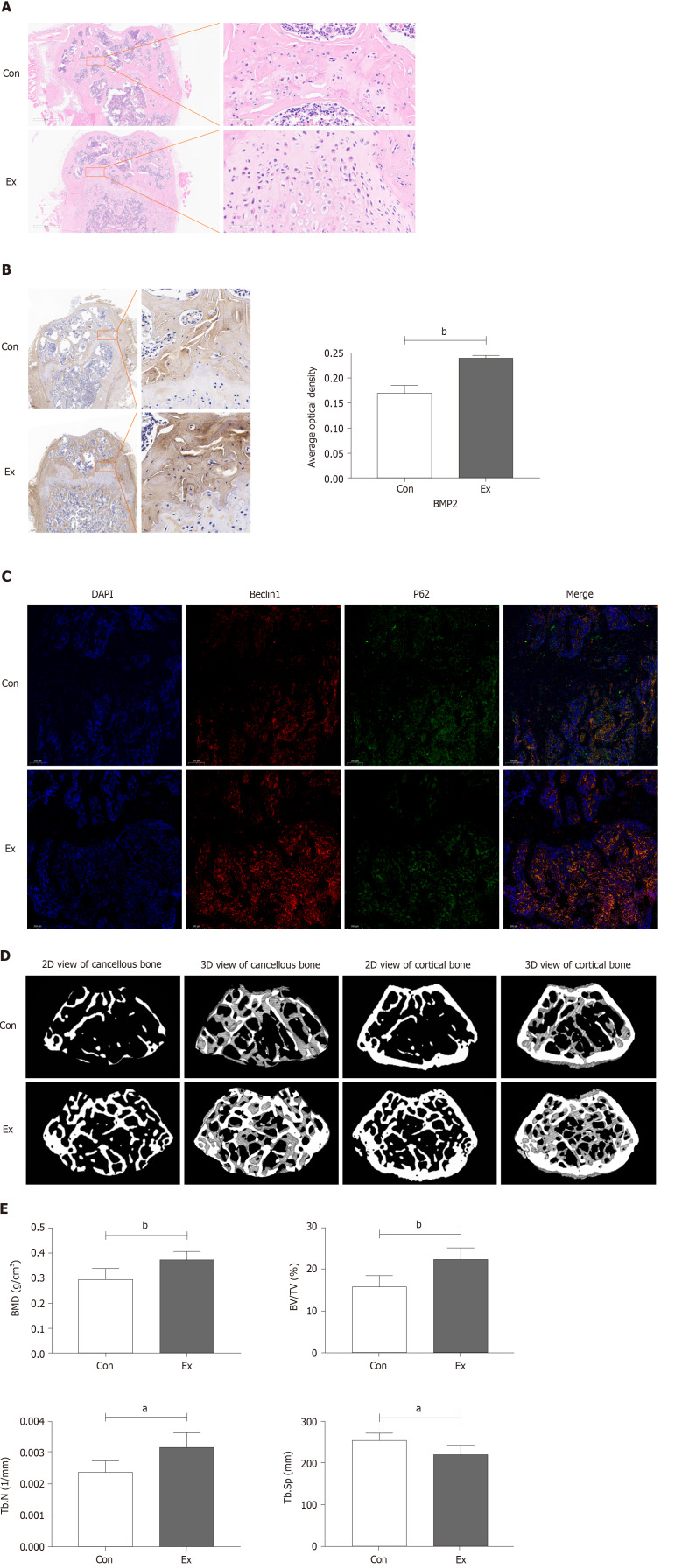
Growth-phase mice were selected to undergo mandatory 8 wk of moderate-intensity treadmill exercise to stimulate bone formation, thereby enhancing peak bone mass. As shown in Figure 1A, compared with the Con group, the femoral growth plate of mice in the Exercise group was significantly thickened, accompanied by distinct chondrocyte stratification: resting-zone chondrocytes, proliferating chondrocytes, and hypertrophic chondrocytes, which is suggestive of an active osteogenic function. Immunohistochemical staining showed that the expression of BMP2, a marker of osteogenic differentiation, was also significantly increased in the Ex group of mice (Figure 1B). A recent study found that exercise may alleviate bone loss in aging mice by modulating autophagy[34]. Therefore, we preliminarily examined the expression of markers of autophagy in the femur and found that the level of autophagy was significantly increased in the femur of mice in the Ex group (Figure 1C). Consistently, micro-CT results showed that 8 wk of moderate-intensity treadmill exercise significantly increased BMD, BV/TV, and Tb.N) and decreased Tb.Sp in the femur of mice (Figure 1D and E). Thus, we found that 8 wk of moderate-intensity treadmill exercise significantly promoted femoral bone formation and increased peak bone mass in growing mice, which may be related to the activation of autophagy.
Figure 1.
Exercise promotes bone formation in growing mice. A: Hematoxylin-eosin staining of mouse femur; B: Changes in bone morphogenetic protein 2 protein expression in mouse femur by immunohistochemical staining; C: Changes in protein expression of Beclin1 and P62 in mouse femur by immunofluorescence staining; D and E: Reconstruction images and bone morphometric indices of changes in the distal femur in mice by micro-CT scanning. n = 6, aP < 0.05, bP < 0.01. Con: Control group; Ex: Treadmill exercise group.
Exercise promotes osteogenic differentiation of mouse BMSCs
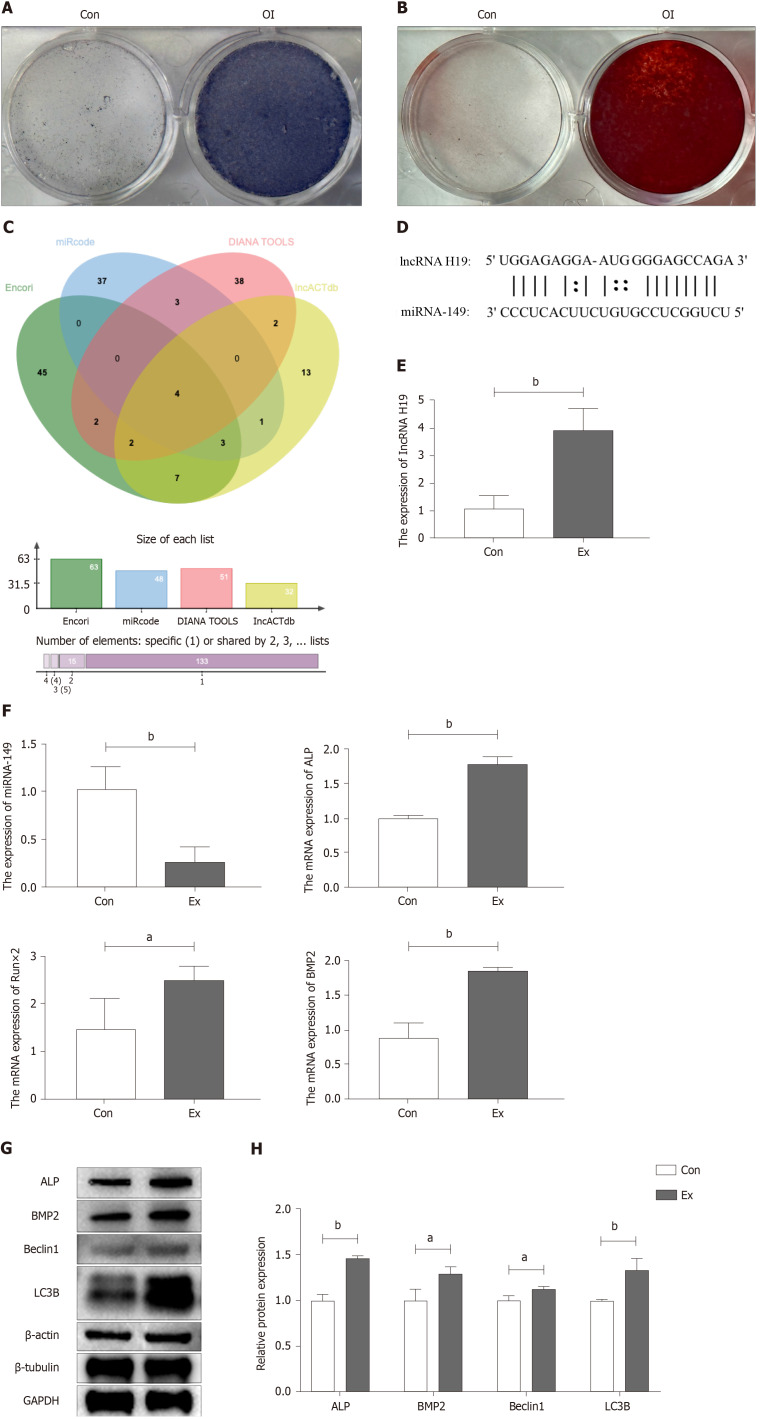
BMSCs from the femur and tibia of Con and Ex group mice were extracted for osteogenic differentiation induction using an osteogenic differentiation medium to verify whether exercise increased peak bone mass in growing mice by promoting osteogenic differentiation of BMSCs. First, we performed stem cell characterization of primary cells extracted from mouse bone marrow. As shown in Figure 2A and B, primary mouse BMSCs subjected to ALP staining and alizarin red staining showed good stem cell properties 7 and 14 d after osteogenic induction of differentiation, respectively. Subsequently, we explored the possible mechanisms by which exercise promotes osteogenic differentiation by bioinformatics analysis to find potential miRNA targets of lncRNA H19. As shown in Figure 2C and D, bioinformatics analysis suggested four potential miRNAs (miR-148a, miR-185, miR-212, and miR-149). Further, RT-qPCR results showed that the expression of miR-149 was significantly down-regulated in Ex-group BMSCs, which was opposite to the expression trend of lncRNA H19 (Figure 2E and F), suggesting that miR-149 may be a direct target gene of lncRNA H19 during the osteogenic differentiation of BMSCs. In addition, we found that compared to the Con group, the mRNA and protein expression levels of osteogenic differentiation markers of BMSCs in the Ex group were significantly higher, including ALP and BMP2 (Figure 2E-H). Notably, autophagy-related protein markers such as Beclin1 and LC3B were also significantly up-regulated in BMSCs from the Ex group (Figure 2G and H). Thus, exercise significantly promotes osteogenic differentiation of BMSCs in growth-phase mice, which may be associated with activation of the lncRNA H19/miR-149 axis and autophagy.
Figure 2.
Exercise promotes osteogenic differentiation of mouse bone mesenchymal stem cells. A: Alkaline phase staining of mouse bone mesenchymal stem cells (BMSCs) at 7 d of osteogenic differentiation induction; B: Alizarin red staining of mouse BMSCs at 14 d of osteogenic differentiation induction; C and D: Identification of potential miRNAs targets of lncRNA H19 by bioinformatics analysis; E and F: Changes in mRNA levels of lncRNA H19, miR-149, and osteogenic differentiation-associated markers after induction of osteogenic differentiation in mouse primary BMSCs by RT-qPCR; G and H: Changes in protein level of osteogenic differentiation-related marker and autophagy-related marker after induction of osteogenic differentiation in mouse primary BMSCs. n = 3, aP < 0.05, bP < 0.01. Con: Control group; OI: Osteogenic differentiation induction; Ex: Treadmill exercise group.
Exercise promotes osteogenic differentiation of BMSCs through upregulation of lncRNA H19/miR-149
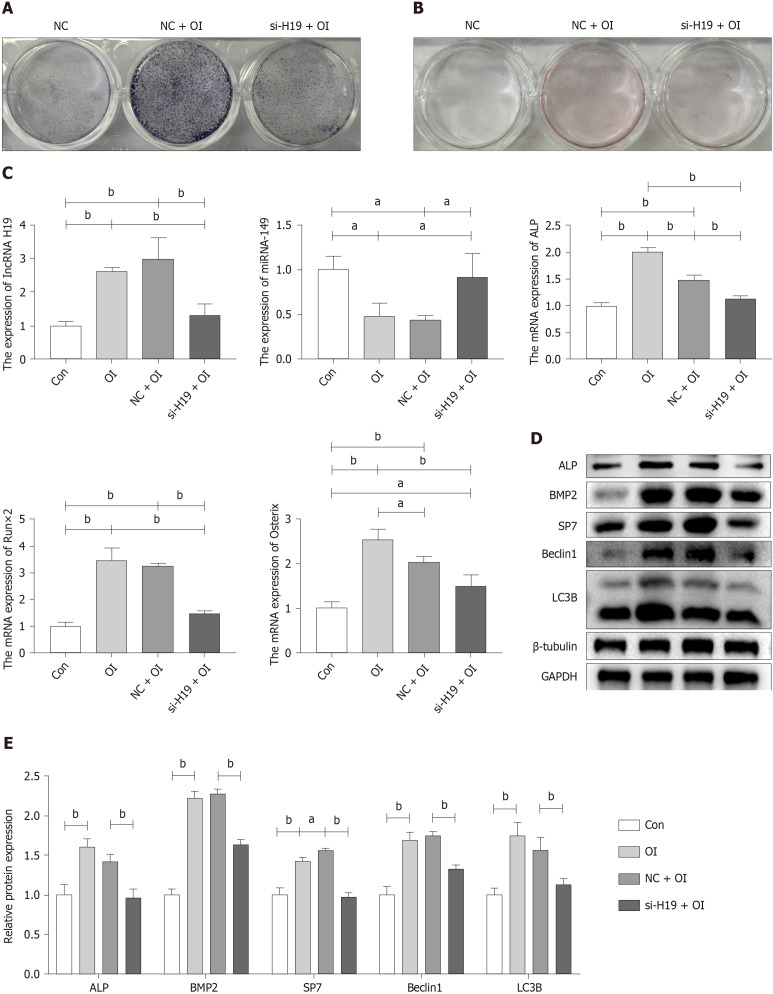
Cell transfection techniques were used to inhibit the expression of lncRNA H19 to verify that the lncRNA H19/miR-149 axis can mediate the process of exercise-promoting osteogenic differentiation in BMSCs. Wild-type mouse BMSCs were extracted for 3 d for osteogenic differentiation induction, as the effective time for si-RNA transfection was 48-72 h. It was found that ALP staining was hampered after lncRNA H19 was knocked down (Figure 3A). However, there was no significant difference in the results of alizarin red staining due to the short time of osteogenic induction of differentiation (Figure 3B). Subsequently, we examined the expression of genes and protein markers related to osteogenic differentiation after the knockdown of lncRNA H19. As shown in Figure 3C-E, inhibition of lncRNA H19 significantly suppressed osteogenic differentiation of BMSCs, characterized by a significant decrease in the expression of markers of osteogenic differentiation such as ALP, Runx2, and BMP2. Meanwhile, the knockdown of lncRNA H19 can significantly upregulate miR-149 expression, which further demonstrates that the lncRNA H19/miR-149 axis may play an important regulatory role in the osteogenic differentiation of BMSCs. In addition, we found that inhibition of lncRNA H19 significantly abolished the activation of Beclin1 and LC3B, resulting in reduced levels of autophagy (Figure 3D and E). The above results suggest that exercise can promote osteogenic differentiation of BMSCs by upregulating the lncRNA H19/miR-149 axis, which may be related to the activation of autophagy.
Figure 3.
Exercise promotes osteogenic differentiation of bone mesenchymal stem cells through upregulation of lncRNA H19/miR-149. A: Alkaline phase staining of bone mesenchymal stem cells (BMSCs) 3 d after osteogenic induction of differentiation; B: Alizarin red staining of BMSCs 3 d after osteogenic induction of differentiation; C: Gene expression changes of lncRNA H19, miR-149 and osteogenic differentiation-related markers in BMSCs after osteogenic differentiation induction and si-lncRNA H19 interference by RT-qPCR; D and E: Protein expression changes of osteogenic differentiation-associated markers and autophagy-associated markers in BMSCs after osteogenic differentiation induction and si-lncRNA H19 interference by WB technique. n = 3, aP < 0.05, bP < 0.01. Con: Control group; NC: Negative control group; OI: Osteogenic differentiation induction group.
DISCUSSION
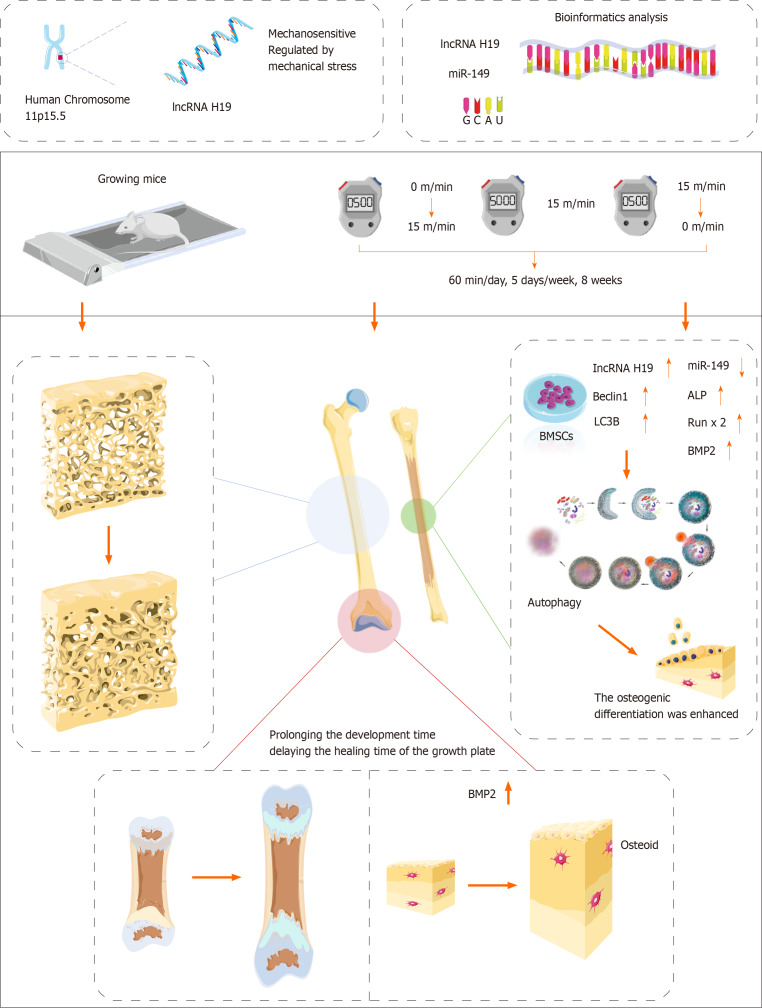
Peak bone mass acquired during adolescence is essential for bone health. The gradual loss of bone mass in adulthood is irreversible[9]. However, exercise is effective in increasing peak bone mass during puberty. More peak bone mass means an increase in relative bone mass in adulthood, which can effectively reduce the risk of osteoporotic fractures[10]. Therefore, it is valuable to explore in depth the potential role of exercise in promoting bone formation during adolescence. In this study, the promotional effect of treadmill exercise on bone formation in growing mice and its underlying mechanisms were explored (Figure 4).
Figure 4.
Summary diagram of this study.
Since mice reach sexual maturity at 6-8 wk and can be defined as adult mice at 12 wk[35]. 4-wk-old growing mice were selected to perform treadmill exercises. Previous studies have shown that short-term programs and/or low-intensity exercise programs are not effective in stimulating bone formation, regardless of gender or age, while high-intensity exercise imposes excessive loads on the bone, causing damage to the bone tissue[35]. Hamann et al[36] suggested that there may be a critical strain threshold to stimulate bone formation. For example, Maurel et al[37] observed no increase in whole-body BMD after exercise, which could be attributed to insufficient intensity of the exercise program. Furthermore, another study demonstrated that a population-based moderate-intensity physical activity intervention program during childhood appears to exert beneficial effects on several musculoskeletal characteristics[38]. In addition, most studies have shown that moderate-intensity exercise with a mean duration of 8 wk or more can promote improvements in bone microarchitecture[39-42]. Therefore, for the present study, moderate-intensity treadmill exercise was chosen as the intervention for growing mice for 8 wk.
Our results showed a significant increase in BMD, BV/TV, and Tb. N and a decrease in trabecular separation in the femur of mice in the Ex group compared to the Con group, suggesting that 8 wk of moderate-intensity treadmill exercise is effective in stimulating bone formation and significantly increasing BMD in growing mice, which contributes to the enhancement of the peak bone mass in adulthood. Notably, in HE staining, we found that the thickness of the growth plate of mice in the Ex group was significantly increased, and the proliferation and differentiation of cells in all layers of the bone growth plate were active. The growth plate, a cartilaginous tissue located between the epiphysis and the diaphysis, is the main differentiation region for longitudinal bone growth and consists mainly of chondrocytes and extracellular matrix[43,44]. During the juvenile period, the proliferation and differentiation of cartilage are balanced with the rate of new bone production, thus ensuring that the growth plate can maintain a certain thickness while the length of the diaphysis increases. With age, the proliferative potential of chondrocytes in the growth plate of adolescent children is gradually exhausted. When the proliferative and osteogenic activity of cartilage ceases, the growth plate is completely ossified and fused, accompanied by a reduction in the width of the growth plate. Simultaneously, the longitudinal growth of the long bones stops[45]. Impaired or premature closure of the bone growth plate function can lead to short stature, limb length incongruity, and abnormal skeletal development in children since continued maturation of bone growth plate cartilage can provide a scaffold for bone deposition[46]. It was found that the proliferation and hypertrophy of chondrocytes in the epiphyseal plate were more pronounced in joints subjected to weight-bearing stress. The stress-affected cell proliferation and differentiation were closely related to the elevated expression level of parathyroid hormone-related protein (PTHrP)[47]. Another study found that short-term cyclic stress can upregulate the expression of PTHrP in growth plate chondrocytes, which in turn affects growth plate development[48]. In the present study, the improvement in the thickness of the distal femoral growth plate by exercise may be an important reason for promoting bone formation and bone growth in growing mice. In addition, the growth plates are more susceptible to injury since they are composed of cartilage, which is weaker than solid bone[44]. It has been shown that shock loading inhibits bone growth throughout adolescence in young rats. This may be due to excessive shock loading, causing damage to the bone growth plate, which in turn inhibits normal bone formation[49]. Therefore, we have to emphasize the necessity of controlling the intensity of exercise when performing exercise interventions in mice during the growth period. Moreover, the timing of growth plate development is the limiting factor in determining height[50,51]. Therefore, we propose the hypothesis that exercise may also promote an increase in BMD and peak bone mass by prolonging the development time/delaying the healing time of the growth plate in mice.
To investigate the effects and potential mechanisms of exercise to promote osteogenesis in growing mice, BMSCs from the femur and tibia of Con and Ex group mice were extracted for further experiments. We found that the expression of lncRNA H19 was significantly higher in BMSCs in the Ex group compared to the Con group, while the mRNA and protein expression levels of ALP and BMP2 were significantly higher, suggesting that exercise significantly promotes the osteogenic differentiation of BMSCs in growth-phase mice, which may be related to the upregulation of lncRNA H19. Subsequently, small interfering RNA was used to inhibit lncRNA H19 expression in BMSCs to verify whether exercise promotes osteogenic differentiation via lncRNA H19. The results showed that si-H19 significantly inhibited osteogenic differentiation of BMSCs by ALP staining, although alizarin red staining showed no significant difference due to the too-short time of osteogenic differentiation. RT-qPCR with WB analysis also further demonstrated that si-H19 significantly inhibited osteogenic differentiation of BMSCs. Therefore, exercise may promote osteogenesis by upregulating lncRNA H19. Since lncRNA H19 can act as a miRNAs sponge to regulate bone formation during osteogenic differentiation[18], we further identified five potential miRNAs targets of lncRNA H19 by bioinformatics analysis. Combined with bioinformatics analysis, we preliminarily found that the expression of miR-149 was decreased during osteogenic differentiation of BMSCs by RT-qPCR, which was opposite to the expression trend of lncRNA H19, suggesting that miR-149 may be a direct target gene of lncRNA H19 during the osteogenic differentiation of BMSCs. It has been reported that miR-149 is involved in the regulation of calcium ions, bone matrix mineralization, and bone resorption, as well as the differentiation and maintenance of bone tissue by targeting multiple pathways and genes[52]. Furthermore, miR-149 was found to be associated with osteosarcoma. miR-149 can target the expression of BMP9 to promote osteosarcoma progression[53]. In the present study, we found that the expression of miR-149 was significantly upregulated after knockdown of lncRNA H19, which further demonstrated that the lncRNA H19/miR-149 axis may play an important regulatory role in the osteogenic differentiation of BMSCs.
It is worth mentioning that in our study, compared with the Con group, the expression level of Beclin1 protein in the femur of mice in the Ex group was significantly increased while the expression of P62 protein was significantly decreased. Furthermore, the expression levels of Beclin1 and LC3B were also significantly up-regulated in the BMSCs of mice in the Ex group. It indicates that exercise may promote bone formation in mice by activating autophagy. Beclin1, p62, and LC3B are regulators of autophagy. High expression of Beclin1 accompanied by a decrease in p62 protein suggests that autophagy is activated, and vice versa[54]. Autophagy is a self-degradation process that balances energy sources in response to cellular stress[55]. Autophagy plays an important role in bone homeostasis. Studies have shown that estradiol can rescue osteoblasts from apoptosis by promoting autophagy through the Er-Erk-mTOR pathway[56]. Additionally, autophagy is closely related to osteoblast differentiation and mineralization. Mineral needle-like structures within the cytoplasm are mainly located in autophagy-like vesicles. Inhibition of autophagy leads to a decrease in the mineralization capacity of osteoblasts, whereas inhibition of autophagic flow prevents the outward transport of minerals from osteoblasts[57,58]. Previous studies have found that under mechanical stress, osteoblasts can promote osteoclastogenesis through autophagy-mediated receptor activator of nuclear factor κB ligand (RANKL) secretion[59]. Recent studies have reported that miRNAs play an important role in autophagy regulation. Wang et al[60] found that miR-140-5p and miR-149 can enhance chondrocyte autophagic activity by regulating the number of autophagic vesicles in human primary chondrocytes. In our study, we found that inhibition of lncRNA H19 significantly down-regulated the expression of Beclin1 and LC3B, leading to a decrease in autophagy levels. This suggests that exercise can promote osteogenic differentiation of BMSCs through up-regulation of the lncRNA H19/miR-149 axis, which may be related to the activation of autophagy. That is, exercise may promote osteogenic differentiation through lncRNA H19/miR-149/autophagy in growth-stage mice. Unfortunately, in this study, we did not provide sufficient evidence to support this hypothesis. In addition, it is well known that exercise inhibits adipogenesis. In HE staining, we found that there was no significant change in adipocytes in the bone marrow cavity of mouse femurs after 8 wk of exercise, which may be because we used mice that were not disease models, such as the osteoporosis model. Therefore, osteoporosis model mice can be selected to further explore whether exercise can inhibit lipogenic differentiation through the lncRNA H19/miR-149 axis while promoting osteogenic differentiation. And, it can also be deeply explored whether this process is similarly related to the regulation of autophagy.
CONCLUSION
In summary, our study demonstrated that moderate intensity running exercise can effectively stimulate bone formation and promote the increase of bone density and bone volume in growing mice, thus enhancing the peak bone mass of mice. Notably, the lncRNA H19/miR-149 axis plays an important regulatory role in the osteogenic differentiation of BMSCs, and this process may be related to the activation of autophagy.
ARTICLE HIGHLIGHTS
Research background
It is well known that exercise promotes bone growth and development. However, the underlying mechanisms by which exercise promotes bone formation are not fully understood.
Research motivation
Our previous findings suggest that the mechanosensitive lncRNA H19 is involved in the regulation of cartilage homeostasis. Therefore, we propose the hypothesis that mechanosensitive lncRNA H19 may be involved in mediating the process of exercise-promoted bone formation. This study will provide more theoretical basis for exercise promoting bone health.
Research objectives
The aim of this study was to investigate whether mechanosensitive lncRNA H19 could promote bone formation by targeting miR-149. This study reveals for the first time the potential regulatory role of the lncRNA H19/miR-149 axis in exercise-promoted bone formation, providing a scientific basis for the promotion of bone health by exercise.
Research methods
The potential role of lncRNA H19/miR-149 axis in exercise-promoted bone formation was fully validated in vivo and in vitro by RT-qPCR, WB, IF, IHC, and micro-CT combined with bioinformatics analysis.
Research results
In vivo, exercise could activate autophagy by promoting the expression of lncRNA H19 and inhibiting the expression of miR-149, thereby promoting bone formation. In vitro, knockdown of lncRNA H19 was able to inhibit autophagy by upregulating miR-149 expression, thereby inhibiting osteogenic differentiation of bone mesenchymal stem cells.
Research conclusions
Exercise can promote autophagy and bone formation through activation of the lncRNA H19/miR-149 axis.
Research perspectives
The potential role of the lncRNA H19/miR-149/autophagy axis in exercise-promoted bone formation was further validated by gain of function and loss of function in animal experiments.
Footnotes
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Beijing Sport University (Approval Number: 2023026A).
Conflict-of-interest statement: The authors declare no conflict of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 22, 2023
First decision: January 24, 2024
Article in press: March 19, 2024
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalaby MN, Egypt S-Editor: Gong ZM L-Editor: A P-Editor: Zhao YQ
Contributor Information
Xu-Chang Zhou, School of Sport Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China.
Dong-Xue Wang, School of Sport Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China.
Chun-Yu Zhang, School of Sport Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China.
Ya-Jing Yang, Department of Acupuncture and Moxibustion, Hubei University of Chinese Medicine, Wuhan 430065, Hubei Province, China.
Ruo-Bing Zhao, School of Sport Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China.
Sheng-Yao Liu, Department of Spinal Surgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Guo-Xin Ni, Department of Rehabilitation Medicine, The First Affiliated Hospital of Xiamen University, Xiamen 361003, Fujian Province, China. nigx@xmu.edu.cn.
Data sharing statement
No additional data are available.
References
- 1.Qin Q, Lee S, Patel N, Walden K, Gomez-Salazar M, Levi B, James AW. Neurovascular coupling in bone regeneration. Exp Mol Med. 2022;54:1844–1849. doi: 10.1038/s12276-022-00899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Bruton A, Matute-Llorente Á, González-Agüero A, Casajús JA, Vicente-Rodríguez G. Plyometric exercise and bone health in children and adolescents: a systematic review. World J Pediatr. 2017;13:112–121. doi: 10.1007/s12519-016-0076-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Cao H, Hua W, Gao L, Yuan Y, Zhou X, Zeng Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Bone Defect Repair. Membranes (Basel) 2022;12 doi: 10.3390/membranes12070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemmler W, Shojaa M, Kohl M, von Stengel S. Effects of Different Types of Exercise on Bone Mineral Density in Postmenopausal Women: A Systematic Review and Meta-analysis. Calcif Tissue Int. 2020;107:409–439. doi: 10.1007/s00223-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutor TW, Kura J, Mattingly AJ, Otzel DM, Yarrow JF. The Effects of Exercise and Activity-Based Physical Therapy on Bone after Spinal Cord Injury. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IWAMOTO J, TAKEDA T, SATO Y. Retraction: Effect of Treadmill Exercise on Bone Mass in Female Rats. Exp Anim. 2022;71:414. doi: 10.1538/expanim.54.1.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barengolts EI, Curry DJ, Bapna MS, Kukreja SC. Effects of endurance exercise on bone mass and mechanical properties in intact and ovariectomized rats. J Bone Miner Res. 1993;8:937–942. doi: 10.1002/jbmr.5650080806. [DOI] [PubMed] [Google Scholar]
- 8.Rosengren BE, Rempe J, Jehpsson L, Dencker M, Karlsson MK. Physical Activity at Growth Induces Bone Mass Benefits Into Adulthood - A Fifteen-Year Prospective Controlled Study. JBMR Plus. 2022;6:e10566. doi: 10.1002/jbm4.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 10.Kannus P, Haapasalo H, Sankelo M, Sievänen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Mustafy T, Londono I, Moldovan F, Villemure I. Isolated Cyclic Loading During Adolescence Improves Tibial Bone Microstructure and Strength at Adulthood. JBMR Plus. 2020;4:e10349. doi: 10.1002/jbm4.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and Girls: the POWER PE study. J Bone Miner Res. 2008;23:1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 13.Jathar S, Kumar V, Srivastava J, Tripathi V. Technological Developments in lncRNA Biology. Adv Exp Med Biol. 2017;1008:283–323. doi: 10.1007/978-981-10-5203-3_10. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Dong R, Diao S, Du J, Fan Z, Wang F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:30. doi: 10.1186/s13287-017-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Liu F, Wang Y, Qu L, Lin A. LncRNAs in tumor metabolic reprogramming and immune microenvironment remodeling. Cancer Lett. 2022;543:215798. doi: 10.1016/j.canlet.2022.215798. [DOI] [PubMed] [Google Scholar]
- 16.Ashrafizadeh M, Rabiee N, Kumar AP, Sethi G, Zarrabi A, Wang Y. Long noncoding RNAs (lncRNAs) in pancreatic cancer progression. Drug Discov Today. 2022;27:2181–2198. doi: 10.1016/j.drudis.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220 doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Hossain MS, Liu D. Involvement of the long noncoding RNA H19 in osteogenic differentiation and bone regeneration. Stem Cell Res Ther. 2021;12:74. doi: 10.1186/s13287-021-02149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Li YL, Wang GL, Li CZ. Regulation of long non-coding RNA in cartilage injury of osteoarthritis. Zhongguo Xiufu Chongjian Waike Zazhi. 2020;34:1486–1491. doi: 10.7507/1002-1892.202002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Kang H. Role of MicroRNAs and Long Non-Coding RNAs in Sarcopenia. Cells. 2022;11 doi: 10.3390/cells11020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue Y, Yue Y, Fan Z, Meng Y, Wen C, An Y, Yao Y, Li X. The long noncoding RNA lnc-H19 is important for endurance exercise by maintaining slow muscle fiber types. J Biol Chem. 2023;299:105281. doi: 10.1016/j.jbc.2023.105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Cao H, Wang M, Zou J, Wu W. Moderate-intensity treadmill running relieves motion-induced post-traumatic osteoarthritis mice by up-regulating the expression of lncRNA H19. Biomed Eng Online. 2021;20:111. doi: 10.1186/s12938-021-00949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G, Reik W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zhang Y, Hu Q, Egranov SD, Xing Z, Zhang Z, Liang K, Ye Y, Pan Y, Chatterjee SS, Mistretta B, Nguyen TK, Hawke DH, Gunaratne PH, Hung MC, Han L, Yang L, Lin C. Functional significance of gain-of-function H19 lncRNA in skeletal muscle differentiation and anti-obesity effects. Genome Med. 2021;13:137. doi: 10.1186/s13073-021-00937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuhlmüller B, Kunisch E, Franz J, Martinez-Gamboa L, Hernandez MM, Pruss A, Ulbrich N, Erdmann VA, Burmester GR, Kinne RW. Detection of oncofetal h19 RNA in rheumatoid arthritis synovial tissue. Am J Pathol. 2003;163:901–911. doi: 10.1016/S0002-9440(10)63450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Xu XY, Shen Y, Ye CF, Hu N, Yao Q, Lv XZ, Long SL, Ren C, Lang YY, Liu YL. Ghrelin protects against obesity-induced myocardial injury by regulating the lncRNA H19/miR-29a/IGF-1 signalling axis. Exp Mol Pathol. 2020;114:104405. doi: 10.1016/j.yexmp.2020.104405. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Liu M, Meng Q. Angelica polysaccharide promotes proliferation and osteoblast differentiation of mesenchymal stem cells by regulation of long non-coding RNA H19: An animal study. Bone Joint Res. 2019;8:323–332. doi: 10.1302/2046-3758.87.BJR-2018-0223.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang G, Kang Y, Huang Z, Zhang Z, Meng F, Chen W, Fu M, Liao W. Identification and Characterization of Long Non-Coding RNAs in Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Cell Physiol Biochem. 2017;42:1037–1050. doi: 10.1159/000478751. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Zhao J, Sun L, Pan Y, Wang H, Zhang WB. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone. 2018;108:62–70. doi: 10.1016/j.bone.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Liang WC, Fu WM, Wang YB, Sun YX, Xu LL, Wong CW, Chan KM, Li G, Waye MM, Zhang JF. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci Rep. 2016;6:20121. doi: 10.1038/srep20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng N, Liao T, Chen XY, Yan ZP, Li JT, Ni GX. Treadmill running induces remodeling of the infrapatellar fat pad in an intensity-dependent manner. J Orthop Surg Res. 2021;16:354. doi: 10.1186/s13018-021-02501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Z, Zeng N, Li J, Liao T, Ni G. Cardiac Effects of Treadmill Running at Different Intensities in a Rat Model. Front Physiol. 2021;12:774681. doi: 10.3389/fphys.2021.774681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu C, Ding H, Shi L, Zhang S, Tong X, Huang M, Liu L, Guan X, Zou J, Yuan Y, Chen X. Exercise improved bone health in aging mice: a role of SIRT1 in regulating autophagy and osteogenic differentiation of BMSCs. Front Endocrinol (Lausanne) 2023;14:1156637. doi: 10.3389/fendo.2023.1156637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Hamann N, Kohler T, Müller R, Brüggemann GP, Niehoff A. The effect of level and downhill running on cortical and trabecular bone in growing rats. Calcif Tissue Int. 2012;90:429–437. doi: 10.1007/s00223-012-9593-6. [DOI] [PubMed] [Google Scholar]
- 37.Maurel DB, Boisseau N, Pallu S, Rochefort GY, Benhamou CL, Jaffre C. Regular exercise limits alcohol effects on trabecular, cortical thickness and porosity, and osteocyte apoptosis in the rat. Joint Bone Spine. 2013;80:492–498. doi: 10.1016/j.jbspin.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, Toyama Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J Bone Miner Metab. 2004;22:26–31. doi: 10.1007/s00774-003-0443-5. [DOI] [PubMed] [Google Scholar]
- 40.Yao Z, Lafage-Proust MH, Plouët J, Bloomfield S, Alexandre C, Vico L. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J Bone Miner Res. 2004;19:1471–1480. doi: 10.1359/JBMR.040517. [DOI] [PubMed] [Google Scholar]
- 41.Kannus P, Sievänen H, Järvinen TL, Järvinen M, Kvist M, Oja P, Vuori I, Jozsa L. Effects of free mobilization and low- to high-intensity treadmill running on the immobilization-induced bone loss in rats. J Bone Miner Res. 1994;9:1613–1619. doi: 10.1002/jbmr.5650091015. [DOI] [PubMed] [Google Scholar]
- 42.Portier H, Benaitreau D, Pallu S. Does Physical Exercise Always Improve Bone Quality in Rats? Life (Basel) 2020;10 doi: 10.3390/life10100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 44.Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, Van Looveren R, Loopmans S, Bultynck G, Vinckier S, Meersman F, Maxwell PH, Rai J, Weis M, Eyre DR, Ghesquière B, Fendt SM, Carmeliet P, Carmeliet G. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565:511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sederquist B, Fernandez-Vojvodich P, Zaman F, Sävendahl L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. J Mol Endocrinol. 2014;53:T35–T44. doi: 10.1530/JME-14-0006. [DOI] [PubMed] [Google Scholar]
- 46.Lui JC, Jee YH, Garrison P, Iben JR, Yue S, Ad M, Nguyen Q, Kikani B, Wakabayashi Y, Baron J. Differential aging of growth plate cartilage underlies differences in bone length and thus helps determine skeletal proportions. PLoS Biol. 2018;16:e2005263. doi: 10.1371/journal.pbio.2005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu T, Yang K, You H, Chen A, Wang J, Xu K, Gong C, Shao J, Ma Z, Guo F, Qi J. Regulation of PTHrP expression by cyclic mechanical strain in postnatal growth plate chondrocytes. Bone. 2013;56:304–311. doi: 10.1016/j.bone.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Ning B, Mustafy T, Londono I, Laporte C, Villemure I. Impact loading intensifies cortical bone (re)modeling and alters longitudinal bone growth of pubertal rats. Biomech Model Mechanobiol. 2023;22:1145–1162. doi: 10.1007/s10237-023-01706-5. [DOI] [PubMed] [Google Scholar]
- 50.Guevara JM, Moncayo MA, Vaca-González JJ, Gutiérrez ML, Barrera LA, Garzón-Alvarado DA. Growth plate stress distribution implications during bone development: a simple framework computational approach. Comput Methods Programs Biomed. 2015;118:59–68. doi: 10.1016/j.cmpb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Ramesh S, Zaman F, Sävendahl L, Madhuri V. Radial shockwave treatment promotes chondrogenesis in human growth plate and longitudinal bone growth in rabbits. Bone. 2022;154:116186. doi: 10.1016/j.bone.2021.116186. [DOI] [PubMed] [Google Scholar]
- 52.Musolino C, Oteri G, Allegra A, Mania M, D'Ascola A, Avenoso A, Innao V, Allegra AG, Campo S. Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann Hematol. 2018;97:1259–1269. doi: 10.1007/s00277-018-3296-7. [DOI] [PubMed] [Google Scholar]
- 53.Xie Z, Xu J, Peng L, Gao Y, Zhao H, Qu Y. miR-149 promotes human osteocarcinoma progression via targeting bone morphogenetic protein 9 (BMP9) Biotechnol Lett. 2018;40:47–55. doi: 10.1007/s10529-017-2445-8. [DOI] [PubMed] [Google Scholar]
- 54.Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther. 2013;14:100–107. doi: 10.4161/cbt.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol. 2017;13:193. doi: 10.1038/nrrheum.2017.17. [DOI] [PubMed] [Google Scholar]
- 56.Yang YP, Hu LF, Zheng HF, Mao CJ, Hu WD, Xiong KP, Wang F, Liu CF. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin. 2013;34:625–635. doi: 10.1038/aps.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S, Cailleteau L, Battaglia S, Farlay D, Dacquin R, Barois N, Jurdic P, Boivin G, Heymann D, Lafont F, Lu SS, Dempster DW, Carle GF, Pierrefite-Carle V. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10:1965–1977. doi: 10.4161/auto.36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li CJ, Xiao Y, Yang M, Su T, Sun X, Guo Q, Huang Y, Luo XH. Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J Clin Invest. 2018;128:5251–5266. doi: 10.1172/JCI99044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Zhao J, Sun W, Wang H, Pan Y, Wang L, Zhang WB. Osteocytes promote osteoclastogenesis via autophagy-mediated RANKL secretion under mechanical compressive force. Arch Biochem Biophys. 2020;694:108594. doi: 10.1016/j.abb.2020.108594. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Hu J, Pan Y, Shan Y, Jiang L, Qi X, Jia L. miR-140-5p/miR-149 Affects Chondrocyte Proliferation, Apoptosis, and Autophagy by Targeting FUT1 in Osteoarthritis. Inflammation. 2018;41:959–971. doi: 10.1007/s10753-018-0750-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.