Abstract
Protein C (PC) is a key component of the vitamin K-dependent coagulation pathway. It exerts anticoagulant effects by inactivating factors V and VIII. Acquired or inherited PC deficiency results in a prothrombotic state, with presentations varying from asymptomatic to venous thromboembolism. However, there has been an increasing number of reports linking PC deficiency to arterial thromboembolic events, such as myocardial infarction and ischemic stroke. This editorial focuses on the association between PC deficiency and thromboembolism, which may provide some insights for treatment strategy and scientific research.
Keywords: Protein C deficiency, Venous thromboembolism, Myocardial infarction, Editorial, Arterial thromboembolism
Core Tip: Protein C (PC) deficiency impairs the balance between the procoagulant and anticoagulant system, which results in venous thromboembolism. However, there has been an increasing number of reports linking the condition to arterial thromboembolic events. A thorough understanding of PC deficiency is essential for the development of new management strategies against PC deficiency-related thromboembolism events.
INTRODUCTION
In this editorial, we comment on the case report by Seo et al[1] published in the recent issue of the World Journal of Clinical Cases. The authors presented a case of unprovoked pulmonary thromboembolism and deep vein thrombosis followed 9 month later by acute myocardial infarction without any underlying major risk factors for atherosclerosis cardiovascular disease, which possesses important clinical implication[1]. Therefore, in this editorial, we discuss the biology and pathophysiology of protein C (PC) and PC deficiency-related venous and arterial thromboembolism (ATE), as well as treatment strategy.
PC AND PC DEFICIENCY
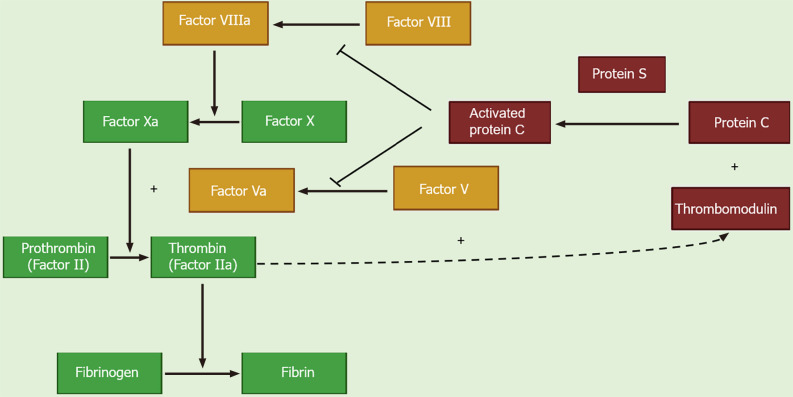
PC is a vitamin K-dependent proenzyme that is synthesized in hepatocytes and circulates in the blood as an inactive zymogen[2]. Thrombin with thrombomodulin cleaves PC, converting it into its activated form, activated PC (APC). Along with its co-factor protein S, APC inhibits thrombin generation by inactivating activated factors V (Va) and VIII (VIIIa)[3]. Both factors Va and VIIIa are required for factor X activation, which then converts prothrombin to thrombin. Factors Va and VIIIa act as substrates for APC, which irreversibly inactivates them through proteolytic activity on cleavage sites, thereby inhibiting their pro-coagulant effect (Figure 1)[4]. In addition to its anticoagulant function, APC also exhibits potent cyto-protective and anti-inflammatory effects and has indirect fibrinolytic properties[5].
Figure 1.
Biological role of protein C.
A PC deficiency impairs the balance between the procoagulant and anticoagulant system and engenders a prothrombotic state. The etiology of PC deficiency may be genetic (heterozygous or homozygous) or acquired, the latter often due to vitamin K antagonist therapy or liver disease. Hereditary PC deficiency is caused by mutation in the protein C (PROC) gene located on chromosome 2q14.3[5]. It has been reported that more than 500 mutations identified throughout the PROC gene length may lead to inherited PC deficiency. The molecular basis of inherited PC deficiency is complicated, as results from a recent study demonstrated that nucleotide variations in the signal peptide and propeptide of PC lead to PC deficiency by differentially affecting the biological process of PC, including posttranscriptional pre-mRNA splicing, translation, and post-translational modifications[6]. Heterozygous PC deficiency is estimated to occur in 0.02%-0.05% of the general population, whereas homozygous PC deficiency is much rarer and can lead to disseminated intravascular coagulation, thrombosis and purpura fulminans that often appears within hours or days after birth[7]. Most cases of inherited PC deficiency in clinical practice are heterozygous deficiencies, with presentations varying from asymptomatic to thromboembolism events.
PC DEFICIENCY AND VENOUS THROMBOEMBOLISM
Venous thromboembolism (VTE) represents the cardinal clinical manifestation of heterozygous PC deficiency. It has been reported that patients with PC deficiency have a 10- to 15-fold higher risk of VTE than wild-type individuals, and nearly 5% of patients with VTE may have heterozygous PC deficiency[8,9]. The risk of VTE among patients with PC deficiency varies, which may be related to both the degree of deficiency and the presence of other acquired or inherited risk factors for thrombosis, such as fracture, immobilization, and surgery. Additionally, a 38% recurrence rate of VTE among patients with PC deficiency and prior VTE has been reported[10]. Therefore, evaluation of PC deficiency should be considered in patients with recurrent VTE.
PC DEFICIENCY AND ATE
Compared to the established association between PC deficiency and VTE, the relationship with ATE remain controversial. A previously large family cohort study has observed a 6.9-fold (95%CI: 2.1-22.2) higher risk of ATE among patients with PC before 55 years of age[11]. PC deficiency was also observed in 12% of reported cases of myocardial infarction with normal coronary arteries[12]. Besides, as seen in the case reported by Seo et al[1], most of the evidence linking PC deficiency to ATE events stems from case reports[7,13]. However, some studies failed to identify the association between PC deficiency and ATE[4]. Therefore, to address the knowledge gap, further large-scale studies are required to investigate the effects of PC deficiency on ATE and explore the underlying mechanisms.
MANAGEMENT OF PC DEFICIENCY
The management of PC deficiency are mostly based on previously reported cases and experiences. For severe PC deficiency cases, lifelong PC replacement therapy may be required[5]. In addition, according to a recent guideline, subcutaneous PC concentrate with or without vitamin K antagonists may be the most appropriate long-term management for severe congenital PC deficiency patients, whereas there are little data available on pharmacokinetics and the most appropriate dosing regimen[14]. For most cases, oral anticoagulants remain the primary treatment option. According to the CHEST Guideline and Expert Panel Report, vitamin K antagonists have been the cornerstone of treatment and secondary prophylaxis in patients with hereditary thrombophilia[15]. Some reported cases suggested a possible role of direct oral anticoagulants in thrombophilic patients, which needs further validation[7]. In addition, there is also a lack of treatment strategies for PC deficiency-related ATE. Hence, more data is required to establish efficacious strategies for the treatment and secondary prophylaxis in patients with PC deficiency manifesting as thromboembolic events.
CONCLUSION
PC deficiency is a risk factor for thrombophilia with higher risks of VTE. Emerging data have linked PC deficiency with increased risk of ATE, which requires further validation in large-scale studies. In addition, future studies are needed to establish efficacious treatment strategy for PC deficiency-related thromboembolic events.
ACKNOWLEDGEMENTS
The authors thank Prof. Gary Tse from Kent and Medway Medical School, Canterbury, Kent for the helpful comments.
Footnotes
Conflict-of-interest statement: The authors declare that they have no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 31, 2023
First decision: January 17, 2024
Article in press: March 26, 2024
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Odhar HA, Iraq; Suravajhala PN, India S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yu HG
Contributor Information
Nan Zhang, Department of Cardiology, Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China.
Dong-Kun Sun, Department of Cardiology, Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China.
Xu Tian, Department of Cardiology, Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China.
Xin-Yu Zheng, Department of Cardiology, Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China.
Tong Liu, Department of Cardiology, Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China. liutongdoc@126.com.
References
- 1.Seo J, Lee J, Shin YH, Jang AY, Suh SY. Acute myocardial infarction after initially diagnosed with unprovoked venous thromboembolism: A case report. World J Clin Cases. 2023;11:7497–7501. doi: 10.12998/wjcc.v11.i30.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouse LH, Comp PC. The regulation of hemostasis: the protein C system. N Engl J Med. 1986;314:1298–1304. doi: 10.1056/NEJM198605153142006. [DOI] [PubMed] [Google Scholar]
- 3.Cooper PC, Pavlova A, Moore GW, Hickey KP, Marlar RA. Recommendations for clinical laboratory testing for protein C deficiency, for the subcommittee on plasma coagulation inhibitors of the ISTH. J Thromb Haemost. 2020;18:271–277. doi: 10.1111/jth.14667. [DOI] [PubMed] [Google Scholar]
- 4.Majid Z, Tahir F, Ahmed J, Bin Arif T, Haq A. Protein C Deficiency as a Risk Factor for Stroke in Young Adults: A Review. Cureus. 2020;12:e7472. doi: 10.7759/cureus.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarvand P, Moser KA. Protein C Deficiency. Arch Pathol Lab Med. 2019;143:1281–1285. doi: 10.5858/arpa.2017-0403-RS. [DOI] [PubMed] [Google Scholar]
- 6.Cao Q, Hao Z, Li C, Chen X, Gao M, Jiang N, Liu H, Shen Y, Yang H, Zhang S, Yang A, Li W, Tie JK, Shen G. Molecular basis of inherited protein C deficiency results from genetic variations in the signal peptide and propeptide regions. J Thromb Haemost. 2023;21:3124–3137. doi: 10.1016/j.jtha.2023.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Yaarubi R, Al Rawahi B, Al Lawati H. Protein C deficiency presenting as an acute infero-posterior ST elevation myocardial infarction in a young man; A case report and focused literature review. Thromb Res. 2020;192:109–112. doi: 10.1016/j.thromres.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, Mackie I, Makris M, Nokes T, Perry D, Tait RC, Walker I, Watson H British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149:209–220. doi: 10.1111/j.1365-2141.2009.08022.x. [DOI] [PubMed] [Google Scholar]
- 9.Knoebl PN. Severe congenital protein C deficiency: the use of protein C concentrates (human) as replacement therapy for life-threatening blood-clotting complications. Biologics. 2008;2:285–296. doi: 10.2147/btt.s1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93–99. [PubMed] [Google Scholar]
- 11.Mahmoodi BK, Brouwer JL, Veeger NJ, van der Meer J. Hereditary deficiency of protein C or protein S confers increased risk of arterial thromboembolic events at a young age: results from a large family cohort study. Circulation. 2008;118:1659–1667. doi: 10.1161/CIRCULATIONAHA.108.780759. [DOI] [PubMed] [Google Scholar]
- 12.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 13.Tiong IY, Alkotob ML, Ghaffari S. Protein C deficiency manifesting as an acute myocardial infarction and ischaemic stroke. Heart. 2003;89:E7. doi: 10.1136/heart.89.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minford A, Brandão LR, Othman M, Male C, Abdul-Kadir R, Monagle P, Mumford AD, Adcock D, Dahlbäck B, Miljic P, DeSancho MT, Teruya J. Diagnosis and management of severe congenital protein C deficiency (SCPCD): Communication from the SSC of the ISTH. J Thromb Haemost. 2022;20:1735–1743. doi: 10.1111/jth.15732. [DOI] [PubMed] [Google Scholar]
- 15.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]