Abstract
Entry of human immunodeficiency virus type 1 (HIV-1) into target cells is mediated by binding of the surface envelope glycoprotein to the CD4 molecule. Interaction of the resulting CD4-glycoprotein complex with α- or β-chemokine receptors, depending on the biological phenotype of the virus, then initiates the fusion process. Here, we show that primary HIV-2 isolates and biological clones, in contrast to those of HIV-1, may use a broad range of coreceptors, including CCR-1, CCR-3, CCR-5, and CXCR-4. The syncytium-inducing capacity of these viruses did not correlate with the ability to infect via CXCR-4 or any other coreceptor. One cell-free passage of the intermediate isolates in mitogen-stimulated, CD8+ cell-depleted peripheral blood mononuclear cells resulted in the outgrowth of variants with CCR-5 only, whereas the coreceptor usage of late and early isolates did not change. Since HIV-2 is less pathogenic in vivo than HIV-1, these data suggest that HIV pathogenicity in vivo is not directly related to the spectrum of coreceptors used in in vitro systems.
Human immunodeficiency virus (HIV) entry is mediated by the binding of its surface glycoprotein to the cellular CD4 molecule, which acts as the primary receptor (7, 18), and the subsequent interaction of the resulting CD4-envelope glycoprotein complex with another specific cellular cofactor (26, 28). Several members of the seven-transmembrane G-protein-coupled receptor family have recently been shown to be possible coreceptors for HIV-1 or HIV-2 (9, 13, 15, 22, 25). Two members of this family, the chemokine receptors CCR-5 and CXCR-4 (also termed LESTR and fusin), are the main coreceptors for macrophage and T-cell line-tropic HIV-1 variants, respectively (1, 2, 4, 8, 11, 12, 24). A small subset of dualtropic HIV-1 strains is also able to use other members of this family, such as the chemokine receptors CCR-2b and CCR-3 (4, 10, 14) or the orphan receptors Bonzo (also termed STRL33) and BOB (9, 17), as coreceptors. Moreover, longitudinal studies have shown a shift toward usage of CXCR-4 as coreceptor for HIV-1 during disease progression, suggesting that the broadening of coreceptor usage contributes to the cytopathic potential of HIV-1 strains in vivo (6). Compared to HIV-1, HIV-2 is less transmissible, is less cytopathic in vivo, and induces generally a slower progression toward AIDS (19). However, we recently showed that rapid progression can be observed in some HIV-2-infected individuals (27). Similar to HIV-1, an inverse correlation was observed between the replication rate of viruses in vitro and the CD4+ T-cell count of the patient from which they originated (27). Depending on the time required to detect virus after cocultivation of patient peripheral blood mononuclear cells (PBMC) with phytohemagglutinin (PHA)-stimulated PBMC and the CD4 count of the patient, we were able to distinguish between early (>500 CD4 cells/μl), intermediate (100 to 500 CD4 cells/μl), and late (<100 CD4 cells/μl) isolates. Studies comparing the requirements for HIV-1 versus HIV-2 entry may contribute to our understanding of the pathogenicity of these human lentiviruses. Here, we have evaluated the capacity of a panel of human cells stably transfected with chemokine receptor genes to support infection with HIV-2 primary isolates from patients at different stages of disease (27) (Table 1). Five of the isolates studied (PH-2-1, RH-2-1, RH-2-2, RH-2-5, and RH-2-7) belong to HIV-2 subtype A, whereas one isolate, RH-2-6, belongs to subtype B (27). The megaglioblastoma astrocytic cell line U87MG, stably transfected with the human CD4 gene and expression plasmids encoding various coreceptors (15), was infected with each of these isolates. Cells expressing both CD4 and chemokine receptors were selected regularly with 250 μg of Geneticin (Gibco BRL) per ml and 1 μg of puromycin (Calbiochem) per ml, respectively, and CD4 expression of these cell lines was confirmed by fluorescence-activated cell sorter analysis (data not shown).
TABLE 1.
HIV-2 primary isolates or biological clones and patient clinical statuses
| Patient | Clinical statusa | CD4 cells/μl | Biological clone | Phenotype | Subtypee |
|---|---|---|---|---|---|
| RH-2-1 | C1 | 600 | NAb | NSI | A |
| PH-2-1 | A3 | 200 | NSI | A | |
| C1 | NSI | ||||
| C12 | NSI | ||||
| E6 | NSI | ||||
| H8 | NSI | ||||
| D5 | SIc | ||||
| H12 | SId | ||||
| RH-2-7 | A3 | 130 | NSI | A | |
| A5 | NSI | ||||
| C9 | NSI | ||||
| C12 | NSI | ||||
| D7 | NSI | ||||
| G12 | NSI | ||||
| RH-2-5 | A3 | 110 | NSI | A | |
| A10 | NSI | ||||
| E4 | NSI | ||||
| E11 | NSI | ||||
| F7 | NSI | ||||
| G7 | NSI | ||||
| RH-2-2 | C3 | 10 | NA | SId | A |
| RH-2-6 | C3 | 10 | NA | SId | B |
Based on Centers for Disease Control and Prevention criteria.
NA, not available.
On PBMC.
On PBMC and MT-2 cells.
Based on nucleotide sequences of reverse transcriptase fragments (27).
Adherent cells (2 × 104 to 4 × 104) were incubated for 7 to 15 h at 37°C in 24-well plates with a viral inoculum of 250 μl containing 2,000 cpm of reverse transcriptase (RT) activity. Cells were then washed once with Dulbecco modified Eagle medium (DMEM)–10% fetal calf serum (FCS) and cultured in 1.5 ml of DMEM–10% FCS. Virus replication was monitored at day 10 or 11 postinfection by quantification of RT activity in 150 μl of culture supernatant. The abilities of HIV-2 primary isolates to infect U87MG CD4 cells expressing different coreceptors are shown in Fig. 1.
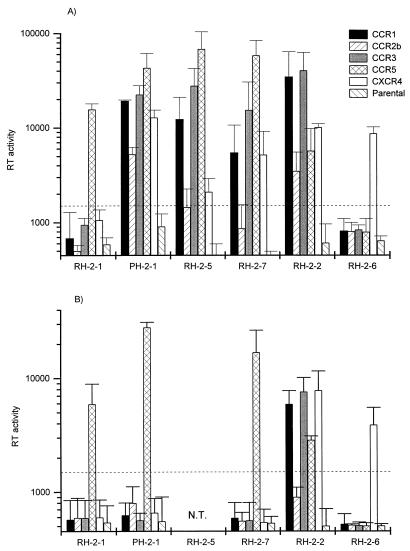
FIG. 1.
Coreceptor usage of HIV-2 primary isolates. Infections were performed with primary isolates cultured for 2 weeks in PHA-stimulated PBMC (A) and then passaged for 1 week in CD8-depleted, PHA-stimulated PBMC (B). Each value represents the mean and standard deviation of three independent experiments and is expressed in counts per minute per 150 μl of supernatant. The cutoff value was set at two times the mean of all background values. N.T., not tested.
The early isolate (RH-2-1) used only CCR-5 as its coreceptor (Fig. 1A). Intermediate, NSI (non-syncytium-inducing) isolates (Table 1) PH-2-1, RH-2-5, and RH-2-7 and late, SI (syncytium-inducing) isolate RH-2-2, all belonging to HIV-2 subtype A, appeared to use a broad range of coreceptors: in addition to CCR-5, both CCR-1 and CCR-3 were efficiently used (Fig. 1A). These isolates were also all able to use CXCR- 4, and some entered U87MG CD4 cells via CCR-2b at different levels of efficiency. This proved not to be dependent on their SI/NSI phenotypes (Fig. 1A). No significant differences in coreceptor usage were observed between intermediate and late subtype A isolates (Fig. 1A and Table 1). The late subtype B SI isolate RH-2-6 (Table 1), however, infected only U87MG CD4 cells expressing the CXCR-4 receptor. In contrast with a previous study (25), these isolates were unable to infect U87MG CD4 cells not transfected with any coreceptor. Therefore, the broad range of coreceptor usage of HIV-2 primary isolates could not be attributed to their ability to infect U87MG CD4 cells via an unidentified coreceptor constitutively expressed by these cells.
Virus isolates were passaged with cell-free infection for 1 week in CD8-depleted, PHA-stimulated PBMC. Progeny viruses were then assayed for coreceptor usage. Replication of NSI isolates thus passaged was detectable only in CCR-5-expressing cells, whereas the range of coreceptors used by SI isolates did not seem to be influenced by this passage (Fig. 1B). However, prolonged cultivation of NSI isolates in U87MG CD4 cells for 4 to 7 days after the standard 11 days showed also a low level of virus production in CCR-1- or CCR-3-expressing cells (data not shown). This indicated that CCR-1- and CCR-3-using variants were still present in the isolates after a 1-week passage in CD8-depleted, PHA-stimulated PBMC. Thus, a short- term passage in CD8-depleted, PHA-stimulated PBMC increased preferentially the frequency of CCR-5-restricted variants present in the NSI isolates.
The selective expansion of a viral subpopulation in CD8-depleted, PHA-stimulated PBMC suggested that the HIV-2 isolates tested consisted of a pool of variants with different coreceptor requirements. In order to determine the actual coreceptor usage of such HIV-2 variants, we isolated 16 biological clones from patients PH-2-1, RH-2-5, and RH-2-7 (Table 1). In short, PBMC from these donors were plated in 96-well round-bottom plates (Coulter) at 2 × 105, 2 × 104, and 2 × 103 per plate with 4 × 106 PHA-stimulated PBMC from seronegative individuals per plate. Each well was further treated individually, and new medium and cells were added once a week, according to standard protocols (27). All wells were monitored after 2 to 3 weeks for the presence of p24 antigen (V5 ELISA; Organon Technika). p24-positive wells of 96-well plates in which less than 5 positive wells were detected were considered clonal. These were further cocultivated with PHA-stimulated PBMC. Before the assessment of their coreceptor usage, these clones were passaged for 1 week on CD8-depleted, PHA-stimulated PBMC. Of the 16 biological clones isolated, two (PH-2-1 D5 and PH-2-1 H12) exhibited an SI phenotype on MT-2 cells and/or PHA-stimulated PBMC, whereas the other 14 were NSI variants (Table 1). The two SI biological clones were obtained from a single individual who progressed rapidly toward AIDS (within 3 years), suggesting that the fusogenic potential of the envelope glycoprotein complex contributed to disease progression in this patient.
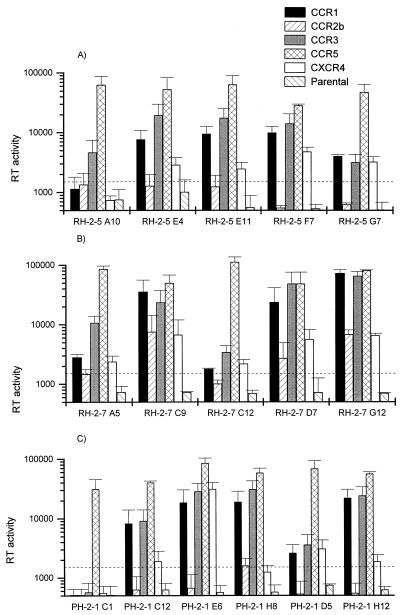
All clones were tested for coreceptor usage by infection of U87MG CD4 cells and appeared to be able to use CCR-5 at very high efficiency (Fig. 2). All but one (PH-2-1 C1) were also able to use at least one other chemokine receptor as a coreceptor. CCR-1, CCR-3, and CXCR-4 were used by 13, 15, and 13 of the 16 clones, respectively. In contrast, CCR-2b was used by only three clones isolated from a single patient: RH-2-7 C9, D7, and G12 (Fig. 2B). However, the HIV-2 biological clones exhibited higher efficiencies of infection via CCR-5 than via the other coreceptors. This was shown by consistently higher RT values in CCR-5-expressing cells and detection of virus production 2 to 4 days earlier in CCR-5-expressing cells (data not shown). Finally, no consistent differences in coreceptor usage between the SI clones PH-2-1 D5 and H12 and the 14 NSI clones were observed.
FIG. 2.
Coreceptor usage of HIV-2 biological clones. Infections were performed with biological clones from patient RH-2-5 (A), patient RH-2-7 (B), and patient PH-2-1 (C). Each value represents the mean and standard deviation of at least three independent experiments and is expressed in counts per minute per 150 μl of supernatant.
It has been stated previously that coreceptor usage of HIV-1 clones is correlated with the viral phenotype: macrophage-tropic, NSI HIV-1 variants exhibit CCR-5-restricted entry, whereas SI T-cell line-tropic isolates can also use CXCR-4 (3, 16, 24). The importance of CCR-5-mediated entry has, moreover, been clearly demonstrated in vivo by the resistance to HIV-1 infection of individuals who have a homozygous deletion in CCR-5 (20, 21, 23). Our experiments showed that all but one (RH-2-6) of the HIV-2 biological clones and primary isolates, of SI or NSI phenotype, can use CCR-5. Therefore, as described for HIV-1, CCR-5 seems to be the main coreceptor for primary HIV-2 strains. However, our results do not show a difference between HIV-2 SI and NSI clones with respect to their usage of either CXCR-4 or any other coreceptor. Thus, in contrast to what has been shown for HIV-1 (3, 12, 16), there seems to be no correlation between any specific coreceptor usage and HIV-2 syncytium-inducing capacity.
A limited set of HIV-1 strains can use chemokine receptors other than CCR-5, such as CCR-2b and CCR-3, as their coreceptors (4, 10, 14). In contrast to HIV-1, almost all of the HIV-2 biological clones and primary isolates used in this study exhibited usage of a broad range of coreceptors, including CCR-1, CCR-3, and/or CXCR-4 in addition to CCR-5. CCR-1 usage by HIV-1 isolates in vitro has never been documented and is also consistently absent in our system (13a). Moreover, 14 of the 16 clones tested, including the two SI clones, were able to use at least three of these chemokine receptors for entry into U87MG CD4 cells. Therefore, and in contrast with previous reports (25), broad coreceptor usage seems to be characteristic of intermediate and late primary HIV-2 variants.
Mitogen-stimulated CD8+ T cells have been shown to produce the natural ligands of CCR-5—RANTES, MIP-1α, and MIP-1β—which may therefore compete with the HIV envelope glycoprotein for binding to CCR-5 (5, 11). The broad coreceptor usage of the unpassaged HIV-2 isolates may therefore have been the result of negative pressure exerted by CD8+ T cells on CCR-5-restricted variants. The preferential selection of CCR-5-restricted viruses within 1 week of cultivation in CD8- depleted, PHA-stimulated PBMC implies, however, that CCR-5-mediated entry is the main mechanism of infection of CD4+ cells in vitro by HIV-2 NSI isolates and that other coreceptor usage may occur when the efficiency of CCR-5-mediated entry has decreased.
It has been hypothesized that broadening of HIV-1 coreceptor usage contributes to the in vivo cytopathogenicity of HIV by increasing the numbers of potential target cells (6). Despite the broad coreceptor usage of HIV-2 isolates in vitro, disease progression in HIV-2-infected individuals is significantly slower than for HIV-1-infected patients (19). Moreover, by using HIV in situ hybridization and in situ histochemistry of lymphoid tissues from early- and intermediate-stage disease patients, we have recently obtained evidence that the number of productively infected cells is significantly lower in HIV-2-infected individuals than in HIV-1-infected individuals (26a). This suggests that the broad coreceptor usage observed in vitro does not add to the in vivo cytopathogenicity of HIV-2, in contrast to syncytium-inducing capacity or viral load. Therefore, additional longitudinal studies are required to further elucidate the relevance of coreceptor usage in disease progression after HIV-2 infection.
Acknowledgments
U87MG CD4 transfected cells were kindly provided by D. Littman.
This work was supported by Dutch AIDS Foundation grant 1022 and European Community Biomed grant PL96-2115.
REFERENCES
- 1.Alkhatib G, Broder C C, Berger E A. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Björndal Å, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 6.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzi P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, Unutmaz D, Kewalramani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 10.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 11.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 12.D’Souza M P, Harden V A. Chemokines and HIV-1 coreceptors. Confluence of two fields generates optimism in AIDS research. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 13a.Guillon, C. Unpublished data.
- 14.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 15.Hill C M, Deng H, Unutmaz D, Kewalramani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 19.Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh C C, Dia M C, Gueye E H, Hellinger J, Gueye-Ndiaye A, Sankalé J, Ndoye I, Mboup S, Essex M. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 20.Paxton W A, Dragic T, Koup R A, Moore J P. The beta-chemokines, HIV type 1 coreceptors, and exposed uninfected persons. AIDS Res Hum Retroviruses. 1996;12:1203–1207. doi: 10.1089/aid.1996.12.1203. [DOI] [PubMed] [Google Scholar]
- 21.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H-H, Du J-G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves J D, McKnight A, Potempa S, Simmons G, Gray P W, Power C A, Wells T, Weiss R A, Talbot S J. CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR-3, and V28 for entry. Virology. 1997;231:130–134. doi: 10.1006/viro.1997.8508. [DOI] [PubMed] [Google Scholar]
- 23.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 24.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sol N, Ferchal F, Braun J, Pleskoff O, Tréboute C, Ansart I, Alizon M. Usage of the coreceptors CCR-5, CCR-3, and CXCR-4 by primary and cell line-adapted human immunodeficiency virus type 2. J Virol. 1997;71:8237–8244. doi: 10.1128/jvi.71.11.8237-8244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 26a.van der Ende, M. E. Submitted for publication.
- 27.van der Ende M E, Schutten M, Ly T D, Gruters R A, Osterhaus A D M E. HIV-2 infection in 12 European residents: virus characteristics and virus progression. AIDS. 1996;10:1649–1655. doi: 10.1097/00002030-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]