Abstract
It is important to study the bacteria that cause endometritis to identify effective therapeutic drugs for dairy cows. In this study, 20% oxytetracycline was used to treat Holstein cows (n = 6) with severe endometritis. Additional 10 Holstein cows (5 for healthy cows, 5 for cows with mild endometritis) were also selected. At the same time, changes in bacterial communities were monitored by high-throughput sequencing. The results show that Escherichia coli, Staphylococcus aureus and other common pathogenic bacteria could be detected by traditional methods in cows both with and without endometritis. However, 16S sequencing results show that changes in the abundance of these bacteria were not significant. Endometritis is often caused by mixed infections in the uterus. Oxytetracycline did not completely remove existing bacteria. However, oxytetracycline could effectively inhibit endometritis and had a significant inhibitory effect on the genera Bacteroides, Trueperella, Peptoniphilus, Parvimonas, Porphyromonas, and Fusobacterium but had no significant inhibitory effect on the bacterial genera Marinospirillum, Erysipelothrix, and Enteractinococcus. During oxytetracycline treatment, the cell motility, endocrine system, exogenous system, glycan biosynthesis and metabolism, lipid metabolism, metabolism of terpenoids, polyketides, cofactors and vitamins, signal transduction, and transport and catabolism pathways were affected.
Subject terms: Developmental biology, Microbiology, Diseases
Introduction
Endometritis is a serious disease that affects reproductive performance, leads to low milk production and a low fertilization rate, increases risk of pregnancy loss, and severely reduces the production and economic benefits of dairy cow farming1–3. At calving the cervix is open, therefore environmental and reproductive tract microorganisms have the opportunity to ascend to and contaminate the uterus4–6. It is common for postpartum cows to have an inflammatory response in the uterus. Most cows can eliminate pathogen infections and resolve the inflammation through endometrial epithelial barrier and congenital immune defense functions within 4 weeks after delivery. However, this process will lead to pathological uterine inflammation due to poor management, physical damage, retention of fetal membranes, hormone disorders, denutrition, low immunity, and damage to the defense system7–9.
Studies have shown that pathogenic bacteria, including Staphylococcus, Streptococcus, Escherichia, Corynebacterium, Pseudomonas, Proteus, Necrobacillus, Pseudomonas aeruginosa, Campylobacter genitalia, Haemophilus, Bacillus pyogenes, Bacterium burgeri, Trueperella, Fusobacterium, and Prevotella, cause endometritis10. They are usually inhibited by antibiotics, hormones, and other drugs in clinical practice11–13.
Oxytetracycline (OTC) has good efficacy as an antibiotic for the treatment of endometritis14–16. OTC is an inexpensive, broad-spectrum antibiotic that is active against a wide variety of bacteria. OTC binds to the 30S ribosomal subunit and prevents the formation of an aminoacyl-tRNA-ribosome complex and further interferes with the ability of bacteria to produce proteins that are essential for bacteria to grow and multiply. However, some strains of bacteria have developed resistance to OTC, which has reduced its effectiveness for treating some types of infections17–19. Therefore, to effectively utilize the efficacy of OTC and treat endometritis, it is important to explore the bacterial communities that cause infection and are sensitive to OTC.
With the development of biotechnology, high-throughput sequencing has been widely used in clinical veterinary fields due to its high throughput, short sequencing time and low cost. When faced with certain clinical statuses, such as increased types of diseases, accelerated pathogen mutations, secondary infections, mixed infections, and latent infections, high-throughput sequencing has shown advantages in the areas of disease surveillance, rapid and accurate diagnosis, pathological research, and drug screening20–22.
This study used high-throughput sequencing to analyze changes in the bacterial community composition of bovine uterus contents in healthy Holstein cows, cows with endometritis, and cows with endometritis treated by OTC. Bacteria at the genus level that are easy or difficult to inhibit with OTC and the pathways that OTC affects were evaluated. These findings provide a theoretical basis for analyzing the pathogenesis of endometritis and the rational use of OTC.
Results
Pathogen identification in cultured bacteria
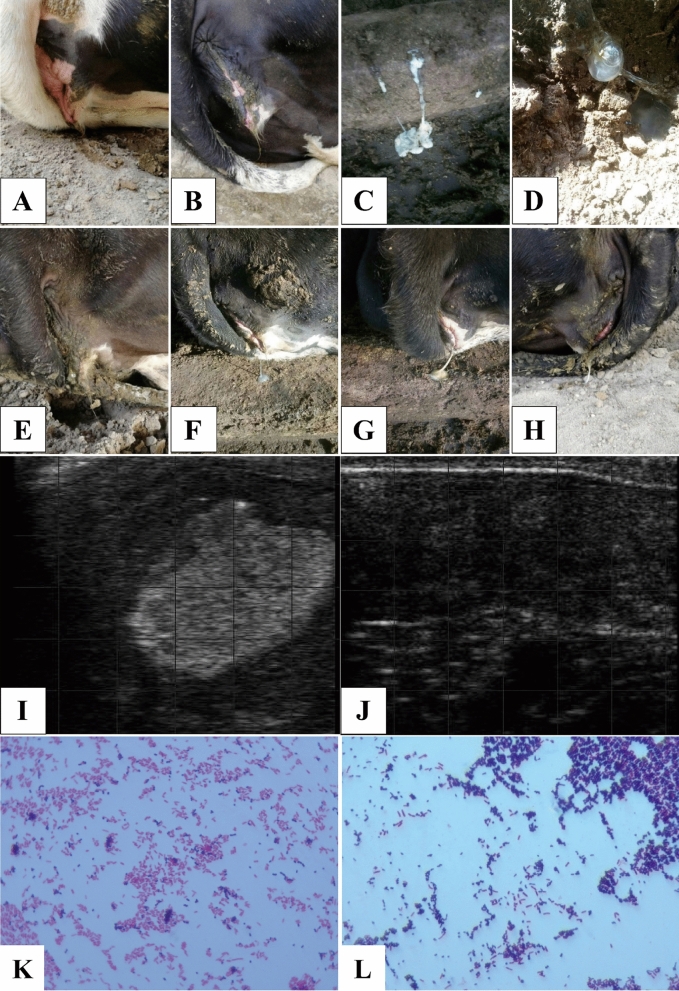
The detection rate of corresponding bacteria with common culture techniques in all four groups is shown in Table 1. Briefly, the bacteria detection rates (the number of positive samples to the number of all samples) between the NT and NC groups were similar. The detection rates of Escherichia coli, Staphylococcus aureus, Staphylococcus haemolyticus, and Streptococcus uberis were greater than 83%, while the detection rates of other bacteria were no less than 33%. The severity of endometritis can be quickly distinguished through visual observation and ultrasound methods (Fig. 1A–J). Microscopic examination results also showed that the cultures in this study had mixed infections of Escherichia coli, Staphylococcus sp., and Streptococcus sp. (Fig. 1K, L). In individuals with severe endometritis (BT group), the detection rate of all mentioned bacteria in Table 1 is relatively higher. After treatment with oxytetracycline (AT group), the detection rate of mentioned bacteria was reduced. However, the related bacteria could still be detected in all samples.
Table 1.
Pathogen identification in uterus.
| Pathogens | Detection rates | |||
|---|---|---|---|---|
| NCa | NTb | BTc | ATd | |
| Escherichia coli | 3/5 | 3/5 | 6/6 | 2/6 |
| Staphylococcus aureus | 3/5 | 2/5 | 6/6 | 2/6 |
| Chryseobacterium indologenes | 2/5 | 2/5 | 2/6 | 1/6 |
| Moraxella osloensis | 2/5 | 3/5 | 3/6 | 1/6 |
| Bacillus cereus | 2/5 | 2/5 | 2/6 | 1/6 |
| Staphylococcus haemolyticus | 2/5 | 2/5 | 5/6 | 2/6 |
| Corynebacterium | 2/5 | 2/5 | 2/6 | 1/6 |
| Enterococcus faecium | 2/5 | 2/5 | 3/6 | 1/6 |
| Streptococcus uberis | 2/5 | 3/5 | 5/6 | 2/6 |
a-dNC = healthy cows (NC group), NT = mild diseased cows, BT = severely ill cows, AT = cows in BT group after OTC treatment.
Figure 1.
Observation, ultrasound, and microscopic imaging of endometritis and bacterial mixed infection of representative examples. Mildly diseased cows had red and swollen vulvae (A), and the vaginal discharge was slightly turbid and could not be drawn into filamentous mucus (B). A large amount of white (C) or yellow (D) purulent secretion with severe malodorous smell could be observed in the vaginal opening of severely diseased cows (E–H). The uterus of diseased cows showed abnormal ultrasound imaging with hyperechoic areas (I), while healthy or cured cows had clear black background (J). (K) Mixed infection with Escherichia and Streptococcus (magnification = 100×). (L) Mixed infection with Escherichia and Staphylococcus (magnification = 100×).
Alpha-diversity of bovine uterine bacterial communities
The sequencing reads of the bacterial 16S rRNA gene resulted in 1,488,649 clean tags from 22 samples. The evaluation results of each sample’s sequencing data are shown in Supplementary Table 1. These high-quality sequences clustered into an average of 1,117 bacterial OTUs. Numbers of identified phyla, classes, orders, families, genera, and species from these OTUs in all samples are shown in Supplementary Table 2.
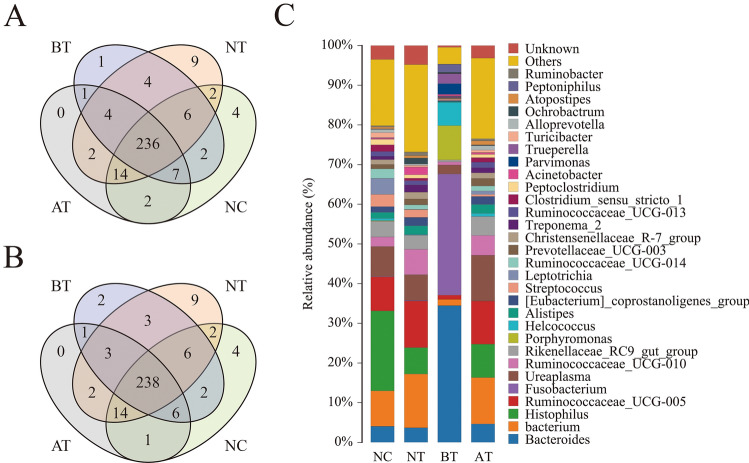
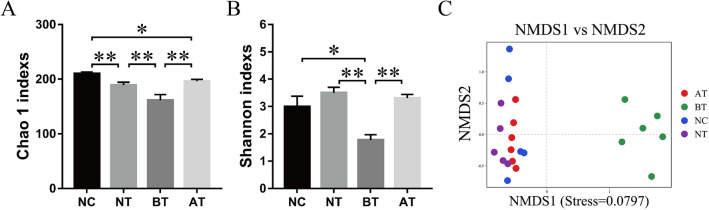
The number of unique/shared genera and species of the bacterial communities across different groups are shown in Fig. 2A,B. The genera Histophilus (20.16%), Bacterium (8.94%), Ruminococcaceae_UCG-005 (8.61%), Ureaplasma (7.52%), and Bacteroides (4.04%) were the 5 most dominantly abundant bacteria in the NC group. Bacterium (13.56%), Ruminococcaceae_UCG-005 (11.77%), Histophilus (6.65%), Ureaplasma (6.47%), and Ruminococcaceae_UCG-010 (6.40%) were the 5 most dominantly abundant bacteria in the NT group. Bacteroides (34.46%), Fusobacterium (30.51%), Porphyromonas (8.65%), Helcococcus (5.85%), and Parvimonas (2.72%) were the 5 most dominantly abundant bacteria in the BT group. Bacterium (11.68%), Ureaplasma (11.50%), Ruminococcaceae_UCG-005 (10.87%), Histophilus (8.43%), and Ruminococcaceae_UCG-010 (5.00%) were the 5 most dominantly abundant bacteria in the AT group (Fig. 2C). The Chao1 and Shannon indexes were calculated for all samples. Alpha-diversity analysis showed significant differences among the four groups (Fig. 3A, B).
Figure 2.
Venn diagram and abundances of the top 30 genera in the four groups. Venn diagrams showing the unique and shared genera of the bacterial communities across different groups at the genus (A) and species (B) levels. (C) Relative abundances of the top 30 bacterial genera in the four groups. Each color represents a genus, and the length of the patch represents the relative abundance ratio of all the bacterial communities in each sample. The other genera are merged in an “others” category. The category “unknown” represents a species that was not annotated with taxonomy.
Figure 3.
Variations in alpha-diversity and NMDS analysis among the four groups. (A) Comparisons of the bacterial community Chao1 indexes within the different groups. (B) Comparisons of the bacterial community Shannon indexes within the different groups. *, P < 0.05; **, P < 0.01 (with Student's t-test). (C) The dots in the figure represent the samples, and the different colors represent the different groups to which they belong. The distance between points indicates the degree of difference. Closer samples on the graph have greater similarity of diversity.
Bacterial beta-diversity
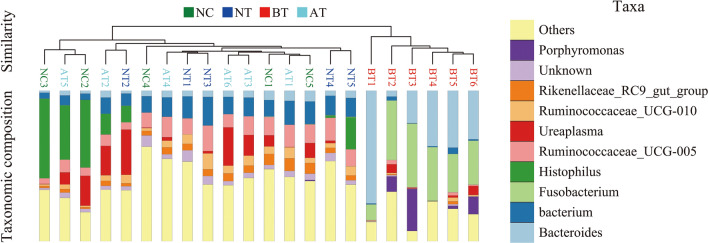
A Bray–Curtis-based NMDS plot of all samples revealed a separation among the four groups (Fig. 3C). The Bray–Curtis-based distance and abundance metrics showed that samples belonging to the BT group had an obviously different distribution compared with that of the other three groups. A UPGMA-based hierarchical clustering analysis confirmed the alpha‐diversity analysis results, which showed that each group had unique communities compared with those of the other groups among the four groups (Fig. 4).
Figure 4.
Bacterial beta diversity based on UPGMA clustering and abundances of the top 10 genera. To optimize the view, the histogram shows only the top 10 most abundant genera; the other genera are merged in an “others” category. “unknown” represents a species that are not annotated with taxonomy. Closer samples with shorter branch lengths have more similar genus-level compositions. Each color represents a genus, and the length of the patch represents the relative abundance ratio of all the bacterial communities in each sample.
Changes in the bacterial community in the uterus before and after treatment with OTC
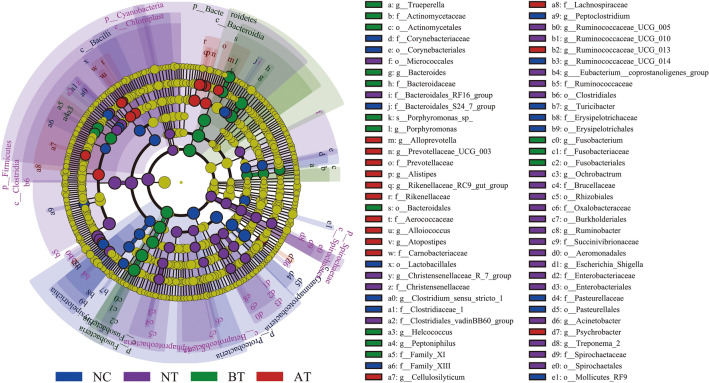
The results of LEfSe analysis indicated that the bacterial communities with significant abundance changes in the four groups included 6 phyla, 10 classes, 15 orders, 24 families, 28 genera, and 1 species (Fig. 5 and Supplementary Table 3). Metagenomic analysis showed that the bacterial composition varied in uteruses with endometritis after OTC treatment.
Figure 5.
Significant changes of bacterial communities in the four groups as determined by LEfSe analysis. Taxonomic representation of statistically consistent differences within the four groups. The taxa with significantly different abundances among groups are represented by colored dots. Colored areas (NC, NT, BT, and AT) mark the most prominent bacteria found in this study. The cladogram was generated by LEfSe and indicates differences at the phylum, class, family, order, genus, and species levels in the four groups. Each successive circle represents a phylogenetic level. Only taxa meeting a linear discriminant analysis (LDA) significance threshold of > 3.5 are shown.
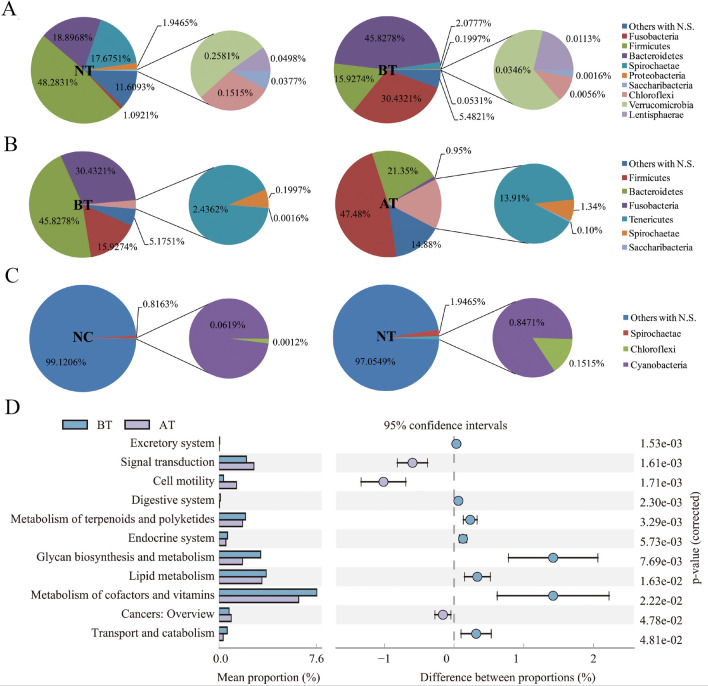
We first clarified the changes in the bacterial communities at the phylum level (Fig. 6A–C). The relative abundance of Fusobacteria, Firmicutes, Bacteroidetes, Spirochaetae, Proteobacteria, Saccharibacteria, Chloroflexi, Verrucomicrobia, and Lentisphaerae varied significantly between the NT and BT groups (P < 0.05). The relative abundance of Fusobacteria, Firmicutes, Spirochaetae, Saccharibacteria, Bacteroidetes, and Tenericutes varied significantly between the BT and AT groups (P < 0.05). The relative abundance of Chloroflexi, Cyanobacteria, and Spirochaetae varied significantly between the NT and NC groups (P < 0.05). No significant bacterial community abundance changes occurred between the NC and AT groups at the phylum level.
Figure 6.
Abundances of bacterial communities changes at phylum level and related metabolic pathways with OTC treatment. (A–C) Relative abundances of bacterial phyla in four groups. Each color represents a phylum. No significantly changed phyla are merged in an “Others with N.S.” category. (D) KEGG pathways associated with significantly different abundances in the bacterial metagenomics profiles of BT and AT groups. The bars represent the proportion of each category in the data. Category differences with a P < 0.05 were considered to be significant.
The top 5 increased/decreased and unique genera in the four groups are shown in Table 2, Supplementary Table 4, and Supplementary Table 5. The relative abundances of Clostridium_sensu_stricto_6 and Tissierella were much higher (25.839 and 10.665 times, respectively) in the NC group than in the NT group, while those of Tepidimonas, Ornithinicoccus, Paludibacter, Serratia, and Rhizobium were much lower (0.016, 0.018, 0.023, 0.042, and 0.043 times, respectively). Sulfuricurvum, Caproiciproducens, and Lactobacillus could not be detected in the NC group, while Paraliobacillus and Amphibacillus were undetectable in the NT group. The abundances of Marinospirillum, Erysipelothrix, Enteractinococcus, Erysipelotrichaceae_UCG-009, and Papillibacter were much lower in the BT group than in the AT group (0.002, 0.005, 0.008, 0.010, and 0.012 times, respectively), while those of Fusobacterium, Porphyromonas, Parvimonas, Peptoniphilus, and Trueperella were much higher (1,548.265, 1,022.113, 415.864, 233.766, and 92.739 times, respectively). Faecalibacterium, hoa5-07d05_gut_group, Salinicoccus, Methylophilus, Proteiniclasticum, Cellulomonas, Amphibacillus, and Phenylobacterium were undetectable in the BT group.
Table 2.
Variations of dominant bacteria composition.
| NC versus NT | BT versus AT | ||
|---|---|---|---|
| Bacteria | Abundance change (times) | Bacteria | Abundance change (times) |
| Tepidimonas | 0.016 | Marinospirillum | 0.002 |
| Ornithinicoccus | 0.018 | Erysipelothrix | 0.005 |
| Paludibacter | 0.023 | Enteractinococcus | 0.008 |
| Serratia | 0.042 | Erysipelotrichaceae_UCG-009 | 0.010 |
| Rhizobium | 0.043 | Papillibacter | 0.012 |
| Tissierella | 10.665 | Trueperella | 92.738 |
| Clostridium_sensu_stricto_6 | 25.839 | Peptoniphilus | 233.766 |
| Sulfuricurvum | + /− | Parvimonas | 415.864 |
| Caproiciproducens | + /− | Porphyromonas | 1022.113 |
| Lactobacillus | + /− | Fusobacterium | 1548.265 |
| Paraliobacillus | −/ + | Faecalibacterium | −/ + |
| Amphibacillus | −/ + | Hoa5-07d05_gut_group | −/ + |
| Salinicoccus | −/ + | ||
| Methylophilus | −/ + | ||
| Proteiniclasticum | −/ + | ||
| Cellulomonas | −/ + | ||
| Amphibacillus | −/ + | ||
| Phenylobacterium | −/ + | ||
" + " represents bacteria can be detected while "−" represents bacteria is undetectable.
BT = severely ill cows, AT = cows in BT group after OTC treatment.
Changes in functional pathways of bacterial communities among the different groups
As shown in Fig. 6D, there were 11 biological pathways (including excretory system, signal transduction, cell motility, digestive system, metabolism of terpenoids and polyketides, endocrine system, glycan biosynthesis and metabolism, lipid metabolism, metabolism of cofactors and vitamins, cancers: overview, and transport and catabolism) involved in bacterial communities with significant abundance changes between the BT and AT groups.
Discussion
OTC has a good therapeutic effect on endometritis, but some bacteria are resistant to OTC. However, most research on the use of oxytetracycline (OTC) in the treatment of endometritis focus on specific flora, especially the culturable bacteria23,24. The inhibition effects of OTC on the metagenomic-based analysis, especially non-culturable bacteria, and its effects on the biological functions of the corresponding bacteria are not well known.
In this study, we isolated and identified several common bacteria that cause endometritis. Overall, our analysis showed that the microbial communities in cows with endometritis are similarly dynamic within either animal groups (Figs. 3, 4, Supplementary Table 4 and 5) as reported by other research25. It is noteworthy that although OTC stimulated the elimination of Bacteroidetes, Fusobacteria, etc., it did not eliminate all bacterial species. On the contrary, some bacteria species increased after OTC administration. The detection rates of bacteria such as Chryseobacterium indologenes, Bacillus cereus, and Corynebacterium were even higher in the NC group than in the BT group. Although some bacteria (e.g. Escherichia coli, Staphylococcus aureus, Staphylococcus haemolyticus, and Streptococcus), which was considered as pathogenic bacteria, has high detection rate or proportion, the individuals have not shown disease characteristics or have been cured. This indicates that identification by traditional bacterial culture methods has limitations for endometritis infection-associated strain identification. In addition, simply analyzing the etiology or treatment of endometritis based on a specific pathogenic bacterial population may also be inaccurate.
At the phylum level, changes in the bacterial communities between the NT group and the BT group indicated that abundances of Fusobacteria, Firmicutes, Bacteroidetes, Spirochaetae, Proteobacteria, Saccharibacteria, Chloroflexi, Verrucomicrobia, and Lentisphaerae changed the most from mild symptoms to severe illness. The changes in bacterial communities between the BT and AT groups indicated that OTC could significantly inhibit Bacteroidetes and Fusobacteria. The changes in bacterial communities between the NC and NT groups indicated that endometritis begins with changes in the abundance of Spirochaetae, Chloroflexi, and Cyanobacteria. These results seem to be contradictory. For example, Streptococcus and Staphylococcus belong to the Firmicutes phylum; Escherichia coli belongs to the Proteobacteria phylum. The abundance of Firmicutes was increased, while Proteobacteria did not change significantly after OTC treatment. This result suggests that exploring the bacteria that might lead to endometritis at the phylum level as a guideline to prevent or cure endometritis is ambiguous. However, since the database is not sufficiently comprehensive, we selected the genus level to further annotate the differences between different groups of bacteria and analyze their biological effects.
At the genus level, Sulfuricurvum, Caproiciproducens, and Lactobacillus were not detected in the uterus of healthy cattle but were in cows in the onset stage of endometritis. The abundances of bacteria such as Tepidimonas, Ornithinicoccus, and Paludibacter increased significantly during the deterioration of endometritis. Interestingly, Amphibacillus and Paraliobacillus could not be detected in the uteruses with endometritis. Amphibacillus was detectable in cows with endometritis until they were cured. Faecalibacterium, hoa5-07d05_gut_group, Salinicoccus, Methylophilus, Proteiniclasticum, Cellulomonas, and Phenylobacterium were undetectable when cows were severely ill, indicating that these bacteria could not survive in a severe endometritis environment.
In addition, we also found that no flora disappeared after OTC treatment, indicating that OTC could not completely remove existing bacteria. However, after treatment with OTC, the relative abundances of Bacteroides, Trueperella, Peptoniphilus, Parvimonas, Porphyromonas, and Fusobacterium decreased to 15.5824%, 1.0783%, 0.4276%, 0.0979%, and 0.0646%, respectively, indicating that OTC has an active inhibitory effect against these bacteria. In contrast, the relative abundance of Marinospirillum, Erysipelothrix, and Enteractinococcus increased tens of thousands of times after OTC treatment, indicating that OTC had no significant inhibitory effect on these bacteria.
Finally, we analyzed the possible biological pathways that OTC affected and its potential effects on the physiological role of uterine bacterial communities. Postpartum insufficient uterine involution, inflammatory secretions in the uterine cavity and poor drainage are important hidden dangers of endometritis. Bacteria lead to endometritis through biological pathways such as the excretory system, endocrine system, signal transduction, and cell motility26–28. The use of OTC can affect the utilization of glycan and lipids in bacterial communities and affect the content of metabolites (including terpenoids and polyketides), thereby affecting the adaptability and reproductive capacity of the communities29–31. In addition, OTC also affects the ability of bacteria to adapt to diverse conditions, which is mediated by regulation of encoded signal transducers (or their fraction in the total protein set)32. These results explain why OTC effectively reduced the production of cytokines, which actively participate in the inflammatory process in the uterus27,33, and had a potential role in responses initiated by recognition proteins, which, in the presence of foreign organisms including bacteria, trigger distinct signal transduction and modulation pathways34. More importantly, we also found that the treatment with OTC had a significant impact on the metabolism of cofactors and vitamins pathway. This result can also explain why the long-term use of OTC causes changes in intestinal communities and causes a vitamin imbalance. Therefore, vitamin supplementation is often used when treating animals with OTC35,36.
In conclusion, this study identified changes in bacteria at the genus level in uteri of cows with endometritis before and after OTC treatment. During OTC treatment, the excretory system, signal transduction, cell motility, digestive system, metabolism of terpenoids and polyketides, endocrine system, glycan biosynthesis and metabolism, lipid metabolism, metabolism of cofactors and vitamins, and transport and catabolism pathways were involved. OTC had a significant inhibitory effect on the genera Bacteroides, Trueperella, Peptoniphilus, Parvimonas, Porphyromonas, and Fusobacterium but had no significant inhibitory effect on bacteria such as Marinospirillum, Erysipelothrix, and Enteractinococcus.
Methods
All methods (including all animal experiments) in this study were performed in accordance with the relevant guidelines and approved by the Institutional Animal Care and Use Committee of Jilin University (IACUC-ID-SY201801026). This study is reported in accordance with ARRIVE guidelines.
Experimental animals
On a dairy farm with 442 cows, a total of 16 Holstein cows with same months of age and parity order were detected. According to the degree of illness, among all the samples, 5 cows were healthy cows (NC group), and 5 cows had mild disease (NT group). Six cows were severely ill (BT group) and became healthy after oxytetracycline hydrochloride (OTC; Aladdin, Shanghai, China) treatment (AT group).
Uterine contents collection
Before sample collection, the anus and vulva area were wiped and disinfected with a 5% potassium permanganate solution, and then the vulvae were disinfected with cotton swabs moistened with 70% alcohol. Endometrial swabs were used to collect the uterine contents as described with some modification25,37. In general, an improved ethylene oxide-sterilized human uterine sampling brush was placed into an infusion cannula, and advanced into the uterus and gently rotated38. After removal, the uterine sampling swabs was rinsed with sterile saline, and the solution samples were collected in two sterile tubes. Bacteria in one tube were stored immediately at − 80 °C until DNA extraction. Bacteria in another tube were prepared for pathogen identification.
Diagnosis of endometritis
Holstein cows with endometritis were identified based the visual observation and ultrasound testing as described with some modification. In general, cows with a foul-smelling and slightly turbid discharged vaginal mucus were diagnosed with mild disease. Cows with fetid and white/yellow vaginal purulent secretions were diagnosed as seriously diseased. Through rectal palpation, the volume of seriously diseased cow's uterine horn was increased and often accompanied by a certain degree of edema, the elasticity of the cervix and uterus deteriorated. Through ultrasound examination, cows with thickened uterine wall and irregular inner edge as well as an obvious fluid sonolucent area in the uterus were identified as diseased cow while healthy or cured cows had clear black background without significant hyperechoic areas (Fig. 1).
Isolation and biochemical identification of endometritis pathogens
Broth medium, MacConkey agar medium, blood agar base medium, and related media were obtained from Qingdao Hope Bio-Technology (Qingdao, China). Bacteria in uterine contents were cultured with the corresponding media at 37 °C incubator for 24 h for Gram staining and microscopic examination. The morphology, staining characteristics and growth characteristics of the colonies were observed and classified. Each sample was subjected to 5 biological replicate tests, and any positive results indicated that the corresponding bacteria existed in the uterine contents. The isolated bacteria obtained were identified based on morphology, Gram staining reactions and different biochemical tests using Bergey’s manual for determinative bacteriology39.
OTC treatment and efficacy evaluation
Briefly, uterine perfusion was performed on a diseased cow with a 20% concentration of OTC (200 g of OTC in 1 L of sterile saline). The vulva was cleaned and disinfected, and then 20 mL of OTC was pushed into the uterus every 2 days by a syringe through an infusion cannula. Four episodes of perfusion was considered a treatment course. One week after stopping the treatment, a rectal examination and an ultrasound examination were performed to verify the curative effect. When a white/yellow stench of vaginal purulent secretions was still found from the orificium vaginae and an inflammatory area was detected by ultrasound examination, the treatment was ineffectual (Fig. 1I). When there was no congestion in the vaginal mucosa, the uterine mucus was clear, and there were abnormal findings on ultrasound, the cows were considered cured (Fig. 1J).
DNA extraction and sequencing
Total bacterial and fungal DNA were extracted from samples using a PowerSoil DNA Isolation Kit (MoBio Laboratories, CA, USA) according to the manufacturer's protocol. Then, DNA was stored at − 80 °C until further processing.
Common primer pairs combined with adapter sequences and barcode sequences were used to amplify the bacterial 16S rRNA gene (forward primer, 5'-ACTCCTACGGGAGGCAGCA-3'; reverse primer, 5'-GGACTACHVGGGTWTCTAAT-3'). PCR amplification was performed in a total volume of 50 μL, which contained 0.2 μL of Q5 High-Fidelity DNA Polymerase (New England Biolabs Inc., MA, USA), 10 μL of buffer, 10 μL of High GC Enhancer, 1 μL of dNTPs, 10 μM each primer and 60 ng of genomic DNA. Thermal cycling conditions were as follows: an initial denaturation at 95 °C for 5 min, followed by 15 cycles at 95 °C for 1 min, 50 °C for 1 min and 72 °C for 1 min, with a final extension at 72 °C for 10 min. PCR products from the first step PCR were purified through VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China). A second round of PCR was then performed in a 40 μL reaction that contained 20 μL of 2 × Phusion HF Mix (Thermo Fisher Scientific, CA, USA), 8 μL of ddH2O, 10 μM each primer and 10 μL of PCR products from the first step. Thermal cycling conditions were as follows: an initial denaturation at 98 °C for 30 s, followed by 10 cycles at 98 °C for 10 s, 65 °C for 30 s min and 72 °C for 30 s, with a final extension at 72 °C for 5 min. Finally, all PCR products were quantified by a Quant-iT™ dsDNA HS Assay Kit (Thermo Fisher Scientific) and pooled together.
High-throughput sequencing analysis of bacterial and fungal genes was performed on the purified, pooled samples using the Illumina HiSeq 2500 platform (2 × 250 paired-end sequencing; Biomarker Technologies Corporation, Beijing, China).
Bioinformatics analyses
Raw sequences were generated from the Illumina HiSeq sequencing platform.Quality control and taxon classification were performed with FLASH v1.2.7 (http://ccb.jhu.edu/software/FLASH/), Trimmomatic (http://www.usadellab.org/cms/?page=trimmomatic), and UCHIME v4.2 (http://drive5.com/usearch/manual/uchime_algo.html). The sequences were further analyzed with the QIIME software package (http://qiime.org/). Operational taxonomic units (OTUs) were selected using a de novo OTU-picking protocol with a 97% identity threshold, and then, a representative sequence was picked for each OTU using Mothur software (https://www.mothur.org/) and the Silva database (https://www.arb-silva.de/) to annotate taxonomic information for each representative sequence.
Alpha-diversity, including Chao1 and Shannon indexes, was first calculated by Mothur software. Then, we compared overall samples between intragroup compositions using the unweighted pair-group method with arithmetic mean (UPGMA, http://genomes.urv.cat/UPGMA/), Venn40, and nonmetric multidimensional scaling (NMDS)41. Species with significant differences in microbial community composition abundance between groups were then analyzed with line discriminant analysis effect size (LEfSe, http://huttenhower.sph.harvard.edu/galaxy)42 and Metastats (http://metastats.cbcb.umd.edu/)43 methods. PICRUSt (http://picrust.github.com/picrust/)44 and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp/)45–47 were used to predict function changes caused by bacterial microbial communities between the BT and AT groups based on the R language platform (R Core Team, https://www.r-project.org/).
Supplementary Information
Author contributions
Conceptualization, H.J., W.L., and J.Z.; Investigation, X.C., J.X., and X.Y.; Data curation, X.C., P.X. and Y.Z.; Formal analysis, X.C., J.X., and X.M.; Methodology, C.C., J.W., M.Z., and H.J.; Project administration, W.L. and J.Z.; Resources, X.Y., and X.M.; Writing—original draft, X.C.; Writing—review & editing, H.J., and W.Y.; Funding acquisition, B.Y., W.L., J.W., and J.Z. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (U20A2053), the China Agriculture Research System of the MOF and MARA (CARS-37), and Research and Demonstration of Integrated Breeding Technology for New Kerqin Beef Cattle (KJXM2020002). The authors have not stated any conflicts of interest.
Data availability
The data presented in this study are available in the article or Supplementary Material. The raw sequencing data generated and analysed during the current study are available in the Sequence Read Archive (SRA) repository (SAMN35443045–SAMN35443066) under BioProject PRJNA976827.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiao-Shi Cai and Hao Jiang.
Contributor Information
Bao Yuan, Email: yuan_bao@jlu.edu.cn.
Jia-Bao Zhang, Email: zjb515@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-59674-4.
References
- 1.Cheong SH, Nydam DV, Galvao KN, Crosier BM, Gilbert RO. Cow-level and herd-level risk factors for subclinical endometritis in lactating Holstein cows. J. Dairy Sci. 2011;94:762–770. doi: 10.3168/jds.2010-3439. [DOI] [PubMed] [Google Scholar]
- 2.Machado VS, et al. Investigation of postpartum dairy cows' uterine microbial diversity using metagenomic pyrosequencing of the 16S rRNA gene. Vet. Microbiol. 2012;159:460–469. doi: 10.1016/j.vetmic.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Santos TM, Bicalho RC. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE. 2012;7:e53048. doi: 10.1371/journal.pone.0053048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garoussi MT, Khosrave AR, Havareshti P. Mycoflora of cervicovaginal fluids in dairy cows with or without reproductive disorders. Mycopathologia. 2007;164:97–100. doi: 10.1007/s11046-007-9031-x. [DOI] [PubMed] [Google Scholar]
- 5.Fourichon C, Seegers H, Malher X. Effect of disease on reproduction in the dairy cow: a meta-analysis. Theriogenology. 2000;53:1729–1759. doi: 10.1016/S0093-691X(00)00311-3. [DOI] [PubMed] [Google Scholar]
- 6.Frazier K, et al. Endometritis in postparturient cattle associated with bovine herpesvirus-4 infection: 15 cases. J. Vet. Diagn. Invest. 2001;13:502–508. doi: 10.1177/104063870101300608. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert RO. Management of reproductive disease in dairy cows. Veterinary Clin. Food Animal Pract. 2016;32:387–410. doi: 10.1016/j.cvfa.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Hammon, D., Evjen, I., Dhiman, T. & Goff, J. Negative energy balance during the periparturient period is associated with uterine health disorders and fever in Holstein cows. Journal Dairy Science87 (2004).
- 9.Carneiro LC, Cronin JG, Sheldon IM. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016;16:1–7. doi: 10.1016/j.repbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Azawi O. Postpartum uterine infection in cattle. Animal Reprod. Sci. 2008;105:187–208. doi: 10.1016/j.anireprosci.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Bicalho ML, Machado VS, Oikonomou G, Gilbert RO, Bicalho RC. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Veterinary Microbiol. 2012;157:125–131. doi: 10.1016/j.vetmic.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Denis-Robichaud J, Dubuc J. Randomized clinical trial of intrauterine cephapirin infusion in dairy cows for the treatment of purulent vaginal discharge and cytological endometritis. J. Dairy Sci. 2015;98:6856–6864. doi: 10.3168/jds.2014-9129. [DOI] [PubMed] [Google Scholar]
- 13.Priest N, et al. The responsiveness of subclinical endometritis to a nonsteroidal antiinflammatory drug in pasture-grazed dairy cows. J. Dairy Sci. 2013;96:4323–4332. doi: 10.3168/jds.2012-6266. [DOI] [PubMed] [Google Scholar]
- 14.Shams-Esfandabadi N, Shirazi A, Ghasemzadeh-Nava H. Pregnancy rate following post-insemination intrauterine treatment of endometritis in dairy cattle. J. Vet. Med. Ser. A. 2004;51:155–156. doi: 10.1111/j.1439-0442.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 15.Barman P, Yadav M, Bangthai A, Kumar H. Antibiogram of bacteria isolated from bovine endometritis. Vet. Res. Int. 2013;1:20–24. [Google Scholar]
- 16.Ghaisari H, Nazifi S, Ahmadi M. The effect of intrauterine cephapirin on treatment of endometritis in commercial dairy cattle. Arch. of Razi Institute. 2005;59:35–45. [Google Scholar]
- 17.Miranda CD, Zemelman R. Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture. 2002;212:31–47. doi: 10.1016/S0044-8486(02)00124-2. [DOI] [Google Scholar]
- 18.Santos T, et al. Antimicrobial resistance and presence of virulence factor genes in Arcanobacterium pyogenes isolated from the uterus of postpartum dairy cows. Veterinary Microbiol. 2010;145:84–89. doi: 10.1016/j.vetmic.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Ndi, O. & Barton, M. Antibiotic resistance in animals–the Australian perspective. Antimicrobial Resistance in the Environment, 265–290 (2012).
- 20.Lewis JD, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18:489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan N, et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2019;2:78. doi: 10.1038/s42003-019-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loging W, Harland L, Williams-Jones B. High-throughput electronic biology: mining information for drug discovery. Nat. Rev. Drug Discov. 2007;6:220–230. doi: 10.1038/nrd2265. [DOI] [PubMed] [Google Scholar]
- 23.Moges N, Regassa F, Yilma T, Unakal CG. Isolation and antimicrobial susceptibility of bacteria from dairy cows with clinical endometritis. J. Reprod. Infertil. 2013;4:04–08. [Google Scholar]
- 24.Sheldon I, Bushnell M, Montgomery J, Rycroft A. Minimum inhibitory concentrations of some antimicrobial drugs against bacteria causing uterine infections in cattle. Veterinary Record. 2004;155:383–387. doi: 10.1136/vr.155.13.383. [DOI] [PubMed] [Google Scholar]
- 25.Tasara T, et al. Interrogating the diversity of vaginal, endometrial, and fecal microbiomes in healthy and metritis dairy cattle. Animals. 2023;13:13. doi: 10.3390/ani13071221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tijani JO, Fatoba OO, Petrik LF. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water Air Soil Pollut. 2013;224:1770. doi: 10.1007/s11270-013-1770-3. [DOI] [Google Scholar]
- 27.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod. Sci. 2013;20:154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 28.Lucy MC, Butler ST, Garverick HA. Endocrine and metabolic mechanisms linking postpartum glucose with early embryonic and foetal development in dairy cows. Animal Int. J. Animal Biosci. 2014;8(Suppl 1):82–90. doi: 10.1017/S1751731114000482. [DOI] [PubMed] [Google Scholar]
- 29.Bibb MJ. Regulation of secondary metabolism in streptomycetes. Curr. Opinion Microbiol. 2005;8:208–215. doi: 10.1016/j.mib.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Kiessling, L. L., Wesener, D. A. & Wangkanont, K. (US Patent App. 14/933,891, 2018).
- 31.Mu C, et al. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids. 2017;49:771–781. doi: 10.1007/s00726-017-2379-4. [DOI] [PubMed] [Google Scholar]
- 32.Galperin MY. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 2005;5:35. doi: 10.1186/1471-2180-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ci X, et al. Oxytetracycline attenuates allergic airway inflammation in mice via inhibition of the NF-kappaB pathway. J. Clin. Immunol. 2011;31:216–227. doi: 10.1007/s10875-010-9481-7. [DOI] [PubMed] [Google Scholar]
- 34.Evans JD. Transcriptional immune responses by honey bee larvae during invasion by the bacterial pathogen, Paenibacillus larvae. J. Invertebrate Pathol. 2004;85:105–111. doi: 10.1016/j.jip.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Kalavathy R, Abdullah N, Jalaludin S, Wong C, Ho Y. Effect of Lactobacillus cultures and oxytetracycline on the growth performance and serum lipids of chickens. Int. J. Poultry Sci. 2008;7:385–389. doi: 10.3923/ijps.2008.385.389. [DOI] [Google Scholar]
- 36.Jukes TH. Antibiotics in Nutrition. A Publication Of Medical Encyclopedia; 2013. [Google Scholar]
- 37.Becker A, et al. The Endometrial Microbiota-16S rRNA Gene Sequence Signatures in Healthy, Pregnant and Endometritis Dairy Cows. Vet. Sci. 2023;10:13. doi: 10.3390/vetsci10030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verstraelen H, et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1–2 region of the 16S rRNA gene. PeerJ. 2016;4:23. doi: 10.7717/peerj.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holt J, Krieg N, Sneath P, Staley J, Williams S. Bergey’s Manual of Determinative Microbiology. Williams and Wilkins; 1994. [Google Scholar]
- 40.Chen H, Boutros PC. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011;12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taguchi YH, Oono Y. Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics. 2005;21:730–740. doi: 10.1093/bioinformatics/bti067. [DOI] [PubMed] [Google Scholar]
- 42.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome biology. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leamer, E. E. Specification searches: Ad hoc inference with nonexperimental data. Vol. 53 (John Wiley & Sons Incorporated, 1978).
- 44.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–D592. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article or Supplementary Material. The raw sequencing data generated and analysed during the current study are available in the Sequence Read Archive (SRA) repository (SAMN35443045–SAMN35443066) under BioProject PRJNA976827.