Abstract
Four new monoclonal antibodies (MAbs) that inhibit human T-cell lymphotropic virus type 1 (HTLV-1)-induced syncytium formation were produced by immunizing BALB/c mice with HTLV-1-infected MT2 cells. Immunoprecipitation studies and binding assays of transfected mouse cells showed that these MAbs recognize class II major histocompatibility complex (MHC) molecules. Previously produced anti-class II MHC antibodies also blocked HTLV-1-induced cell fusion. Coimmunoprecipitation and competitive MAb binding studies indicated that class II MHC molecules and HTLV-1 envelope glycoproteins are not associated in infected cells. Anti-MHC antibodies had no effect on human immunodeficiency virus type 1 (HIV-1) syncytium formation by cells coinfected with HIV-1 and HTLV-1, ruling out a generalized disruption of cell membrane function by the antibodies. High expression of MHC molecules suggested that steric effects of bound anti-MHC antibodies might explain their inhibition of HTLV-1 fusion. An anti-class I MHC antibody and a polyclonal antibody consisting of several nonblocking MAbs against other molecules bound to MT2 cells at levels similar to those of class II MHC antibodies, and they also blocked HTLV-1 syncytium formation. Dose-response experiments showed that inhibition of HTLV-1 syncytium formation correlated with levels of antibody bound to the surface of infected cells. The results show that HTLV-1 syncytium formation can be blocked by protein crowding or steric effects caused by large numbers of immunoglobulin molecules bound to the surface of infected cells and have implications for the structure of the cellular HTLV-1 receptor(s).
Human T-cell lymphotropic virus type 1 (HTLV-1) is a type C retrovirus and the etiologic agent of adult T-cell leukemia (43, 56, 59) and HTLV-1-associated myelopathy or tropical spastic paraparesis (15, 17, 49, 61). Although HTLV-1 shows tropism primarily for T cells, it can infect a variety of cell types including cells from some nonhuman species (6, 9, 27, 46, 48, 60, 62). Infection by free HTLV-1 tends to be highly inefficient, and the virus appears to be transmitted primarily by the cell-to-cell route (37). The HTLV-1 envelope glycoprotein is synthesized as a 61-kDa precursor which is cleaved into surface (gp46) and transmembrane (gp21) proteins (40, 57). gp46 is thought to serve as the virus attachment protein, as does gp120 for human immunodeficiency virus (HIV) (40, 57). Although previous reports have identified host cell molecules which might potentially mediate virus binding (9, 14), the cellular receptor for HTLV-1 has not been definitively identified. A recent study in which affinity chromatography was carried out with a gp46 peptide has provided evidence that the heat shock protein HSC70 binds directly to gp46 and may serve as a virus receptor (47).
gp21 contains an N-terminal hydrophobic fusion domain and likely serves as a fusion protein similar to HIV gp41 (12, 61). Like many other retroviruses, HTLV-1 can induce syncytium formation between infected cells and certain uninfected cell types (28, 39). However, there are no data to indicate that virus transmission or virus persistence in vivo depends on syncytium formation. It is thought that cell-cell fusion involves the same receptors and occurs in a manner similar to virus-cell fusion. For this reason, HTLV-1 syncytium assays have been used to screen for cell surface molecules that may serve as virus receptors (13, 14, 25, 29). Monoclonal antibodies (MAbs) against a number of membrane proteins including members of the tetraspanner family (30, 31) have been found to block syncytium formation. My colleagues and I recently reported that expression of the cell adhesion molecule vascular cell adhesion molecule 1 (VCAM-1) on uninfected cells can confer sensitivity to HTLV-1-mediated syncytium formation (25). In this previous study, we were not able to block HTLV-1 cell fusion with MAbs against the major VCAM-1 counterreceptor VLA-4 (25). Others have reported that MAbs to other adhesion molecules including intercellular adhesion molecule 3 (ICAM-3) also block HTLV-1 syncytium formation (29). We have demonstrated that adhesion molecules also facilitate HIV type 1 (HIV-1) infection and syncytium formation (16, 24). Thus, adhesion molecules may be important accessory molecules for retroviruses generally.
Earlier studies on accessory molecules involved in HTLV-1 biology have been extended by immunizing mice with HTLV-1-infected cells and screening for MAbs that block VCAM-1-supported HTLV-1 syncytium formation. Four new MAbs that completely block HTLV-1-mediated cell fusion have been generated. The MAbs were all determined to be specific for class II major histocompatibility complex (MHC) molecules. These MAbs had no effect on syncytium formation induced by HIV-1. Studies on the mechanism by which the MAbs mediate this effect have revealed a novel mode of antibody blockade of virus-induced cell fusion: protein crowding at the infected cell surface resulting in steric blockade of critical receptor-ligand interactions.
MATERIALS AND METHODS
Cells.
The following cell lines were obtained from the American Type Culture Collection (Manassas, Va.): U937, K562, and MJ. Cell lines obtained from the National Institutes of Health AIDS Research and Reference Reagent Program included MT2 and the H9 line infected with HIV-1RF and HIV-1MN. CEMx174 cells were obtained from Janice Clements (Johns Hopkins University). All of the above cell lines were maintained in cRPMI (RPMI 1640 supplemented with 10 mM HEPES, 2 mM l-glutamine, and 10% fetal bovine serum). MT2 and U937 cell lines chronically infected with HIV-1MN and HIV-1RF, respectively, were established as previously described (41). Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell lines were established with supernatants from the marmoset line B95.8 as previously described (45). Construction of the pCEP4VCAM-1 expression vector and establishment of the K562/VCAM-1 stable transfectant line were described previously (25). Mouse epithelial (L) cell lines transfected with human MHC class II genes were kindly provided by Robert Karr (University of Iowa) (32). These cells were maintained in Dulbecco’s minimum essential medium supplemented with 10% fetal bovine serum, 10 mM HEPES, and 300 μg of G418 per ml.
Antibodies.
New MAbs against the HTLV-1-producing cell line MT2 were produced as previously described (21, 34). Briefly, female BALB/c mice received four biweekly intraperitoneal injections of 107 MT2 cells in phosphate-buffered saline (PBS). Two weeks after the third intraperitoneal injection, 107 MT2 cells were injected intravenously, and the fusion was carried out 4 days later with isolated splenocytes (21, 34). Supernatants from the resulting hybridoma lines were screened for inhibition of syncytium formation between MT2 and K562/VCAM-1 cells. Hybridomas scoring positive in this assay were subcloned twice by limiting dilution. Several antibodies that blocked fusion were identified, and four were selected for further characterization, designated MT.M1, MT.M2, MT.M3, and MT.M4. All four of these new MAbs were determined to be of the immunoglobulin G1 (IgG1k) isotype (MonoAb ID kit; Zymed). A MAb against VCAM-1 (CD106; clone 51-10C9) was obtained from Pharmingen, San Diego, Calif. Anti-ICAM-1 (CD54) MAb 84H10 and anti-CD29 MAb 4B4 were supplied by AMAC (Westbrook, Maine) and Coulter Immunology (Hialeah, Fla.), respectively. Anti-HTLV-1 gp46 MAb was obtained from Cellular Products, Buffalo, N.Y. The TS2/9.1 hybridoma (anti-CD58) was obtained from the American Type Culture Collection. All other MAbs used in this study were produced in my laboratory as previously reported (specificity in parentheses): H52 and PLM-2 (CD18) (21, 22), H5C6 (CD63) (4), U9.M2 and H4C4 (CD44) (5, 18), MHM.33, MHM.36, H53 (class II MHC) (35, 42), H4A3 and H4B4 (LAMPS, CD107a,b) (36), MHM.5 (class I MHC) (11), and H5H5 (CD43) (41). MT.M5 (IgG1k) was produced in the same fusion as the new antibodies described in this study. Immunoprecipitation, binding to transfected cells and recombinant proteins, and functional studies showed this antibody to be specific for ICAM-1 (CD54). All antibodies were used in the form of hybridoma culture supernatants (10 to 30 μg of IgG per ml) or purified IgG in the appropriate buffer or medium at a concentration of 20 μg/ml.
Vectorial cell labeling and immunoprecipitation.
MT2 cells were surface labeled with 125I, and immunoprecipitations were performed as reported elsewhere (18, 21). Briefly, 5 × 107 cells were washed three times with PBS and resuspended in 0.5 ml of PBS. One unit each of lactoperoxidase and glucose oxidase (Boehringer Mannheim), 1 mCi of carrier-free [125I]NaI (Amersham), and 25 μl of 1% dextrose were then added followed by incubation for 20 min at 21°C. After being washed twice with PBS, the cells were incubated on ice for 45 min in 50 mM Tris (pH 7.5)–5 mM EDTA–150 mM NaCl (TEN) containing 1% Nonidet P-40 (NP-40) and protease inhibitors (2 μg each of leupeptin, soybean trypsin inhibitor, antipain, aprotinin, and chymostatin per ml) and 1 mM phenylmethylsulfonyl fluoride. Detergent-insoluble material was removed by centrifugation (100,000 × g, 45 min). Where indicated, the cell lysate was precleared of nonspecific binding proteins with two cycles of addition of 10 μl of normal rabbit serum and 100 μl of Pansorbin (10% fixed Staphylococcus aureus [SaC]; Calbiochem), incubation for 2 h on ice, and centrifugation (10,000 × g, 5 min). Immunoprecipitation was carried out in two steps. Hybridoma culture supernatants and control antibodies were mixed with cell lysates and incubated for 15 h at 0°C. Ten micrograms of affinity-purified rabbit anti-mouse Ig (Jackson ImmunoResearch, Avondale, Pa.) was then added, followed by incubation for 2 h at 0°C. Fifty microliters of Pansorbin was then added, and immune complexes were pelleted after 20 min. The immune complexes were washed twice with 2 M KCl in TEN–0.5% NP-40 and once with 50 mM Tris (pH 8.0)–0.5% NP-40 before analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (18, 21). Protein bands were visualized by autoradiography. Immunoprecipitations to look for protein-protein interactions were carried out similarly, except that the detergent CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate) was substituted for NP-40 in lysis and wash buffers and high salt (2 M KCl) was omitted from wash buffers.
Flow cytometry.
Flow cytometry analysis was performed as previously described (18). Briefly, washed cells were resuspended at 2 × 106/ml in PBS containing 5% normal goat serum (PBS-NGS). One hundred microliters of cells was mixed with 100 μl of MAb at 20 μg/ml in PBS-NGS and incubated for 45 min on ice. The cells were washed twice with cold PBS and resuspended in 100 μl of PBS-NGS containing 25 μg of fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Jackson ImmunoResearch). After 45 min on ice, the cells were washed twice with cold PBS, resuspended in 0.5 ml of 2% paraformaldehyde in PBS, and analyzed on an Epics Profile II flow cytometer. Nonviable cells were excluded from analysis of 5,000 cells. Isotype-matched murine myeloma proteins were included as negative controls.
Radiolabeling of MAbs and competition binding assay.
MAbs were labeled with radioiodinated Bolton-Hunter reagent (Amersham) and desalted by Sephadex G-25 filtration as previously described. Specific activity ranged from 1 × 107 to 5 × 107 cpm/μg (22). Competitive MAb binding assays were performed as previously reported (23).
Syncytium assay.
Syncytium assays were carried out as described in a previous report (25). All cells were washed with cRPMI and resuspended in cRPMI at a density of 2 × 106/ml. Fifty microliters of MT2 cells was added to duplicate or triplicate wells of a flat-bottom 96-well plate and mixed with 100 μl of cRPMI or MAb (20 μg of IgG per ml or undiluted hybridoma supernatants). After incubation for 30 min at ambient temperature, 50 μl of K562/VCAM-1 cells was added. The contents of the wells were mixed, and the plates were incubated for 15 h at 37°C under 5% CO2. Syncytium formation was scored by counting syncytia in two random high-power fields (HPFs) from each well after disrupting cell clumps by gentle pipetting and allowing the cells to settle for 45 min (25). Photomicroscopy was carried out on an Olympus CK2 microscope. Syncytium assays with HIV-1-infected MT2 cells and SupT1 or HIV-1-infected U937 cells and MT2 cells were carried out in similar fashion. When HIV-1 syncytia were scored by counting, this was carried out after only 4 to 6 h of incubation since HIV-1 syncytium formation occurs rapidly and considerable cell lysis and low viability are seen after 15 h (24). All syncytium formation assays reported here were carried out at least twice with similar results.
RESULTS
New MAbs block HTLV-1 syncytium formation.
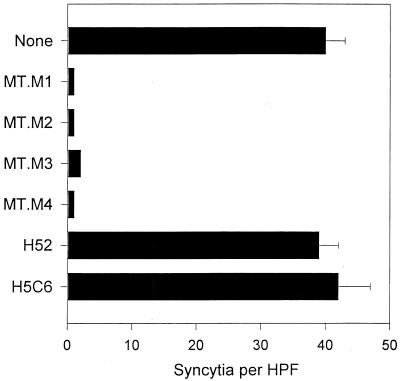
We have described the high fusion activity between HTLV-1-infected cells and cells expressing the adhesion molecule VCAM-1 (25). We wished to identify the structures on the infected cells involved in VCAM-1-mediated cell fusion. A series of new MAbs with anti-HTLV-1 fusion activity were generated by immunizing BALB/c mice with the HTLV-1-infected cell line MT2. The resulting hybridomas were screened by testing their culture supernatants in syncytium assays with MT2 and K562 cells transfected with VCAM-1 (25). Several hybridomas secreted antibodies that blocked syncytium formation. Four antibodies with strong inhibitory activity were chosen for further study, designated MT.M1, MT.M2, MT.M3, and MT.M4. Figure 1 shows a typical result when these antibodies were tested in HTLV-1 syncytium assays. The antibodies reproducibly blocked syncytium formation by more than 90%, and in most cases, inhibition was complete. Control antibodies such as anti-CD18 (H52) and anti-CD63 (H5C6) had no effect (Fig. 1).
FIG. 1.
New MAbs against MT2 cells block HTLV-1 syncytium formation. MAbs produced against MT2 cells (MT.M series) were tested for inhibition of HTLV-1-induced syncytium formation between K562/VCAM-1 and MT2 cells as described in Materials and Methods. MAbs H52 and H5C6 recognize CD18 and CD63, respectively, and were used as isotype-matched negative controls.
New syncytium-inhibiting MAbs recognize class II MHC.
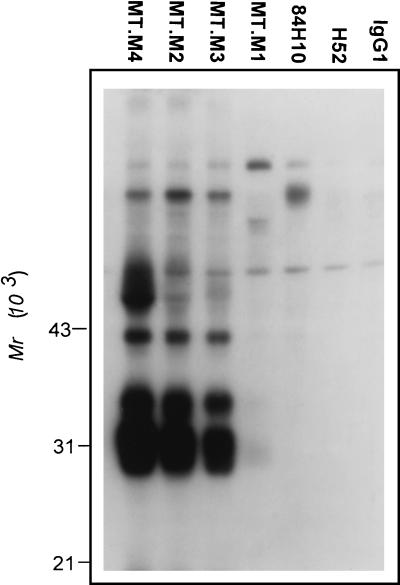
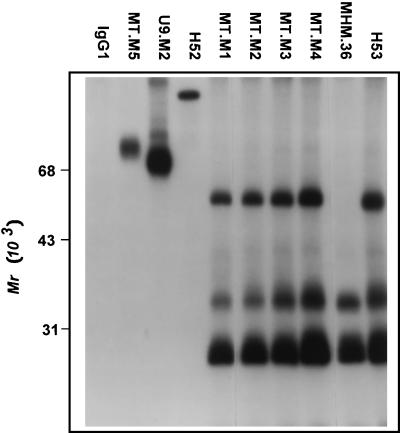
Vectorial radioiodination of MT2 cells and immunoprecipitation analysis were performed to identify the structures recognized by the new antibodies. Figure 2 shows the result of the first study in which the preclearing step was omitted to ensure that any protein complexes would not be missed. As seen in Fig. 2, MT.M2, M3, and M4 MAbs all precipitated strong bands at 28, 33, and 40 kDa. An additional band at approximately 60 kDa is also present, being much stronger in the case of MT.M4. Weak bands at similar molecular weights were apparent in the MT.M1 MAb lane after prolonged exposure. This pattern of precipitated bands suggested that the MAbs might recognize class II MHC molecules. The immunoprecipitation experiment was thus repeated with inclusion of the preclearing step and the addition of well-characterized antibodies against class II MHC. The results are shown in Fig. 3. All four new MAbs precipitated bands in a pattern exactly matching that of the previously described class II antibody H53 (42). The expected α (28 kDa) and β (33 kDa) subunits and stable dimers (60 kDa) are seen in these lanes. A second characterized class II antibody recognized only detergent-sensitive dimers (MHM.36 [35]). Control antibodies against CD54 (MT.M5), CD44 (U9.M2), and CD18 (H52) precipitated bands of appropriate sizes (Fig. 3).
FIG. 2.
Immunoprecipitation with MT.M MAbs from lysates of vectorially iodinated MT2 cells. MT2 cells were surface labeled with 125I, and immunoprecipitation analysis was carried out as described in Materials and Methods. In this experiment, the cell lysate was not precleared with normal rabbit serum and SaC. Control antibodies used were anti-CD54 (84H10), anti-CD18 (H52), and isotype-matched myeloma protein (IgG1). Migration positions of molecular weight standards are indicated.
FIG. 3.
Immunoprecipitation with MT.M MAbs from lysates of vectorially iodinated MT2 cells. MT2 cells were surface labeled with 125I, and immunoprecipitation analysis was carried out as described in Materials and Methods. In this experiment, the cell lysate was precleared with normal rabbit serum and SaC to remove nonspecific binding proteins. Antibody controls consisted of myeloma control (IgG1), anti-CD54 (MT.M5), anti-CD44 (U9.M2), anti-CD18 (H52), and anti-class II MHC (MHM.36 and H53).
The immunoprecipitation studies strongly indicated that the new syncytium-inhibiting antibodies recognized class II MHC molecules. To confirm this specificity, the antibodies were tested by flow cytometry against a panel of mouse epithelial (L) cells transfected with human MHC class II genes (32). The results are shown in Table 1. As expected, the MHM.33 MAb bound to all three transfected lines, indicating a specificity for HLA-DP, DQ, and DR as previously shown (42). Two of the new antibodies, MT.M2 and MT.M3, showed a similar binding pattern. Two of the antibodies, MT.M1 and MT.M4, recognized DP and DR but did not bind to DQ (Table 1). None of the antibodies bound to control L cells, and all bound to CEMx174 cells, a human EBV-transformed B-cell–T-cell hybridoma which expresses high levels of class II MHC molecules (19). These data confirmed that the new HTLV-1 syncytium-blocking MAbs recognized class II molecules on MT2 cells.
TABLE 1.
HTLV-1 syncytium-inhibiting MAbs recognize class II MHCa
| MAb | Cell line:
|
||||
|---|---|---|---|---|---|
| CEMx174 | L25.4 (DP4α DQ4β) | L54.4 (DQ7α DQ3.2β) | L89.2 (DRα DR4β) | L cells | |
| MHM.33 | 100.0 | 97.0 | 73.3 | 92.4 | 0.5 |
| MT.M1 | 100.0 | 99.1 | 2.8 | 95.5 | 0.6 |
| MT.M2 | 99.7 | 88.9 | 21.6 | 91.3 | 1.0 |
| MT.M3 | 100.0 | 100.0 | 95.6 | 99.6 | 1.5 |
| MT.M4 | 100.0 | 99.0 | 3.1 | 86.7 | 0.6 |
MAbs were tested for binding to the indicated cell lines as described in Materials and Methods. Data shown are the percentages of cells staining positive with a MAb relative to an isotype-matched myeloma IgG used as a negative control. MAb MHM.33 recognizes a framework determinant on human class II MHC molecules.
Class II MHC molecules are expressed at high levels on HTLV-1-infected cells.
The expression of MHC class I and II molecules on HTLV-1-infected cell lines MT2 and MJ was examined by flow cytometry. Assuming that MAbs bind to surface proteins with similar affinities and that the secondary polyclonal antibody binds equally well to all primary MAbs, flow cytometry under saturating conditions provides good estimates of the relative expression of membrane proteins. As seen in Table 2, expression of MHC class I and II proteins was extremely high on both cell lines. Indeed, this assay was carried out under saturating conditions and indicated that MHC molecules were expressed at levels approximately 50 times higher (mean channel fluorescence [MCF], ∼1,000 versus 20) than those of a typical membrane protein such as CD63 (MAb H5C6). The only exception was class I MHC on MJ cells, where expression was approximately one-half that of class II MHC. These data are in agreement with other studies showing high expression of MHC antigens on HTLV-1-transformed cells (33, 55). The results also confirm that the K562/VCAM-1 cells express neither class I nor class II molecules. This indicates that antibodies against MHC antigens block syncytium formation at the level of the HTLV-1-infected cells only.
TABLE 2.
MJ cells also express high levels of MHC class II moleculesa
| MAb | MT2
|
MJ
|
K562/VCAM-1
|
|||
|---|---|---|---|---|---|---|
| % Pos | MCF | % Pos | MCF | % Pos | MCF | |
| H53 | 99.8 | 976 | 99.9 | 949 | 1.4 | 14 |
| MT.M1 | 99.7 | 768 | 99.9 | 672 | 0.3 | 15 |
| MT.M2 | 99.3 | 876 | 99.9 | 921 | 9.6 | 19 |
| MT.M3 | 99.4 | 893 | 99.9 | 985 | 8.0 | 19 |
| MT.M4 | 99.6 | 948 | 99.8 | 887 | 5.8 | 18 |
| H5C6 | 89.4 | 21 | 99.8 | 39 | 94.2 | 62 |
| MHM.5 | 99.3 | 923 | 99.9 | 441 | 0.9 | 14 |
The indicated MAbs were tested for binding to MJ, MT2, and K562/VCAM-1 cells by flow cytometry as described in Materials and Methods. Data shown are percent positive cells relative to an isotype-matched myeloma IgG (% Pos) and the intensity of staining, shown as MCF. MAbs H53, H5C6, and MHM.5 recognize class II MHC, CD63, and class I MHC, respectively.
Inhibition of syncytium formation by class I and II MHC antibodies is a common feature of HTLV-1-infected cells.
Class I and II MHC MAbs were tested for their effect on syncytium formation by the MT2 and MJ cell lines in parallel. The results are shown in Fig. 4. Both MT.M2 and MT.MT4 inhibited syncytium formation by both MT2 and MJ cells by 90% or more. Similar results were obtained with other class II MHC antibodies as well. The class I MHC antibody MHM.5 inhibited fusion of MT2 cells extremely well but only partially inhibited fusion of MJ cells (Fig. 4). Interestingly, this antibody bound to high levels on MT2 cells (MCF, 923) but less so on MJ cells (MCF, 441). These results showed that inhibition of HTLV-1 syncytium formation by MHC-specific antibodies was not restricted to MT2 cells.
FIG. 4.
Inhibition of HTLV-1 syncytium formation by class II MHC-specific MAbs is not limited to MT2 cells. Class I (MHM.5) and class II (MT.M2 and MT.M4) MAbs were tested for inhibition of syncytium formation between K562/VCAM-1 cells and either MT2 or MJ cells as described in Materials and Methods. Data reported are numbers of syncytia as percentages of the positive controls (fusion in the absence of inhibitor). Control mean syncytium levels for MT2 and MJ cells were 31 and 18, respectively. Numbers shown next to the bars are intensities of MAb staining (MCF) of the corresponding cell line (MJ or MT2) in flow cytometry studies which were run on the same cell preparations used in the syncytium assays. Anti-CD18 MAb H52 was used a negative control.
Class II MHC molecules are not associated with HTLV-1 gp46.
The fact that the antibodies blocked fusion at the level of the infected cells suggested a possible association between MHC molecules and virus glycoproteins. Flow cytometry analysis under saturating conditions indicated that the MHC molecules outnumbered the gp46 molecule by as much as 30 to 1 (MCF, ∼1,000 and ∼30, respectively). Thus, it might be possible for every cell surface gp46 molecule to associate with multiple MHC molecules. Coimmunoprecipitation analysis was performed as described in Materials and Methods with CHAPS detergent. In several experiments, the anti-gp46 antibody precipitated the expected virus band but not bands corresponding to proteins of MHC class I (40 and 12 kDa) or MHC class II (28 and 33 kDa) as seen in control samples in which MHC antibodies were used (data not shown).
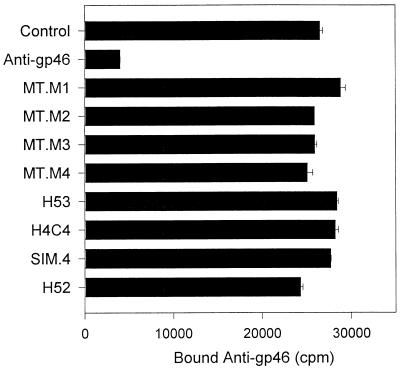
In another approach to demonstrating an association between the glycoproteins of HTLV-1 and MHC molecules, anti-gp46 MAb was radioiodinated and it competed with MHC antibodies for binding to MT2 cells. As shown in Fig. 5, binding of labeled anti-gp46 was blocked by unlabeled anti-gp46 but not by any of the competing MHC MAbs. Antibodies against other surface proteins including CD4 (SIM.4), CD18 (H52), and CD44 (H4C4) also failed to block binding of 125I-anti-gp46.
FIG. 5.
Anti-class II MHC MAbs do not block binding of anti-gp46 MAb. Purified anti-gp46 MAb from Cellular Products was iodinated with 125I-Bolton-Hunter reagent and used in MAb competitive binding assays as described in Materials and Methods. Approximately 10 ng (100,000 cpm) of radiolabeled anti-gp46 was added to 200,000 MT2 cells in the presence of the indicated unlabeled MAbs (2 μg each, except for unlabeled anti-gp46, for which 1 μg was used). Data shown are mean bound counts per minute of radiolabeled anti-gp46 in duplicate tubes.
Taken together, the coimmunoprecipitation and competitive binding studies indicate that HTLV-1 glycoproteins and MHC molecules are not closely associated on the cell surface.
MHC antibodies do not have a generalized effect on cell fusion.
Binding of MHC antibodies to HTLV-1-infected cells may have blocked syncytium formation by a generalized nonspecific effect on the cell membrane or cytoskeleton. Such an effect by the antibodies would result in inhibition of fusion mediated by viral glycoproteins other than HTLV-1 gp46/gp20. To test this idea, MT2 cells already infected with HTLV-1 were superinfected with HIV-1 (MN strain), yielding a single cell line expressing both HIV-1 and HTLV-1 glycoproteins. This cell line, MT2/HIV-1MN, forms syncytia when mixed with VCAM-1-positive cells through HTLV-1 gp46 or when mixed with CD4/CXCR4-positive cells through HIV-1 gp120. As shown in Fig. 6, class II MHC antibody MT.M1 completely blocked fusion between MT2/HIV-1MN and K562/VCAM-1 cells but had no effect on fusion between MT2/HIV-1MN and SupT1 cells (VCAM-1 negative, CD4/CXCR4 positive). SupT1 cells did not fuse to MT2 cells in the absence of HIV-1 infection. Similar results were obtained with the other MHC class II antibodies and the class I MHC antibody (data not shown). Fusion between MT2/HIV-1MN and SupT1 cells was completely blocked by anti-CD4 antibody SIM.7 (data not shown). The amount of gp46 on MT2/HIVMN cells was similar to that of HIV-1 gp120 (MCF, 46 versus 60 by flow cytometry), indicating that differential inhibition of HTLV-1 syncytium formation was not due to lower HTLV-1 envelope protein expression. The above results showed that blockade of syncytium formation by anti-MHC antibodies was specific to HTLV-1.
FIG. 6.
Anti-class II MHC MAb blocks HTLV-1 syncytium formation but not HIV-1 syncytium formation. HTLV-1-infected MT2 cells were chronically infected with HIV-1MN. MT2/HIV-1MN cells were mixed with K562/VCAM-1 or SupT1 cells in the presence of class II MHC MAb MT.M1. Identical results were obtained with all other class II MHC MAbs tested. HIV-1 syncytium formation was completely blocked by anti-CD4 MAb (SIM.7) (data not shown). SupT1 cells do not fuse with MT2 cells that do not express HIV-1 glycoproteins.
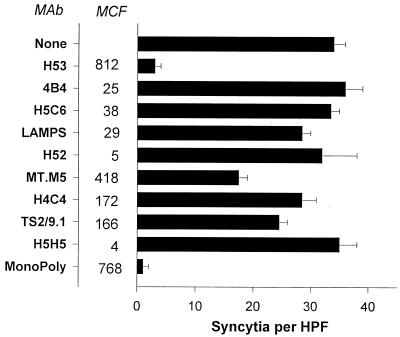
HTLV-1 syncytium formation is blocked by a monoclonal “polyclonal” antibody.
An earlier assay showed that class I antibody MHM.5 bound to MJ cells approximately half as well as it did to MT2 cells and blocked syncytium formation by MJ cells much less than it did syncytium formation by MT2 cells. This result suggested that inhibition of HTLV-1 syncytium formation by MHC antibody might be occuring through a steric or protein crowding mechanism. Such a model predicts that any antibody regardless of specificity that binds to the HTLV-1-infected cells in sufficient numbers should block HTLV-1 syncytium formation. To test this idea, a monoclonal polyclonal antibody was generated by combining several MAbs with distinct specificities, each of which bound to the cells at low to moderate levels. The monoclonal polyclonal antibody (MonoPoly) and each of the individual antibodies were then tested for their effect on HTLV-1 syncytium formation. As shown in Fig. 7, MAbs against CD29 (4B4), CD63 (H5C6), CD107a/b (LAMPS), CD18 (H52), CD44 (H4C4), CD58 (TS2/9.1), and CD43 (H5H5) all bound to the MT2 cells with MCF values of less than 200 and had no effect on HTLV-1 syncytium formation. Anti-CD54 (MT.M5) bound with an MCF of 418 and blocked fusion by approximately 50%. The antibody against MHC class II bound with an MCF value of 812 and blocked syncytium formation by 90%. The MonoPoly bound with an MCF value of 768, and it too blocked syncytium formation by more than 90%, even though none of the individual MAbs other than MT.M5 had any effect on fusion. A control mouse IgG at a concentration (250 μg/ml) greater than the total IgG concentration in the MonoPoly antibody had no effect on fusion (data not shown). The MonoPoly antibody was also tested for inhibition of fusion mediated by HIV-1. MT2 cells chronically infected with HIV-1MN were mixed with either SupT1 or K562/VCAM-1 cells to allow HIV-1- and HTLV-1-mediated syncytium formation, respectively, to occur. As seen in Fig. 8, like the MHC class I and II antibodies (MHM.5 and H53) the MonoPoly antibody blocked HTLV-1 syncytium formation but had no effect on HIV-1-driven fusion, which was completely blocked by an anti-CD4 MAb (SIM.7). These results confirmed that for inhibition of HTLV-1 syncytium formation the antibody specificity was not important but only the level of antibody binding to the infected cells.
FIG. 7.
HTLV-1 syncytium formation is blocked by a monoclonal polyclonal antibody. The indicated MAbs were examined for binding to MT2 cells by flow cytometry under saturating conditions. Their specificities are given in Materials and Methods. The intensity of staining of each antibody is shown as MCF. All of the MAbs shown except H53 were mixed to produce the monoclonal polyclonal antibody (MonoPoly). The concentration of each MAb in the MonoPoly preparation was equal to its concentration when used alone (10 to 20 μg/ml). The antibodies were also tested for inhibition of syncytium formation between MT2 and K562/VCAM-1 cells as described in Materials and Methods. A control mouse IgG at a concentration (250 μg/ml) greater than the total IgG concentration of the MonoPoly antibody had no effect on syncytium formation.
FIG. 8.
Monoclonal polyclonal antibody blocks HTLV-1 syncytium formation but not HIV-1 syncytium formation. The monoclonal polyclonal (MonoPoly) antibody described for Fig. 7 was tested for inhibition of HTLV-1 and HIV-1 syncytium formation as described in Materials and Methods. For this purpose, MT2/HIV-1MN cells were mixed with either SupT1 or K562/VCAM-1 in the presence of the indicated antibodies. The antibodies were also tested for binding to MT2/HIV-1MN cells by flow cytometry. The intensity of staining is shown as MCF. MAb SIM.7 is specific for human CD4.
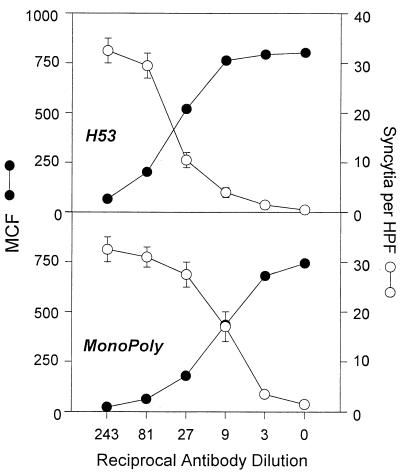
Inhibition of HTLV-1 syncytium formation by MHC MAbs correlates with number of antibody molecules bound to infected cells.
The prozone or protein crowding model of HTLV-1 syncytium inhibition also predicts that there should be a correlation between the number of antibody molecules bound and blockade of cell fusion. This idea was tested by carrying out a dose-response experiment with anti-class II MHC and the MonoPoly antibodies. The results are shown in Fig. 9. The number of syncytia formed between MT2 and K562/VCAM-1 cells was inversely proportional to the MCF of antibody binding to MT2 cells. In the case of both the class II MAb and the MonoPoly antibody, the threshold for inhibition of fusion seemed to be an MCF value of approximately 500 or 75% maximal binding. These results support a model for antibody inhibition of HTLV-1 cell fusion based on protein crowding or prozone effects.
FIG. 9.
Dose-response effect of anti-class II MHC and monoclonal polyclonal antibodies on HTLV-1 syncytium formation. The MonoPoly antibody and anti-class II MHC MAb H53 were tested for inhibition of HTLV-1 syncytium formation at the indicated dilutions. The antibodies were tested at these same dilutions for staining of the MT2 cells used in the syncytium assay. Intensity of antibody staining (MCF) is plotted versus the mean syncytia per HPF at the indicated antibody dilutions. H53 was used in the form of hybridoma culture supernatant. Syncytium formation in the absence of antibody was 35 ± 4 per HPF.
DISCUSSION
Although elegant work by several investigators has found good candidates, the cellular receptor for HTLV-1 has still not been definitively identified (1, 13, 14, 30, 31, 47). In an earlier study, we reported that the adhesion molecule VCAM-1 could support syncytium formation mediated by HTLV-1 (25). We were unable to inhibit this effect by blocking VLA-4, the major VCAM-1 counterreceptor, on the HTLV-1-infected cells. We therefore set out to identify possible nonintegrin molecules on the infected cells that might interact with VCAM-1. We immunized BALB/c mice with HTLV-1-infected cells and generated a panel of four MAbs that inhibited HTLV-1-mediated cell fusion. Immunoprecipitation studies and binding assays on transfected mouse cells showed that all four antibodies recognized class II MHC molecules on the surface of the HTLV-1-infected cells. Previously generated well-characterized MAbs against class II MHC were also found to block HTLV-1 syncytium formation. Since the K562 cells used as fusion partners in syncytium assays do not express class II MHC, it was clear that the antibodies mediated their inhibitory effect at the level of the HTLV-1-infected cells.
There are no previous reports of VCAM-1 binding to class II MHC antigens, and no evidence has been found for such an interaction. I therefore examined the possibility that HTLV-1 gp46 may be associated with class II MHC molecules on HTLV-1-infected cells. Such an association might have allowed anti-MHC class II antibodies to block the binding of gp46 to its receptor(s). Coimmunoprecipitation and antibody competition assays indicated that class II MHC molecules were not associated with HTLV-1 glycoproteins on virus-infected cells. However, it is possible that gp46-class II MHC interactions do occur but are disrupted by even the mild detergents used in this study. In a previous study, the results demonstrated that binding of MHC class II-specific MAbs to monocytes induced transmembrane signals including a rise in intracellular Ca2+ levels (42). We therefore wondered whether the anti-class II MHC MAbs blocked HTLV-1 syncytium formation by a nonspecific effect on cell membrane function or organization. The antibodies were tested for inhibition of HTLV-1- and HIV-1-mediated cell fusion by MT2 cells coinfected with both HTLV-1 and HIV-1 viruses. The class II antibodies blocked HTLV-1 syncytium formation but not HIV-1 mediated fusion, indicating that the antibodies did not have a generalized effect on the cell membrane that prevented fusion. Because of the poor infectivity of cell-free HTLV-1 particles, we have been unable to test the effect of class II MHC antibodies on HTLV-1 infection by free particles. Interestingly, Arthur and colleagues have presented evidence that cell-free HIV-1 may be neutralized by class II MHC antibodies (3). We have been unable to demonstrate neutralization of cell-free HIV-1 by class II MHC antibodies (data not shown).
The extremely high levels of MHC antigen expression on HTLV-1-infected cells suggested that MAb inhibition of fusion might occur through steric hindrance of HTLV-1 envelope glycoproteins. Class I MHC molecules were found to be expressed at levels similar to those of class II MHC proteins, and an antibody against class I MHC also blocked HTLV-1 syncytium formation. Moreover, a polyclonal antibody made by mixing several noninhibitory MAbs bound to the HTLV-1-infected cells at levels similar to those of class II MHC MAbs, and it too blocked HTLV-1 but not HIV-1 syncytium formation. Dose-response experiments showed that inhibition of HTLV-1 syncytium formation correlated with the absolute level of antibody binding regardless of the antibody specificity. These data provide strong evidence that cell fusion induced by HTLV-1, the principal mode of transmission for this virus, is subject to inhibition by protein crowding or prozone effects.
Previous work on the expression of MHC class II molecules by EBV-transformed cells showed that these cells express more than 5 million MHC molecules per cell by Scatchard analysis (20). MT2 cells were compared to the same EBV-transformed cells used in the aforementioned studies in saturating MAb binding assays and found to have equivalent amounts of surface MHC class II molecules (data not shown). This finding is consistent with previous work showing that HTLV-1 infection results in increased expression of several host cell membrane proteins including adhesion molecules and MHC proteins (33, 55, 58). The footprint of an antibody Fab region is approximately 5,000 to 7,000 Å2 (2, 44). This means that class II MHC, class I MHC, or the MonoPoly antibodies physically cover as much as 350 μm2 of the cell surface when bound at saturating levels. Thus, these antibodies may cover more than 95% of the total surface area of a typical large lymphoid cell line such as MT2, which ranges from 10 to 12 μm in diameter. Cell membrane projections such as pseudopodia may substantially increase the total surface area of the cells such that the area covered by the antibodies may be somewhat smaller than the calculated percentage. It is reasonable to assume that if a region of the membrane were inaccessible to the antibodies it would also be inaccessible to membrane from other cells. Thus, antibodies may not need to completely cover the cell membrane but only accessible surfaces to mediate steric blockade of HTLV-1 fusion. Under saturating conditions, antibodies are bound univalently to cell surface proteins, leaving the Fc and unbound Fab regions free to flop around at the cell surface. This probably increases the steric hindrance effects of these large globular proteins.
The HTLV-1 envelope glycoprotein is relatively small and hydrophobic especially compared to that of other human retroviruses such as HIV-1 (7). This may explain why HTLV-1 is subject to prozone blockade of syncytium formation but HIV-1 is not (data not shown). Many critical cellular receptors involved in immune function are also small molecules projecting only a short distance from the cell membrane and in some cases not extending beyond the glycocalyx (50, 52). Interactions of these molecules with their counterreceptors are facilitated by adhesion molecules which take on an extended rod conformation that allows them to easily find their ligands (50, 51). The relative smallness or shortness of the HTLV-1 glycoprotein could be similarly compensated for by a cellular receptor that assumes an extended rod conformation and projects far above the cell membrane. Such a molecule could presumably reach through the nonspecific protein barrier represented by anti-MHC antibodies as did the anti-gp46 MAb or as did CD4 in binding to HIV-1 gp120. The results obtained in the current study imply that the cellular receptor for HTLV-1 on the target cells used in this system is not such a molecule. Another possibility raised by the data is that, unlike HIV-1 gp120 which binds to its receptor with a very high affinity, HTLV-1 gp46 may bind to its receptor(s) with a very low affinity. In such a case, even the increased avidity afforded by thousands of gp46 molecules on the cell surface may not be sufficient to overcome the steric barrier created by MHC antibodies.
Steric inhibition of HTLV-1 syncytium formation may provide an insight into the dominance of the cell-cell mode of transmission for this virus (8). We demonstrated in a previous study that the cell adhesion molecule VCAM-1, the ligand of integrins VLA-4 and α4β7, can confer sensitivity to HTLV-1 syncytium formation (25). Other investigators have shown that antibodies against ICAM-3, a ligand of integrin LFA-1, can block HTLV-1 syncytium formation by certain cell types (29). There is no evidence that these molecules directly interact with viral proteins. The lack of such interactions along with the fact that at least two distinct adhesion receptor-ligand pairs can affect HTLV-1 syncytium formation (25, 29) indicates that adhesion molecules mediate their effect by facilitating the interaction between gp46 and its cellular receptor. The involvement of cell adhesion molecules in the interaction of gp46 with its receptor supports the model of a “sterically disadvantaged” gp46 molecule. Cell adhesion molecules are known to facilitate interactions between smaller cell surface receptors on T cells and other cell types (50). Among other mechanisms, these molecules appear to trigger changes in membrane topology that result in a clearing away of taller, charged molecules, thus allowing shorter receptors and ligands to find each other (50). HTLV-1 may take advantage of this phenomenon to bind its receptor and promote its transmission by the cell-cell route. Interestingly, HTLV-1 upregulates expression of receptors in three major families of adhesion molecules including selectins (26, 54), integrins (10), and Ig supergene family members (38, 53). This fact supports the notion of an important role for adhesion molecules in the intercellular transmission of HTLV-1.
Other investigators have taken a similar approach to identifying the cellular receptor(s) for HTLV-1 as that taken in this study. MAbs that block HTLV-1 fusion or infection have been produced against a number of cellular structures including members of the large tetraspanner family (29–31). The findings suggest that the results of such studies may need to be reevaluated in the context of the level of cell surface expression of the molecules in question. Data obtained with the MonoPoly antibody indicate that any MAb regardless of specificity that bound to a very highly expressed molecule on HTLV-1-infected cells would test positive for inhibition of HTLV-1 syncytium formation. It thus appears that there are at least three mechanisms by which MAbs can block HTLV-1-mediated syncytium formation: (i) direct blockade of gp46 interaction with its receptor(s), (ii) blockade of accessory adhesion molecules such as VCAM-1 or ICAM-3, and (iii) antibody-mediated protein crowding at the cell surface resulting in indirect blockade of gp46 binding to its receptor(s).
REFERENCES
- 1.Agadjanyan M G, Ugen K E, Wang B, Williams W V, Weiner D B. Identification of an 80-kilodalton membrane glycoprotein important for human T-cell leukemia virus type I and type II syncytium formation and infection. J Virol. 1994;68:485–493. doi: 10.1128/jvi.68.1.485-493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amzel L M, Poljak R J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- 3.Arthur L O, Bess J W, Sowder R C, Benveniste R E, Mann D L, Chermann J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 4.Azorsa D O, Hyman J A, Hildreth J E K. CD63/Pltgp40: a platelet-activation antigen identical to the stage-specific, melanoma-associated antigen ME491. Blood. 1991;78:280–284. [PubMed] [Google Scholar]
- 5.Belitsos P C, Hildreth J E K, August J T. Homotypic cell aggregation induced by anti-CD44(Pgp-1) monoclonal antibodies and related to CD44(Pgp-1) expression. J Immunol. 1990;144:1661–1670. [PubMed] [Google Scholar]
- 6.Clapham P, Nagy K, Cheinsong-Popov R, Exley M, Weiss R A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983;222:1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- 7.Delamarre L, Rosenberg A R, Pique C, Pham D, Callebaut I, Dokhelar M C. The HTLV-1 envelope glycoproteins: structure and functions. J Acquired Immune Defic Syndr Hum Retroviral. 1996;13:S85–S91. doi: 10.1097/00042560-199600001-00015. [DOI] [PubMed] [Google Scholar]
- 8.Delamarre L, Rosenberg A R, Pique C, Pham D, Dokhelar M C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J Virol. 1997;71:259–266. doi: 10.1128/jvi.71.1.259-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhawan S, Streicher H Z, Wahl L M, Miller N, Louie A T, Goldfarb I S, Jackson W L, Casali P, Notkins A L. Model for studying virus attachment. II. Binding of biotinylated human T cell leukemia virus type I to human blood mononuclear cells. Potential targets for human T cell leukemia virus type I infection. J Immunol. 1991;147:102–108. [PubMed] [Google Scholar]
- 10.Dhawan S, Weeks B S, Abbasi F, Gralnick H R, Notkins A L, Klotman M E, Yamada K M, Klotman P E. Increased expression of alpha 4 beta 1 and alpha 5 beta 1 integrins on HTLV-I-infected lymphocytes. Virology. 1993;197:778–781. doi: 10.1006/viro.1993.1656. [DOI] [PubMed] [Google Scholar]
- 11.Ellis S A, Taylor C, Hildreth J E K, McMichael A J. An HLA class I specific monoclonal antibody that fails to bind to all HLA-A antigens. Hum Immunol. 1985;13:13–19. doi: 10.1016/0198-8859(85)90023-0. [DOI] [PubMed] [Google Scholar]
- 12.Essex M. Immunopathogenesis of HTLV. AIDS Res Hum Retroviruses. 1992;8:719–724. [PubMed] [Google Scholar]
- 13.Fukudome K, Furuse M, Imai T, Nishimura M, Takagi S, Hinuma Y, Yoshie O. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1-positive T cells. J Virol. 1992;66:1394–1401. doi: 10.1128/jvi.66.3.1394-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavalchin J, Fan N, Lane M J, Papsidero L, Poiesz B J. Identification of a putative cellular receptor for HTLV-I by a monoclonal antibody, MAb 34-23. Virology. 1993;194:1–9. doi: 10.1006/viro.1993.1228. [DOI] [PubMed] [Google Scholar]
- 15.Gessain A, Gout O. Chronic myelopathy associated with human T-lymphotropic virus type I (HTLV-I) Ann Intern Med. 1992;117:933–946. doi: 10.7326/0003-4819-117-11-933. [DOI] [PubMed] [Google Scholar]
- 16.Gomez M B, Hildreth J E K. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–4632. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg S J. Human retroviruses and demyelinating diseases. Neurol Clin. 1995;13:75–97. [PubMed] [Google Scholar]
- 18.Guo M L, Hildreth J E K. HIV-induced loss of CD44 expression in monocytic cell lines. J Immunol. 1993;151:2225–2236. [PubMed] [Google Scholar]
- 19.Guo M L, Hildreth J E K. HIV acquires functional adhesion receptors from host cells. AIDS Res Hum Retroviruses. 1995;11:1007–1013. doi: 10.1089/aid.1995.11.1007. [DOI] [PubMed] [Google Scholar]
- 20.Hildreth J E. Immune recognition of human cell surface antigens. Ph.D. dissertation. Oxford, United Kingdom: University of Oxford; 1982. [Google Scholar]
- 21.Hildreth J E K, August J T. The human lymphocyte function-associated (HLFA) antigen and a related macrophage differentiation antigen (HMac-1): functional effects of subunit-specific monoclonal antibodies. J Immunol. 1985;134:3272–3280. [PubMed] [Google Scholar]
- 22.Hildreth J E K, Holt V, August J T, Pescovitz M D. Monoclonal antibodies against porcine LFA-1: species cross-reactivity and functional effects of β-subunit-specific antibodies. Mol Immunol. 1989;26:883–895. doi: 10.1016/0161-5890(89)90145-4. [DOI] [PubMed] [Google Scholar]
- 23.Hildreth J E K, Hyman J A. Production and characterization of monoclonal anti-CD18 anti-idiotype antibodies. Mol Immunol. 1989;26:1155–1167. doi: 10.1016/0161-5890(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 24.Hildreth J E K, Orentas R J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 25.Hildreth J E K, Subramanium A, Hampton R A. Human T-cell lymphotropic virus type 1 (HTLV-1)-induced syncytium formation mediated by vascular cell adhesion molecule-1: evidence for involvement of cell adhesion molecules in HTLV-1 biology. J Virol. 1997;71:1173–1180. doi: 10.1128/jvi.71.2.1173-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraiwa N, Hiraiwa M, Kannagi R. Human T-cell leukemia virus-1 encoded Tax protein transactivates alpha 1→3 fucosyltransferase Fuc-T VII, which synthesizes sialyl Lewis X, a selectin ligand expressed on adult T-cell leukemia cells. Biochem Biophys Res Commun. 1997;231:183–186. doi: 10.1006/bbrc.1997.6068. [DOI] [PubMed] [Google Scholar]
- 27.Hiramatsu K, Masuda M, Yoshikura H. Mode of transmission of human T-cell leukemia virus type I (HTLV I) in a promyelocytic leukemia HL60 cell. Int J Cancer. 1986;37:601–606. doi: 10.1002/ijc.2910370420. [DOI] [PubMed] [Google Scholar]
- 28.Hoshino H, Shimoyama M, Miwa M, Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci USA. 1983;80:7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ida H, Eguchi K, Mizokami A, Yamashita I, Origuchi T, Takashima H, Shimada H, Kawabe Y, Nakamura T, Nagataki S. CD18 and CD50(ICAM-3) mAb block human T-cell lymphotropic virus type I (HTLV-I)-induced syncytium formation. In: Schlossman S F, Boumsell L, Gilks W, et al., editors. Leukocyte typing. V. White cell differentiation antigens. Oxford, United Kingdom: Oxford University Press; 1995. pp. 1598–1599. [Google Scholar]
- 30.Imai T, Fukudome K, Takagi S, Nagira M, Furuse M, Fukuhara N, Nishimura M, Hinuma Y, Yoshie O. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol. 1992;149:2879–2886. [PubMed] [Google Scholar]
- 31.Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with CD4 or CD8 in T cells. J Immunol. 1993;151:6470–6481. [PubMed] [Google Scholar]
- 32.Klohe E P, Watts R, Bahl M, Alber C, Yu W, Anderson R, Silver J, Gregersen P K, Karr R W. Analysis of the molecular specificities of anti-class II monoclonal antibodies by using L cell transfectants expressing HLA class II molecules. J Immunol. 1988;141:2158–2164. [PubMed] [Google Scholar]
- 33.Lehky T J, Cowan E P, Lampson L A, Jacobson S. Induction of HLA class I and class II expression in human T-lymphotropic virus type I-infected neuroblastoma cells. J Virol. 1994;68:1854–1863. doi: 10.1128/jvi.68.3.1854-1863.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S-L, Derr D, Hildreth J E K. A monoclonal antibody against a novel 20-kDa protein induces cell adhesion and cytoskeleton-dependent morphologic changes. J Immunol. 1992;149:2549–2559. [PubMed] [Google Scholar]
- 35.Makgoba M W, Hildreth J E K, McMichael A J. Identification of a human Ia antigen that is different from HLA-DR and DC antigens. Immunogenetics. 1983;17:623–635. doi: 10.1007/BF00366130. [DOI] [PubMed] [Google Scholar]
- 36.Mane S M, Marzella L, Bainton D F, Holt V K, Cha Y, Hildreth J E K, August J T. Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys. 1989;268:360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- 37.Markham P D, Salahuddin S Z, Kalyanaraman V S, Popovic M, Sarin P, Gallo R C. Infection and transformation of fresh human umbilical cord blood cells by multiple sources of human T-cell leukemia-lymphoma virus (HTLV) Int J Cancer. 1983;31:413–420. doi: 10.1002/ijc.2910310404. [DOI] [PubMed] [Google Scholar]
- 38.Mori N, Murakami S, Oda S, Eto S. Human T-cell leukemia virus type I tax induces intracellular adhesion molecule-1 expression in T cells. Blood. 1994;84:350–351. . (Letter.) [PubMed] [Google Scholar]
- 39.Nagy K, Clapham P, Cheingsong-Popov R, Weiss R A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients’ sera. Int J Cancer. 1983;32:321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- 40.Nagy K, Weiss R A, Clapham P, Cheingsong-Popov R. Biological properties of human T-cell leukemia virus envelope antigens. In: Gallo R C, Essex M, Gross L, editors. Human T-cell leukemia viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. pp. 121–131. [Google Scholar]
- 41.Orentas R J, Hildreth J E K. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 42.Orentas R J, Reinlib L, Hildreth J E K. Anti-class II MHC antibody induces multinucleated giant cell formation from peripheral blood monocytes. J Leukocyte Biol. 1992;51:199–209. doi: 10.1002/jlb.51.3.199. [DOI] [PubMed] [Google Scholar]
- 43.Poiesz B J, Ruscetti F, Mier J, Woods A, Gallo R. Detection and isolation of type-C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poljak R J, Amzel L M, Phizackerley R P. Studies on the three-dimensional structure of immunoglobulins. Prog Biophys Mol Biol. 1976;31:67–93. doi: 10.1016/0079-6107(78)90005-6. [DOI] [PubMed] [Google Scholar]
- 45.Rowe M, Hildreth J E K, Rickinson A B, Epstein M A. Monoclonal antibodies to Epstein-Barr virus-induced, transformation-associated cell surface antigens: binding patterns and effect upon virus-specific T-cell cytotoxicity. Int J Cancer. 1982;29:373–381. doi: 10.1002/ijc.2910290403. [DOI] [PubMed] [Google Scholar]
- 46.Ruscetti F R. Immunopathology associated with human lymphotropic viruses. Surv Synth Pathol Res. 1985;4:216–226. doi: 10.1159/000156975. [DOI] [PubMed] [Google Scholar]
- 47.Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y. 71-kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J Virol. 1998;72:535–541. doi: 10.1128/jvi.72.1.535-541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saida T, Saida K, Funauchi M, Nishiguchi E, Nakajima M, Matsuda S, Ohta M, Ohta K, Nishitani H, Hatanaka M. HTLV-I myelitis: isolation of virus, genomic analysis, and infection in neural cell cultures. Ann N Y Acad Sci. 1988;540:636–638. doi: 10.1111/j.1749-6632.1988.tb27196.x. [DOI] [PubMed] [Google Scholar]
- 49.Scaravilli F. Neuropathology of HIV-1 and HTLV-1 infection. Bailliere’s Clin Neurol. 1992;1:211–238. [PubMed] [Google Scholar]
- 50.Shaw A S, Dustin M L. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 51.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 52.Springer T A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka Y, Fukudome K, Hayashi M, Takagi S, Yoshie O. Induction of ICAM-1 and LFA-3 by Tax1 of human T-cell leukemia virus type 1 and mechanism of down-regulation of ICAM-1 or LFA-1 in adult-T-cell-leukemia cell lines. Int J Cancer. 1995;60:554–561. doi: 10.1002/ijc.2910600421. [DOI] [PubMed] [Google Scholar]
- 54.Tatewaki M, Yamaguchi K, Matsuoka M, Ishii T, Miyasaka M, Mori S, Takatsuki K, Watanabe T. Constitutive overexpression of the L-selectin gene in fresh leukemic cells of adult T-cell leukemia that can be transactivated by human T-cell lymphotropic virus type 1 Tax. Blood. 1995;86:3109–3117. [PubMed] [Google Scholar]
- 55.Uno H, Matsuoka H, Suzuki M, Tsuda K, Tsubouchi H. Altered expression of class I HLA antigen on peripheral mononuclear cells in patients with adult T-cell leukemia: inverse relationship with natural killer susceptibility. Cancer Epidemiol Biomark Prev. 1995;4:367–372. [PubMed] [Google Scholar]
- 56.Weiss R A. Retroviruses and human cancer. Semin Cancer Biol. 1992;3:321–328. [PubMed] [Google Scholar]
- 57.Weiss R A, Clapham P, Nagy K, Hoshino H. Envelope properties of human T-cell leukemia viruses. Curr Top Microbiol Immunol. 1985;115:235–246. doi: 10.1007/978-3-642-70113-9_15. [DOI] [PubMed] [Google Scholar]
- 58.Xu X, Heidenreich O, Nerenberg M. Role of kinases in HTLV-I transformation. J Investig Med. 1996;44:113–123. [PubMed] [Google Scholar]
- 59.Yamaguchi K, Takatsuki K. Adult T cell leukemia-lymphoma. Bailliere’s Clin Haematol. 1993;6:899–915. doi: 10.1016/s0950-3536(05)80183-0. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto N, Matsumoto T, Koyanagi Y, Tanaka Y, Hinuma Y. Unique cell lines harboring both Epstein-Barr virus and adult T-cell leukaemia virus, established from leukemia patients. Nature. 1982;299:367–369. doi: 10.1038/299367a0. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida M. Tenth anniversary perspectives on AIDS. Host-HTLV type I interaction at the molecular level. AIDS Res Hum Retroviruses. 1994;10:1193–1197. doi: 10.1089/aid.1994.10.1193. [DOI] [PubMed] [Google Scholar]
- 62.Yoshikura H, Nishida J, Kitamura Y, Takaku F, Ikeda S. Isolation of HTLV derived from Japanese adult T-cell leukemia patients in human diploid fibroblasts strain IMR90 and the biological characters of the infected cells. Int J Cancer. 1984;33:745–749. doi: 10.1002/ijc.2910330606. [DOI] [PubMed] [Google Scholar]